Paper card-like electrochemical platform as a smart point-of-care device for reagent-free glucose measurement in tears†
Luca
Fiore
ab,
Ankita
Sinha
 c,
Narjiss
Seddaoui
a,
Jessica
di Biasio
a,
Federico
Ricci
d,
Goran M.
Stojanovic
c,
Narjiss
Seddaoui
a,
Jessica
di Biasio
a,
Federico
Ricci
d,
Goran M.
Stojanovic
 c and
Fabiana
Arduini
c and
Fabiana
Arduini
 *ab
*ab
aDepartment of Chemical Science and Technologies, University of Rome ‘‘Tor Vergata’’, via della Ricerca Scientifica 1, Rome 00133, Italy. E-mail: Fabiana.arduini@uniroma2.it
bSENSE4MED, Via Bitonto 139, Rome 00133, Italy
cFaculty of Technical Sciences, University of Novi Sad, 21000 Novi Sad, Serbia
dOphthalmology Unit, Department of experimental medicine and surgery, University of Rome “Tor Vergata”, Rome, Italy
First published on 9th March 2023
Abstract
This communication describes the development of polyvinyl chloride electrochemical system in which a paper layer loaded with reagents is inserted into the device, demonstrating a new concept of a paper card-like pad for a reagent-free and easy measurement of the target analyte in solution. This device detects glucose in artificial tears in the range of 0.2–2 mM with a detection limit of 50 μM by simply adding the artificial tears to the paper card-like pad. The novel configuration goes beyond the state of the art, widening the application range of paper in the design of smart analytical devices.
In the last decade, paper-based electrochemical (bio)sensors have attracted huge interest from the scientific community and industrial sectors for their sustainability, easy fabrication, facile design of multiplex analyses, and cost-effective waste management. Since the first example reported by Henry's group1 in which a paper-based electrochemical biosensing platform was developed for detecting glucose, lactate, and uric acid in serum, several configurations have been described in the literature.2–10 For instance, origami paper-based electrochemical biosensors11 have been fabricated by exploiting the foldability of paper, delivering a one-hand laboratory analytical tool, for (i) treating the sample, (ii) containing the reagents, and (iii) carrying out the measurement. Furthermore, as recently highlighted,12 the use of this eco-friendly support in the development of paper-based electrochemical (bio)sensors boosted the use of paper beyond the “simple” use of this sustainable material, indeed the paper network:
– serves as a reactor in which the nanomaterial was synthesized, allowing for nanomaterial-decorated paper ready to be used as a support, already modified with nanomaterials, for printing the electrochemical cell;13
– is used as a reservoir to contain the reagents by loading the paper network with a few μL of the reagent solution. In that case, after the solvent volatilization, the reagents remain inside the cellulose network, and the addition of the liquid sample dissolves them, delivering a reagent-free device;14
– is used as a preconcentrator by adding several aliquots of the sample through several steps by increasing the sensitivity of the measurement easily;15
– allows for the direct sampling of the analyte in aerosol without no additional sampling device, overcoming the limitations of the polyester- or alumina-based printed electrochemical sensors able to measure only liquid samples;16,17
– is used to print sensors for detecting the parameters and the target analyte in the solid sample, like the potential, pH, and chloride content in concrete-based structures;18,19
– easily collects sweat by using the capillarity properties of paper, even when combined with a Kapton-based sensor.20
In this communication, we demonstrate for the first time that paper serves as a card to insert in polyvinyl chloride (PVC)-based electrochemical cells for developing novel point-of-care devices. In detail, the paper card-like pad contains both electrolytes and a biocomponent for biosensing application, and thus the task required by the end-user is limited to the insertion of the paper card-like pad wetted with the sample to be analyzed. This setup allows for using PVC-based electrochemical cell for several measurements because the replacement of the paper card-like pad is the only requirement for different analyses. This approach avoids the waste of the printed electrochemical cell for different measurements.
Screen-printed biosensors for glucose detection are the most important of printed electrochemical sensors, being used daily by diabetic patients, significantly impacting their quality of life. In addition, this type of device has found a relevant position in the market, considering that the self-monitoring blood glucose device market surpassed USD 19 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of over 10% between 2021 and 2027, due to the growing prevalence of diabetes.21
However, in the last few years, the need for non-invasive analytical tools has opened the door to other devices as well, including optical, microwave, and electrochemical methods using other biofluids such as saliva, tears, sweat, and interstitial fluids.22
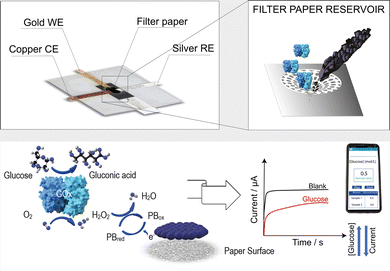
Herein, we use a paper card-like pad previously functionalized with the electrochemical mediator Prussian blue nanoparticles, carbon black, the enzyme glucose oxidase (GOx), and phosphate salts to detect glucose in artificial tears by simply inserting the paper card-like pad into a PVC-based electrochemical cell and adding the sample to be analyzed (Fig. 1). In detail, the paper card-like pad serves as a disposable and environmentally friendly component of this hybrid device, because it can be incinerated after use, while the PVC-based electrochemical cell can be reused for glucose monitoring, being conceived as an electrochemical cell where only the paper card-like pad is replaced between measurements.
The PVC-based electrochemical cell shown in Fig. S1 (ESI†) was produced by the xurography method using a cutter plotter and a card laminator (Fig. S2, ESI†). The electrochemical cell was initially developed with a three-electrode system produced with silver, aluminum, or gold foils (Fig. S3, ESI†). Using silver- and aluminum-based electrodes, no correct electrochemical behaviour was observed when tested using cyclic voltammetry with 140 μL of 5 mM ferro/ferricyanide solution in 0.1 M KCl as the electrochemical probe loaded onto the paper card-like pad, while the typical behaviour constituting anodic–cathodic peaks was observed using the system produced with gold electrodes (Fig. S4, ESI†). To fabricate a reliable and cost-effective PVC-based electrochemical cell, the pseudoreference electrode was fabricated using silver foil, the working electrode with gold foil, and the counter electrode with copper foil as shown in Fig. S1 (ESI†).
This novel configuration constituting a PVC-based electrochemical cell with insertion of a paper card-like pad was characterized over the scan range of 0.05–0.150 V s−1 by loading 80 μL of 5 mM ferro/ferricyanide solution in 0.1 M KCl on the paper card-like pad. The current of the anodic and cathodic peaks increases linearly with the square root of the scan rate, indicating a semi-infinite linear diffusion-controlled current (Fig. S5A, ESI†). Using the Nicholson method23 and considering an alpha value equal to 0.5 (owing to the fact that the ipa/ipc value is very close to unity), the heterogeneous rate constant (k0) was calculated in the case of potassium ferricyanide and found to be equal to 8.54 × 10−4 cm s−1.
This value is lower than the ones obtained using printed electrochemical sensors, unmodified and modified with carbon black and carbon nanotubes, tested in the solution of potassium ferricyanide.24 This behaviour is ascribed to the lower diffusion of the redox probe through the paper network. Because hydrogen peroxide is the enzymatic by-product of the glucose biosensor, the paper card-like pad has been modified by drop-casting with carbon black-Prussian blue nanoparticles (CB-PBNPs) dispersion to obtain a paper card-like pad containing the electrochemical mediator able to electrocatalyze the reduction of hydrogen peroxide at a potential close to 0 V vs. Ag pseudoreference.25 The selection of this dispersion was carried out considering that i) the (CB)-PBNPs dispersion is an effective and sensitive electrochemical mediator and ii) the use of dispersion allows for an easy mass-production of the modified paper card-like pad by drop-casting with automatic dispensing machines. The cyclic voltammograms obtained by adding 80 μL of phosphate buffer solution 0.05 M + KCl 0.1 M, pH = 7.4, to the PVC paper-card device, in which the paper card was previously modified with CB-PBNPs dispersion, are shown in Fig. S5B (ESI†). The results obtained highlighted the typical behaviour with a couple of redox peaks ascribed to the oxidation of Prussian white to Prussian blue and the reduction of Prussian blue to Prussian white. This electrochemical mediator is able to electrocatalyze the reduction of hydrogen peroxide because, in the presence of hydrogen peroxide, Prussian white is oxidized by this compound and it is consequently reduced at the working electrode surface, by supplying reductive current proportional to the amount of hydrogen peroxide present in the sample analyzed.25
The first parameter investigated was the amount of dispersion to be loaded onto the paper card-like pad. As reported in Fig. S6 (ESI†) the amount of 20 μL gave the best sensitivity and repeatability. In addition, 20 μL was selected, taking also into account the coverage of the paper card-like pad (Fig. S7, ESI†); indeed this amount allows for better coverage of the paper pad. Successively, the amount of the GOx enzyme to be immobilized on the CB-PBNPs paper card-like pad was optimized and chosen to be equal to 1.2 U because it represented the best result in terms of repeatability (Fig. S8, ESI†).
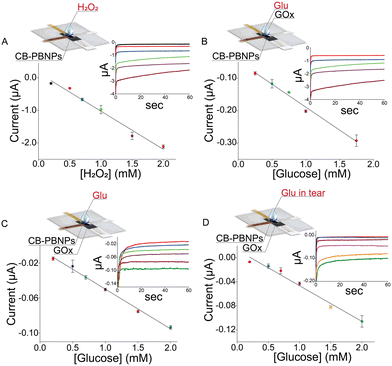
The suitability of this device containing the CB-PBNPs paper card-like pad was first evaluated by analyzing different concentrations of hydrogen peroxide in the range between 0.2 mM and 10 mM. Fig. 2A clearly shows a linearity of up to 2 mM, described by the following equation: y = (−0.15 ± 0.06) − (1.17 ± 0.01) x, R2 = 0.973, with a sensitivity of 1.17 μA mM−1.
To demonstrate the effectiveness of the reagent-free device approach, calibration curves were carried out by adding glucose together with the GOx enzyme to the device (i.e. the biosensing system in which the enzyme is added together with the sample) (Fig. 2B) or by adding glucose to the device, in which the card is already loaded with the GOx enzyme (i.e. the biosensor in which the enzyme is immobilized onto the paper and it is necessary to add only the sample) (Fig. 2C).
The calibration curve obtained using GOx in solution was described by the following equation: y = (−0.048 ± 0.006) − (0.141 ± 0.006) x, R2 = 0.988, with a detection limit (LOD) equal to 0.11 mM (calculated as 3 times the standard deviation of y-residual/slope of the curve) with a RSD% equal to 2%, testing 1 mM glucose. In the case of immobilized GOx, the calibration curve was described by the following equation: y = (−0.004 ± 0.002) – (0.046 ± 0.001) x, R2 = 0.985, with a LOD equal to 0.13 mM and a RSD% equal to 3%, testing 1 mM glucose. The results obtained showed better sensitivity in the case of the enzyme in solution due to the better capability of the enzyme to react with its substrate, i.e. glucose, in solution. Considering the concentration level (mM) of the glucose in the tears and the easy analysis using a reagent-free device, the configuration with the immobilized enzyme was selected for further studies.
After the analytical characterisation of this device in standard solutions, we tested the paper card-like device in the matrix. To assess the reliability of the sensor to detect glucose in human tears, the calibration curve was carried out in artificial tears. We first tested the artificial tears prepared according to a previous study26 to evaluate if the compounds present in the artificial tears serve as interferents. In detail, ammonium bicarbonate (2.9 mM) and potassium bicarbonate (23.1 mM) were added to deionized water with the aim of preparing a buffer stock. Then, 10 μL of histamine solution (90 μM) and 1 mL of mixture solution of peroxidase (1.32 μM), IgG (0.2 μM), sodium citrate (0.3 mM), lysozyme (0.2 mM), urea (62.7 mM), and sialic acid (11.4 mM) were added into 8.89 mL of the buffer stock to prepare the artificial tear solution.
By analysing the artificial tear solution, we observed that the current is close to the value of the phosphate buffer solution (i.e. 0.005 μA), demonstrating that no interference of compounds is usually present in tears. Then several amounts of glucose were added to the artificial tear solution for constructing the calibration curve, described by the following equation: y = (−0.013 ± 0.003) − (0.060 ± 0.003) x, R2 = 0.984, with a LOD equal to 0.05 mM and RSD% equal to 6%, testing 1 mM glucose. As highlighted in Fig. 2D, the linearity ranged between 0.2 and 2 mM, which is the concentration usually detected in tears.27 To evaluate the accuracy, considering the absence of the certified samples, we have followed 96/23/CE, which approved the recovery study to evaluate the trueness of the analytical system in the absence of the certified sample. Thus, the artificial tear was spiked with glucose to have final concentrations of 0.5, 1.0, and 1.5 mM. Testing these solutions with the developed device, recovery values equal to 90 ± 12%, 94 ± 4%, and 107 ± 3% were obtained, respectively, demonstrating the good accuracy and thus the reliability of this novel point-of-care device.
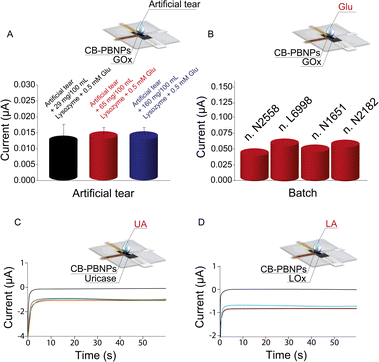
Because tears are characterized by high variability in the content of components in the function of diseases and their type (i.e. basal, reflex, and emotional), we have also evaluated the effect of the different amounts of lysozyme, which is a well-established component present in significantly different amounts in basal and reflex tears.28 In detail, 80 μL of tears with two different amounts of lysozyme (65 mg/100 mL and 160 mg/100 mL) were used to simulate basal and reflex tears, respectively. To evaluate the recovery, 0.5 mM of glucose was selected as the level of concentration to test. As reported in Fig. 3A, a comparable increase in the reduction current was observed when these different tears were spiked with glucose as well as the artificial tear composition used in the entire work. Recovery values were found to be equal to 92 ± 8% and 94 ± 5%, respectively, for simulated basal and reflex tears, demonstrating the absence of the lysozyme effect.
Additionally, the robustness was evaluated by varying the paper batches. Three different paper batches, namely, N1651, L6998, and N2182, were purchased from Cordenons SpA (Milano, Italy) and used to fabricate another three different types of the paper card-like PVC device. In detail, three different paper layers belonging to these three different batches were modified with CB-PBNPs and GOx, as previously described, and then used for the detection of 1 mM of glucose. As shown in Fig. 3B, the variation in the intensity of the current obtained with different batches, compared with the one obtained with the batch used in the entire work N2558, is within the experimental error range, demonstrating the robustness of the developed paper card-like device.
To further demonstrate its versatility, the paper card-like pad was modified with uricase or lactate oxidase for the detection of uric acid and lactate, respectively. As shown in Fig. 3C and D, adding 80 μL of uric acid or lactate (1 mM) allows for an increase in reduction current. This behaviour is ascribed to the enzymatic reaction with the consequent production of hydrogen peroxide, which is reduced at the electrode surface, demonstrating the capability of this device to be used in combination with other oxidase enzymes, widening its application range.
In this communication, we reported the first example of a paper layer (paper card-like pad) integrated into a PVC-based electrochemical cell. The paper card-like pad is able to contain the following:
(i) an electrochemical mediator for sensitive measurements of the GOx enzyme by-product, i.e. hydrogen peroxide, at a potential close to 0 vs. Ag pseudoreference;
(ii) the immobilized GOx enzyme for sensitive and selective glucose detection in artificial tears.
The novel idea relies on the use of paper as a pad preloaded with reagents combined with a plastic-based fluidic system, delivering a reagent-free device also in the case of plastic-based fluidic analytical tools. This new concept was demonstrated in the case of this fluidic device, but can be enlarged to microfluidic systems. The use of paper integrated into the microfluidic device will be able to furnish different reagents in different zones of the microfluidic device. Furthermore, this configuration will avoid the undesirable gas bubble problem which usually occurs in microfluidic systems, thanks to the exploitation of the capillarity of the paper to manage the flow.
The analytical platform reported here was successfully applied for glucose detection in artificial tears, with the advantages of being faster, requiring only one enzyme, no light environmental dependence, and reuse of the electrodes, when compared with other paper-based optical or electrochemical sensing systems (Table S1, ESI†).
Finally, this device paves the way for novel hybrid paper-based point-of-care devices, considering its potentiality to be extended to other analytes in different biological fluids including sweat and saliva.
This study has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement no. 854194 and the Project E-Crome (A0375-2020-36563), Lazio Innova, Regione Lazio.
Conflicts of interest
There are no conflicts to declare.References
- A. Dungchai, W. Chailapakul and C. S. Henry, Anal. Chem., 2009, 81, 5821 CrossRef PubMed.
- E. Noviana, C. P. McCord, K. M. Clark, I. Jang and C. S. Henry, Lab Chip, 2020, 20, 9 RSC.
- E. Noviana and C. S. Henry, Curr. Opin. Electrochem., 2020, 23, 1 CrossRef CAS.
- N. A. Meredith, C. Quinn, D. M. Cate, T. H. Reilly, J. Volckens and C. S. Henry, Analyst, 2016, 141, 1874 RSC.
- T. Gebretsadik, T. Belayneh, S. Gebremichael, W. Linert, M. Thomas and T. Berhanu, Analyst, 2018, 144, 2467 RSC.
- E. Noviana, D. B. Carrão, R. Pratiwi and C. S. Henry, Anal. Chim. Acta, 2020, 1116, 70 CrossRef CAS PubMed.
- V. N. Ataide, L. F. Mendes, L. I. Gama, W. R. de Araujo and T. R. Paixão, Anal. Methods, 2020, 12, 1030 RSC.
- W. Mazurkiewicz, M. Podrażka, E. Jarosińska, K. K. Valapil, M. Wiloch, M. Jönsson-Niedziółka and E. Witkowska Nery, ChemElectroChem, 2020, 7, 2939 CrossRef CAS.
- V. Caratelli, E. Di Meo, N. Colozza, L. Fabiani, L. Fiore, D. Moscone and F. Arduini, J. Mater. Chem. B, 2022, 10, 9021 RSC.
- M. Baharfar, M. Rahbar, M. Tajik and G. Liu, Biosens. Bioelectron., 2020, 167, 112506 CrossRef CAS PubMed.
- N. Colozza, V. Caratelli, D. Moscone and F. Arduini, Biosensors, 2021, 11, 328 CrossRef CAS PubMed.
- F. Arduini, Curr. Opin. Electrochem., 2022, 35, 101090 CrossRef CAS.
- N. Bagheri, V. Mazzaracchio, S. Cinti, N. Colozza, C. DiNatale, P. A. Netti, M. Saraji, S. Roggero, D. Moscone and F. Arduini, Anal. Chem., 2021, 93, 5225 CrossRef CAS PubMed.
- G. Scordo, D. Moscone, G. Palleschi and F. Arduini, Sens. Actuators, B, 2018, 258, 1015 CrossRef CAS.
- N. Bagheri, S. Cinti, E. Nobile, D. Moscone and F. Arduini, Talanta, 2021, 232, 122474 CrossRef CAS PubMed.
- N. Colozza, K. Kehe, G. Dionisi, T. Popp, A. Tsoutsoulopoulos, D. Steinritz, D. Moscone and F. Arduini, Biosens. Bioelectron., 2019, 129, 15 CrossRef CAS PubMed.
- D. Maier, E. Laubender, A. Basavanna, S. Schumann, F. Güder, G. A. Urban and C. Dincer, ACS Sens., 2019, 4, 2945 CrossRef CAS PubMed.
- N. Colozza, S. Tazzioli, A. Sassolini, L. Agosta, M. G. di Monte, K. Hermansson and F. Arduini, Anal. Chem., 2021, 93, 14369 CrossRef CAS PubMed.
- N. Colozza, A. Sassolini, L. Agosta, A. Bonfanti, K. Hermansson and F. Arduini, ChemElectroChem, 2020, 7, 2274 CrossRef CAS.
- V. Mazzaracchio, L. Fiore, S. Nappi, G. Marrocco and F. Arduini, Talanta, 2021, 222, 121502 CrossRef CAS PubMed.
- https://www.gminsights.com/industry-analysis/self-monitoring-blood-glucose-devices-market?gclid=CjwKCAjwiJqWBhBdEiwAtESPaK4fyjycbECbshhpHAP2Eyb38CpjdM9nt-jav5-5Od9QZPwOyOBlTBoCFeoQAvD_BwE, accessed on 25th February 2023.
- L. Tang, S. J. Chang, C. J. Chen and J. T. Liu, Sensors, 2020, 20, 6925 CrossRef CAS PubMed.
- R. S. Nicholson, Anal. Chem., 1965, 37, 1351 CrossRef CAS.
- E. V. Suprun, F. Arduini, D. Moscone, G. Palleschi, V. V. Shumyantseva and A. I. Archakov, Electroanalysis, 2012, 24, 1923 CrossRef CAS.
- S. Cinti, F. Arduini, G. Vellucci, I. Cacciotti, F. Nanni and D. Moscone, Electrochem. Commun., 2014, 47, 63 CrossRef CAS.
- M. Park, H. Jung, Y. Jeong and K. H. Jeong, ACS Nano, 2017, 11, 438 CrossRef CAS PubMed.
- H. Lee, Y. J. Hong, S. Baik, T. Hyeon and D. H. Kim, Adv. Healthcare Mater., 2018, 7, 1701150 CrossRef PubMed.
- R. N. Stuchell, R. L. Farris and I. D. Mandel, Ophthalmology, 1981, 88, 858 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06561d |
| This journal is © The Royal Society of Chemistry 2023 |