Highly efficient exciplex-based OLEDs incorporating a novel electron donor†
Qi-Sheng
Tian
a,
Xiang-Dong
Zhu
a and
Liang-Sheng
Liao
 *ab
*ab
aJiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, Suzhou, Jiangsu 215123, China. E-mail: lsliao@suda.edu.cn
bInstitute of Organic Optoelectronics, Jiangsu Industrial Technology Research Institute (JITRI), Wujiang, Suzhou, Jiangsu 215211, China
First published on 13th April 2020
Abstract
A novel triphenylamine (TPA) derivative molecule 4,4,8,8,12,12-hexaphenyl-8,12-dihydro-4H-benzo[9,1]quinolizino[3,4,5,6,7-defg]acridine (DEX) is presented as an electron donor to form an effective exciplex system with an electron acceptor molecule ((1,3,5-triazine-2,4,6-triyl)tris(benzene-3,1-diyl))tris(diphenylphosphine oxide) (PO-T2T) for fabricating high performance exciplex-based organic light-emitting diodes (OLEDs). If the DEX:PO-T2T exciplex itself is used as an emitting layer without any doped luminescent emitter in an OLED, it can exhibit green emission with a maximum power efficiency (PEmax), current efficiency (CEmax), and external quantum efficiency (EQEmax) of 44.6 lm W−1, 36.0 cd A−1, and 11.2%, respectively. The high device performance indicates that the green exciplex would have balanced charge transport properties, a broad recombination zone, and a suitable triplet energy level (ET), which is therefore expected to serve as an ideal host for a red phosphorescent emitter. Indeed, due to the desirable thermal and electrical properties of donor DEX, an optimized exciplex-based red phosphorescent OLED (PhOLED), doped with iridium(III) bis(2-methyldibenzo[f,h]quinoxaline)acetylacetonate (Ir(MDQ)2(acac)), achieved an impressive PEmax, CEmax, and EQEmax of 46.1 lm W−1, 36.0 cd A−1, and 24.5% as well as a low operating voltage (2.4 V at 0.2 mA cm−2). Remarkably, both an excellent EQE over 22% and low operating voltage of 3.4 V remained even at a high brightness of 1000 cd m−2. The high EQE and low efficiency roll-off reveal that the new exciplex system using novel donor DEX enables improved OLED performance, which paves the way to practical applications of OLEDs.
Introduction
Organic light-emitting diodes (OLEDs) have opened a reliable avenue in optoelectronic devices due to their inherent characteristics, such as high-contrast, high-efficiency, and flexibility.1–3 The development of conventional fluorescent material-based OLEDs is impeded by the limited internal quantum efficiency (IQE) of 25% without being capable of utilization of the remaining 75% noraditive triplet excitons.4 Over the past decades, 2nd generation OLEDs employing phosphorescent emitters have offered striking EQE via harvesting both singlet and triplet excitons for 100% internal quantum efficiency (IQE).5–7 Recently, thermally activated delayed fluorescence (TADF) materials commonly exhibit typical intramolecule charge transfer (ICT) behavior, which show an effective reverse intersystem crossing (RISC) process and small singlet–triplet energy splitting (ΔEST).8–10 So, they have the ability to efficiently utilize both singlet and triplet excitons by converting triplet excitons from the triplet excited state (T1) into the singlet excited state (S1). Thereby, phosphorescent and TADF materials are widely considered as promising strategies to sufficiently utilize excitons for achieving high performance devices.Exciplex systems comprising an electron donor and an electron acceptor can contribute to intermolecular charge transfer accompanied by effective RISC and a small ΔEST.11–13 So the exciplex is considered as another type of efficient TADF emitter to fabricate high-efficiency exciplex-based fluorescent OLEDs.13,14 It's worth noting that an exciplex is generally a combination of an electron-transporting material and a hole-transporting material, which can realize balanced charge transport, a negligible injection barrier, and a broad recombination zone. Therefore, several exciplex forming hosts have been developed and they played a crucial role to reduce the operating voltage in devices for enhancing the power efficiency (PE).15–17 Ideally, an exciplex system can be not only regarded as the emitting layer to fabricate efficient fluorescent OLEDs, but also serve as a co-host to enhance the performance of emitters. Thus, designing an effective exciplex system is in demand for the development of OLEDs.
However, it is relatively rare to select an electron donor for fabricating an exciplex system in order to achieve high performance OLEDs, because most researchers pay more attention to designing electron acceptor materials, e.g., star-shaped 1,3,5-triazine/cyano hybrid molecule CN-T2T,18 heptazine derivative HAP-3MF,13 benzimidazole-triazine-based electron acceptor PIM-TRZ,19 and isomeric N-linked benzoimidazole materials (iTPyBIB, iTPBIPy, and iTPyBIPy).20 These electron acceptor materials have shown significant roles in highly efficient exciplex systems. In contrast, the common electron donors widely utilized in exciplex systems are mainly restricted to commercial materials, such as 1,3-di-9-carbazolylbenzene (mCP),15 1,1-bis[4-[N′,N′-di(p-tolyl)amino]-phenyl]cyclohexane (TAPC),17 4,4′,4′′-tris[3-methylphenyl(phenyl)amino] triphenylamine (m-MTDATA),11 and N,N,N-tris(4-(9-carbazolyl)phenyl)amine (TCTA).21 It is therefore necessary to develop more appropriate electron donor materials to match suitable electron acceptor materials in fabricating effective exciplex systems for obtaining high performance OLEDs with high EQE and PE, and low operating voltage. With the expectation to design ideal electron donor materials, the following prerequisites should be mentioned: (i) obtaining a suitable ET of the donor for ensuring effective energy transfer; (ii) achieving satisfactory highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels to match well with the acceptor material and the adjacent charge transport materials for ensuring the formation of an exciplex; and (iii) realizing low cost, an uncomplicated synthesis procedure, and good thermal properties.
Reported herein is a new triphenylamine (TPA) derivative-based electron donor molecule 4,4,8,8,12,12-hexaphenyl-8,12-dihydro-4H-benzo[9,1]quinolizino[3,4,5,6,7-defg]acridine (DEX) that shows a desirable triplet energy level (ET = 2.75 eV), good thermal stability, a facile synthetic process, and appropriate HOMO and LUMO levels. The electron donor DEX and an electron-transporting molecule ((1,3,5-triazine-2,4,6-triyl)tris(benzene-3,1-diyl))tris(diphenylphosphine oxide) (PO-T2T) were selected to fabricate a suitable exciplex system. An exciplex-based fluorescent OLED was first proposed and it can exhibit green emission peaking at 520 nm. Importantly, the green exciplex-based OLED can realize a maximum EQE (EQEmax) and PE (PEmax) and low operating voltage (at 0.2 mA cm−2) of 11.2%, 48.1 lm W−1 and 2.5 V, respectively, which are superior to those of traditional fluorescent OLEDs. Furthermore, the green emission exciplex was proved as a promising co-host in devices to reduce the operating voltage and to broaden the exciton recombination zone for improving the performance of red phosphorescent emitters, such as iridium(III) bis(2-methyldibenzo[f,h]quinoxaline)acetylacetonate Ir(MDQ)2(acac). Finally, benefiting from the exciplex forming co-host, the optimized red phosphorescent OLED (PhOLED) with Ir(MDQ)2(acac) as the emitter shows remarkable performance with an EQEmax, PEmax, and operating voltage (at 0.2 mA cm−2) of 24.5%, 46.1 lm W−1 and 2.4 V, respectively. These superior results of the efficient green and red exciplex-based OLEDs can be ascribed to the properties of DEX, which has a suitable ET, good photophysical characteristics, better carrier transport capability, and suitable frontier molecular orbitals (FOMs) as well as a reasonable device structure to facilitate charge injection and recombination.
Results and discussion
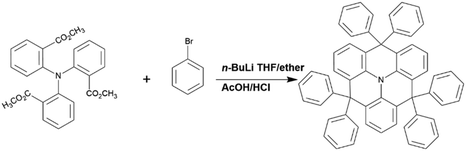
The synthetic route and chemical structure of the donor DEX are outlined in Scheme 1. The specific steps of the synthetic process are expressed in the Experimental section. It clearly shows that the synthetic process is particularly uncomplicated and convenient, proving that it has the practical potential for fabrication and applications. For investigating the electronic structure of DEX, density functional theory (DFT) calculations were applied at the B3LYP/6-31G(d) level. The HOMO/LUMO FMO distributions and energy levels are depicted in Fig. 1. The HOMO energy level (−5.03 eV) and LUMO energy level (−2.03 eV) of DEX can be calculated. From the ground state geometry, a singlet energy level (ES) and ET of 3.1 and 2.6 eV can be found, respectively.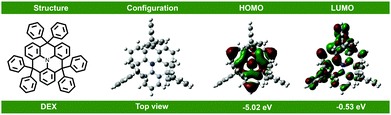 | ||
| Fig. 1 Chemical structure, calculated molecular HOMO and LUMO distributions, and energy levels of DEX. | ||
To explore the electrochemical properties of DEX, the HOMO energy level was estimated through ultraviolet photospectroscopy (UPS) measurements (Fig. 2a). The UV-vis absorption and photoluminescence (PL) spectra of DEX in dilute CH2Cl2 solution at room temperature are shown in Fig. 2b. From the UPS curves we can conclude that DEX has a HOMO energy level of −5.8 eV, which is helpful to transfer holes.22 The maximum absorption at a wavelength around 300 nm is ascribed mainly to the π–π* intramolecular transition. The onset of the UV-vis absorption edge of DEX is at about 344 nm, so the optical bandgap (Eg) can be estimated to be 3.6 eV. Hence, the LUMO energy level of DEX can be calculated according to the equation Eg = −(EHOMO − ELUMO) as ca. −2.2 eV. Meanwhile, the electrochemical properties of DEX were investigated by cyclic voltammetry (CV); from the reversible oxidation curve we can conclude that DEX is electrochemically stable (shown in Fig. S4, ESI†). The wavelength of the peak emission of the phosphorescence spectrum at 77 K is 450 nm, indicating that the ET of DEX is about 2.75 eV. The high T1 level of DEX is beneficial to ensure energy transfer from the host to the emitters effectively.23 The device performance is susceptible to the thermal properties of the organic material. As shown in Fig. S3 (ESI†), DEX exhibits distinguished thermal stability with a high decomposition temperature (Td) of 397 °C (refers to 5% weight loss), which was calculated from thermogravimetric analysis (TGA). The high Td indicates that DEX has excellent thermal properties, which is ascribed to its rigid molecular structure. The detailed photophysical properties are summarized in Table 1.
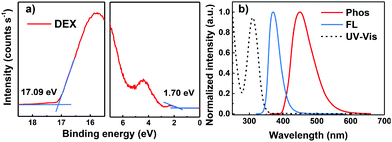 | ||
| Fig. 2 (a) UPS spectrum of the DEX film, and (b) UV-vis absorption in CH2Cl2 and the fluorescence spectrum in toluene (300 K) as well as the phosphorescence (toluene, 77K) spectrum of DEX. | ||
| Molecule | Abs λmaxa [nm] | PL λmaxa [nm] | T d [°C] | E g [eV] | E T [eV] | HOMOe [eV] | LUMOf [eV] |
|---|---|---|---|---|---|---|---|
| a Tested in toluene solution at room temperature. b T d: Decomposition temperature. c E g: Optical band gap energy was measured from the corresponding absorption onset. d E T: Measured in a toluene glass matrix at 77 K. e HOMO energy level was calculated from UPS data. f LUMO level was obtained from the results of the HOMO and Eg. | |||||||
| DEX | 344 | 370 | 397 | 3.6 | 2.7 | −5.8 | −2.2 |
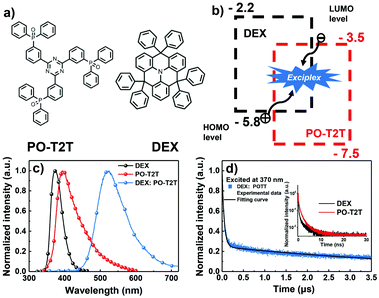
In order to fabricate an exciplex system with an effective intermolecular charge transfer process, a considerable offset (>0.4 eV) between the HOMO/LUMO energy levels of the donor/acceptor should be achieved.24 Hence, an electron-transporting material PO-T2T was chosen to form an exciplex with DEX owing to its ideal HOMO/LUMO (−3.5/−7.5 eV) energy levels.15 Thus the energy level offset between DEX and PO-T2T is large enough to form an exciplex. The molecular configurations of DEX and PO-T2T are depicted in Fig. 3a. The exciplex forming process and energy level diagrams are illustrated in Fig. 3b.
To verify the formation of the DEX:PO-T2T exciplex, the PL spectrum of exciplex DEX:PO-T2T with peak emission at a wavelength of 520 nm was observed (shown in Fig. 3c). It is clearly found that the PL of the exciplex is red shifted compared with those of DEX (370 nm) and PO-T2T (410 nm), which means that an exciplex was generated.25 The photo energy level of exciplex (ES,ex) DEX:PO-T2T was experimentally measured to be 2.38 eV according to the PL peak emission (520 nm), which is consistent with the energy difference between the HOMO energy level (−5.8 eV) of DEX and the LUMO energy level (−3.5 eV) of PO-T2T.23 The triplet energy level of the exciplex (ET,ex) was estimated to be 2.3 eV based on previously reported exciplex-based work, which means that the exciplex system has a dramatically negligible ΔEST.26–28 These results demonstrate that electron–hole coupling was generated under external excitation. Additionally, the transient fluorescence decay curves of DEX:PO-T2T and the pristine molecules are shown in Fig. 3d. DEX and PO-T2T only exhibit decay lifetimes of a few nanoseconds. In contrast, prompt and delayed components could be observed in the DEX:PO-T2T transient decay curve. The exciplex exhibits a short and long decay lifetime of 30 ns and 1.2 μs, respectively. The aforementioned results reveal certain evidence that the formation of exciplex DEX:PO-T2T possessing small ΔEST with an effective RISC process is achieved.
To further study the potential advantages of the DEX:PO-T2T exiplex system, a DEX:PO-T2T exciplex-based OLED was first fabricated to investigate its electroluminescence (EL) properties. For better comparison, a classic hole-transporting material 4,4,4-tris(N-carbazolyl)triphenylamine (TCTA) (ET = 2.76), which has HOMO and LUMO levels of −5.8 and −2.4 eV, respectively, was employed to fabricate an exciplex with PO-T2T.29 As shown in Fig. S5 (ESI†), the exciplex TCTA:PO-T2T shows a broad spectrum with a wavelength of 520 nm, which is red shifted compared with TCTA (400 nm) and PO-T2T, indicating the formation of an exciplex. The ET values of exciplexes DEX:PO-T2T (2.3 eV) and TCTA:PO-T2T are very close according to their emission peaks (520 nm). The devices were designed with the structure of ITO/HAT-CN (10 nm)/TAPC (30 nm)/DEX or TCTA (10 nm)/DEX:PO-T2T or TCTA:PO-T2T (20 nm)/PO-T2T (45 nm)/Liq (2 nm)/Al (120 nm). Hexaaza-triphenylene hexacarbonitrile (HAT-CN) and lithium quinolate (Liq) were employed as hole and electron-injecting layers (HIL and EIL), respectively.25TAPC can serve as a hole-transporting layer (HTL) owing to its suitable HOMO energy level (−5.6 eV) and high ET (2.98 eV),30 which matches well with DEX and TCTA, so that the holes can smoothly transfer from the anode to the emission zone for generating excitons with electrons. The additional 10 nm DEX or TCTA was introduced as an electron-blocking layer to prevent the undesirable formation of exciplex TAPC:PO-T2T.31
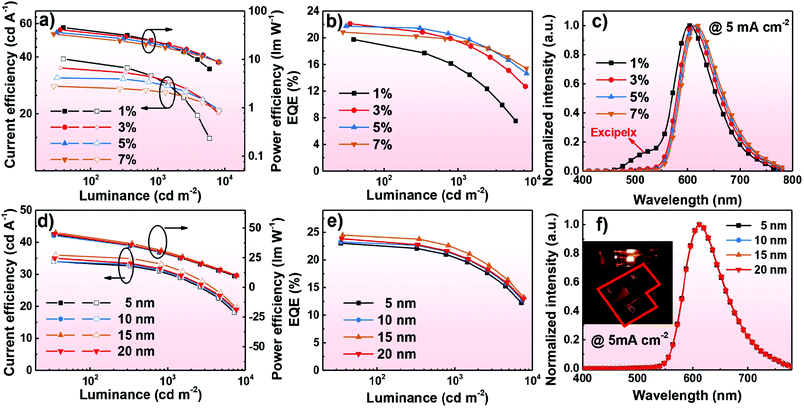
The EL characteristics of the exciplex DEX:PO-T2T (Green1) and TCTA:PO-T2T (Green2) OLEDs are presented in Fig. 4. The exciplex-based OLEDs realized typical green emission with a peak at 520 nm, which is consistent with the PL one. So all excitons generated on DEX:PO-T2T and TCTA:PO-T2T are sufficiently used for the emission of the exciplex without energy loss. As the current density–voltage–luminance (J–V–L) curves show (Fig. 4a), low operating voltages of 2.6 and 3.5 V at a luminance of 100 and 1000 cd m−2 were achieved in Green1. Notably, these values are lower than that of the TCTA-based OLED, indicating that DEX has better carrier transport properties compared with TCTA.32 The low operating voltages of both devices are due to the good injection and the direct recombination of holes from the HOMO of DEX or TCTA and electrons from the LUMO of PO-T2T. Because of the low operating voltages of the DEX-based device, an excellent PEmax and maximum CE (CEmax) of 44.1 lm W−1 and 36 cd A−1 were achieved. For the TCTA-based OLED, a PEmax and CEmax of 17.4 lm W−1 and 15.0 cd A−1 can be realized. Finally, the DEX and TCTA-based devices exhibited an EQEmax of 11.2% and 4.0%, respectively. The substantial difference in the performance between the two devices is mainly ascribed to the superior properties of DEX, having good carrier transport and a rigid molecular structure. Additionally, though they have the same HOMO levels, the LUMO level of DEX (−2.2 eV) is higher than that of TCTA (−2.4 eV), which means that electrons are blocked more effectively to recombine with holes for generating excitons and then emitting light in the DEX based exciplex system. Particularly, it should be mentioned that the high EQE of 11.2% is among the excellent efficiencies of exciplex-based OLEDs without any luminescent dopant (see Table S1, ESI†). The detailed EL performances of Green1 and Green2 are listed in Table 2.
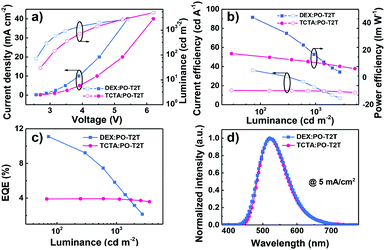 | ||
| Fig. 4 (a) J–V–L plots, (b) CE–luminance–PE plots, (c) EQE–luminance plots, and (d) EL (at 5 mA cm−2) spectra of DEX:PO-T2T and TCTA:PO-T2T exciplex-based devices. | ||
| Devicesa | V [V] | η CE [cd A−1] | η PE [lm W−1] | η ext [%] | CIEd [x, y] |
|---|---|---|---|---|---|
| a Devices in this work. b Voltages at 0.2 mA cm−2. c Efficiencies of the maximum and at a brightness of 1000 cd m−2. d CIE coordinates measured at 5 mA cm−2. | |||||
| Green1 | 2.5 | 36.0, 18.2 | 44.1, 16.8 | 11.2, 5.8 | 0.29, 0.55 |
| Green2 | 2.7 | 15.0, 14.3 | 17.5, 11.4 | 4.0, 3.8 | 0.29, 0.55 |
| Red1 | 2.7 | 39.0, 31.5 | 45.3, 24.2 | 19.7, 16.0 | 0.54, 0.42 |
| Red2 | 2.7 | 35.0, 31.0 | 40.8, 24.1 | 22.1, 19.6 | 0.60, 0.38 |
| Red3 | 2.7 | 31.0, 29.2 | 36.2, 21.8 | 21.7, 20.4 | 0.62, 0.37 |
| Red4 | 2.6 | 28.0, 26.4 | 33.3, 19.9 | 20.8, 19.6 | 0.63, 0.36 |
| Red5 | 2.4 | 34.0, 30.5 | 44.2, 27.7 | 23.0, 20.9 | 0.62, 0.37 |
| Red6 | 2.4 | 34.0, 31.2 | 43.7, 27.7 | 23.3, 21.3 | 0.62, 0.37 |
| Red7 | 2.4 | 36.0, 33.1 | 46.1, 39.7 | 24.5, 22.4 | 0.62, 0.37 |
| Red8 | 2.4 | 35.0, 31.3 | 45.2, 28.1 | 23.8, 21.4, | 0.62, 0.37 |
The highly efficient DEX:PO-T2T exciplex system is expected to be employed as a co-host for improving the efficiencies of PhOLEDs. According to the above results of the DEX:PO-T2T exciplex system, we believe that the exciplex should act well as a host for red emitters. The energy-level diagram and chemical structures of each material in the exciplex-based red PhOLEDs are shown in Fig. 5a–c.33,34 Red PhOLEDs were fabricated with different electron-injection layers (EILs) corresponding to different emitting layers. The exciplex DEX:PO-T2T was first successfully used as a co-host for the red phosphorescent emitter Ir(MDQ)2(acac)-based OLED with the configuration of ITO/HAT-CN (10 nm)/TAPC (30 nm)/DEX (10 nm)/DEX:PO-T2T:Ir(MDQ)2(acac) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, X wt%, X = 1, 3, 5, and 7, 20 nm)/PO-T2T (50 nm)/Liq (2 nm)/Al (120 nm); X = 1, 3, 5, and 7 correspond to the devices R1, R2, R3, and R4. Different doping ratios of the emitter were used to investigate the EL performance of the red devices. The EL characteristics of the red devices are shown in Fig. 6a–c. These devices show low onset voltages (at 0.2 mA cm−2) and operating voltages (at 1000 cd m−2) of 2.7 and 4.5 V, respectively (as shown in Fig. S6a, ESI†). As shown in Fig. 6c, the emission peak of Ir(MDQ)2(acac) is red-shifted with the doping ratio increase, indicting the effect of intermolecular interactions and strong polarization.35 At a low doping concentration of 1 wt%, the device Red1 showed red emission peaking at 604 nm corresponding to Commission Internationale de l’Eclairage (CIE) coordinates of (0.54, 0.42). With increasing the doping concentration of the emitter, decreased intermolecular distances of the emitter will cause intense intermolecular interactions, and thereby red-shifted emission can be observed in devices Red2–Red4. Particularly, red emission with CIE coordinates of (0.63, 0.36) can be achieved in device Red4 at a high doping concentration of 7 wt%. The detailed results of device R1–R4 are summarized in Table 2. Notably, additional exciplex emission was observed in the Red1 device with a low doping ratio of 1 wt%, which illustrates that the undesirable phenomenon of inadequate energy transfer was generated.21,27,36 An EQEmax, CEmax, and PEmax of 19.7%, 39.0 cd A−1, and 45.3 lm W−1 were presented in the Red1 device with an emission peak of 604 nm. With the doping ratio changes, an excellent EQEmax of 22.1%, 21.7%, and 20.8% was achieved for the R2, R3, and R4 devices, and they can maintain high EQEs of about 20% at a high brightness of 1000 cd m−2. The low efficiency roll-off is due to the sufficient utilization of excitons without energy loss and the broad recombination zone in the constructive exciplex forming co-host. It's worth noting that device R3 (doping ratio of 5 wt%) can achieve desirable performance with CIE coordinates of (0.62, 0.37). The detailed characteristics of device R1–4 are given in Table 2.
1, X wt%, X = 1, 3, 5, and 7, 20 nm)/PO-T2T (50 nm)/Liq (2 nm)/Al (120 nm); X = 1, 3, 5, and 7 correspond to the devices R1, R2, R3, and R4. Different doping ratios of the emitter were used to investigate the EL performance of the red devices. The EL characteristics of the red devices are shown in Fig. 6a–c. These devices show low onset voltages (at 0.2 mA cm−2) and operating voltages (at 1000 cd m−2) of 2.7 and 4.5 V, respectively (as shown in Fig. S6a, ESI†). As shown in Fig. 6c, the emission peak of Ir(MDQ)2(acac) is red-shifted with the doping ratio increase, indicting the effect of intermolecular interactions and strong polarization.35 At a low doping concentration of 1 wt%, the device Red1 showed red emission peaking at 604 nm corresponding to Commission Internationale de l’Eclairage (CIE) coordinates of (0.54, 0.42). With increasing the doping concentration of the emitter, decreased intermolecular distances of the emitter will cause intense intermolecular interactions, and thereby red-shifted emission can be observed in devices Red2–Red4. Particularly, red emission with CIE coordinates of (0.63, 0.36) can be achieved in device Red4 at a high doping concentration of 7 wt%. The detailed results of device R1–R4 are summarized in Table 2. Notably, additional exciplex emission was observed in the Red1 device with a low doping ratio of 1 wt%, which illustrates that the undesirable phenomenon of inadequate energy transfer was generated.21,27,36 An EQEmax, CEmax, and PEmax of 19.7%, 39.0 cd A−1, and 45.3 lm W−1 were presented in the Red1 device with an emission peak of 604 nm. With the doping ratio changes, an excellent EQEmax of 22.1%, 21.7%, and 20.8% was achieved for the R2, R3, and R4 devices, and they can maintain high EQEs of about 20% at a high brightness of 1000 cd m−2. The low efficiency roll-off is due to the sufficient utilization of excitons without energy loss and the broad recombination zone in the constructive exciplex forming co-host. It's worth noting that device R3 (doping ratio of 5 wt%) can achieve desirable performance with CIE coordinates of (0.62, 0.37). The detailed characteristics of device R1–4 are given in Table 2.
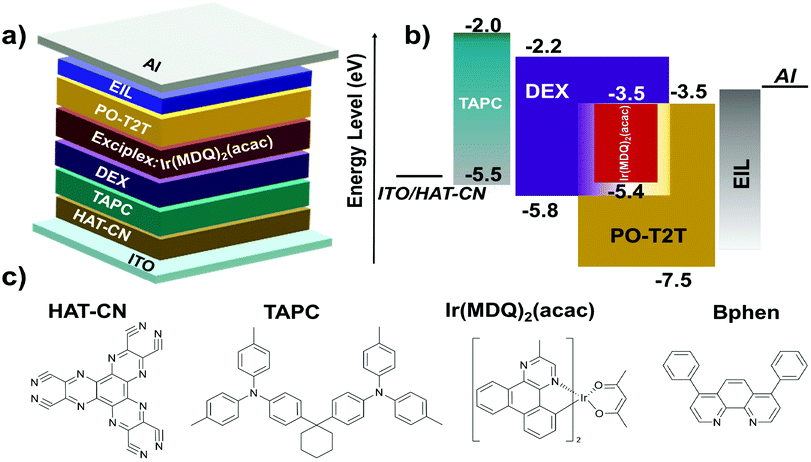 | ||
| Fig. 5 (a) The optimized device structure of the red PhOLED, (b) energy-level diagram of the red PhOLED, and (c) chemical structures of the related materials in the red PhOLED. | ||
For further enhancing the efficiency of red OLEDs, n-doping technology was proposed in our devices. It is an effective method to enhance the electron injection and transport for decreasing the energy consumption.25,33 As a result, the LiH material with a 0.1 wt% doping ratio was doped into 4,7-diphenyl-1,10-phenanthroline (Bphen) to serve as an electron-injection layer. Hence, devices R5–8 were fabricated with structures of ITO/HAT-CN (10 nm)/TAPC (30 nm)/DEX (10 nm)/DEX:PO-T2T:Ir(MDQ)2(acac) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 wt%, 20 nm)/PO-T2T (X nm, X = 5, 10, 15, and 20)/Bphen: 0.1 wt% LiH (50–X nm)/Al (120 nm). We changed the thickness of PO-T2T to prevent the undesirable phenomenon where the lithium ions migrating into the emitting layer can seriously cause exciton quenching.37 The emitter doping ratio was fixed at 5 wt%. From the J–V–L curves displayed in Fig. S6b (ESI†), the onset voltages of devices R5–8 are approximately at 2.4 V and low operating voltages of 3.4 V can be realized at a high brightness of 1000 cd m−2. These operating voltages of devices R5–8 are all lower than that of R1–4, indicting the achievement of better charge injection and transport after introducing n-doping technology. The EL characteristics of R5–8 are demonstrated in Fig. 6d–f. All devices show sole emission with a peak at 612 nm, demonstrating that effective energy transfer can be achieved. In the end, the n-doping based devices can obtain high EQEs over 23% and PE over 44 lm W−1. Remarkably, a high performance EQEmax, PEmax, and CEmax of 24.5%, 46.1 lm W−1, and 36.0 cd A−1 were presented in device R7 and it can also reach a high EQE, PE, and CE of 22.4%, 39.7 lm W−1, and 22.4 cd A−1 at 1000 cd m−2, so the 15 nm PO-T2T is effective to prevent exciton quenching. The detailed characteristics of devices R5–8 are summarized in Table 2. Notably, the device efficiencies of R7 are comparable with other reported red devices employing the same dopant (see Table S2, ESI†). The high EQEs and low efficiency roll-off suggest that the exciplex forming co-host using DEX has balanced charge transport and efficient utilization of excitons for radiative emission.
1, 5 wt%, 20 nm)/PO-T2T (X nm, X = 5, 10, 15, and 20)/Bphen: 0.1 wt% LiH (50–X nm)/Al (120 nm). We changed the thickness of PO-T2T to prevent the undesirable phenomenon where the lithium ions migrating into the emitting layer can seriously cause exciton quenching.37 The emitter doping ratio was fixed at 5 wt%. From the J–V–L curves displayed in Fig. S6b (ESI†), the onset voltages of devices R5–8 are approximately at 2.4 V and low operating voltages of 3.4 V can be realized at a high brightness of 1000 cd m−2. These operating voltages of devices R5–8 are all lower than that of R1–4, indicting the achievement of better charge injection and transport after introducing n-doping technology. The EL characteristics of R5–8 are demonstrated in Fig. 6d–f. All devices show sole emission with a peak at 612 nm, demonstrating that effective energy transfer can be achieved. In the end, the n-doping based devices can obtain high EQEs over 23% and PE over 44 lm W−1. Remarkably, a high performance EQEmax, PEmax, and CEmax of 24.5%, 46.1 lm W−1, and 36.0 cd A−1 were presented in device R7 and it can also reach a high EQE, PE, and CE of 22.4%, 39.7 lm W−1, and 22.4 cd A−1 at 1000 cd m−2, so the 15 nm PO-T2T is effective to prevent exciton quenching. The detailed characteristics of devices R5–8 are summarized in Table 2. Notably, the device efficiencies of R7 are comparable with other reported red devices employing the same dopant (see Table S2, ESI†). The high EQEs and low efficiency roll-off suggest that the exciplex forming co-host using DEX has balanced charge transport and efficient utilization of excitons for radiative emission.
Conclusions
In conclusion, we fabricated an efficient green exciplex system by combining a new electron donor molecule DEX and an electron acceptor PO-T2T. Thanks to the advantages of the photophysical and electrochemical properties of DEX, the exciplex-based green OLED achieved a low operating voltage of 2.6 V at 100 cd m−2 as well as an excellent PEmax and EQEmax of 44.1 lm W−1 and 11.2%. The DEX:PO-T2T exciplex as an emitter provides improved performance compared to the TCTA-based device, which is ascribed to the better carrier transport abilities and the rigid molecular structure of DEX. Furthermore, the red PhOLED employing the DEX:PO-T2T exciplex forming co-host system achieved a PEmax, CEmax, and EQEmax of 46.1 lm W−1, 36.0 cd A−1, and 24.5% and low operating voltages (2.7 V at 100 cd m−2 and 3.4 V at 1000 cd m−2). Therefore, it is believed that the proposed new electron donor DEX is beneficial to design an efficient exciplex system, which provides a reliable way to develop highly efficient OLEDs.Experimental
Material synthesis
General methods
All chemicals and reagents employed in our work were obtained from commercial resources without further purification. In the synthetic routes, tetrahydrofuran (THF) and dichloromethane (CH2Cl2) were purified by a PURE SOLV (Innovative Technology) purification system. 1H NMR and 13C NMR spectra were measured with a Bruker 600 spectrometer at room temperature. UV-vis absorption spectra were realized through a PerkinElmer Lambda 750 spectrophotometer. Photoluminescence (PL) spectra and phosphorescent spectra were measured on a Hitachi F-4600 fluorescence spectrophotometer. Differential scanning calorimetry (DSC) was performed on a TA DSC 2010 unit at a heating rate of 10 °C min−1 under nitrogen. Thermogravimetric analysis (TGA) was performed by using a TA SDT 2960 instrument with a heating rate of 10 °C min−1 under nitrogen; the temperature at 5% weight loss was used as the decomposition temperature (Td). Cyclic voltammetry was performed on a CHI600 voltammetric analyzer connected to a three-electrode system in which a platinum working electrode, a Pt wire counter electrode, and an Ag/AgCl reference electrode were employed. Ferrocenium–ferrocene (Fc+/Fc) was used as an internal standard. Nitrogen-bubbled 0.1 mol L−1 tetrabutylammonium hexafluorophosphate ([Bu4N]PF6) dichloromethane solution served as a supporting electrolyte.Device fabrication and measurement
The OLEDs were fabricated on indium tin oxide (ITO)-covered glass substrates (15 Ω square−1). All substrates were ultrasonically cleaned with acetone, ethanol and deionized water for 15 min before treating with UV-ozone for 15 min. All devices were made in a thermal deposition chamber under a pressure of 4 × 10−4 Pa. HAT-CN and Liq were deposited with a rate of 0.2 Å s−1, and 8 Å s−1 for Al; other organic materials were treated with a rate of 2 Å s−1. Finally, the devices were encapsulated with glass sheets at room temperature. The electroluminescence (EL) information of all devices was measured using a PR 655 photometer connected to a constant current source (Keithley 2400 SourceMeter). Transient PL decays were obtained on a time-resolved fluorescence spectrometer (PL-TCSPC) of HORIB-FM-2015.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (Grant No. 51773141, 61961160731, 21572152 and 51873139) and the National Key R&D Program of China (Grant No. 2016YFB0400700). This project is also funded by the Collaborative Innovation Center of Suzhou Nano Science and Technology (Nano-CIC), by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and by the “111” Project of the State Administration of Foreign Experts Affairs of China.Notes and references
- C. W. Tang and S. A. VanSlyke, Organic electroluminescent diodes, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- L. S. Liao, K. P. Klubek and C. W. Tang, High-efficiency tandem organic light-emitting diodes, Appl. Phys. Lett., 2003, 84, 167–169 CrossRef.
- G. Schwartz, M. Pfeiffer, S. Reineke, K. Walzer and K. Leo, Harvesting triplet excitons from fluorescent blue emitters in white organic light-emitting diodes, Adv. Mater., 2007, 19, 3672–3676 CrossRef CAS.
- B. B. Li, L. Gan, X. Y. Cai, X. L. Li, Z. H. Wang, K. Gao, D. C. Chen, Y. Cao and S. J. Su, An effective strategy toward high-efficiency fluorescent OLEDs by radiative coupling of spatially separated electron-hole pairs, Adv. Mater. Interfaces, 2018, 5, 1800025 CrossRef.
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, Nearly 100% internal phosphorescence efficiency in an organic light-emitting device, J. Appl. Phys., 2001, 90, 5048–5051 CrossRef CAS.
- K. S. Yook and J. Y. Lee, Organic materials for deep blue phosphorescent organic light-emitting diodes, Adv. Mater., 2012, 24, 3169–3190 CrossRef CAS PubMed.
- H. Shin, J. H. Lee, C. K. Moon, J. S. Huh, B. Sim and J. J. Kim, Sky-blue phosphorescent OLEDs with 34.1% external quantum efficiency using a low refractive index electron transporting layer, Adv. Mater., 2016, 28, 4920–4925 CrossRef CAS PubMed.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- Y. Liu, G. H. Xie, K. L. Wu, Z. H. Luo, T. Zhou, X. Zeng, J. Yu, S. L. Gong and C. L. Yang, Boosting reverse intersystem crossing by increasing donors in triarylboron/phenoxazine hybrids: TADF emitters for high-performance solution-processed OLEDs, J. Mater. Chem. C, 2016, 4, 4402–4407 RSC.
- L. Gan, Z.-D. Xu, Z.-H. Wang, B.-B. Li, W. Li, X.-Y. Cai, K.-K. Liu, Q.-M. Liang and S. J. Su, Utilizing a spiro TADF moiety as a functional electron donor in TADF molecular design toward efficient “multichannel” reverse intersystem crossing, Adv. Funct. Mater., 2019, 29, 1808088 CrossRef.
- K. Goushi, K. Yoshida, K. Sato and C. Adachi, Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion, Nat. Photonics, 2012, 6, 253–258 CrossRef CAS.
- H. Shin, S. Lee, K. H. Kim, C. K. Moon, S. J. Yoo, J. H. Lee and J. J. Kim, Blue phosphorescent organic light-emitting diodes using an exciplex forming co-host with the external quantum efficiency of theoretical limit, Adv. Mater., 2014, 26, 4730–4734 CrossRef CAS PubMed.
- J. Li, H. Nomura, H. Miyazaki and C. Adachi, Highly efficient exciplex organic light-emitting diodes incorporating a heptazine derivative as an electron acceptor, Chem. Commun., 2014, 50, 6174–6176 RSC.
- K. H. Kim, S. J. Yoo and J. J. Kim, Boosting triplet harvest by reducing nonradiative transition of exciplex toward fluorescent organic light-emitting diodes with 100% internal quantum efficiency, Chem. Mater., 2016, 28, 1936–1941 CrossRef CAS.
- J. H. Lee, S. H. Cheng, S. J. Yoo, H. Shin, J. H. Chang, C. I. Wu, K. T. Wong and J. J. Kim, An Exciplex Forming Host for Highly Efficient Blue Organic Light Emitting Diodes with Low Driving Voltage, Adv. Funct. Mater., 2015, 25, 361–366 CrossRef CAS.
- W. Liu, J. X. Chen, C. J. Zheng, K. Wang, D. Y. Chen, F. Li, Y. P. Dong, C. S. Lee, X. M. Ou and X. H. Zhang, Novel strategy to develop exciplex emitters for high-performance OLEDs by employing thermally activated delayed fluorescence materials, Adv. Funct. Mater., 2016, 26, 2002–2008 CrossRef CAS.
- X. K. Liu, Z. Chen, C. J. Zheng, M. Chen, W. Liu, X. H. Zhang and C. S. Lee, Nearly 100% triplet harvesting in conventional fluorescent dopant-based organic light-emitting devices through energy transfer from exciplex, Adv. Mater., 2015, 27, 2025–2030 CrossRef CAS.
- W. Y. Hung, P. Y. Chiang, S. W. Lin, W. C. Tang, Y. T. Chen, S. H. Liu, P. T. Chou, Y. T. Hung and K. T. Wong, Balance the carrier mobility to achieve high performance exciplex OLED using a triazine-based acceptor, ACS Appl. Mater. Interfaces, 2016, 8, 4811–4818 CrossRef CAS.
- B. Liang, J. Wang, Z. Cheng, J. Wei and Y. Wang, Exciplex-based electroluminescence: over 21% external quantum efficiency and approaching 100 lm W−1 power efficiency, J. Phys. Chem. Lett., 2019, 10, 2811–2816 CrossRef CAS.
- J. Hu, C. Y. Zhao, T. M. Zhang, X. P. Zhang, X. D. Cao, Q. J. Wu, Y. H. Chen, D. Zhang, Y. T. Tao and W. Huang, Isomeric N-linked benzoimidazole containing new electron acceptors for exciplex forming hosts in highly efficient blue phosphorescent OLEDs, Adv. Opt. Mater., 2017, 5, 1700036 CrossRef.
- H. G. Kim, K. H. Kim, C. K. Moon and J. J. Kim, Harnessing triplet excited states by fluorescent dopant utilizing codoped phosphorescent dopant in exciplex host for efficient fluorescent organic light emitting diodes, Adv. Opt. Mater., 2017, 5, 1600749 CrossRef.
- W. Song, I. Lee and J. Y. Lee, Host engineering for high quantum efficiency blue and white fluorescent organic light-emitting diodes, Adv. Mater., 2015, 27, 4358–4363 CrossRef CAS PubMed.
- X. K. Liu, Z. Chen, C. J. Zheng, C. L. Liu, C. S. Lee, F. Li, X. M. Ou and X. H. Zhang, Prediction and design of efficient exciplex emitters for high-efficiency, thermally activated delayed-fluorescence organic light-emitting diodes, Adv. Mater., 2015, 27, 2378–2383 CrossRef CAS.
- V. Jankus, P. Data, D. Graves, C. McGuinness, J. Santos, M. R. Bryce, F. B. Dias and A. P. Monkman, Highly efficient TADF OLEDs: how the emitter-host interaction controls both the excited state species and electrical properties of the devices to achieve near 100% triplet harvesting and high efficiency, Adv. Funct. Mater., 2014, 24, 6178–6186 CrossRef CAS.
- Q. S. Tian, L. Zhang, Y. Hu, S. Yuan, Q. Wang and L. S. Liao, High-performance white organic light-emitting diodes with simplified structure incorporating novel exciplex-forming host, ACS Appl. Mater. Interfaces, 2018, 10, 39116–39123 CrossRef CAS PubMed.
- S. F. Wu, S. H. Li, Y. K. Wang, C. C. Huang, Q. Sun, J. J. Liang, L. S. Liao and M. K. Fung, White organic LED with a luminous efficacy exceeding 100 lm W−1 without light out-coupling enhancement techniques, Adv. Funct. Mater., 2017, 27, 1701314 CrossRef.
- Z. B. Wu, L. Yu, F. C. Zhao, X. F. Qiao, J. S. Chen, F. Ni, C. L. Yang, T. Ahamad, S. M. Alshehri and D. G. Ma, Precise exciton allocation for highly efficient white organic light-emitting diodes with low efficiency roll-off based on blue thermally activated delayed fluorescent exciplex emission, Adv. Opt. Mater., 2017, 5, 1700415 CrossRef.
- X. K. Liu, W. Chen, H. Thachoth Chandran, J. Qing, Z. Chen, X. H. Zhang and C. S. Lee, High-performance, simplified fluorescence and phosphorescence hybrid white organic light-emitting devices allowing complete triplet harvesting, ACS Appl. Mater. Interfaces, 2016, 8, 26135–26142 CrossRef CAS PubMed.
- S. J. Su, E. Gonmori, H. Sasabe and J. Kido, Highly Efficient Organic Blue-and White-Light-Emitting Devices Having a Carrier- and Exciton-Confining Structure for Reduced Efficiency Roll-Off, Adv. Mater., 2008, 20, 4189–4194 CAS.
- K. Udagawa, H. Sasabe, F. Igarashi and J. Kido, Simultaneous realization of high EQE of 30%, low drive voltage, and low efficiency roll-off at high brightness in blue phosphorescent OLEDs, Adv. Opt. Mater., 2016, 4, 86–90 CrossRef CAS.
- X. K. Liu, Z. Chen, J. Qing, W. J. Zhang, B. Wu, H. L. Tam, F. Zhu, X. H. Zhang and C. S. Lee, Remanagement of singlet and triplet Excitons in single-emissive-layer hybrid white organic light-emitting devices using thermally activated delayed fluorescent blue exciplex, Adv. Mater., 2015, 27, 7079–7085 CrossRef CAS PubMed.
- L. Zhang, Y. X. Zhang, Y. Hu, X. B. Shi, Z. Q. Jiang, Z. K. Wang and L. S. Liao, Highly efficient blue phosphorescent organic light-emitting diodes employing a host material with small bandgap, ACS Appl. Mater. Interfaces, 2016, 8, 16186–16191 CrossRef CAS PubMed.
- Z. Bin, G. Dong, P. Wei, Z. Liu, D. Zhang, R. Su, Y. Qiu and L. Duan, Making silver a stronger n-dopant than cesium via in situ coordination reaction for organic electronics, Nat. Commun., 2019, 10, 866 CrossRef PubMed.
- X. Tang, X. Y. Liu, Z. Q. Jiang and L. S. Liao, High-quality white organic light-emitting diodes composed of binary emitters with color rendering index exceeding 80 by utilizing color remedy strategy, Adv. Funct. Mater., 2019, 29, 1807541 CrossRef.
- Y. J. Cho, S. K. Jeon and J. Y. Lee, Molecular engineering of high efficiency and long lifetime blue thermally activated delayed fluorescent emitters for vacuum and solution processed organic light-emitting diodes, Adv. Opt. Mater., 2016, 4, 688–693 CrossRef CAS.
- D. Y. Zhou, S. H. Zamani, Q. Wang, L. S. Liao and H. Aziz, Host to guest energy transfer mechanism in phosphorescent and fluorescent organic light-emitting devices utilizing exciplex-forming hosts, J. Phys. Chem. C, 2014, 118, 24006–24012 CrossRef CAS.
- J. H. Lee, M. H. Wu, C. C. Chao, H. L. Chen and M. K. Leung, High efficiency and long lifetime OLED based on a metal-doped electron transport layer, Chem. Phys. Lett., 2005, 416, 234–237 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qm00116c |
| This journal is © the Partner Organisations 2020 |