Recent progress in ammonia synthesis based on photoelectrocatalysis
Pengyan
Li
a,
Yumin
Liu
 a,
Muhammad Asim
Mushtaq
ab and
Dongpeng
Yan
a,
Muhammad Asim
Mushtaq
ab and
Dongpeng
Yan
 *ab
*ab
aBeijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, and Key Laboratory of Radiopharmaceuticals, Ministry of Education, Beijing Normal University, Beijing 100875, People's Republic of China
bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing, 100029, People's Republic of China. E-mail: yandp@bnu.edu.cn
First published on 27th June 2023
Abstract
Photoelectrocatalytic (PEC) ammonia synthesis from nitrogen and water is a promising approach for energy development and N-neutralization goal under mild conditions. Although significant progress has been made in the past few decades, the mechanisms underlying the synergistic effect between light and electricity are still challenging. One particular line of study is to improve the performances of PEC catalysts, such as selectivity, yield, and stability, etc. Here we review the recent progress in PEC ammonia synthesis. We first provide a systematic description of the driven bias in PEC ammonia processes, involving electrochemical apparatus, photovoltaic voltage, and chemical potential. The various strategies, including vacancy engineering, ion doping, frustrated Lewis pair design, heterojunction construction, cocatalyst loading and single atom synthesis to fabricate new catalysts, are then outlined. The performance and mechanism of PEC N2 reduction are further summarized, followed by the current challenge and future prospects. This would guide both the productiveness and mechanism of NH3 synthesis based on advanced PEC systems.
1. Introduction
Ammonia synthesis provides an effective pathway to obtain fertilizers, medicines, chemicals and explosives from air, which is significant to human survival and industrial development.1,2 In nature, microorganisms depend on Mo–Fe protease to achieve the conversion of atmospheric N2 to multipurpose NH3.3 However, azotase is extremely sensitive to environmental oxygen, so the evolution of rhizobium cultivation technology became the key to industrialization of biological N2-fixing. As a paragon of artificial nitrogen fixation, the Haber–Bosch (H–B) reaction has contributed to the global economy in which Fe-based compounds show excellent catalytic performances under harsh conditions (temperatures: 300–500 °C, pressures: 150–300 atm) with N2 and H2 as feed gases. However, catalytic N2 hydrogenation consumes mass heat, and the production of feedstock H2 derived from methane steam reforming results in serious carbon emissions with more than 1% of global emissions,4 furthermore, the whole H–B craft requires a large plant infrastructure. Therefore, it is essential to find alternative approaches for sustainable N-neutralization under ambient conditions.5–10 Electrocatalysis and/or photocatalysis has become one of the promising strategies for realizing the N cycle due to its cost advantage, environmental friendliness, and significant N2 conversion efficiency.Electrocatalytic N2 reduction in water involves electron-coupled proton transfer along with various product distributions (NH3, N2H2, N2H4, and H2).11–13 Generally, the bias can induce the first electron transfer progress (N2 + e− ↔ N2−: −3.37 V vs. RHE, pH = 14) and the first-H addition reaction (N2 + H+ + e− ↔ N2H+: −3.20 V vs. RHE) that are not favourable thermodynamically, and accomplish continuously the following reduction.14,15 However, the deficient sites and hydrogen evolution reaction (HER) result in minimal yield and low Faradaic efficiency (FE) in electrocatalytic ammonia synthesis.16–19 Most studies have proved that Bi-based materials and layered double hydroxides (LDHs) have the ability to undergo photocatalytic N2 reduction even if the band structures do not meet the requirements of the first electron transfer and the first-H addition, which contribute to considerable N2 activation, accelerated charge separation, and desired electron generation with strong reducibility.20 Currently, the integration of electrocatalysis and photocatalysis serves as an effective way to improve the yield and selectivity of NH3 by virtue of the bias of electrocatalysis and the abundant sites of photocatalysts (Fig. 1).21–23
PEC N2 reduction is a process in which catalysts are excited under irradiation to generate carriers, and then carriers are separated into electrons and holes, finally, electrons migrate rapidly and participate in N2 reduction on the surface sites assisted with the bias. In addition, the photoelectric synergy is reflected in the coexistence of photochemical and electrochemical reactions. Therefore, PEC NH3 synthesis is considered as a promising approach that possesses advantages from photocatalysis and electrocatalysis simultaneously to efficiently complete N2 conversion.24–26 A classical double chamber contains a photocathode (i.e. catalysts), anode (i.e. counter electrode), electrolyte, N2 and proton exchange membrane for PEC N2 fixation. Candidate photocathodes include inorganic semiconductors with suitable bandgaps (g-C3N4, BiOI, TiO2, Cu2O, W18O49)27,28 and metal modifications,29,30 metal–organic frameworks with adjustable structures,31 and single atoms with remarkable active sites.32 In consideration of the fact that the more negative position of the conduction band (CB) minimum and the enhanced capacity of electron reduction are favourable to N2 reduction in the PEC strategy, vacancy engineering, heteroatom-doping, heterostructure construction, and single atom synthesis are promising approaches to improve N2 reduction ability. What's more, the extended light response capacity to the near-infrared region is a potential idea for solar-based economy in PEC N2 reduction.33,34
Chemisorption is a crucial step for N2 reduction because appropriate adsorption can destroy the molecular symmetry and enhance the proton affinity,35 thus promoting N2 hydrogenation. However, the bond energy (941 kJ mol−1) and cleavage energy (410 kJ mol−1) indicate that N2 activation is challenging (Fig. 2a). Many studies have investigated activation mechanisms, such as vacancy engineering,27,36 large specific surface area, transition metal (TM)/non-metal doping,25 and thermal field coupling. The empty d orbital of the TMs can capture lone pair electrons from N2, and the occupied d orbital donates electrons to the π* orbital, thus producing a weak N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond through the ‘acceptor–donor’ route.37 In addition to metals, B-based molecules with empty orbitals and lone pair electrons have similar electronic structures to TMs, so N2 activation could be achieved through the ‘acceptor–donor’ pathway of B-containing catalysts (Fig. 2b and c).37–40
N bond through the ‘acceptor–donor’ route.37 In addition to metals, B-based molecules with empty orbitals and lone pair electrons have similar electronic structures to TMs, so N2 activation could be achieved through the ‘acceptor–donor’ pathway of B-containing catalysts (Fig. 2b and c).37–40
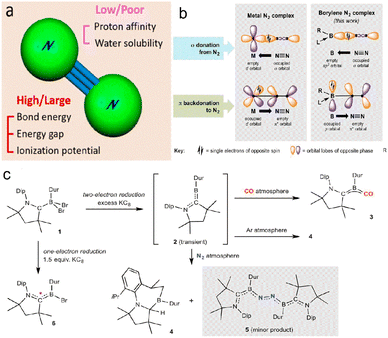 | ||
| Fig. 2 (a) N2 molecular structure,41 Copyright 2020, American Chemical Society. (b) Mechanism of metal activation N2 (left) and boron activation N2 (right), (c) Outcomes of various reduction reactions of dihaloborane adduct 1, including generation of a transient dicoordinate borylene species (2) and its reactionwith dinitrogen. Dip, 2,6-diisopropylphenyl; Dur, 2,3,5,6-tetramethylphenyl.38 Copyright 2018, American Association for the Advancement of Science. | ||
The research studies of electrocatalytic and photocatalytic ammonia synthesis are flourishing;42–45 however, systematic summarizations of PEC N2 reduction are rather rare. Here, we review the various bias-driven photocatalytic ammonia processes, involving electrochemical apparatus, photovoltaic voltage and chemical potential. The various strategies and mechanistic understanding based on calculations and experiments are then outlined, furthermore, we summarize the methods of NH3 quantification, and highlight the necessity of nitrogen source determination.
2. Merging the solar strategy and the electrical strategy for PEC N2 reduction
There are synergistic effects between light and electricity, which promote the PEC progress by taking advantage of both photocatalysis and electrocatalysis.46,47 In particular, the electro-coupled photocatalysis strategy avoids NH3 oxidation, enhances the reduction ability of electrons, and boosts the carrier separation capacity, while photo-assisted electrocatalysis is more outstanding than single electrocatalysis progress, which contributes to the equilibrium of electron distribution under irradiation.48 Besides, the synergistic effect is displayed in the form of the coexistence of photochemical and electrochemical processes, that is, the potential of electrons is determined by bias in electrocatalysis, and the reduction ability of photoelectrons depends on the minimum CB of semiconductors for photocatalysis.492.1 PEC N2 reduction assisted by electrochemical apparatus
Generally, in the PEC process, light is absorbed to initiate the catalytic reaction directly, and the applied bias is used to promote the migration of the photogenerated electrons by changing the band bending.49–51 Upon quenching photogenerated holes located on the valence band (VB),52 regulating the H+ concentration on the surface of the catalyst,27 and increasing the accumulation of electrons in the catalytic site, the catalytic performance of ammonia synthesis would be significantly improved. Electrocatalysis enhanced by photo-excitation and photocatalysis promoted by external bias occur simultaneously in PEC N2 reduction, which make the catalysis more effective than the individual electrocatalytic or photocatalytic nitrogen fixation. It was reported that black phosphorus on indium tin oxide showed extraordinary PEC N2 reduction properties, which benefit from the coexistence of electrocatalysis and photocatalysis.482.2 Solar-driven and chemical bias-driven PEC N2 reduction
Photocatalysts (such as g-C3N4, TiO2, BiVO4, etc.) exhibit inefficient electrocatalytic performance due to poor conductivity, and there is a obvious side reaction (HER) for the electrochemical apparatus-assisted PEC strategy. The current studies have proved that PEC N2 fixation can be carried out without electrochemical apparatus, and the formed anode photovoltage contributes negative potential to N2 reduction in the form of compensation voltage.53–55 The photovoltage from plasma-induced semiconductors shows huge potential for efficient ammonia synthesis in PEC cells without apparatus. Ali et al. described a solar-driven PEC cell over plasmon-assisted Si for atmospheric N2 reduction and produced a high yield (13.3 mg m−2 h−1) under 2 sun radiation for the first time.22 Lee et al. reported that the photovoltage generated from Au modified ordered Si nanoarrays can reduce nitrate to ammonia with a FE of 95.6% at 0.2 V vs. RHE.56 Li et al. fabricated a photovoltaic cell to realize PEC N2 fixation with a high NH3 yield (at the level of μmol h−1 or even mmol h−1) over the TiO2/Au photocathode.57 Inspired by the successful construction of Au-loaded black silicon, Wu's group synthesized Ag-loaded black silicon for PEC nitrogen fixation without external bias,25 and the catalysts achieved a high FE (40.6%) and NH3 yield (2.87 μmol h−1 cm−2). The perovskite-based photocathode (TiOx/CdS/Cu2ZnSnS4) achieved NH3 production from NO3− reduction with a FE of 89.1% (Fig. 3).58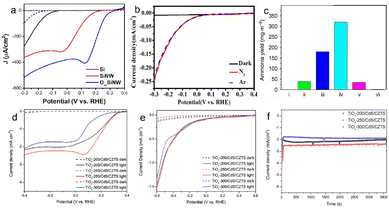 | ||
| Fig. 3 The enhanced current density or improved NH3 yield under light irradiation compared to that in the dark. (a) J–V plots of planar Si (black), Si nanowire (red), and highly ordered Si nanowire (blue) photocathodes for NO3− reduction in Ar-filled K2SO4 with KNO3. Dashed lines represent dark current,56 Copyright 2022, Wiley-VCH. (b) ammonia yield on various substrates: (i) P-type Si, (ii) black Si, (iii) Au/black Si, (iv) Au/black Si/Cr, and (v) Au/Si/Cr under photo-radiation, (vi) Au/black Si/Cr under dark conditions,22 Copyright 2016, Nature Publishing Group. (c) Linear sweep voltammetry (LSV) of the Ag/black Si electrode under N2, Ar and dark conditions,25 Copyright 2020, American Chemical Society. (d) The LSV of TiOx/CdS/Cu2ZnSnS4 in KNO3 and H2SO4 solution, (e) the LSV of TiOx/CdS/Cu2ZnSnS4 and in K2SO4 solution, (f) the chronoamperometry curves of TiOx/CdS/Cu2ZnSnS4 with different spray coating temperatures at 0 V vs. RHE.58 Copyright 2022, Wiley-VCH. | ||
Photocatalytic N2 fixation is completed under the action of chemical bias induced by pH. Oshikiri's group was committed to research chemical bias-driven PEC N2 fixation over Au/SrTiO3 to improve NH3 selectivity.30,59 The photo-excited hot electrons from Au were transferred to the CB of SrTiO3 to induce nitrogen reduction, and resulted in a hole on the SrTiO3 surface for the oxidation reaction. There is an accelerated reaction under the action of chemical bias that was generated when the anode potentials move negatively and the cathode potentials move positively.
3. Synthesis and performance of advanced catalysts for PEC N2 reduction
3.1 Vacancy engineering
Thermal vibration may cause atoms to deviate from the equilibrium position or escape from the surface lattice, and form vacancies.60 As one of the most common defects on atomic-scale surfaces, vacancies can promote charge separation, anchor single atoms, and act as adsorption sites by breaking N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N.61–63 Therefore, the creation of vacancies significantly improved PEC performance for ammonia synthesis.
N.61–63 Therefore, the creation of vacancies significantly improved PEC performance for ammonia synthesis.
The methods for constructing oxygen vacancies have been widely studied, and the nitrogen fixation performance has been significantly improved. BiOF, BiOCl, BiOBr and BiOI are metallic compounds consisting of a [Bi2O2]+ layer and a F/Cl/Br/I atomic layer through Van Der Waals interaction. The induced dipole action between the layered structures is favourable to form an electric field, thus passivating the recombination of photogenerated carriers.65 Due to improved electronic availability, the Ovs located on the BIOX (X = F, Cl, Br, I) surface is used as a reaction site to facilitate N2 reduction.66 Bai et al. synthesized Ovs-BiOI photocathodes by the in situ electrodeposition method (Fig. 4a),24 and the adsorption capacity of R-BiOI to N2 was significantly enhanced after introducing Ovs (Fig. 4b). The ammonia production rate is 1400 μmol m−2 h−1 (Fig. 4c) at 0.4 V vs. RHE, which is 1.3 times higher that of the anaerobic catalyst. Similarly, Lin et al. prepared an Ovs-BiOBr/TiO2 photoelectrode, and the Ovs elevated the capacity of capturing photoelectrons. The bandgap of Ovs-BiOBr was reduced to 2.76 eV, indicating that Ovs can broaden the light absorption range (Fig. 4d). The Ovs is in favor of the formation of quasi-continuous defects that transport electrons under low-energy light irradiation, and Ovs-BiOBr/TiO2 increases the NH3 yield, which is 3.3 fold of the BiOBr/TiO2 photocathode.27 Mao et al. prepared LixMoO3 nanosheets with Ovs through the lithiation strategy, and there are more abundant Ovs and Mo5+ in LixMoO3 than in MoO3 (Fig. 4e).64 Density functional theory (DFT) calculations confirmed that LixMoO3 nanosheets were favourable for the activation of N2 and the formation of *N2H (Fig. 4f). The experiments confirm that the effective NH3 yield of LixMoO3 nanosheets is 3.48 μg cm−2 h−1, 9 times that of MoO3 nanosheets. The LDH systems with an abundance of surface oxygen vacancies or coordinatively unsaturated metal cations in ammonia synthesis were popular in N2 reduction. Zhang et al. demonstrated a simple pretreatment of ZnCr-LDH, ZnAl-LDH, and NiAl-LDH nanosheets with aqueous NaOH, thus enhancing the concentration of oxygen vacancies to significantly improve photocatalytic activity for N2 reduction to NH3.67
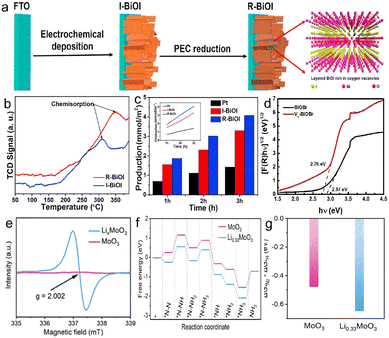 | ||
| Fig. 4 (a) Schematic diagram of the synthesis process, of the I-BiOI/R-BiOI, (b) N2-TPD profiles of the I-BiOI/R-BiOI, (c) production rate of ammonia,24 Copyright 2019, Elsevier. (d) the bandgap of OVS-BiOBr and BiOBr,27 Copyright 2022, Elsevier. (e) EPR of MoO3 and LixMoO3, (f) free energy on oxygen vacancies (OVS) of MoO3 (010) and Li0.33MoO3 (010), (g) Gibbs free energy difference between *N2 and *H over MoO3 (010) and Li0.33MoO3 (010).64 Copyright 2022, Elsevier. | ||
Nitrogen vacancies (Nvs) exhibit enhanced performance for PEC N2 reduction. Li et al. used 3-amino-1,2,4-triazole treated with NaOH as a precursor to prepare g-C3N5 with Nvs,68 and the Nvs realized charge separation by accepting electrons of g-C3N5. BiOBr/g-C3N5 heterostructures with Nvs are synthesized to realize the double-electron transfer mechanism (DETM). The DETM delays the recombination of carriers to ensure high-quality electrons, which is manifested through increased ammonia yield (BiOBr: 2.5 μg mg−1 h−1; Nvs-g-C3N5: 14.8 μg mg−1 h−1, Nvs-g-C3N5/BiOBr: 29.4 μg mg−1 h−1).
3.2 Heteroatom doping
Metal/non-metal ion doping could adjust the electronic structure, redistribute the electron density, and construct surface defects.69,70 The unoccupied orbitals of the TMs capture lone pair electrons of N2 molecules due to matched orbital energy and symmetry, while the occupied d orbitals donate electrons in reverse to the anti-bonding orbitals of the N2 molecule. Therefore, the strong triple bond of N2 is weakened when the TM–N σ-bond is formed, thus promoting the activation of N2. The most studied metal doping for photocatalytic nitrogen fixation is an iron ion with abundant unfilled 3d orbitals, variable valence and considerable cost. Schrauzer et al. improved the nitrogen-fixing activity of TiO2 by doping Fe, Co, Mo or Cr, and found that 2%Fe-doped TiO2 exhibited the best performance in photocatalytic NH3 synthesis.71 Vu et al. synthesized Fe-W18O49 nanorods with Au modification by two-step approach, in which the Fe existed in the form of Fe3+/Fe2+(Fig. 5a).21 Fe-W18O49 enhanced light absorption ability and improved the separation capacity of electron–hole pairs, and achieved a high yield of NH3 (9.82 μg h−1 cm−1) under PEC conditions.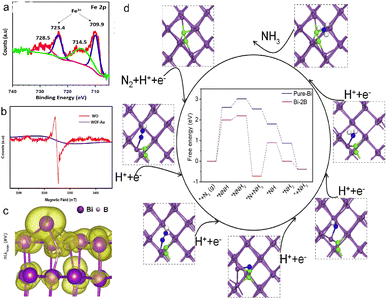 | ||
| Fig. 5 (a) Fe 2p XPS of WOF-Au, (b) EPR of WO and WOF-Au,21 Copyright 2020, American Chemical Society. (c) Partial charge density of the Bi-2B (012) facet, (d) N2 reduction progress on Bi-2B (012).72 Copyright 2020, Elsevier. | ||
The doping of non-metallic atoms (such as B, P, N, etc.) is used to increase the VB position and reduce the bandgap through orbital hybridization or defect engineering.73 Furthermore, non-metallic doping avoids the formation of recombination centers in N2 reduction.74,75 Xu et al. synthesized B-doped Bi from layered BiOBr nanosheets with sodium borohydride (NaBH4) as a reductant,72 and the injection of B atoms enhanced the polarization of Bi (012), and Bi provided electrons to the neighbouring B atoms, resulting in the change of the Bi oxidation state and surface geometry (Fig. 5c). The Bi surface with a high curvature promoted N2 adsorption, and the accumulation of electrons on Bi facilitated the entry of electrons into the anti-bonding orbital of *NNH, thus promoting the elongation of the N–N bond of *NNH. The energy barrier of the potential-determining step was reduced to 2.00 eV, showing that B doping had a positive role in N2 reduction (Fig. 5d). The B-doped Bi showed excellent PEC N2 reduction performance (NH3 yield: 29.2 mg gcat.−1 h−1), far exceeding that of bismuth (10.6 mg gcat.−1 h−1).
As a burgeoning class of two-dimensional materials, graphene is sought in the field of photocatalysis and electrocatalysis. Nitrogen doping can regulate the optical bandgap of graphene to endow favourable optical and electrical features through expanded π electron delocalization.76 The introduction of nitrogen affects both the crystal structure and electronic structure of graphene, in addition, the polarity, electronic donor property, conductivity and chemical stability of graphene can be improved.77,78 Nitrogen doping into carbon supporter generates regulated charge density, resulting in complex reactions related to the multi-proton and electron transfer in N2 reduction. Meanwhile, studies have shown that the graphene lattice that is rich in nitrogen dopants contributes to the formation of n-type semiconductors.79 Generally, nitrogen doped reduced graphene oxide (RGO) shows potential for p–n heterojunction construction, which is conducive to the formation of an electric field. Paramanik et al. constructed the composite of CoTiO3 and N-RGO, where an electron is confined in the atomic layer when N-RGO grows on the surface of CoTiO3. In addition, N-RGO/CoTiO3 achieves a shorter diffusion distance of electrons, rapid charge transfer and small interface resistance.80 In addition, the synergistic interaction between the rod-like structure of CoTiO3 and the rough surface of N-RGO contributes to the overall improvement of the catalytic performance. The optimized 1N-RGO/CoTiO3 has a quenching rate of 54.89% for photogenerated carriers, and possesses an electron lifetime of 3.0 ms. The photocatalytic and PEC activities are more than 1.7 mmol−1 h−1 and 16 μg cm−1 h−1, respectively.
Although nonmetallic doped catalysts have improved performance, the mechanism and stability still need to be further explored in view of the fact that the introduced elements may become the quenching sites of electrons and holes,62 so it is indispensable to study the stabilization strategy for doping non-metallic elements.
3.3 Frustrated Lewis pairs (FLPs)
Metal-free catalytic ammonia synthesis with high selectivity and ideal yield faces challenges until the breakthrough of N2 activation based on the frustrated Lewis pairs (FLPs) in 2018.81 FLPs with an electrophilic acid and nucleophilic base are favorable for the breaking of N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N through the electron transfer between the unoccupied orbitals of electrophilic acid and the nonbonding orbitals of nucleophilic base (Fig. 6a). Stephan et al. reported the space blockage in inhibiting the capture of N2 in the synthesis of Ph2CN2B(C6F5)3 with carbene, N2 and borane as reactants.82 Dai et al. presented boron carbon nitride (BCN) with the abundant unsaturated B (Lewis acid sites) and N atoms (base sites), and the results showed that FLPs adsorb N2 molecules to form six membered ring intermediates, which reduces the energy of N
N through the electron transfer between the unoccupied orbitals of electrophilic acid and the nonbonding orbitals of nucleophilic base (Fig. 6a). Stephan et al. reported the space blockage in inhibiting the capture of N2 in the synthesis of Ph2CN2B(C6F5)3 with carbene, N2 and borane as reactants.82 Dai et al. presented boron carbon nitride (BCN) with the abundant unsaturated B (Lewis acid sites) and N atoms (base sites), and the results showed that FLPs adsorb N2 molecules to form six membered ring intermediates, which reduces the energy of N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N breaking.83 Chen et al. proposed an alternative strategy based on FLP catalysts to stabilize N2H* intermediates, that is, substituting two-dimensional black phosphorus co-doped with TMs and boron atoms for FLPs.84 Zhu et al. designed a variety of FLPs that have the ability to activate N2, and exhibit low ΔG values of −37.5 to −51.0 kcal mol−1 (Fig. 6e).81
N breaking.83 Chen et al. proposed an alternative strategy based on FLP catalysts to stabilize N2H* intermediates, that is, substituting two-dimensional black phosphorus co-doped with TMs and boron atoms for FLPs.84 Zhu et al. designed a variety of FLPs that have the ability to activate N2, and exhibit low ΔG values of −37.5 to −51.0 kcal mol−1 (Fig. 6e).81
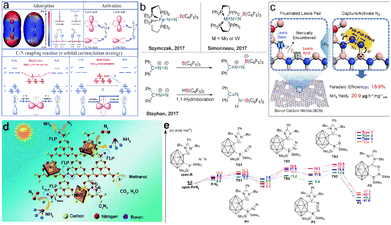 | ||
| Fig. 6 (a) Illustration of artificial frustrated Lewis pairs to adsorb and activate N2, C–N coupling reaction,85 Copyright 2021, the Royal Society of Chemistry. (b) Lewis acidic boranes for N–N activation,86 Copyright 2017, Wiley-VCH. (c) Schematic illustration of BCN for electrochemical nitrogen reduction,83 Copyright 2022, Wiley-VCH. (d) Mechanism of over 10B/10Mo–C3N4 in the photocatalytic N2 fixation strategy,87 Copyright 2020, the Royal Society of Chemistry. (e) Gibbs energy over carborane-based FLPs for N2 reduction.81 Copyright 2022, American Chemical Society. | ||
3.4 Heterojunction construction
Different from the inherent band structure of single-phase catalysts, heterojunction materials have the advantages of regulating the threshold of light absorption, accelerating the separation of carriers by changing the band structure, and passivating the recombination of electrons and holes. A typical heterojunction is an interface formed through the contact of two different semiconductors, which is mainly divided into types I, II and III (Fig. 7a–c). In type I, the VB and the CB of one semiconductor are more negative than those of another semiconductor, and there is no photocatalytic activity because the carriers cannot be effectively separated under photoexcitation. In type III heterojunction, the band positions of one semiconductor are all above the band positions of another semiconductor, and the formed interface has almost no catalytic activity in type III. For type II, the band positions are staggered between the two semiconductors, thus forming an upward or downward bending of the band,89 which causes the charge carriers to migrate in the opposite direction, thus prolonging the life of electrons and holes and improving the redox ability. Although effective charge separation is accomplished in constructed type II systems, the main disadvantage is the lower oxidation and poorer reduction capabilities.90 The Z-scheme heterojunctions achieve rapidly separated carriers and strong redox ability, which can be attributed to the recombination of holes on the VB for one semiconductor and electrons on the CB for another semiconductor (Fig. 7d and e). The Z-scheme heterojunctions are divided into semiconductor–semiconductor (that is the S-scheme) and semiconductor–mediator–semiconductor depending on whether there is an electronic mediator between two semiconductors. Researchers have focused on the construction of different heterojunction nanocomposites,91 aiming to boost the utilization of ultraviolet light or near-infrared light and to promote carrier separation for PEC nitrogen fixation.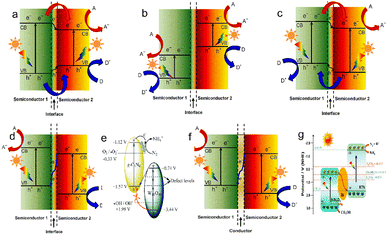 | ||
| Fig. 7 Band structure of (a) type I, (b) type II, (c) type III, and (d) Z-scheme heterojunction in semiconductor–semiconductor and for example, (e) g-C3N4/W18O49,33 Copyright 2017, the Royal Society of Chemistry. (f) Z-scheme in the semiconductor–electron mediator–semiconductor. A: electron acceptor, D: electron donor; and for example, (g) Bi-Bi2O3/KTa0.5Nb0.5O3.88 Copyright 2022, the Royal Society of Chemistry. | ||
Owing to the controllable chemical environment, metal–organic frameworks (MOFs) are used in the preparation of heterostructures as photoelectrodes.92,93 Liu et al. used the in situ chemical etching strategy to form Cu-MOFs on the Cu2O surface with an organic ligand (H3BTC), and the Cu-MOF amount can be determined by etching time (Fig. 8a).94 The establishment of the Cu-MOF/Cu2O heterostructure improves the carrier density and charge separation efficiency (Fig. 8b). Meanwhile, the NH3 yield of Cu-MOF/Cu2O is increased to 7.2 mmol m−2 h−1 due to unsaturated Cu(II) sites, which is 5 times that of Cu2O (3.7 mmol m−2 h−1) and 3.9 times that of Cu-MOFs (1.9 mmol m−2 h−1). Phthalocyanine copper is used for ammonia synthesis due to strong photo-responsiveness. However, the surface of phthalocyanine copper lacks an active site and does not have the desired surface reaction kinetics. Li et al. prepared a phthalocyanine copper/CeO2 heterostructure by the chemical deposition method, and CeO2 is coated on the phthalocyanine copper surface.95 UV-Vis absorption and electrochemical measurement make it clear that the phthalocyanine copper/CeO2 heterostructure has a wide absorption range and a high electrochemical activity area. The PEC N2 reduction performance reaches the best with an NH3 yield of 1.16 μmol h−1 cm−2.
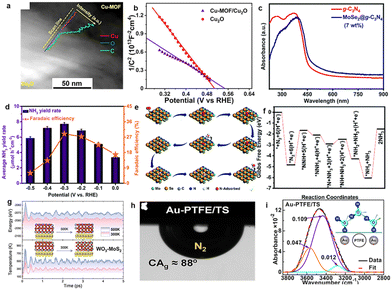 | ||
| Fig. 8 (a) TEM images and EDS spectra of Cu-MOF/Cu2O, (b) Mott–Schottky plot (frequency: 800 Hz),94 Copyright 2021, Elsevier. (c) optical bandgap energy of g-C3N4 and MoSe2@g-C3N4, (d) equivalent NH3 yield and FE at different potentials on MoSe2@g-C3N4 heterojunctions, (e) associative distal pathway for PEC N2 reduction, (f) Gibbs free energy on MoSe2@g-C3N4 heterojunctions,26 Copyright 2020, the Royal Society of Chemistry. (g) Fluctuation of temperature and energy against the time of WO3-MoS2 at 300 K and 500 K,96 Copyright 2022, American Chemical Society. (h) The image of an underwater N2 bubble on the Au-PTFE/TS surface, (i) original (black line) and deconvoluted (red line) spectra of interfacial water at the Au-PTFE/TS surface.97 Copyright 2019, Elsevier. | ||
The C atom inserted into the Mo lattice shows a narrow metal band and exhibits similar Fermi level densities to noble metals, so Mo2C is expected to perform well in catalysis.98 However, Mo2C is a matrix with poor electrical conductivity,99 so it is necessary to consider the optimization of carrier migration for PEC N2 reduction. Li et al. took graphite as a unit of the core–shell heterostructure, and wrapped it on the Mo2C cluster surface based on in situ hydrothermal and calcination treatment, and effectively broke through the limitation of Mo2C conductivity.100 The graphitized carbon can accelerate electron transfer, which is conducive to the activation of N2, with an NH3 yield of 6.6 μg h−1 mg−1. In addition, MoSe2 and the two-dimensional material g-C3N4 are assembled to form heterojunctions. Mushtaq et al. synthesized layered flower-like MoSe2-g-C3N4,26 and the Mo–N bond between MoSe2 and g-C3N4 enhances the internal conductivity of MoSe2. The absorption capacity of MoSe2-g-C3N4 is enhanced (Fig. 8c), and the MoSe2-g-C3N4 hybrid shows efficient PEC N2 reduction performance with an FE of 28.91% and a yield of 7.72 mmol h−1 cm−2, respectively (Fig. 8d). The associative distal pathway and Gibbs free energy on MoSe2@g-C3N4 heterojunctions are shown in Fig. 8e and f. The results show that N2 is well adsorbed and activated on the MoSe2-g-C3N4 (7 wt%) hybrid surface.
WO3 is widely used as a photo/electrocatalyst due to its tunable composition, favourable stability and abundant availability. Guo et al. simulated a heterojunction constructed by WO3 and metal dichalcogenides (MoS2, WSe2), and proved that heterojunctions are stable in thermodynamics through calculation (Fig. 8g). In particular, optimized WO3-MoS2 has the lowest onset potential (0.25 V),96 and its catalytic performance is enhanced by re-positioning the d-band center that is precisely controlled to a higher energy level. Furthermore, in order to improve the N2 reduction activity, porous skeleton structures are used in heterojunction construction engineering. Multiple scattering and absorption of light in the porous structure are conducive to the efficient capture of sunlight.101–103 Porous CdS was obtained by growing CdS on Mo doped WO3 hollow microspheres based on the hydrothermal method. The Mo-WO3@CdS heterostructure104 achieved a FE of 36.72% and an NH3 yield of 38.99 μg h−1 mg−1. The DFT analysis suggests that Mo–W is the catalytic center of N2 reduction, and CdS promotes the provision of electrons, thus accelerating PEC N2 hydrogenation. Meanwhile, the development of porous structures provides the basis for functionalized heterogeneous structures. The Au modified porous polytetrafluoroethylene (PTFE) skeleton dispersed on a silicon-based electrode was synthesized. The silicon and the porous PTFE skeleton were used as the light absorber and gas diffusion channels, respectively, while Au was used as the reaction site.97 Attenuated total reflection and caption-bubble measurements demonstrated that the porous framework of Teflon and Au created a gas-hydrophilic structure, which promoted N2 enrichment and controlled proton activity in aqueous solution (Fig. 8h and i). The optimal ammonia production rate of the photocathode is about 18.9 μg cm−2 h−1, and the FE is 37.8% at −0.2 V vs. RHE. The LDH plays a key role in the fields of photocatalysis/electrocatalysis and energy conversion due to easily controllable composition and abundance of surface oxygen vacancies or coordinatively.105 Yan et al. constructed a CoVP@NiFeV-LDH heterojunction that has shown excellent electrocatalytic performance in the N2 reduction reaction. The new three-dimensional hollow hierarchical structure provides abundant active sites for nitrogen adsorption and reduction to NH3.106
3.5 Cocatalyst loading
Noble metal catalysts have the advantages of high catalytic activity, stability and selectivity. For effective utilization of noble metals, it is necessary to load them onto the host matrix. For example, black silicon serves as a carrier, and it can also enhance light absorption and suppress reflection.107,108 Ali et al. used plasma-enhanced black silicon decorated with Au nanoparticles (NPs) to achieve photoelectrically driven nitrogen conversion (Fig. 9a).22 When black silicon is replaced by unetched silicon, NH3 production is 11% of that generated by Au/black Si/Cr. Black silicon provides a large surface area for Au loading. Au-coated black silicon has a higher ammonia yield (more than 13 mg m−2 h−1) than black silicon. Wang et al. realized the PEC process of converting nitrogen into ammonia, when black silicon was used to enhance light absorption, and silver was used as an active site with a FE of 55.05% at −0.1 V (vs. RHE).25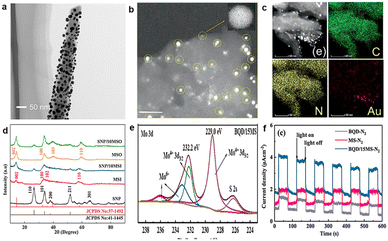 | ||
| Fig. 9 (a) Corresponding TEM images of Au-coated silicon nanowires,22 Copyright 2016, Nature Publishing Group. (b) TEM images of Au/g-C3N4, (c) mapping of N, C, and Au,109 Copyright 2021, Wiley-VCH. (d) XRD patterns of SnO2/10MoS2, SnO2/10MoS2 (silicomolybdic acid is not a precursor), MoS2 (silicomolybdic acid is not a precursor) and SnO2,110 Copyright 2022, Elsevier. (e) The XPS spectra of Bi2S3 QDs/15MoS2, (f) photocurrent with a light on–off cycle.111 Copyright 2022, Elsevier. | ||
Porous structures are also excellent to disperse and stabilize metal NPs. Peramaiah et al. deposited Au NPs on porous g-C3N4 with N defects, and then coated it on the n+np+-Si photocathode surface (Fig. 9b and c).109 Silicon plays the roles of light collection and electrical management, Au is the active center to promote the conversion of N2 to NH3, and porous g-C3N4 with N-defects provides more N2 adsorption sites. Each N atom is alternately associated with the first and second hydrogenation; then, the third hydrogen is added to destroy the N–N bond; finally, the distal N atom is reduced thoroughly with the release of the first NH3 molecule. Au/g-C3N4 exhibits excellent PEC N2 reduction performance with a FE of up to 61.8% and an NH3 yield of 13.8% μg h−1 cm−2. MoS2 is an excellent carrier due to its well-dispersed layered structure, high conductivity and large specific surface area. Ye et al. dispersed TiO2 NPs on MoS2 nanosheets by the hydrothermal method, and the addition of MoS2 leads to a smaller bandgap (2.98 eV) than that of TiO2, and expedites the separation of electrons and holes.112 Enhanced charge transfer maintains higher photoelectric voltage and slower decay behaviour. The results show that the NH3 yield and FE are 1.42 × 10–6 mol h−1 cm−2 and 65.52%, respectively, for MoS2@TiO2. Hydrophilic SnO2 is uniformly dispersed on the surface of hydrophobic MoS2, which leads to a change in the growth direction of MoS2, so as to induce the growth of the (002) crystal plane. The improved transfer and separation of carriers are attributed to the strong interaction between SnO2 and MoS2, with an NH3 yield of 19.6 at −0.3 V μg h−1 mg−1 and a FE of 40.34%, much higher than those of individual SnO2 and MoS2 (Fig. 9d).
Quantum dots (QDs) possess superior properties that play important roles in N2 reduction,113 such as small size, strong photostability, multiple excitation, exposed sites and electronic peculiarity. For example, Bi2S3 QDs have a high absorption coefficient and narrow bandgap.114 Gao et al. grew Bi2S3 QDs on MoS2 through hydrothermal and solvothermal processes for photocatalytic reduction of N2 to NH3.111 Bi2S3 QDs were dispersed on the MoS2 surface, and the agglomeration of Bi2S3 QDs can be inhibited. The shift of binding energy indicates that there is an interaction between Bi2S3 QD and MoS2 (Fig. 9e), thus leading to electron transfer from Bi2S3 to MoS2 and accelerating the carrier separation (Fig. 9f). The FE of the Bi2S3 QD/15MoS2 photocathode reaches 33%, and the NH3 yield is 19 μg h−1 mg−1. Carbon quantum dots (CQDs) are narrow-gap semiconductor materials, and Hu et al. synthesized CQD-modified hydrogenated mesoporous SrTiO3 (CQDs/STO) based on the small size and good conductivity.115 The ammonia yield of CQDs/STO is 143 μmol g−1 h−1 and is 7 times that of STO without a sacrificial agent. Furthermore, heterogeneous CQD/STO with a defective STO (200) surface can promote catalytic N2 conversion.
3.6 Other advanced catalysts and strategies for nitrogen fixation
Single atomic alloy catalysts have broad application prospects in electro-catalytic nitrogen reduction because of the highly exposed active sites. However, there are limited comprehensive experiments and theoretical research on the conversion of N2 to nitrogen-containing molecules. Chuk et al. reported that the yield of NH3 in the nitrogen reduction reaction was 111.9 μg h−1 mg−1 using single atom PdFe alloy.116 In addition, compared with the pristine Ru, the use of Ru–Cu alloy showed that the addition of Cu improved the FE of NH3 (31%) through enhanced N2 adsorption and a reduced H barrier.117 Li-mediated N2 reduction is flexible in the electrocatalytic approach, which has practical significance for realizing the nitrogen cycle; however, the yield and FE are not optimistic. Du et al. studied the properties of the interface generated by the imide-based Li salt electrolytes. The constructed interface can provide a substantial NH3 yield of 150 ± 20 nmol s−1 cm−2, and a FE of up to 100%.118 In addition, this group has realized the ammonia synthesis by the phosphonium cation and isopropanol.119 Therefore, the development of non-metallic materials with high nitrogen fixation efficiency is of great potential. Recently, both experiments and theoretical calculations have suggested that traditional semiconductor materials are promising photoelectrocatalysts for N2 reduction under ambient conditions, as listed in Table 1.| Catalyst | Irradiation source | Bias [V] | Rate of NH3 | Ref. |
|---|---|---|---|---|
| BiVO4/PANI | 300 W Xe lamp | −0.1 V vs. RHE | 0.055 μmol cm−2 h−1 | 52 |
| BiOI | 100 mW cm−2 | +0.4 V vs. RHE | 0.140 μmol cm−2 h−1 | 24 |
| CQDs/SrTiO3 | 300 W Xe lamp | −0.3 V vs. RHE | 1.915 μmol cm−2 h−1 | 115 |
| Cu2O | 300 W Xe lamp | +0.4 V vs. RHE | 0.423 μmol cm−2 h−1 | 49 |
| PANI-ASSM/CdS-Co3S4 | 40 W LED | −0.75 V vs. RHE | 12.33 μmol cm−2 h−1 | 120 |
| Vo-BiOBr/TiO2 | 300 W Xe lamp | −0.2 V vs. RHE | 1.475 μmol cm−2 h−1 | 27 |
| Cu-MOF/Cu2O | 300 W Xe lamp | +0.5 V vs. RHE | 0.716 μmol cm−2 h−1 | 94 |
| MoSe2@g-C3N4 | 150 W Xe lamp | −0.3 V vs. RHE | 7.72 μmol cm−2 h−1 | 26 |
| Fe-doped W18O49 | simulated solar light | −0.65 V vs. Ag/AgCl, | 0.577 μmol cm−2 h−1 | 21 |
| SnO2/MoS2 | 300 W Xe lamp | −0.3 V vs. RHE | 19.6 μg h−1 mgcat.−1 | 110 |
| B-doped Bi nanorolls | 300 W Xe lamp | +0.48 V vs. RHE | 29.2 mg h−1 gcat.−1 | 72 |
| Mo-doped WO3@CdS | 300 W Xe lamp | −0.3 V vs. RHE | 38.99 μg h−1 mgcat.−1 | 101 |
| Mo2C/C | 100 mW cm−2 | +0.2 V vs. Ag/AgCl | 6.6 μg h−1 mgcat.−1 | 121 |
4. Mechanism of PEC N2 reduction for ammonia synthesis
It is generally believed that PEC N2 reduction on a heterogeneous catalyst surface undergoes two mechanisms, namely the associative reaction and dissociative reaction (Fig. 10a).23 For dissociation, the triple bond of N2 is cracked before hydrogenation, and two separate N atoms are reduced to form NH3 independently through hydrogenation steps, which is similar to the H–B process that requires more energy. In the association pathway, two nitrogen atom centers are combined together when the N2 molecule is hydrogenated, and NH3 is released with the breaking of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond. The hydrogenation in the association mechanism can take place in two possible ways, namely distal and alternative pathways. When N2 appears in the mode of terminal coordination, hydrogenation is inclined to occur on the nitrogen far from the catalysts, thus leading to the generation of NH3 molecules, and leaving the metal nitrogen unit to react with H+ to generate another NH3 molecule. The alternative route requires that two nitrogen centers alternate between two N atoms on the catalytic surface. After the first NH3 is removed, the second NH3 will be released immediately. Our group has reported the possible route (distal pathway) of NH3 synthesis on the MoSe2-g-C3N4 surface based on the Gibbs free energy,26 that is, the adsorbed N2 is hydrogenated by H+ adsorption and electron transfer to form an N2H* group at the Mo site. And then H+ + e− reacts with N to form N2H*. The adsorption of H+ on the pre-hydrogenated N site of N2H2* yields an NH3 molecule, leaving the remaining N at the Mo atom. After that, another three H+ ions are coupled to the electron, hydrogenating the remaining N* molecule to give a second NH3.
N bond. The hydrogenation in the association mechanism can take place in two possible ways, namely distal and alternative pathways. When N2 appears in the mode of terminal coordination, hydrogenation is inclined to occur on the nitrogen far from the catalysts, thus leading to the generation of NH3 molecules, and leaving the metal nitrogen unit to react with H+ to generate another NH3 molecule. The alternative route requires that two nitrogen centers alternate between two N atoms on the catalytic surface. After the first NH3 is removed, the second NH3 will be released immediately. Our group has reported the possible route (distal pathway) of NH3 synthesis on the MoSe2-g-C3N4 surface based on the Gibbs free energy,26 that is, the adsorbed N2 is hydrogenated by H+ adsorption and electron transfer to form an N2H* group at the Mo site. And then H+ + e− reacts with N to form N2H*. The adsorption of H+ on the pre-hydrogenated N site of N2H2* yields an NH3 molecule, leaving the remaining N at the Mo atom. After that, another three H+ ions are coupled to the electron, hydrogenating the remaining N* molecule to give a second NH3.
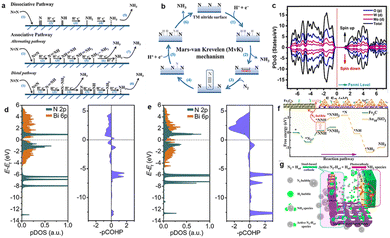 | ||
| Fig. 10 (a) Dissociative, distal and alternating pathways for N2 reduction, (b) MvK mechanism on the TM nitride surface,132 (c) atom projected density of states (PDOS) of Mo-WO3,104 Copyright 2022, Elsevier. (d and e) PDOS and projected crystal orbital Hamilton populations of the N atoms (*NNH) on Bi (012) and (e) Bi-2B (012),72 Copyright 2021, Elsevier. (f) Free energy and optimized geometric structure of distal mechanisms on the Fe3C sites and Au1+ sites of the Au/SiO2/Si photocathode, (g) schematic representation of the catalytic and enhanced mechanism of the PEC NRR on the Au/SiO2/Si photocathode.133 Copyright 2021, American Chemical Society. | ||
Researchers proposed a new reaction mechanism in recent years, namely Mars–van-Krevelen (MvK), based on transition metal nitrides (TMNs: V–N, Zr–N, Nb–N) as shown in Fig. 10.122 The lattice N atoms of TMNs are hydrogenated to obtain NH3 by lattice nitrogen reduction, followed by the regeneration of lattice nitrogen. Importantly, N2 activation on TMNs requires a smaller overpotential for the MvK mechanism, which is more conducive to the formation of NH3 than association and dissociation reactions.
DFT studies are used for energy assessment and catalyst design on the basis of experimental results, including the calculations of adsorption energy, Gibbs free energy,75,123,124 the density of states analysis, the Bader charge analysis, and the crystal orbital Hamilton population analysis. In addition, advanced in situ monitoring technologies are meaningful for verifying the reaction mechanisms, dynamics of molecular catalyst interactions, and revealing the structure of intermediates, especially for in situ observation of catalytic reaction intermediates under practical operation, such as synchrotron radiation, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning tunnelling microscopy (STM), X-ray absorption spectroscopy (XAS), diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and surface-enhanced infrared absorption spectroscopy (SEIRAS).125–128 Yang et al. used in situ XPS and in situ XAS to reveal that VN0.7O0.45 was the active site rather than VN, and they monitored that the catalyst underwent passivation due to a change in VN0.7O0.45 to form VN.129 According to in situ SEIRAS, the possible nitrogen reduction pathway on the Ru surface in acidic solution was deduced.130 Feng et al. observed that adsorbed H2O molecules were located at the Ovs site based on operando STM equipment, which accelerated the electrocatalytic HER process, and provided a rich route for revealing the kinetic process of nitrogen fixation.131 Various in situ characterization techniques can be used not only independently, but also in combination to obtain accurate and multi-dimensional research results. In the future, in situ characterization technology will make a breakthrough in cost and universality, which further deepens our understanding of catalytic mechanisms.
The selectivity of NH3 from N2 and H2O is challenging against the HER (and/or N2H4), and numerous computational simulations and experimental attempts have been conducted to elucidate the essence of selectivity. Singh et al. provided some guidance that the selectivity between NH3 and H2 can be determined through a competition between the binding energies of N and H. They found that the generation rate of ammonia is of zero-order at electron and proton concentrations, while the rate of H2 is of first-order at a potential more negative than Us (Us refers to the potential to favourable H adsorption).134 And the strategies of proton/electron availability have been proposed due to the significant impact of the proton concentration on HER performance, so employing an aprotic solvent,135 blanketing the catalyst with a hydrophobic protection layer,136 and designing photoelectrocatalysts are several potential solutions to selectively block the proton transfer to the catalyst surface, thus hindering H2 evolution. Increased loadings of H+ and e− equivalents can directly translate to greater N2H4 yields, and the powerful reductant KC8 and strong Brønsted acid can deliver H+/e− equivalents for the necessary proton-coupled electron transfer to improve the selectivity of N2H4.137 In addition, regulating the adsorption strength of N and H towards catalytic sites can alter the selectivity of NH3. It is reported that the adsorption energy of H on Ru is smaller than that on N, which indicates that N atoms are preferably adsorbed on H atoms, thus obtaining higher NH3 selectivity.138
5. Detection and quantification of nitrogen sources for ammonia
The accurate quantification of ammonia is restricted by many factors, such as trace NH3 yield intrinsically, ammonia pollution from the environment, tedious ammonia separation from electrolytes, solution pH, and interferants. Serious concerns exist on a reliable quantitative method of ammonia concentration for N2 reduction, and we should maintain a rigorous scientific attitude and strive to accurately quantify NH3 based on different catalytic systems (Fig. 11). Currently, spectrophotometry and ion chromatography are common methods with the advantage of simplicity. In addition, the use of ion-selective electrodes (ISEs) and colorimetric NH3 assay kits, and nuclear magnetic resonance (NMR) methods can significantly improve the sensitivity, however, the experimental cost is increased to a certain extent.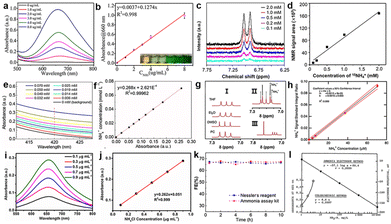 | ||
| Fig. 11 Calibration curves for NH3 quantification. (a) the UV-Vis absorption, (b) the corresponding calibration curve for the indophenol blue method,147 Copyright 2019, Wiley-VCH. (c) 1H NMR spectra of 15NH4+ standard solutions (0.1–2.0 mM), (d) the corresponding calibration curve for the NMR method,49 Copyright 2020, American Chemical Society. (e) UV-Vis of standard solutions, and (f) the corresponding calibration curve with Nessler's reagent,145 Copyright 2019, Nature Publishing Group. (g) (I) 1H NMR of NH4+ in 100 μM NH4+ in various solvents, (II) NMR spectra of a mixture of 14NH4++15NH4+, (III) NMR of the LiClO4/THF electrolyte after N2 reduction, (h) calibration curve to determine the NH4+ concentration,144 Copyright 2019, American Chemical Society. (i) UV-Vis absorption spectra and (j) the calibration curve using salicylic acid,104 Copyright 2022, Elsevier. (k) FE of ammonia measured using Nessler's reagent or an enzymatic ammonia assay kit,145 Copyright 2019, Nature Publishing Group. (l) The ion-selective electrode method.148 Copyright 1969, Springer Nature. | ||
The universal chemical chromogenic approaches are indophenol blue (IB) and Nessler's reagent based on the calibration curve, and they all have limitations. The IB test is usually used under neutral and acidic conditions because the generated indophenol dye could react with other amine groups under alkaline conditions.139 Sodium salicylate is more steady than IB because salicylate can prevent the generation of toxic substances.140 Nessler's method has wide pH applicability, however, some sacrificial agents (alcohol, glucose) are oxidized to carbonyl easily, thus resulting in misleading results.35 Ion chromatography has high sensitivity and reliable results, among which the distraction of pH and sacrificial agents are avoided through appropriate detection methods.141,142 The ISE determines the concentration of ions in solution by a membrane potential based on the Nernst equation, which can be divided into the NH4+-selective electrode and the NH3-selective electrode. The concentrated solution can further improve the accuracy of NH3 detection when choosing an ISE.143 The resonance signal obtained from 1H can be qualitatively and quantitatively detected by NH4+.144 Nielander et al. reported a frequency-selective pulse NMR method for measuring NH3 concentration in electrolytes and quantified the common Berthelot method. The reliability of the results can be greatly improved by using various methods simultaneously to realize the quantitative determination of ammonia. Hao et al. used a colorimetric method with Nessler's reagent, an enzymatic NH3 assay kit (Sigma-Aldrich), and NMR to quantify ammonia accurately;145 they found that the FEs measured using Nessler's approach or the enzymatic ammonia assay kit are persuasive. Zhang et al. used Nessler's approach, ion chromatography and the indole phenol method to quantify NH3, and the results showed that the three methods had good coordination at low concentrations (0–500 μg L−1). However, Nessler's approach and ion chromatography are more suitable for the quantification of ammonia than the indole phenol method at high concentrations.146
In order to scale up the PEC ammonia synthesis technology, the most essential progress is to confirm the true role of the catalysts. The primary task of removing nitrogen pollution is to ensure that the feed gases and catalysts are not polluted by nitrogen containing substances, including nitrate, nitrite and other contaminants before the experiment. Considering serious ammonia pollution in the environment, the 15N2 labeling experiment is necessary to provide direct evidence,149 especially for N-containing catalyst systems.123 However, many researchers often ignore the problem of 15N2 pollution, so it is only used as a qualitative method. Therefore, we need to accurately present the nitrogen fixation performance of the catalysts from multiple views. In addition, the background experiment is another piece of evidence to assist in proving the role of catalysts. The development of a combined technology of ammonia synthesis and ammonia detection can avoid ammonia pollution and realize the in-situ detection and online quantification of ammonia.
6. Conclusion
The above achievements have proved that PEC N2 reduction is a promising route to realize the N-cycle goal under environmental conditions, and multiple catalytic strategies are proposed to improve the yield and selectivity of NH3. However, there are fundamental challenges as follows:(I) Disclosure of the essence of photo-electrical synergy. The catalytic performance of PEC N2 reduction is more excellent than that of individual photocatalysis or electrocatalysis; however, this collaborative technology causes obstacles to the mechanistic understanding and revelation of photogenic/electrical effects. Most studies show that light irradiation promotes the electrocatalytic process, and bias accelerates the separation of photogenerated carriers, and few works reveal the coexistence of photocatalytic and electrocatalytic processes.
(II) Reliable methods in consideration of NH3 quantification and N-source determination. Although the spectral analysis is maneuverable, the accuracy usually is disturbed by pH, ionic strength and sacrificial agents. Therefore, it is necessary to combine it with other quantitative experiments, such as ion chromatography and NMR, to obtain accurate NH3 concentrations. In addition, researchers judge the rationality of catalytic performance by testing the recommended benchmark catalyst.
(III) Rigorous mechanistic insights. The active sites, free energies and possible reaction paths are revealed on the basis of theoretical exploration. The available theoretical methods, catalyst model and computing power are the driving force behind accurate calculations.
(IV) Advanced in situ characterization. It is difficult to predict the reconstruction of the surface structure, including the change of valence states, the identification of reaction intermediates and active centers, and real-time monitoring of reaction processes. For example, in situ surface enhanced infrared absorption spectroscopy and high-resolution electron energy loss spectroscopy showed potential for understanding the N2 reduction pathway due to its high sensitivity to the catalyst surface. In conclusion, reasonable catalysts are designed based on calculation and experiment, which will promote the rapid development of PEC N2 reduction to a large extent under environmental conditions in the future. The advantages of PEC N2 reduction are higher than those of individual photocatalysis or electrocatalysis. The correlation between PEC performance and the structures could be established at the molecular level to achieve industrial capabilities. In addition, strengthening durability testing is the key to proving the practicality at the industrial level. Finally, large-scale synthesis of catalysts should be considered.
Author contributions
Pengyan Li: methodology, investigation, and writing – original draft. Yumin Liu: supervision. Muhammad Asim Mushtaq: supervision. Dongpeng Yan: supervision and writing – review & editing. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22288201 and 22275021) and the Beijing Municipal Natural Science Foundation (Grant No. JQ20003).References
- S. Chen, D. Liu and T. Peng, Fundamentals and recent progress of photocatalytic nitrogen–fixation reaction over semiconductors, Sol. RRL, 2020, 5, 2000487 CrossRef.
- L. Wang, W. Wu, K. Liang and X. Yu, Advanced strategies for improving the photocatalytic nitrogen fixation performance: a short review, Energy Fuels, 2022, 36, 11278–11291 CrossRef CAS.
- R. Shi, X. Zhang, G. I. N. Waterhouse, Y. Zhao and T. Zhang, The journey toward low temperature, low pressure catalytic nitrogen fixation, Adv. Energy Mater., 2020, 10, 2000659 CrossRef CAS.
- J. Lee, L. L. Tan and S. P. Chai, Heterojunction photocatalysts for artificial nitrogen fixation: fundamentals, latest advances and future perspectives, Nanoscale, 2021, 13, 7011–7033 RSC.
- Z. Zhao, H. Ren, D. Yang, Y. Han, J. Shi, K. An, Y. Chen, Y. Shi, W. Wang, J. Tan, X. Xin, Y. Zhang and Z. Jiang, Boosting nitrogen activation via bimetallic organic frameworks for photocatalytic ammonia synthesis, ACS Catal., 2021, 11, 9986–9995 CrossRef CAS.
- H. Liu, P. Wu, H. Li, Z. Chen, L. Wang, X. Zeng, Y. Zhu, Y. Jiang, X. Liao, B. S. Haynes, J. Ye, C. Stampfl and J. Huang, Unravelling the effects of layered supports on Ru nanoparticles for enhancing N2 reduction in photocatalytic ammonia synthesis, Appl. Catal., B, 2019, 259, 118026 CrossRef CAS.
- Y. Gao, S. Zhang, X. Sun, W. Zhao, H. Zhuo, G. Zhuang, S. Wang, Z. Yao, S. Deng, X. Zhong, Z. Wei and J.-G. Wang, Computational screening of O-functional MXenes for electrocatalytic ammonia synthesis, Chin. J. Catal., 2022, 43, 1860–1869 CrossRef CAS.
- P. Y. Li, L. Liu, W. An, H. Wang, H. X. Guo, Y. H. Liang and W. Q. Cui, Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C–C coupling photothermal catalytic reduction of CO to ethanol, Appl. Catal., B, 2020, 266, 118618 CrossRef CAS.
- X.-Y. Dao and W.-Y. Sun, Single- and mixed-metal–organic framework photocatalysts for carbon dioxide reduction, Inorg. Chem. Front., 2021, 8, 3178–3204 RSC.
- M. Yang, C. H. Zhang, N. W. Li, D. Luan, L. Yu and X. W. D. Lou, Design and synthesis of hollow nanostructures for electrochemical water splitting, Adv. Sci., 2022, 9, e2105135 CrossRef PubMed.
- Q. Liu, T. Xu, Y. Luo, Q. Kong, T. Li, S. Lu, A. A. Alshehri, K. A. Alzahrani and X. Sun, Recent advances in strategies for highly selective electrocatalytic N2 reduction toward ambient NH3 synthesis, Curr. Opin. Electrochem., 2021, 29, 100766 CrossRef CAS.
- W. Song, L. Yue, X. Fan, Y. Luo, B. Ying, S. Sun, D. Zheng, Q. Liu, M. S. Hamdy and X. Sun, Recent progress and strategies on the design of catalysts for electrochemical ammonia synthesis from nitrate reduction, Inorg. Chem. Front., 2023, 3489–3514 RSC.
- Q. Liu, L. Xie, J. Liang, Y. Ren, Y. Wang, L. Zhang, L. Yue, T. Li, Y. Luo, N. Li, B. Tang, Y. Liu, S. Gao, A. A. Alshehri, I. Shakir, P. O. Agboola, Q. Kong, Q. Wang, D. Ma and X. Sun, Ambient ammonia synthesis via electrochemical reduction of nitrate enabled by NiCo2O4 Nanowire Array, Small, 2022, 18, e2106961 CrossRef PubMed.
- J. Han, Z. Liu, Y. Ma, G. Cui, F. Xie, F. Wang, Y. Wu, S. Gao, Y. Xu and X. Sun, Ambient N2 fixation to NH3 at ambient conditions: Using Nb2O5 nanofiber as a high-performance electrocatalyst, Nano Energy, 2018, 52, 264–270 CrossRef CAS.
- Z. Du, J. Liang, S. Li, Z. Xu, T. Li, Q. Liu, Y. Luo, F. Zhang, Y. Liu, Q. Kong, X. Shi, B. Tang, A. M. Asiri, B. Li and X. Sun, Alkylthiol surface engineering: an effective strategy toward enhanced electrocatalytic N2-to-NH3 fixation by a CoP nanoarray, J. Mater. Chem. A, 2021, 9, 13861–13866 RSC.
- D. Bao, Q. Zhang, F. L. Meng, H. X. Zhong, M. M. Shi, Y. Zhang, J. M. Yan, Q. Jiang and X. B. Zhang, Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle, Adv. Mater., 2017, 29, 1604799 CrossRef PubMed.
- H. Ma, Z. Chen and Z. Wang, Electroreduction of nitrogen to ammonia on nanoporous gold, Nanoscale, 2021, 13, 1717–1722 RSC.
- Z. Y. Niu, L. Jiao, T. Zhang, X. M. Zhao, X. F. Wang, Z. Tan, L. Z. Liu, S. Chen and X. Z. Song, Boosting electrocatalytic ammonia synthesis of Bio-inspired porous Mo-doped hematite via nitrogen activation, ACS Appl. Mater. Interfaces, 2022, 14, 55559–55567 CrossRef CAS PubMed.
- X. Xu, X. Liu, J. Zhao, D. Wu, Y. Du, T. Yan, N. Zhang, X. Ren and Q. Wei, Interface engineering of MoS2@Fe(OH)3 nanoarray heterostucture: Electrodeposition of MoS2@Fe(OH)3 as N2 and H+ channels for artificial NH3 synthesis under mild conditions, J. Colloid Interface Sci., 2022, 606, 1374–1379 CrossRef CAS PubMed.
- X. Niu, A. Shi, D. Sun, S. Xiao, T. Zhang, Z. Zhou, X. A. Li and J. Wang, Photocatalytic ammonia synthesis: mechanistic insights into N2 activation at oxygen vacancies under visible light excitation, ACS Catal., 2021, 11, 14058–14066 CrossRef CAS.
- M. H. Vu, C. C. Nguyen and T. O. Do, Synergistic effect of Fe doping and plasmonic Au nanoparticles on W18O49 nanorods for enhancing photoelectrochemical nitrogen reduction, ACS Sustainable Chem. Eng., 2020, 8, 12321–12330 CrossRef CAS PubMed.
- M. Ali, F. Zhou, K. Chen, C. Kotzur, C. Xiao, L. Bourgeois, X. Zhang and D. R. MacFarlane, Nanostructured photoelectrochemical solar cell for nitrogen reduction using plasmon-enhanced black silicon, Nat. Commun., 2016, 7, 11335 CrossRef CAS PubMed.
- H. Zheng, S. Zhang, X. Liu and A. P. O'Mullane, The application and improvement of TiO2 (titanate) based nanomaterials for the photoelectrochemical conversion of CO2 and N2 into useful products, Catal. Sci. Technol., 2021, 11, 768–778 RSC.
- Y. Bai, H. Bai, K. Qu, F. Wang, P. Guan, D. Xu, W. Fan and W. Shi, In-situ approach to fabricate BiOI photocathode with oxygen vacancies: understanding the N2 reduced behavior in photoelectrochemical system, Chem. Eng. J., 2019, 362, 349–356 CrossRef CAS.
- B. Wang, L. Yao, G. Xu, X. Zhang, D. Wang, X. Shu, J. Lv and Y.-C. Wu, Highly efficient photoelectrochemical synthesis of ammonia using plasmon-enhanced black silicon under ambient conditions, ACS Appl. Mater. Interfaces, 2020, 12, 20376–20382 CrossRef CAS PubMed.
- M. A. Mushtaq, M. Arif, X. Fang, G. Yasin, W. Ye, M. Basharat, B. Zhou, S. Yang, S. Ji and D. Yan, Photoelectrochemical reduction of N2 to NH3 under ambient conditions through hierarchical MoSe2@g-C3N4 heterojunctions, J. Mater. Chem. A, 2021, 9, 2742–2753 RSC.
- S. Lin, Y. Chen, J. Fu, L. Sun, Q. Jiang, J.-F. Li, J. Cheng, C. Lin and Z.-Q. Tian, Photoelectrocatalytic nitrogen fixation with Vo-BiOBr/TiO2 heterostructured photoelectrode as photocatalyst, Int. J. Hydrogen Energy, 2022, 47, 41553–41563 CrossRef CAS.
- H. Chen, J. Liang, K. Dong, L. Yue, T. Li, Y. Luo, Z. Feng, N. Li, M. S. Hamdy, A. A. Alshehri, Y. Wang, X. Sun and Q. Liu, Ambient electrochemical N2-to-NH3 conversion catalyzed by TiO2 decorated juncus effusus-derived carbon microtubes, Inorg. Chem. Front., 2022, 9, 1514–1519 RSC.
- M. Nazemi and M. A. El-Sayed, Managing the nitrogen cycle via plasmonic (photo)electrocatalysis: toward circular economy, Acc. Chem. Res., 2021, 54, 4294–4304 CrossRef CAS PubMed.
- T. Oshikiri, K. Ueno and H. Misawa, Plasmon-induced ammonia synthesis through nitrogen photofixation with visible light irradiation, Angew. Chem., Int. Ed., 2014, 53, 9802–9805 CrossRef CAS PubMed.
- J. Wang, Z. Zhang, S. Qi, Y. Fan, Y. Yang, W. Li and M. Zhao, Photo-assisted high performance single atom electrocatalysis of the N2 reduction reaction by a Mo-embedded covalent organic framework, J. Mater. Chem. A, 2021, 9, 19949–19957 RSC.
- M. Fritz, S. Rupp, C. I. Kiene, S. Kisan, J. Telser, C. Wurtele, V. Krewald and S. Schneider, Photoelectrochemical conversion of dinitrogen to benzonitrile: selectivity control by electrophile- versus proton-coupled electron transfer, Angew. Chem., Int. Ed., 2022, 61, e202205922 CAS.
- H. Liang, J. Li and Y. Tian, Construction of full-spectrum-driven Ag–g-C3N4/W18O49 heterojunction catalyst with outstanding N2 photofixation ability, RSC Adv., 2017, 7, 42997–43004 RSC.
- H. Liang, H. Zou and S. Hu, Preparation of the W18O49/g-C3N4 heterojunction catalyst with full-spectrum-driven photocatalytic N2 photofixation ability from the UV to near infrared region, New J. Chem., 2017, 41, 8920–8926 RSC.
- Y.-G. Liu, M. Tian, J. Hou and H.-Y. Jiang, Research progress and perspectives on active sites of photo- and electrocatalytic nitrogen reduction, Energy Fuels, 2022, 36, 11323–11358 CrossRef CAS.
- H.-J. Chen, Z.-Q. Xu, S. Sun, Y. Luo, Q. Liu, M. S. Hamdy, Z.-S. Feng, X. Sun and Y. Wang, Plasma-etched Ti2O3 with oxygen vacancies for enhanced NH3 electrosynthesis and Zn–N2 batteries, Inorg. Chem. Front., 2022, 9, 4608–4613 RSC.
- C. Ling, X. Niu, Q. Li, A. Du and J. Wang, Metal-free single atom catalyst for N2 fixation driven by visible light, J. Am. Chem. Soc., 2018, 140, 14161–14168 CrossRef CAS PubMed.
- M.-A. Légaré, G. Bélanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels and H. Braunschweig, Nitrogen fixation and reduction at boron, Science, 2018, 359, 896–900 CrossRef PubMed.
- B. Xu, H. Beckers, H. Ye, Y. Lu, J. Cheng, X. Wang and S. Riedel, Cleavage of the N identical with N triple Bond and unpredicted formation of the cyclic 1,3-diaza-2,4-diborete (FB)2 N2 from N2 and fluoroborylene BF, Angew. Chem., Int. Ed., 2021, 60, 17205–17210 CrossRef CAS PubMed.
- J. Yu, S. Xiong, B. Wang, R. Wang, B. He, J. Jin, H. Wang and Y. Gong, Constructing boron-doped graphitic carbon nitride with 2D/1D porous hierarchical architecture and efficient N2 photofixation, Colloids Surf., A, 2023, 656, 130481 CrossRef CAS.
- L. Shi, Y. Yin, S. Wang and H. Sun, Rational catalyst design for N2 reduction under ambient conditions: strategies toward enhanced conversion efficiency, ACS Catal., 2020, 10, 6870–6899 CrossRef CAS.
- J. Li, X. Zhu, T. Wang, Y. Luo and X. Sun, An Fe2O3 nanoparticle-reduced graphene oxide composite for ambient electrocatalytic N2 reduction to NH3, Inorg. Chem. Front., 2019, 6, 2682–2685 RSC.
- M. Wang, S. Liu, T. Qian, J. Liu, J. Zhou, H. Ji, J. Xiong, J. Zhong and C. Yan, Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential, Nat. Commun., 2019, 10, 341 CrossRef CAS PubMed.
- M. Nazemi, S. R. Panikkanvalappil and M. A. El-Sayed, Enhancing the rate of electrochemical nitrogen reduction reaction for ammonia synthesis under ambient conditions using hollow gold nanocages, Nano Energy, 2018, 49, 316–323 CrossRef CAS.
- X. Shen, S. Liu, X. Xia, M. Wang, H. Ji, Z. Wang, J. Liu, X. Zhang, C. Yan and T. Qian, Interfacial microextraction boosting nitrogen feed for efficient ambient ammonia synthesis in aqueous electrolyte, Adv. Funct. Mater., 2022, 32, 2109422 CrossRef CAS.
- B. G. K. Rambabu, A. Hai, N. Ponpandian, J. E. Schmidt, D. D. Dionysiou, M. A. Haija and F. Banat, Dual-functional paired photoelectrocatalytic system for the photocathodic reduction of CO2 to fuels and the anodic oxidation of furfural to value-added chemicals, Appl. Catal., B, 2021, 298, 120520 CrossRef.
- X. X. Jiang, X. De Hu, M. Tarek, P. Saravanan, R. Alqadhi, S. Y. Chin and M. M. Rahman, Tailoring the properties of g-C3N4 with CuO for enhanced photoelectrocatalytic CO2 reduction to methanol, J. CO2 Util., 2020, 40, 101222 CrossRef CAS.
- D. Liu, J. Wang, S. Bian, Q. Liu, Y. Gao, X. Wang, P. K. Chu and X. F. Yu, Photoelectrochemical synthesis of ammonia with black phosphorus, Adv. Funct. Mater., 2020, 30, 2002731 CrossRef CAS.
- Y. J. Jang, A. E. Lindberg, M. A. Lumley and K.-S. Choi, Photoelectrochemical nitrogen reduction to ammonia on cupric and cuprous oxide photocathodes, ACS Energy Lett., 2020, 5, 1834–1839 CrossRef CAS.
- P. Li, T. Zhang, M. A. Mushtaq, S. Wu, X. Xiang and D. Yan, Research progress in organic synthesis by means of photoelectrocatalysis, Chem. Rec., 2021, 841–857 CrossRef CAS PubMed.
- P. B. Pati, R. W. Wang, E. Boutin, S. Diring, S. Jobic, N. Barreau, F. Odobel and M. Robert, Photocathode functionalized with a molecular cobalt catalyst for selective carbon dioxide reduction in water, Nat. Commun., 2020, 11, 3499 CrossRef CAS PubMed.
- Y. Bai, H. Bai, Z. Fang, X. Li, W. Fan and W. Shi, Understanding the Z-scheme heterojunction of BiVO4/PANI for photoelectrochemical nitrogen reduction, Chem. Commun., 2021, 57, 10568–10571 RSC.
- M. Zhang, X. Xuan, W. Wang, C. Ma and Z. Lin, Anode photovoltage compensation–enabled synergistic CO2 photoelectrocatalytic reduction on a flower–like graphene–decorated Cu foam cathode, Adv. Funct. Mater., 2020, 30, 2005983 CrossRef CAS.
- Y. Zhao, G. Brocks, H. Genuit, R. Lavrijsen, M. A. Verheijen and A. Bieberle-Hütter, Boosting the performance of WO3/n–Si heterostructures for photoelectrochemical water splitting: from the role of Si to interface engineering, Adv. Energy Mater., 2019, 9, 1900940 CrossRef.
- S. Y. Chae, J. Y. Choi, Y. Kim, D. Le Tri Nguyen and O. S. Joo, Photoelectrochemical CO2 reduction with a rhenium organometallic redox mediator at semiconductor/aqueous liquid junction interfaces, Angew. Chem., Int. Ed., 2019, 58, 16395–16399 CrossRef CAS PubMed.
- H. E. Kim, J. Kim, E. C. Ra, H. Zhang, Y. J. Jang and J. S. Lee, Photoelectrochemical nitrate reduction to ammonia on ordered silicon nanowire array photocathodes, Angew. Chem., Int. Ed., 2022, 61, e202204117 CAS.
- C. Li, T. Wang, Z. J. Zhao, W. Yang, J. F. Li, A. Li, Z. Yang, G. A. Ozin and J. Gong, Promoted fixation of molecular nitrogen with surface oxygen vacancies on plasmon-enhanced TiO2 photoelectrodes, Angew. Chem., Int. Ed., 2018, 57, 5278–5282 CrossRef CAS PubMed.
- S. Zhou, K. Sun, C. Y. Toe, J. Yin, J. Huang, Y. Zeng, D. Zhang, W. Chen, O. F. Mohammed, X. Hao and R. Amal, Engineering a kesterite-based photocathode for photoelectrochemical ammonia synthesis from NOx reduction, Adv. Mater., 2022, 34, e2201670 CrossRef PubMed.
- T. Oshikiri, K. Ueno and H. Misawa, Selective dinitrogen conversion to ammonia using water and visible light through plasmon-induced charge separation, Angew. Chem., Int. Ed., 2016, 55, 3942–3946 CrossRef CAS PubMed.
- P. Zhou, Y. Chao, F. Lv, J. Lai, K. Wang and S. Guo, Designing noble metal single-atom-loaded two-dimension photocatalyst for N2 and CO2 reduction via anion vacancy engineering, Sci. Bull., 2020, 65, 720–725 CrossRef CAS PubMed.
- H. Li, J. Li, Z. Ai, F. Jia and L. Zhang, Durch sauerstoff-leerstellen vermittelte photokatalyse mit BiOCl: reaktivität, selektivität und ausblick, Angew. Chem., Int. Ed., 2018, 130, 128–145 CrossRef.
- Y. Nosaka, M. Matsushita, J. Nishino and A. Nosaka, Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds, Sci. Technol. Adv. Mater., 2016, 6, 143–148 CrossRef.
- X. Xue, R. Chen, H. Chen, Y. Hu, Q. Ding, Z. Liu, L. Ma, G. Zhu, W. Zhang, Q. Yu, J. Liu, J. Ma and Z. Jin, Oxygen vacancy engineering promoted photocatalytic ammonia synthesis on ultrathin two-dimensional bismuth oxybromide nanosheets, Nano Lett., 2018, 18, 7372–7377 CrossRef CAS PubMed.
- Y. Mao, H. Zhang, W. Jiang, R. Zhao, Y. Liu, Z. Wang, P. Wang, Z. Zheng, K. Song, W. Wei, Y. Dai, J.-H. He, H. Cheng and B. Huang, An integrated Si photocathode with lithiation-activated molybdenum oxide nanosheets for efficient ammonia synthesis, Nano Energy, 2022, 102, 107639 CrossRef CAS.
- D. Zhang, J. Li, Q. Wang and Q. Wu, High {001} facets dominated BiOBr lamellas: facile hydrolysis preparation and selective visible-light photocatalytic activity, J. Mater. Chem. A, 2013, 1, 8622–8629 RSC.
- D. Liu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Surface engineering of g-C3N4 by stacked BiOBr sheets rich in oxygen vacancies for boosting photocatalytic performance, Angew. Chem., Int. Ed., 2020, 59, 4519–4524 CrossRef CAS PubMed.
- Y. Zhao, L. Zheng, R. Shi, S. Zhang, X. Bian, F. Wu, X. Cao, G. I. N. Waterhouse and T. Zhang, Alkali etching of layered double hydroxide nanosheets for enhanced photocatalytic N2 reduction to NH3, Adv. Energy Mater., 2020, 10, 2002199 CrossRef CAS.
- M. Li, Q. Lu, M. Liu, P. Yin, C. Wu, H. Li, Y. Zhang and S. Yao, Photoinduced charge separation via the double-electron transfer mechanism in nitrogen vacancies g-C3N5/BiOBr for the photoelectrochemical nitrogen reduction, ACS Appl. Mater. Interfaces, 2020, 12, 38266–38274 CrossRef CAS PubMed.
- M. Soleilhavoup and G. Bertrand, Borylenes: an emerging class of compounds, Angew. Chem., Int. Ed., 2017, 56, 10282–10292 CrossRef CAS PubMed.
- P. X. Sun, Z. Cao, Y. X. Zeng, W. W. Xie, N. W. Li, D. Luan, S. Yang, L. Yu and X. W. D. Lou, Formation of super-assembled TiO(x)/Zn/N-doped carbon inverse opal towards dendrite-free Zn anodes, Angew. Chem., Int. Ed., 2022, 61, e202115649 CAS.
- G. N. Schrauzer and T. D. Guth, Photolysis of water and photoreduction of nitrogen on titanium dioxide, J. Am. Chem. Soc., 1977, 99, 7189–7193 CrossRef CAS.
- F. Xu, F. Wu, K. Zhu, Z. Fang, D. Jia, Y. Wang, G. Jia, J. Low, W. Ye, Z. Sun, P. Gao and Y. Xiong, Boron doping and high curvature in Bi nanorolls for promoting photoelectrochemical nitrogen fixation, Appl. Catal., B, 2021, 284, 119689 CrossRef CAS.
- M. Lv, X. Sun, S. Wei, C. Shen, Y. Mi and X. Xu, Ultrathin lanthanum tantalate perovskite nanosheets modified by nitrogen doping for efficient photocatalytic water splitting, ACS Nano, 2017, 11, 11441–11448 CrossRef CAS PubMed.
- J. Cao, N. Li and X. Zeng, Exploring the synergistic effect of B–N doped defective graphdiyne for N2 fixation, New J. Chem., 2021, 45, 6327–6335 RSC.
- X. Yu, P. Han, Z. Wei, L. Huang, Z. Gu, S. Peng, J. Ma and G. Zheng, Boron-doped graphene for electrocatalytic N2 reduction, Joule, 2018, 2, 1610–1622 CrossRef CAS.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, Nitrogen-doped graphene quantum dots with oxygen-rich functional groups, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS PubMed.
- P. Zhai, T. C. Wei, Y. H. Chang, Y. T. Huang, W. T. Yeh, H. Su and S. P. Feng, High electrocatalytic and wettable nitrogen-doped microwave-exfoliated graphene nanosheets as counter electrode for dye-sensitized solar cells, Small, 2014, 10, 3347–3353 CrossRef CAS PubMed.
- X. Peng, D. Chen, X. Yang, D. Wang, M. Li, C. C. Tseng, R. Panneerselvam, X. Wang, W. Hu, J. Tian and Y. Zhao, Microwave-assisted synthesis of highly dispersed PtCu nanoparticles on three-dimensional nitrogen-doped graphene networks with remarkably enhanced methanol electrooxidation, ACS Appl. Mater. Interfaces, 2016, 8, 33673–33680 CrossRef CAS PubMed.
- N.-I. Kim, R. A. Afzal, S. R. Choi, S. W. Lee, D. Ahn, S. Bhattacharjee, S.-C. Lee, J. H. Kim and J.-Y. Park, Highly active and durable nitrogen doped-reduced graphene oxide/double perovskite bifunctional hybrid catalysts, J. Mater. Chem. A, 2017, 5, 13019–13031 RSC.
- L. Paramanik, S. Sultana and K. M. Parida, Photocatalytic and photo-electrochemical ammonia synthesis over dimensional oriented cobalt titanate/nitrogen-doped reduced graphene oxide junction interface catalyst, J. Colloid Interface Sci., 2022, 625, 83–99 CrossRef CAS PubMed.
- C. Dai, Y. Huang and J. Zhu, Predicting dinitrogen activation by carborane-based frustrated lewis pairs, Organometallics, 2022, 41, 1480–1487 CrossRef CAS.
- C. Tang, Q. Liang, A. R. Jupp, T. C. Johnstone, R. C. Neu, D. Song, S. Grimme and D. W. Stephan, 1,1-hydroboration and a borane adduct of diphenyldiazomethane: a potential prelude to FLP-N2 chemistry, Angew. Chem., Int. Ed., 2017, 56, 16588–16592 CrossRef CAS PubMed.
- W. Lin, H. Chen, G. Lin, S. Yao, Z. Zhang, J. Qi, M. Jing, W. Song, J. Li, X. Liu, J. Fu and S. Dai, Creating frustrated lewis pairs in defective boron carbon nitride for electrocatalytic nitrogen reduction to ammonia, Angew. Chem., Int. Ed., 2022, 61, e202207807 CAS.
- Z. Chen, J. Zhao, Y. Jiao, T. Wang and L. Yin, Achieving efficient N2 electrochemical reduction by stabilizing the N2H* intermediate with the frustrated Lewis pairs, J. Energy Chem., 2022, 66, 628–634 CrossRef CAS.
- M. Yuan, J. Chen, Y. Xu, R. Liu, T. Zhao, J. Zhang, Z. Ren, Z. Liu, C. Streb, H. He, C. Yang, S. Zhang and G. Zhang, Highly selective electroreduction of N2 and CO2 to urea over artificial frustrated Lewis pairs, Energy Environ. Sci., 2021, 14, 6605–6615 RSC.
- R. L. Melen, A step closer to metal-free dinitrogen activation: a new chapter in the chemistry of frustrated Lewis pairs, Angew. Chem., Int. Ed., 2018, 57, 880–882 CrossRef CAS PubMed.
- Y. Ran, X. Yu, J. Liu, J. Cui, J. Wang, L. Wang, Y. Zhang, X. Xiang and J. Ye, Polymeric carbon nitride with frustrated Lewis pair sites for enhanced photofixation of nitrogen, J. Mater. Chem. A, 2020, 8, 13292–13298 RSC.
- L. Chen, J. Wang, X. Li, C. Zhao, X. Hu, Y. Wu and Y. He, A novel Z-scheme Bi-Bi2O3/KTa0.5Nb0.5O3 heterojunction for efficient photocatalytic conversion of N2 to NH3, Inorg. Chem. Front., 2022, 9, 2714–2724 RSC.
- R. Marschall, Semiconductor composites: strategies for enhancing charge carrier separation to improve photocatalytic activity, Adv. Funct. Mater., 2014, 24, 2421–2440 CrossRef CAS.
- M. Asadi, K. Kim, C. Liu, A. V. Addepalli, P. Abbasi, P. Yasaei, P. Phillips, A. Behranginia, J. M. Cerrato, R. Haasch, P. Zapol, B. Kumar, R. F. Klie, J. Abiade, L. A. Curtiss and A. Salehi-Khojin, Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid, Science, 2016, 353, 467–470 CrossRef CAS PubMed.
- J. Liang, Q. Liu, A. A. Alshehri and X. Sun, Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis, Nano Res. Energy, 2022, 1, e9120010 CrossRef.
- S. Zhao, Y. Wang, J. Dong, C.-T. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. M. Khattak, N. A. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao and Z. Tang, Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution, Nat. Energy, 2016, 1, 16184 CrossRef CAS.
- L. Feng, S. Yuan, L. L. Zhang, K. Tan, J. L. Li, A. Kirchon, L. M. Liu, P. Zhang, Y. Han, Y. J. Chabal and H. C. Zhou, Creating hierarchical pores by controlled linker thermolysis in multivariate metal-organic frameworks, J. Am. Chem. Soc., 2018, 140, 2363–2372 CrossRef CAS PubMed.
- Y. Liu, H. Bai, Q. Zhang, Y. Bai, X. Pang, F. Wang, Y. Yang, J. Ding, W. Fan and W. Shi, In-situ decoration of unsaturated Cu sites on Cu2O photocathode for boosting nitrogen reduction reaction, Chem. Eng. J., 2021, 413, 127453 CrossRef CAS.
- X. Li, W. Fan, Y. Bai, Y. Liu, F. Wang, H. Bai and W. Shi, Photoelectrochemical reduction of nitrate to ammonia over CuPc/CeO2 heterostructure: Understanding the synergistic effect between oxygen vacancies and Ce sites, Chem. Eng. J., 2022, 433, 133225 CrossRef CAS.
- H. Guo, H. Zhang, J. Zhao, P. Yuan, Y. Li, Y. Zhang, L. Li, S. Wang and R. Song, Two-dimensional WO3-transition-metal dichalcogenide vertical heterostructures for nitrogen fixation: a photo(Electro) catalysis theoretical strategy, J. Phys. Chem. C, 2022, 126, 3043–3053 CrossRef CAS.
- J. Zheng, Y. Lyu, M. Qiao, R. Wang, Y. Zhou, H. Li, C. Chen, Y. Li, H. Zhou, S. P. Jiang and S. Wang, Photoelectrochemical synthesis of ammonia on the aerophilic-hydrophilic heterostructure with 37.8% efficiency, Chem, 2019, 5, 617–633 CAS.
- S. T. Gyama, Preparation and catalytic properties of transition metal carbides and nitrides, Catal. Today, 1992, 15, 179–200 CrossRef.
- X. Li, M. Deng, W. Zhang, Q. Gao, H. Wang, B. Yuan, L. Yang and M. Zhu, Mo2C/N-doped carbon nanowires as anode materials for sodium-ion batteries, Mater. Lett., 2017, 194, 30–33 CrossRef CAS.
- L. Liu, W. An, H. Wang, H. Guo, Y. Liang and W. Cui, Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C-C coupling photothermal catalytic reduction of CO2 to ethanol, Appl. Catal., B, 2020, 266, 118618 CrossRef.
- F. You, J. Wan, J. Qi, D. Mao, N. Yang, Q. Zhang, L. Gu and D. Wang, Lattice distortion in hollow multi-shelled structures for efficient visible-light CO2 reduction with a SnS2/SnO2 Junction, Angew. Chem., Int. Ed., 2020, 59, 721–724 CrossRef CAS PubMed.
- M. Luo, Y. Sun, L. Wang and S. Guo, Tuning multimetallic ordered intermetallic nanocrystals for efficient energy electrocatalysis, Adv. Energy Mater., 2017, 7, 1602073 CrossRef.
- G. Yasin, S. Ibraheem, S. Ali, M. Arif, S. Ibrahim, R. Iqbal, A. Kumar, M. Tabish, M. A. Mushtaq, A. Saad, H. Xu and W. Zhao, Defects-engineered tailoring of tri-doped interlinked metal-free bifunctional catalyst with lower gibbs free energy of OER/HER intermediates for overall water splitting, Mater. Today Chem., 2022, 23, 100634 CrossRef CAS.
- M. A. Mushtaq, A. Kumar, G. Yasin, M. Arif, M. Tabish, S. Ibraheem, X. Cai, W. Ye, X. Fang, A. Saad, J. Zhao, S. Ji and D. Yan, 3D interconnected porous Mo-doped WO3@CdS hierarchical hollow heterostructures for efficient photoelectrochemical nitrogen reduction to ammonia, Appl. Catal., B, 2022, 317, 121711 CrossRef CAS.
- Y. Zhao, Y. Zhao, G. I. N. Waterhouse, L. Zheng, X. Cao, F. Teng, L. Z. Wu, C. H. Tung, D. O'Hare and T. Zhang, Layered-double-hydroxide nanosheets as efficient visible-light-driven photocatalysts for dinitrogen fixation, Adv. Mater., 2017, 29, 1703828 CrossRef PubMed.
- M. Arif, G. Yasin, L. Luo, W. Ye, M. A. Mushtaq, X. Fang, X. Xiang, S. Ji and D. Yan, Hierarchical hollow nanotubes of NiFeV-layered double hydroxides@CoVP heterostructures towards efficient, pH-universal electrocatalytical nitrogen reduction reaction to ammonia, Appl. Catal., B, 2020, 265, 118559 CrossRef CAS.
- S. Koynov, M. S. Brandt and M. Stutzmann, Black nonreflecting silicon surfaces for solar cells, Appl. Phys. Lett., 2006, 88, 203107 CrossRef.
- J. Oh, H. C. Yuan and H. M. Branz, An 18.2%-efficient black-silicon solar cell achieved through control of carrier recombination in nanostructures, Nat. Nanotechnol., 2012, 7, 743–748 CrossRef CAS PubMed.
- K. Peramaiah, V. Ramalingam, H. C. Fu, M. M. Alsabban, R. Ahmad, L. Cavallo, V. Tung, K. W. Huang and J. H. He, Optically and electrocatalytically decoupled Si photocathodes with a porous carbon nitride catalyst for nitrogen reduction with over 61.8% faradaic efficiency, Adv. Mater., 2021, 33, e2100812 CrossRef PubMed.
- H. Yang, C. Nan, N. Gao, W. Zhou, F. Gao, D. Dong, D. Dou, Y. Liu, Z. Liang and D. Yang, Three-phase interface of SnO2 nanoparticles loaded on hydrophobic MoS2 enhance photoelectrochemical N2 reduction, Electrochim. Acta, 2022, 430, 141086 CrossRef CAS.
- N. Gao, H. Yang, D. Dong, D. Dou, Y. Liu, W. Zhou, F. Gao, C. Nan, Z. Liang and D. Yang, Bi2S3 quantum dots in situ grown on MoS2 nanoflowers: an efficient electron-rich interface for photoelectrochemical N2 reduction, J. Colloid Interface Sci., 2022, 611, 294–305 CrossRef CAS PubMed.
- W. Ye, M. Arif, X. Fang, M. A. Mushtaq, X. Chen and D. Yan, Efficient photoelectrochemical route for the ambient reduction of N2 to NH3 based on nanojunctions assembled from MoS2 nanosheets and TiO2, ACS Appl. Mater. Interfaces, 2019, 11, 28809–28817 CrossRef CAS PubMed.
- X. Xu, X. Tian, B. Sun, Z. Liang, H. Cui, J. Tian and M. Shao, 1 T-phase molybdenum sulfide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions, Appl. Catal., B, 2020, 272, 118984 CrossRef CAS.
- X. Li, H. Liu, D. Luo, J. Li, Y. Huang, H. Li, Y. Fang, Y. Xu and L. Zhu, Adsorption of CO2 on heterostructure CdS(Bi2S3)/TiO2 nanotube photocatalysts and their photocatalytic activities in the reduction of CO2 to methanol under visible light irradiation, Chem. Eng. J., 2012, 180, 151–158 CrossRef CAS.
- Y. Hu, Z. L. Zhao, R. Ahmad, M. Harb, L. Cavallo, L. M. Azofra, S. P. Jiang and X. Zhang, A bifunctional catalyst based on a carbon quantum dots/mesoporous SrTiO3 heterostructure for cascade photoelectrochemical nitrogen reduction, J. Mater. Chem. A, 2022, 10, 12713–12721 RSC.
- X. Li, P. Shen, Y. Luo, Y. Li, Y. Guo, H. Zhang and K. Chu, PdFe single-atom alloy metallene for N2 electroreduction, Angew. Chem., Int. Ed., 2022, 61, e202205923 CAS.
- C. Kim, J. Y. Song, C. Choi, J. P. Ha, W. Lee, Y. T. Nam, D. M. Lee, G. Kim, I. Gereige, W. B. Jung, H. Lee, Y. Jung, H. Jeong and H. T. Jung, Atomic-scale homogeneous Ru−Cu alloy nanoparticles for highly efficient electrocatalytic nitrogen reduction, Adv. Mater., 2022, 34, e2205270 CrossRef PubMed.
- H. L. Du, M. Chatti, R. Y. Hodgetts, P. V. Cherepanov, C. K. Nguyen, K. Matuszek, D. R. MacFarlane and A. N. Simonov, Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency, Nature, 2022, 609, 722–727 CrossRef CAS PubMed.
- H.-L. Du, K. Matuszek, R. Y. Hodgetts, K. N. Dinh, P. V. Cherepanov, J. M. Bakker, D. R. MacFarlane and A. N. Simonov, The chemistry of proton carriers in high-performance lithium-mediated ammonia electrosynthesis, Energy Environ. Sci., 2023, 16, 1082–1090 RSC.
- R. Karimi, F. Yousefi, M. Ghaedi, K. Dashtian and G. Yasin, Unveiling charge dynamics of Co3S4 nanowalls/CdS nanospheres n-n heterojunction for efficient photoelectrochemical Cr(VI) detoxification and N2 fixation, J. Environ. Chem. Eng., 2022, 10, 108549 CrossRef CAS.
- X. Li, W. Fan, D. Xu, J. Ding, H. Bai and W. Shi, Boosted photoelectrochemical N2 reduction over Mo2C in situ coated with graphitized carbon, Langmuir, 2020, 36, 14802–14810 CrossRef CAS PubMed.
- Y. Abghoui, A. L. Garden, J. G. Howalt, T. Vegge and E. Skúlason, Electroreduction of N2 to ammonia at ambient conditions on mononitrides of Zr, Nb, Cr, and V: A DFT guide for experiments, ACS Catal., 2015, 6, 635–646 CrossRef.
- Z. Geng, Y. Liu, X. Kong, P. Li, K. Li, Z. Liu, J. Du, M. Shu, R. Si and J. Zeng, Achieving a record-high yield rate of 120.9 mug NH3 mgcat.−1 h−1 for N2 electrochemical reduction over Ru single-atom catalysts, Adv. Mater., 2018, e1803498 CrossRef PubMed.
- B. H. R. Suryanto, C. S. M. Kang, D. Wang, C. Xiao, F. Zhou, L. M. Azofra, L. Cavallo, X. Zhang and D. R. MacFarlane, Rational electrode–electrolyte design for efficient ammonia electrosynthesis under ambient conditions, ACS Energy Lett., 2018, 3, 1219–1224 CrossRef CAS.
- T. B. Yuan, Z. Hu, Y. X. Zhao, J. J. Fang, J. Lv, Q. H. Zhang, Z. B. Zhuang, L. Gu and S. Hu, Two-dimensional amorphous SnOx from liquid metal: mass production, phase transfer, and electrocatalytic CO2 reduction toward formic acid, Nano Lett., 2020, 20, 2916–2922 CrossRef CAS PubMed.
- C. Xu, X. Zhang, M.-N. Zhu, L. Zhang, P.-F. Sui, R. Feng, Y. Zhang and J.-L. Luo, Accelerating photoelectric CO2 conversion with a photothermal wavelength-dependent plasmonic local field, Appl. Catal., B, 2021, 298, 120533 CrossRef CAS.
- M. P. Kou, W. Liu, Y. Y. Wang, J. D. Huang, Y. L. Chen, Y. Zhou, Y. Chen, M. Z. Ma, K. Lei, H. Q. Xie, P. K. Wong and L. Q. Ye, Photocatalytic CO2 conversion over single-atom MoN2 sites of covalent organic framework, Appl. Catal., B, 2021, 291, 120146 CrossRef CAS.
- L. Li, C. Tang, B. Xia, H. Jin, Y. Zheng and S.-Z. Qiao, Two-dimensional mosaic bismuth nanosheets for highly selective ambient electrocatalytic nitrogen reduction, ACS Catal., 2019, 9, 2902–2908 CrossRef CAS.
- X. Yang, J. Nash, J. Anibal, M. Dunwell, S. Kattel, E. Stavitski, K. Attenkofer, J. G. Chen, Y. Yan and B. Xu, Mechanistic insights into electrochemical nitrogen reduction reaction on vanadium nitride nanoparticles, J. Am. Chem. Soc., 2018, 140, 13387–13391 CrossRef CAS PubMed.
- Y. Yao, H. Wang, X.-Z. Yuan, H. Li and M. Shao, Electrochemical nitrogen reduction reaction on ruthenium, ACS Energy Lett., 2019, 4, 1336–1341 CrossRef CAS.
- H. Feng, Z. Xu, L. Ren, C. Liu, J. Zhuang, Z. Hu, X. Xu, J. Chen, J. Wang, W. Hao, Y. Du and S. X. Dou, Activating titania for efficient electrocatalysis by vacancy engineering, ACS Catal., 2018, 8, 4288–4293 CrossRef CAS.
- Z. Yan, M. Ji, J. Xia and H. Zhu, Recent advanced materials for electrochemical and photoelectrochemical synthesis of ammonia from dinitrogen: one step closer to a sustainable energy future, Adv. Energy Mater., 2019, 10, 1922020 Search PubMed.
- J. Zheng, Y. Lyu, J.-P. Veder, B. Johannessen, R. Wang, R. De Marco, A. Huang, S. P. Jiang and S. Wang, Electrochemistry-assisted photoelectrochemical reduction of nitrogen to ammonia, J. Phys. Chem. C, 2021, 125, 23041–23049 CrossRef CAS.
- A. R. Singh, B. A. Rohr, J. A. Schwalbe, M. Cargnello, K. Chan, T. F. Jaramillo, I. Chorkendorff and J. K. Nørskov, Electrochemical ammonia synthesis—the selectivity challenge, ACS Catal., 2016, 7, 706–709 CrossRef.
- D. V. Yandulov and R. R. Schrock, Catalytic reduction of dinitrogen to ammonia at a single molybdenum center, Science, 2003, 301, 76–78 CrossRef CAS PubMed.
- H. K. Lee, C. S. L. Koh, Y. H. Lee, C. Liu, I. Y. Phang, X. Han, C.-K. Tsung and X. Y. Ling, Favoring the unfavored: selective electrochemical nitrogen fixation using a reticular chemistry approach, Sci. Adv., 2018, 4, eaar3208 CrossRef PubMed.
- P. J. Hill, L. R. Doyle, A. D. Crawford, W. K. Myers and A. E. Ashley, Selective catalytic reduction of N2 to N2H4 by a simple Fe complex, J. Am. Chem. Soc., 2016, 138, 13521–13524 CrossRef CAS PubMed.
- Y. Abghoui, A. L. Garden, V. F. Hlynsson, S. Bjorgvinsdottir, H. Olafsdottir and E. Skulason, Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design, Phys. Chem. Chem. Phys., 2015, 17, 4909–4918 RSC.
- H. Ali, M. Masar, A. C. Guler, M. Urbanek, M. Machovsky and I. Kuritka, Heterojunction-based photocatalytic nitrogen fixation: principles and current progress, Nanoscale Adv., 2021, 3, 6358–6372 RSC.
- N. Zhang, L. Li, Q. Shao, T. Zhu, X. Huang and X. Xiao, Fe-doped BiOCl nanosheets with light-switchable oxygen vacancies for photocatalytic nitrogen fixation, ACS Appl. Energy Mater., 2019, 2, 8394–8398 CrossRef CAS.
- T. Wang, C. Feng, J. Liu, D. Wang, H. Hu, J. Hu, Z. Chen and G. Xue, Bi2WO6 hollow microspheres with high specific surface area and oxygen vacancies for efficient photocatalysis N2 fixation, Chem. Eng. J., 2021, 414, 128827 CrossRef CAS.
- K. Hu, Z. Huang, L. Zeng, Z. Zhang, L. Mei, Z. Chai and W. Shi, Recent advances in MOF–based materials for photocatalytic nitrogen fixation, Eur. J. Inorg. Chem., 2021, 2022, e202100748 Search PubMed.
- D. Yan, H. Li, C. Chen, Y. Zou and S. Wang, Defect engineering strategies for nitrogen reduction reactions under ambient conditions, Small Methods, 2018, 3, 1800331 CrossRef.
- A. C. Nielander, J. M. McEnaney, J. A. Schwalbe, J. G. Baker, S. J. Blair, L. Wang, J. G. Pelton, S. Z. Andersen, K. Enemark-Rasmussen, V. Čolić, S. Yang, S. F. Bent, M. Cargnello, J. Kibsgaard, P. C. K. Vesborg, I. Chorkendorff and T. F. Jaramillo, A versatile method for ammonia detection in a range of relevant electrolytes via direct nuclear magnetic resonance techniques, ACS Catal., 2019, 9, 5797–5802 CrossRef CAS.
- Y.-C. Hao, Y. Guo, L.-W. Chen, M. Shu, X.-Y. Wang, T.-A. Bu, W.-Y. Gao, N. Zhang, X. Su, X. Feng, J.-W. Zhou, B. Wang, C.-W. Hu, A.-X. Yin, R. Si, Y.-W. Zhang and C.-H. Yan, Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water, Nat. Catal., 2019, 2, 448–456 CrossRef CAS.
- Y. Zhao, R. Shi, X. Bian, C. Zhou, Y. Zhao, S. Zhang, F. Wu, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates?, Adv. Sci., 2019, 6, 1802109 CrossRef PubMed.
- J. Yuan, X. Yi, Y. Tang, M. Liu and C. Liu, Efficient photocatalytic nitrogen fixation: enhanced polarization, activation, and cleavage by asymmetrical electron donation to N≡N bond, Adv. Funct. Mater., 2019, 30, 1906983 CrossRef.
- A. LeDuy and R. Samson, Testing of an ammonia ion selective electrode for ammonia nirogen measureture in the methanogenic sludge, Biotechnol. Lett., 1982, 4, 303–306 CrossRef CAS.
- H. Wang, L. Wang, Q. Wang, S. Ye, W. Sun, Y. Shao, Z. Jiang, Q. Qiao, Y. Zhu, P. Song, D. Li, L. He, X. Zhang, J. Yuan, T. Wu and G. A. Ozin, Ambient electrosynthesis of ammonia: electrode porosity and composition engineering, Angew. Chem., Int. Ed., 2018, 57, 12360–12364 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2023 |