Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unveiling the photocatalytic potential of graphitic carbon nitride (g-C3N4): a state-of-the-art review
Mahmoud A. Ahmed
 *a,
Safwat A. Mahmoud
b and
Ashraf A. Mohamed
*a,
Safwat A. Mahmoud
b and
Ashraf A. Mohamed
 a
a
aChemistry Department, Faculty of Science, Ain Shams University, Cairo-11566, Egypt. E-mail: mahmoudmahmoud_p@sci.asu.edu.eg
bPhysics Department, Faculty of Science, Northern Border University, Arar, 13211, Saudi Arabia
First published on 15th August 2024
Abstract
Graphitic carbon nitride (g-C3N4)-based materials have emerged as promising photocatalysts due to their unique band structure, excellent stability, and environmental friendliness. This review provides a comprehensive and in-depth analysis of the current state of research on g-C3N4-based photocatalysts. The review summarizes several strategies to improve the photocatalytic performance of pristine g-C3N4, e.g., by creating heterojunctions, doping with non-metallic and metallic materials, co-catalyst loading, tuning catalyst morphology, metal deposition, and nitrogen-defect engineering. The review also highlights the various characterization techniques employed to elucidate the structural and physicochemical features of g-C3N4-based catalysts, as well as their applications of in photocatalytic degradation and hydrogen production, emphasizing their remarkable performance in pollutants' removal and clean energy generation. Furthermore, this review article investigates the effect of operational parameters on the catalytic activity and efficiency of g-C3N4-based catalysts, shedding light on the key factors that influence their performance. The review also provides insights into the photocatalytic pathways and reaction mechanisms involving g-C3N4 based photocatalysts. The review also identifies the research gaps and challenges in the field and presents prospects for the development and utilization of g-C3N4-based photocatalysts. Overall, this comprehensive review provides valuable insights into the synthesis, characterization, applications, and prospects of g-C3N4-based photocatalysts, offering guidance for future research and technological advancements in this rapidly growing field.
1 Introduction
The discharge of pollutants into aquatic environments has increased significantly as a result of the manufacturing sector's growth.1,2 Because of their hazardous characteristics and possible carcinogenicity, organic pollutants found in both air and water are especially concerning.3 The chemical processing industries, building materials, textile production, and coatings used in indoor furniture are the main producers of these pollutants.4,5 Exposure to organic pollutants, whether indoors or outdoors, has been associated with various adverse health effects including hypertension, renal damage, Alzheimer's disease, nausea, epilepsy, mental confusion, and vomiting.4–6 Furthermore, the mutagenic and carcinogenic impacts of these pollutants are noteworthy.7,8 Moreover, organic pollutants, such as dyes, pesticides, pharmaceuticals, phenols, and others, significantly impact the receiving water bodies by changing key variables like unpleasant odors, color, toxicity levels, biochemical oxygen demand (BOD), and chemical oxygen demand (COD). Some of these organic pollutants have long half-life times, (bio)accumulate, are not easily degraded, and damage the marine flora and fauna, aquatic lives, and ultimately human health. In addition to these environmental concerns, the global community also faces a pressing challenge in terms of ensuring energy security.9 Fossil fuels are limited resources, and using them to produce energy increases harm to the atmosphere by emitting various pollutants, including carbon dioxide.10 This has spurred a global effort to explore technologies that promote the utilization of renewable energy sources and address environmental challenges.11On the other hand, water purification has been achieved using conventional techniques such as reverse osmosis, adsorption, membrane filtration, precipitation, coagulation, ion exchange, and biological treatments.12–17 However, when handling complicated pollutants with a variety of chemical and physical features, these conventional approaches have limits in terms of efficiency and energy usage, as well as the increased risk of generating secondary pollutants.18 Nevertheless, in advanced oxidation processes (AOP), photocatalysis approach has emerged as a cost-effective, trustworthy, and environmentally benign alternative.19–22 This approach utilizes solar radiation to facilitate various applications, including treating pollutants, facilitating chemical reactions, and splitting water to produce hydrogen.23–27 The efficient utilization of solar photocatalysis holds significant research value in terms of improving the environment and reducing greenhouse gas emissions. Typically, a photocatalytic process involves stages, such as harnessing visible light, exciting photocarriers, segregating and migrating photo-induced charge carriers to active sites, and facilitating the redox process on the photocatalyst surface.28,29 These redox processes are responsible for generating reactive species, such as superoxide radicals (˙O2−), and hydroxyl radicals (˙OH) which play a key role in the overall photocatalytic process30,31
Recently, two-dimensional (2D) compounds like graphitic carbon nitride (g-C3N4), graphene, boron nitride, and transition-metal dichalcogenides, with excellent features have been widely employed in chemical sensors, electronic and optical devices, energy storage and generation, as well as environmental remediation.32–34 In particular, g-C3N4, a metal-free polymer semiconductor containing tri-s-triazine units, has garnered a great deal of interest due to its potential uses in photochemistry and photocatalysis.35
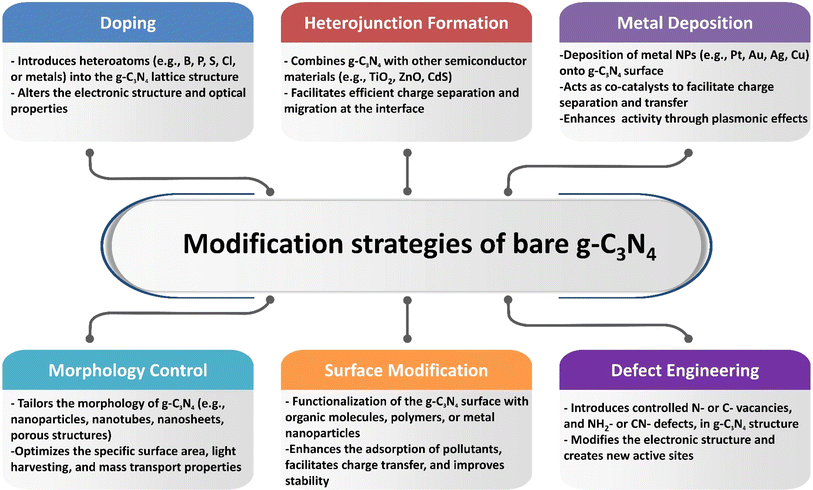
Graphitic carbon nitride (g-C3N4) is regarded as one of the first organic conjugated polymers, having been discovered in 1834.36 There are five primary phases that g-C3N4 may be categorized into: the cubic phase, the pseudo-cubic phase, the graphitic phase with minimal compressibility and remarkable hardness that is comparable to a diamond, the α-phase, and the β-phase.37 Research communities have become quite excited by g-C3N4-based materials as photocatalysts because of its non-toxicity, high visible light harvesting, π-conjugated assembly, increased profusion, and chemical and thermal durability.38,39 The optical bandgap of g-C3N4 at 2.7 eV (460 nm), with VB and CB potentials at −1.09 and +1.56 V (vs. NHE), respectively, make g-C3N4 attractive material for overall water splitting.40,41 Furthermore, the widespread usage of g-C3N4-based materials as a visible-light-driven photocatalyst is mostly due to its easy synthesis process from readily accessible, affordable precursors.42,43 Additionally, g-C3N4 has a powerful electrical conductivity and distinct conjugated structure due to the graphitic stacking of g-C3N4 layers connected by tertiary amines.44,45 The presence of carbon and nitrogen atoms with distinct valence states results in the creation of multiple band structures; therefore, pristine g-C3N4 has shown promise as a photocatalyst, but it also has limitations that must be addressed.43,46 One major limitation is its low photocatalytic activity, attributed to its wide bandgap energy, which limits its absorption of the solar spectrum.47 Additionally, the performance of photocatalytic techniques is further decreased by the quick coupling of photo-generated charge carriers in g-C3N4.48 It also has limited charge carrier mobility, hindering efficient charge transfer. Other limitations of pristine g-C3N4 are its relatively low specific surface area and lack of stability under photocatalytic conditions, as prolonged exposure to light and reactive species can degrade its performance over time. To overcome these limitations, different modification approaches were adopted to enhance the performance of pure g-C3N4 including heterojunctions, doping, co-catalyst loading, tuning morphology, metal deposition, and defect engineering.49–52
Heterostructure development has emerged as the most promising approach to improve the photocatalytic activity of g-C3N4. One of the advantageous properties of g-C3N4 is its tunable band gap, which allows precise control over the energy levels of its highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO).53 This tunability significantly impacts the photoelectronic performance of g-C3N4 as a photocatalytic nanosheet. By constructing hetero structures, the band gap of g-C3N4 can be effectively modified, leading to expanded light harvesting and promoting the separation of hole–electron pairs.54,55 This modification approach involves the intentional introduction of metal, nonmetal, or other nanomaterials into the structure, offering a means to enhance the photocatalytic performance of g-C3N4.
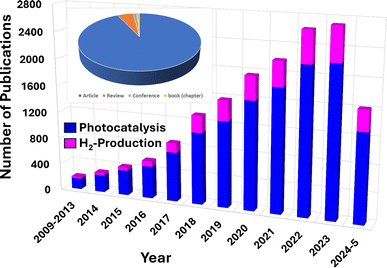
Thus, recent research has highlighted the potential of g-C3N4 composites in effectively removing various pollutants from wastewater, such as dyes, oil spills, heavy metal ions, pesticides, microplastics, phenols, and pharmaceuticals.54,56–59 Moreover, there is increasing research interest in utilizing g-C3N4-composites for hydrogen generation.60 The number of publications focusing on pollutant removal and H2-production using g-C3N4 nanocomposites has shown a notable increase over the last few years, as seen in Fig. 1. Initially, there were only a few publications per year, indicating limited attention to the topic. However, since 2017, there has been a rapid upward trend in both citations and publications, signifying a growing interest in the field, where documents on photocatalysis were almost five times higher than those on H2-production. Most of these publications consist of journal articles (93%), with a smaller fraction being reviews (4.9%), and conference articles (1.1%), as shown in Fig. 1. This indicates a scarcity of dedicated and updated review papers, which are essential for providing interested researchers and the scientific community with a comprehensive and up-to-date evaluation of g-C3N4-composites' application as photocatalysts.
This comprehensive review aims at providing a detailed examination of the synthesis methods of g-C3N4-based photocatalysts, along with their applications in environmental remediation, e.g., organic pollutants' degradation and hydrogen production. Additionally, the review highlights the characterization techniques used to understand the crystal structure, morphology, surface area, nanoparticle distribution, and compositional properties of g-C3N4-based photocatalysts. Moreover, the review describes the mechanisms and factors influencing the photocatalytic performance of g-C3N4-based photocatalysts in organic pollutant degradation, providing insights into the identification of key intermediates and reactive species involved in the photocatalytic degradation processes. It further investigates the strategies employed to enhance the efficiency and selectivity of g-C3N4-based photocatalysts, including the utilization of metal cocatalysts, co-doping techniques, heterojunction formation, and surface modification. Additionally, the review assesses the g-C3N4-based photocatalysts' application in hydrogen production through water splitting, evaluating their performance in terms of hydrogen evolution rate, stability, and selectivity, while discussing the underlying mechanisms of photogenerated charge separation and transfer.
2 Modification of g-C3N4 for improved photocatalytic activity
Composite g-C3N4 photocatalysts have gained significant attention in recent years due to their potential for efficient and sustainable energy conversion and environmental remediation. The g-C3N4 modification with other materials allows for improved light absorption, better charge separation, and boosted catalytic performance, resulting in enhanced photocatalytic activity.Several approaches have been applied to modify pristine graphitic carbon nitride and improve its photocatalytic performance, such as creating heterojunctions, doping with non-metallic and metallic materials, co-catalyst loading, tuning catalyst morphology, metal deposition, and nitrogen-defect engineering, as shown in Scheme 1.49–52,61,62 When it comes to the fabrication of g-C3N4 composites as photocatalysts, two main approaches are commonly employed based on the crystallization process: in situ crystallization and ex situ crystallization.
2.1. Synthesis of g-C3N4 composites by in situ crystallization
In situ crystallization: the g-C3N4 composite is fabricated by incorporating the other material during the polymerization process of g-C3N4 itself. This approach involves the co-condensation of a precursor monomer of g-C3N4 with other components, which subsequently polymerizes and crystallizes simultaneously.63,64 During the in situ crystallization process, the precursor monomers of g-C3N4, typically urea, thiourea, melamine, cyanamide, or dicyanamide are combined with the desired components, such as metal precursors or carbon-based materials. The mixture is then subjected to thermal treatment under specific temperature and atmosphere conditions. The heating process triggers the polymerization and condensation of the monomers into a layered g-C3N4 structure, thereby incorporating the additional components into the composite. In situ crystallization offers several advantages, including uniform distribution of the composite components and good interfacial interaction between g-C3N4 and the additional material. This approach allows for control over the composition and structure of the composite, leading to improved photocatalytic performance.65,662.2. Synthesis of g-C3N4 composites by ex situ crystallization
In ex situ Crystallization, g-C3N4 is synthesized separately, and subsequently, other materials are introduced or deposited onto its surface to form the composite.67 To fabricate the ex situ composite, various methods can be utilized. For example, metal nanoparticles or metal oxide precursors can be deposited onto the surface of pre-prepared g-C3N4 through methods like impregnation, photo-deposition, or chemical reduction. Carbon-based materials, such as graphenes or carbon nanotubes, can also be integrated with pre-formed g-C3N4 through solution mixing or deposition techniques. Ex situ crystallization offers advantages such as precise control over the loading amount and distribution of the additional material. It allows for flexibility in choosing the post-treatment conditions for efficient deposition or integration of the composite components, resulting in improved photocatalytic performance. The choice between in situ and ex situ crystallization depends on the specific composite design, the compatibility of the materials, and the desired properties. In situ crystallization allows for simultaneous formation of the g-C3N4 composite during the polymerization process, while ex situ crystallization offers flexibility in introducing and controlling the deposition of other materials onto pre-formed g-C3N4.682.3. Modification of g-C3N4 by metal-deposition
Metal deposition involves the introduction of metal nanoparticles or tiny thin films onto the surface of g-C3N4 through various deposition techniques, such as physical vapor deposition or chemical methods (e.g., impregnation, electrochemical deposition).69 In this process, the metal species are not incorporated into the lattice structure of g-C3N4 but rather exist as separate entities on the surface. The incorporation of metals onto g-C3N4 as a composite photocatalyst offers critical prospects for improving its light absorption, charge separation, catalytic activity, and overall photocatalytic performance. The localized surface plasmon resonances, catalytic properties, and synergistic effects of noble metals contribute to the enhanced efficiency and selectivity of photocatalytic reactions. For instance, a facile immobilization of noble metals (Ag, Au, and Pd) onto g-C3N4 using a simple ultrasonication technique was described.70 In this method, g-C3N4 (0.5 g) was dispersed in DI water through ultrasonication for 1 hour. The metal precursor was then mixed with the previous suspension, followed by reduction using NaBH4 with continuous stirring for 1 hour. After noble metals' deposition, XRD examination showed a modest drop in the diffraction intensity of the g-C3N4 (100) plane. This implies that the presence of metal atoms prevented the formation of g-C3N4 crystals.70 Furthermore, Ag/g-C3N4 photocatalyst was synthesized by using an infrared-assisted heating strategy to deposit AgNO3 salt onto the g-C3N4. The presence of Ag nanoparticles on the surface of g-C3N4 facilitates the capture of electrons generated by g-C3N4 and their subsequent utilization in degrading methyl orange or producing H2 from H+.71 In another investigation, researchers employed ultrasonication-assisted liquid exfoliation to create g-C3N4 nanosheets from bulk g-C3N4.72 After that Au was deposited on g-C3N4 via green photoreduction of Au(III). TEM analysis verified the good exfoliation of bulk g-C3N4 (Fig. 2a). However, numerous Au NPs ranging from 5 to 20 nm were formed on the nanosheets, as depicted in (Fig. 2b). Additionally, DRS results demonstrated that the Au NPs/g-C3N4 composite exhibited an absorption peak at 550 nm, indicative of the surface plasmon resonance band specific to colloidal gold (Fig. 2c). Hence, the presence of Au NPs served as electron sinks, facilitating the separation of photogenerated electron/hole pairs.72 Moreover, Ag NPs/g-C3N4 composite was synthesized using an environmentally friendly chemical approach, as depicted in (Fig. 2d).73 The deposition of Ag NPs onto the g-C3N4 surface resulted in a slight reduction in the BET surface area, as shown in (Fig. 2d). XPS analysis further confirmed the existence of metallic silver on the g-C3N4 surface. Furthermore, chemical impregnation of single Pd atoms onto g-C3N4 enhanced its photocatalytic activity.75 The presence of single Pd atoms and their coordination structure in the composite were confirmed using HAADF-STEM (high-angle annular dark-field scanning transmission electron microscopy) and XAFS (X-ray absorption fine structure) analyses. The powerful interaction between the Pd- and surrounding N-atoms facilitated the production of photogenerated electrons, leading to the promotion of the photocatalytic performance of the composite.75 However, the noble metal's cost prevents its extensive use in real applications. Studies have been performed on various transition metals, including Fe, Cu, W, Zn, Mo, Zr, etc.76–80 For example, the incorporation of cobalt into g-C3N4 thorough a one-step thermal polycondensation approach suppressed the growth of the g-C3N4 crystals and resulted in a larger specific surface area with the formation of abundant Co–Nx active sites.81 It Also reduced the band gap energy and facilitated more efficient separation of photogenerated electrons and holes.81 Furthermore, the Fe/g-C3N4 composites were fabricated with various initial concentrations of FeCl3, resulting in samples labeled FCN-0.5, FCN-1, FCN-2, and FCN-3 representing 0.5%, 1%, 2%, and 3% Fe, respectively.74 The DRS revealed an enhanced visible-light range absorption and a redshift for Fe/g-C3N4 composites. As the Fe content increased, the optical band gap gradually shifted to lower energy, indicating the incorporation of Fe ions into the g-C3N4 lattice and altering its electronic structure. This redshift in absorption promoted the production of more electron–hole pairs under sunlight, ultimately enhancing the photocatalytic features. Additionally, the Nyquist plots illustrated clear differences in the semicircle diameter between bulk g-C3N4, pure g-C3N4, and FCN-2 nanosheets, with the FCN-2 nanosheets displaying a significantly smaller semicircle diameter compared to the others (Fig. 2e).74 Moreover, the Co/gC3N4 composite was fabricated through an in situ calcination strategy.82 Initially, 30 g of melamine was mixed with 50 mL of DI water. Subsequently, Co(NO3)2 was added to the suspension under sonication for 10 minutes, maintaining a weight ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. The resulting mixture was then calcined in a Muffle furnace at 550 °C for 1 hour at a heating rate of 10 °C min−1.82 Co/g-C3N4 had a surface area of 25.6 m2 g−1, featuring a larger amount of mesopores compared to g-C3N4 (surface area: 18.2 m2 g−1). The SEM image showed a mixed morphology in Co/g-C3N4, consisting of cobalt oxide grains with an irregular polygonal crystal shape and g-C3N4 sheets.
0.5. The resulting mixture was then calcined in a Muffle furnace at 550 °C for 1 hour at a heating rate of 10 °C min−1.82 Co/g-C3N4 had a surface area of 25.6 m2 g−1, featuring a larger amount of mesopores compared to g-C3N4 (surface area: 18.2 m2 g−1). The SEM image showed a mixed morphology in Co/g-C3N4, consisting of cobalt oxide grains with an irregular polygonal crystal shape and g-C3N4 sheets.
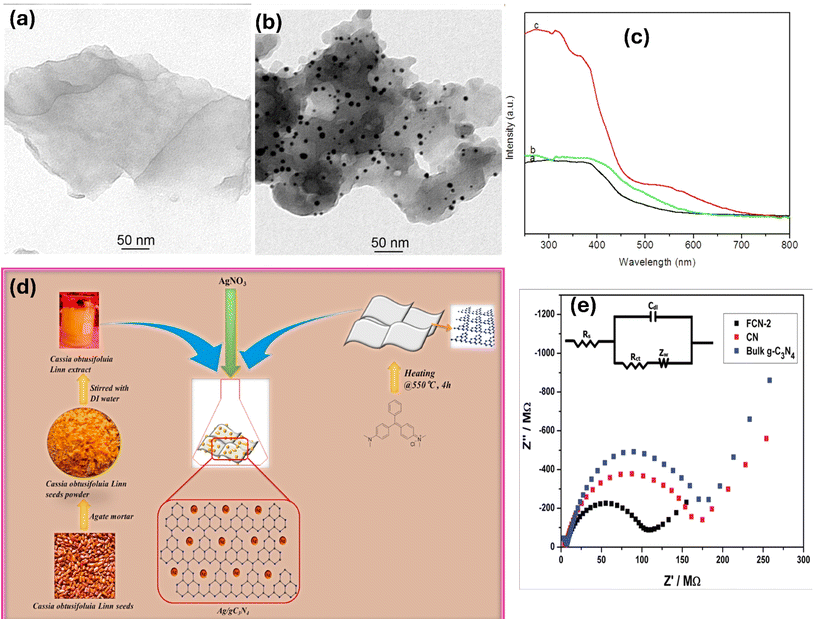 | ||
| Fig. 2 Tem image of (a) g-C3N4, (b) Au/g-C3N4, (c) DRS data of g-C3N4 nanosheets, bulk g-C3N4, and AuNP/g-C3N4 nanohybrids reprinted with the permission of ref. 72, copyright 2024, American Chemical Society; (d) synthesis of Ag/g-C3N4 via green route, reprinted with the permission of ref. 73, copyright 2024, Elsevier; and (e) EIS of the g-C3N4, and pure and Fe-doped g-C3N4 nanosheets, reprinted with the permission of ref. 74, copyright 2024, RSC. | ||
2.4. Modification of g-C3N4 by non-metallic and metallic doping
Doping involves introducing dopant into the lattice structure of g-C3N4 by substituting carbon or nitrogen atoms with dopant atoms. This process modifies the electronic structure and properties of g-C3N4 by altering the band structure, charge carrier mobility, and recombination rates. Non-metal and metal doping are the two primary types of elemental doping of g-C3N4. Non-metal doping has gained significant attention as a means to preserve the metal-free property of g-C3N4. Non-metals possess high ionization energies and electronegativities, allowing them to form covalent bonds by gaining electrons during reactions with other compounds.83–86 This characteristic makes non-metals a suitable option for doping g-C3N4, as they do not introduce metal ions with varying chemical states, which could be affected by thermal variations. Various non-metal dopants, including phosphorus, sulphur, carbon, nitrogen, oxygen, boron, and halogens, have been extensively investigated for their efficacy in doping g-C3N4.87–89A facile method was employed to synthesize metal-free boron and oxygen-doped g-C3N4 with carbon vacancy.90 In this method, a mixture of g-C3N4 and varying amounts of H3BO3 (1%, 2.5%, 5%, and 10%) was ground and transferred to a crucible for calcination at 500 °C for 2 hours. The resulting B and O doped g-C3N4 exhibited distinct morphological characteristics compared to pristine g-C3N4, featuring loose and irregular tissue-like structures. SEM images revealed that the B and O dopants caused a modification in the morphology by dividing the bulk layers of g-C3N4 into smaller layers.90
Phosphorus-doped g-C3N4 was fabricated via a simple poly-condensation strategy using dicyandiamide (or cyanoguanidine) as the precursor and 1-butyl-3-methylimidazolium hexafluorophosphate as the phosphorus source.91 The hexafluorophosphate ions reacted with amine groups upon raising the temperature, incorporating phosphorus into the C–N framework. Analysis confirmed the formation of P–N bonds, with phosphorus likely substituting corner or bay carbon positions. Even at low doping levels, the electronic structure of g-C3N4 was significantly altered, leading to reduced optical band gap energy and increased electrical conductivity.91 Furthermore, P-doped g-C3N4 was synthesized via a thermal polymerization method, where the P atoms were successfully introduced into the g-C3N4 lattice, resulting in modified electronic properties and improved suppressions of charge carrier recombination.92 Moreover, a co-condensation approach, without the use of templates, was followed to synthesize P-doped g-C3N4 nanoflowers with in-plane mesopores, where the introduced phosphorus species exhibited strong chemical bonding with neighboring carbon and nitrogen atoms, leading to a forced planar coordination within the carbon nitride framework.93
Furthermore, a single-pot pyrolysis method was employed to synthesize sulfur-doped graphitic carbon nitride porous rods (S-pg-C3N4) by heating a complex of melamine and trithiocyanuric acid at various temperatures.94 The characterization results demonstrated that S-pg-C3N4 exhibited a porous rod structure with a significantly higher surface area (ranging from 20 to 52 m2 g−1) when compared to bulk g-C3N4. Additionally, it was observed that the surface area of the S-pg-C3N4 samples increased as the heating temperature was raised.94 On the other hand, the synthesis of oxygen-doped g-C3N4 using a facile H2O2 hydrothermal method was reported.95 XPS analysis revealed the successful doping of oxygen into the g-C3N4 lattice, resulting in the formation of N–C–O bonds, where oxygen atoms were directly bonded to sp2-hybridized carbon. Notably, the oxygen doping induced a downshift of the conduction band (CB) minimum by 0.21 eV without altering the valence band (VB) maximum. This oxygen doping-induced modulation of the electronic and band structure of g-C3N4 and led to various beneficial effects, including an increase in visible light absorption, extended surface area and enhanced photogenerated separation efficiency.95 Otherwise, using a hydrothermal synthesis, sulfur fluoride-doped carbon nitride (F-SCN) was effectively synthesized.96 The incorporation of fluorine and sulfur into the carbon nitride lattice resulted in a notable improvement in the photocatalytic performance by enhancing the separation of electron–hole pairs and facilitating efficient charge transfer.96
On the other hand, the g-C3N4 structure has been modified via metal doping.97–100 For example, mesoporous graphitic-carbon-nitride nanosheets doped with zinc ions (Zn-mpg-C3N4) were reported.101 The surface area and porosity of g-C3N4 were improved by PEG-1500, whereas the electrical features of the g-C3N4 increased when zinc was incorporated into the g-C3N4 structure.
2.5. Modification of g-C3N4 by creating heterojunctions
Heterojunctions in g-C3N4-based photocatalysts can be classified into several types based on their structural configurations and electronic band alignments, each offering unique advantages and functionalities for photocatalytic applications. Heterojunctions are typically formed by hybridizing g-C3N4 with other materials, e.g., semiconductors or carbon materials, in a composite form. When these materials are nearby in a heterojunction, they maintain their distinct crystal structures and electrical properties. Different types of heterojunctions, such as Type-I, Type-II, p–n junctions, and Z and S schemes, can be used to create these connections.Conversely, misalignment of the conduction and valence band boundaries among the two materials results in the creation of Type II heterojunctions, where the two semiconductors are interfaced while one semiconductor has a lower conduction band and the other has a higher valence band. An inherent electric field that is generated by the energy level movement at the interface may facilitate charge separation and boost charge migration across the junction. The CB potential of g-C3N4 typically around −1.1 eV, significantly lower than that of many other photocatalysts. Consequently, when exposed to irritation, e− excited in the CB of g-C3N4 can swiftly move to the CB of a secondary photocatalyst with a greater potential. In parallel, the generated holes will move in the opposite direction. The creation of a Type II junction allows for the spatial separation of photogenerated electrons and holes, which prevents them from recombining and allows them to participate in desired redox reactions efficiently. This separation of charges leads to an increased lifetime of the charge carriers and enhances the photocatalytic activity of the system. Moreover, the band alignment in Type II heterojunctions can promote interfacial charge transfer processes, such as electron or hole transfer from one component to another, further improving the overall photocatalytic efficiency. This synergistic effect between different semiconductor materials in the heterojunction structure enables better utilization of solar energy and enhances the photocatalytic performance of g-C3N4-based systems. This phenomenon can be validated through specific analytical techniques like steady-state/time-resolved photoluminescence (PL) spectra, photocurrent measurements, and EIS measurements. Different types of semiconductor substances have been used in combination with g-C3N4 to create Type II heterojunctions to reduce the recombination of the generated charges, such as TiO2, ZnO, Fe2O3, MoO3, WO3, ZnTe, CdS, MoS2, ZnIn2S4, Bi2WO6, and others.104–107 For instance, various hierarchical heterojunctions of BixOyIz/g-C3N4, such as g-C3N4/BiOI, g-C3N4/Bi4O5I2, and g-C3N4/Bi5O7I have been successfully developed.108 The g-C3N4/BiOI is synthesized using a direct precipitation method, while g-C3N4/Bi4O5I2 and g-C3N4/Bi5O7I are obtained through in situ calcination transformation of g-C3N4/BiOI at different temperatures. The g-C3N4/BiOI and g-C3N4/Bi4O5I2 heterojunctions are classified as Type-I, while g-C3N4/Bi5O7I is categorized as a Type-II heterojunction. Notably, g-C3N4/Bi5O7I exhibited significantly improved performance compared to g-C3N4/BiOI and g-C3N4/Bi4O5I2. The promoted activity of g-C3N4/Bi5O7I can be attributed to its surface area, promote charge separation and transfer performance, and robust charge carrier density resulting from the formation of a Type-II heterojunction.
It is worthy to mention that metal oxides heterostructures can not only enhance the visible light absorption ability of g-C3N4 due to their unique band structures but also facilitate the separation and transfer of photogenerated electron–hole pairs, as well as improve the stability and reusability of g-C3N4 photocatalysts. The metal oxides act as protective layers, preventing the photocorrosion of g-C3N4 and enhancing its durability under harsh reaction conditions. This is particularly advantageous for long-term applications and practical implementation. The method used to incorporate the metal oxide into g-C3N4 can significantly impact the distribution and interaction between the two components, which ultimately affects the photocatalytic efficiency. For instance, TiO2 is a widely favored photocatalyst due to its excellent chemical stability, affordability, and suitable valence band (VB) and conduction band (CB) positions that facilitate redox reactions.115,116 Thus, a highly efficient heterojunction photocatalyst was developed by combining TiO2 nanotubes with g-C3N4 through a thermal deposition approach.117 In this process, a solution containing 100 mg of TiO2 nanotubes and 4 mg of g-C3N4 in 20 mL of distilled water was subjected to stirring at 80 °C for 6 hours. The HRTEM analysis confirmed the close attachment between TiO2 and g-C3N4, indicating a strong solid interaction and successful formation of the heterojunction.117 In a separate study, an S-scheme heterojunction of mesoporous/macro TiO2/g-C3N4 was fabricated using a straightforward chemical vapor deposition technique.118 The research revealed that by adjusting the melamine dosage, the microstructure of the samples could be readily controlled.118 Similarly, ZnO/g-C3N4 photocatalyst, consisting of ZnO loaded onto g-C3N4, was fabricated using an ex situ crystallization strategy.119 The images revealed that ZnO particles were present on the g-C3N4 layers, distinguishing it from pure g-C3N4 (Fig. 3a and b).119 XPS analysis confirmed the presence of Zn in the modified catalyst, indicating the successful combination of ZnO with g-C3N4 (Fig. 3c). Moreover, coral-like WO3/g-C3N4 were fabricated using a wet chemistry strategy, with different mass ratios of WO3 to g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 3
3, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). TEM images revealed that g-C3N4 appeared as ribbon-like sheets, surrounded by plate-like particles of WO3.121 The measurements of the crystallographic particle spacing between 0.20 and 0.39 nm suggest the existence of tiny crystalline zones in the g-C3N4 nanosheets. This close contact between g-C3N4 and WO3 facilitates the good separation of photo-excited carriers.121 Further, TiO2/g-C3N4 composites containing 20–50% TiO2 by weight were fabricated using a hydrothermal process by dispersing TiOSO4 in DI water, followed by the addition of g-C3N4 and ultrasonication for 30 minutes.120 The mixture was then heated in an autoclave at 180 °C for 4 hours. The resulting powder was dried at 65 °C. XRD patterns of the composites displayed peaks from both g-C3N4 and TiO2, with no shifting in the TiO2 peaks demonstrating that the TiO2 lattice structure was not impacted by the coupling with g-C3N4 (Fig. 3d). This lack of influence on the lattice structure is beneficial for photocatalytic activity. Moreover, among the composites, 40% TiO2/g-C3N4 had the lowest bandgap energy at 2.89 eV (Fig. 3e).120 In another study, MoO3/g-C3N4 was fabricated by combining 0.01 g of Mo2N with varying quantities of g-C3N4 and the resulting mixtures were subjected to calcination at 350 °C for 240 minutes.122
1). TEM images revealed that g-C3N4 appeared as ribbon-like sheets, surrounded by plate-like particles of WO3.121 The measurements of the crystallographic particle spacing between 0.20 and 0.39 nm suggest the existence of tiny crystalline zones in the g-C3N4 nanosheets. This close contact between g-C3N4 and WO3 facilitates the good separation of photo-excited carriers.121 Further, TiO2/g-C3N4 composites containing 20–50% TiO2 by weight were fabricated using a hydrothermal process by dispersing TiOSO4 in DI water, followed by the addition of g-C3N4 and ultrasonication for 30 minutes.120 The mixture was then heated in an autoclave at 180 °C for 4 hours. The resulting powder was dried at 65 °C. XRD patterns of the composites displayed peaks from both g-C3N4 and TiO2, with no shifting in the TiO2 peaks demonstrating that the TiO2 lattice structure was not impacted by the coupling with g-C3N4 (Fig. 3d). This lack of influence on the lattice structure is beneficial for photocatalytic activity. Moreover, among the composites, 40% TiO2/g-C3N4 had the lowest bandgap energy at 2.89 eV (Fig. 3e).120 In another study, MoO3/g-C3N4 was fabricated by combining 0.01 g of Mo2N with varying quantities of g-C3N4 and the resulting mixtures were subjected to calcination at 350 °C for 240 minutes.122
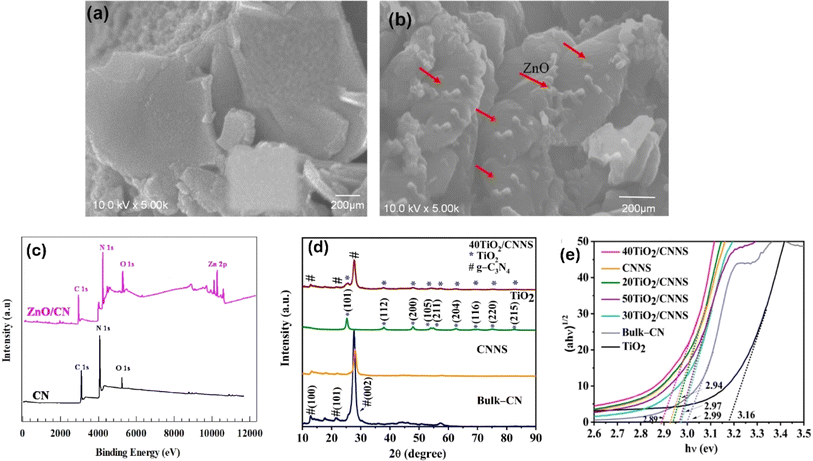 | ||
| Fig. 3 Surface morphology of (a) g-C3N4 and (b) ZnO/g-C3N4 and (c) XPS of g-C3N4 ZnO/g-C3N4, reprinted with the permission of ref. 119, copyright 2024, Elsevier; (d) PXRD patterns of bulk-g-C3N4 (CN), g-C3N4 nanosheets (CNNS), TiO2, and 40TiO2/CNNS, (e) Tauc plot displaying band gaps of g-C3N4, TiO2 and their composites.120 | ||
Metal sulfides is another type of semiconductor materials, greatly enhancing the efficiency of photocatalysis.123–126 Metal sulfides possess band structures that meet the thermodynamic requirements for water splitting and exhibit improved responses to sunlight due to the formation of a less negatively charged valence band through the (S-3p) orbitals.127 These advantageous properties of metal sulfides significantly contribute to the superior photocatalytic performance of g-C3N4/metal sulfide heterojunction systems.112,128 The incorporation of metal sulfides allows for the creation of customizable band structures, thereby providing tangible benefits for the desired photocatalytic reaction. In a study, CdS/g-C3N4 core/shell nanowires were synthesized using a combination of solvothermal and chemisorption methods.112 Transmission electron microscopy (TEM) analysis revealed that g-C3N4 was effectively coated onto CdS nanowires, establishing intimate contact between the two materials. Additionally, the composite exhibited a higher surface area compared to pure CdS.112 In another investigation, a one-step solvothermal strategy was utilized to synthesize ultra-thin g-C3N4 (UCN) and incorporate NiS onto the surface of ZnIn2S4 (ZIS).129 The resulting ternary compound, NiS/ZnIS/UCN, was designed to possess dual great-speed charge transfer channels. By combining these materials, the composite achieved improved efficiency in H2 generation through enhanced charge transfer.129 It is evident from the TEM picture of NiS/ZIS/UCN that some NiS is loaded onto the surface of ZIS and UCN, implying that the heterojunction ternary compound of NiS/ZIS/UCN has been well constructed.129 In another work, a series of CoS2/g-C3N4 were fabricated through a photodeposition strategy.130 The size of the CoS2 species could be adjusted, ranging from single atom to nanometer scale, allowing for control over the photocatalytic features. The synthesis process involved mixing 20 mg of g-C3N4 with a solution containing 1 mL of 15.2 mg mL−1 thiourea aqueous solution, 1 mL of 5 mg mL−1 Co(CH3COO)2, 4 mL of ultrapure water, and 4 mL of absolute ethanol. The mixture was evacuated to remove air and then irradiated using a 300 W Xenon lamp to facilitate the deposition of CoS2 onto the g-C3N4 surface.130 In another work, a solvothermal approach was utilized to create a heterostructure photocatalyst made of g-C3N4/Bi2S3/CuS.131 Further, NiS/g-C3N4, CdS/g-C3N4, and CdS/NiS/g-C3N4 were created via a simple and dependable chemical deposition technique.126 In another study, g-C3N4 was coated with ternary NiCo2S4 using a solvent evaporation technique.132 Whereby, 30 mL of ethanol was used to dissolve sulphide nanoparticles and g-C3N4 nanosheets, and the mixture was then ultrasonicated for 30 minutes to create a homogenous suspension. Subsequently, the solvent evaporated at 70 °C, yielding a ZnCo2S4/g-C3N4 photocatalyst. The ZnCo2S4 nanoparticles, which are in very near proximity to the 2D g-C3N4 flakes, have a median size of around 20 nm, as determined by TEM investigation (Fig. 4a–d). Moreover, EDS analysis, on the other hand, confirmed that C, N, Zn, Co, and S coexist in the composite and that the atomic ratios of Zn, Co, and S are around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, which is in agreement with the ZnCo2S4 theoretical chemical ratio (Fig. 4e).132
4, which is in agreement with the ZnCo2S4 theoretical chemical ratio (Fig. 4e).132
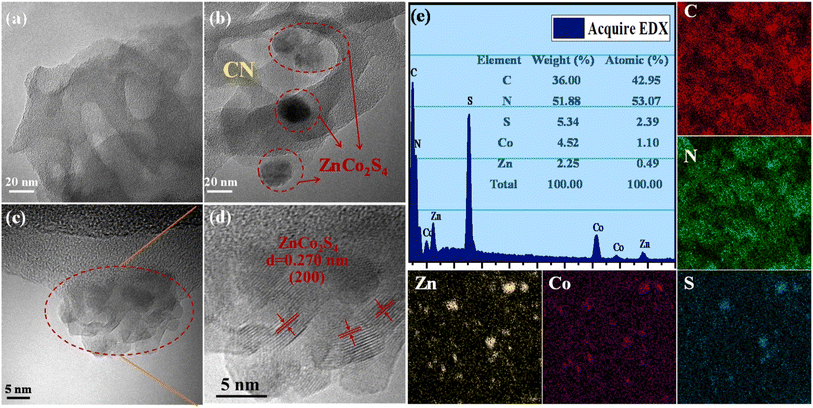 | ||
| Fig. 4 TEM images of (a) CN and (b) ZnCo2S4/CN, HRTEM images of (c–d) ZnCo2S4/CN, and (e) EDS spectrum of ZnCo2S4/CN and elemental mapping analysis, reprinted with the permission of ref. 132, copyright 2024, Elsevier. | ||
Pioneering studies constructed heterostructure with other different types of semiconductors, such as phosphides, carbonates, nitrides, halides, among others.133–137 For instance, Ag2CO3/g-C3N4 heterojunctions were fabricated using an ultrasonic method, where Ag2CO3 was sonochemically targeted and fixed to the g-C3N4 active centers.135
Carbon materials including graphene, carbon nanofibers, carbon nanodots, carbon nanotubes, and other forms of carbon materials, have gained significant attention for coupling with g-C3N4 in heterojunctions.138–141 Carbon materials possess symmetrical molecule arrangements with unique conjugated structures, offering superior photon excitation, high surface area, thermodynamic stability, and electron transmission.142–145 The creation of carbon-induced g-C3N4 photocatalysts presents a viable route for sustained improvements in photocatalytic technology as well as renewable carbon materials as an ecologically benign alternative to metal-based materials. Enhancement of photocatalytic processes has been obtained by modifications of carbon-induced g-C3N4 photocatalysts by several techniques such as junction interaction, surface reconstruction, cocatalyst effects, local electric modification, and more.146–149 For instance, g-C3N4/GO (graphene oxide)-wrapped melamine sponge (MS) monolith was developed through successful design and fabrication (Fig. 5).150 The g-C3N4 was uniformly distributed on the GO, ensuring efficient utilization of incident light and effective contact with pollutants. By acting as a bridge, GO facilitated the connection between the g-C3N4 and MS components. In another instance, g-C3N4/GO nanocomposite was synthesized by loading g-C3N4 onto GO using an electrostatic self-assembly approach.151 Furthermore, a unique protonated g-C3N4/GO aerogel (p-CN/GOA) was synthesized by a direct frozen-drying technique (Fig. 6a).152 The protonating treatment caused a significant change in the surface electric charge of g-C3N4, converting it from negative to positive (p-CN), which allowed for powerful self-assembly with the negative surface of GO. This assembly facilitated the transfer of photogenerated charge carriers. The stacking of p-CN blocks, which were several microns in size, were uniformly attached to the GO nanosheet due to the abundant surface functional groups of GO (Fig. 6c). While TEM confirmed the excellent loading of p-CN onto GO (Fig. 6d), providing further evidence of the combination between p-CN and GOA.152 In order to enhance the efficiency of underwater photocatalysis for g-C3N4, a composite consisting of g-C3N4 and carbon nanotubes (CNT) was fabricated using an in situ solvothermal approach.153 This composite had great surface area and improved light absorption capacity. The findings demonstrate that CNT and g-C3N4 exhibit good compatibility with each other. The g-C3N4 can grow directly on the surface of CNT, forming a stable composite structure.153 Another study used a straightforward water bath approach to construct g-C3N4 that had been enhanced with carbon nanotubes (CNTs).154 The morphological study showed that two materials were mixed together and that CNTs were wrapped in a lot of g-C3N4. This mixture promoted the movement of photogenerated electrons and aided in their separation efficiency.154 Further, carbon fibers (CF), graphene (GN), and CNTs were introduced to modify g-C3N4 through a solvothermal approach.155 The development morphology of the synthetic composites varied significantly depending on the utilized carbon substrate as shown in Fig. 7.155 The poor physicochemical features (e.g., SBET, particle size, pore volume, adsorptive properties, … etc.), the limited photocatalytic catalytic activity, and stability and poor light-harvesting of pristine g-C3N4 are marginally boosted by proper modification and application of modified g-C3N4. The superior photocatalytic performance of modified g-C3N4 over pristine g-C3N4 is illustrated by various examples shown in Tables 1 and 2.
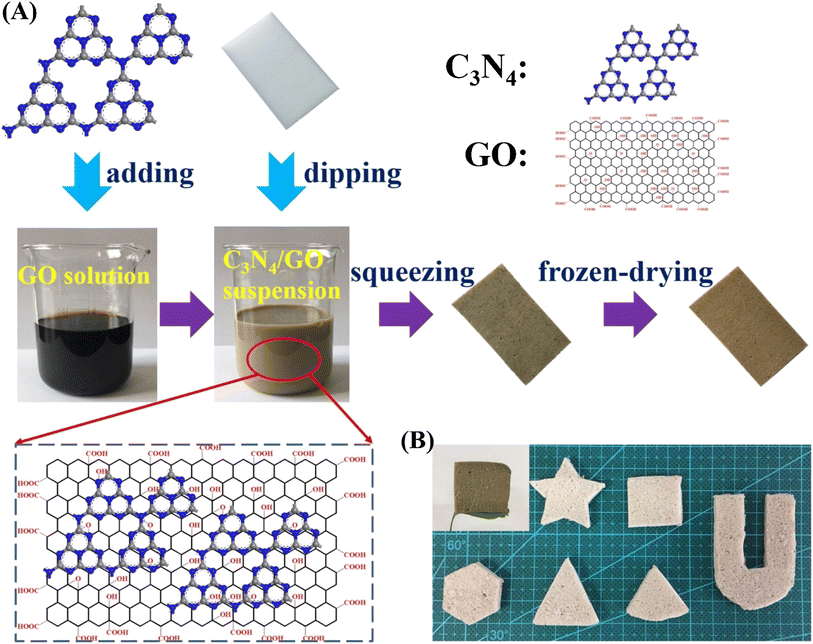 | ||
| Fig. 5 (a) Schematic illustration of the preparation of g-C3N4/GO-wrapped sponge; (B): image of different shapes of g-C3N4/GO-wrapped sponge, reprinted with the permission of ref. 150, copyright 2024, Elsevier. | ||
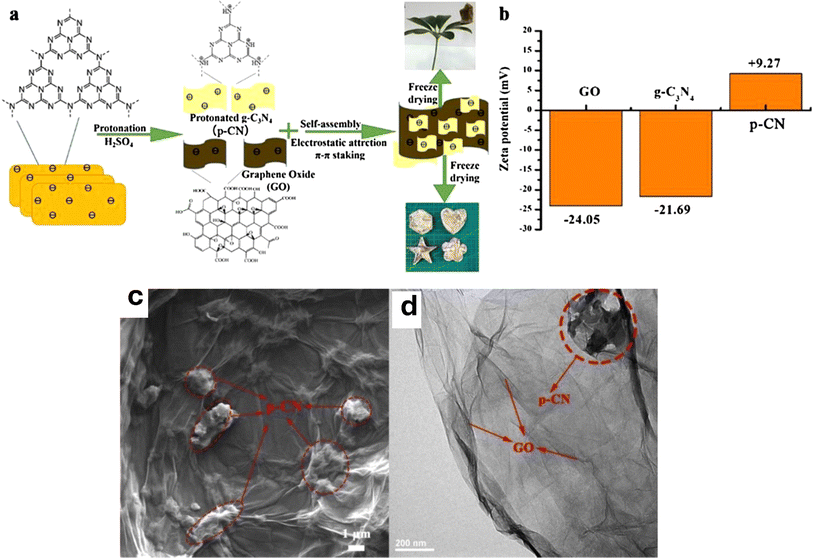 | ||
| Fig. 6 (a) Schematic of the fabrication of p-CN/GOA; (b) zeta potential of GO, g-C3N4 and p-CN, (c) the SEM of p-CN/GOA; (d) the TEM of p-CN/GOA, reprinted with the permission of ref. 152, copyright 2024, Elsevier. | ||
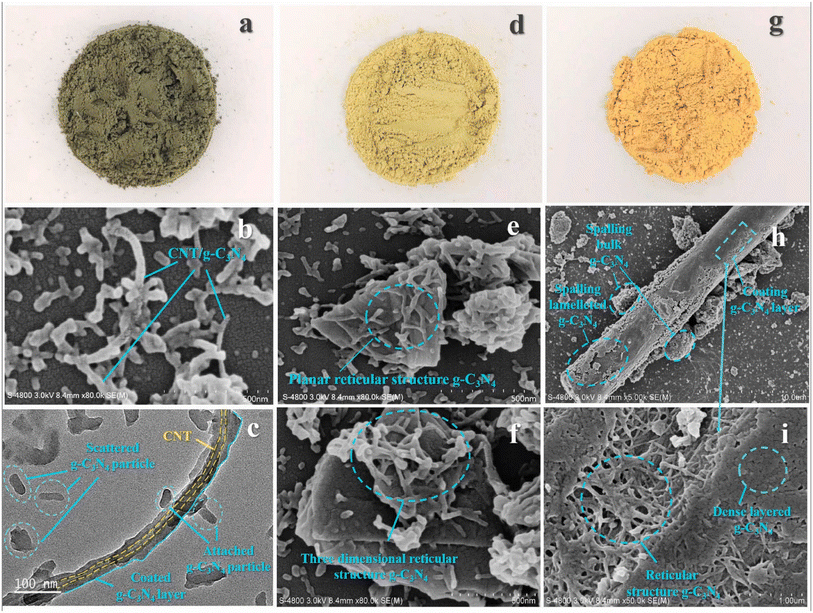 | ||
| Fig. 7 Macro shots of (a–c) CNT/g-C3N4, GN/g-C3N4 and CF/g-C3N4. SEM images of (d–f) CNT/g-C3N4, GN/g-C3N4 and CF/g-C3N4. TEM images of (g–i) CNT/g-C3N4, GN/g-C3N4 and CF/g-C3N4, reprinted with the permission of ref. 155, copyright 2024, Elsevier. | ||
| Photocatalyst composite | Pollutant | Initial concentration (mg L−1) | Catalyst dose (mg) | Light source | Irradiation time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a g-C3N4, graphitic carbon nitride; rGO, reduced graphene oxide; MWCNTs, multi-walled carbon nanotube; carbon dots (CDs) -BC, biochar; TC, tetracycline; RhB, rhodamine B, MB, Methylene blue; MO, methyl orange; TC, tetracycline; CV, crystal violet; DZN, diazinon; IMD, imidacloprid; atrazine, ATZ. | |||||||
| rGO–g-C3N4 | RhB | 10 | 8.0 mg | 1000 W Xe lamp | 100 | 75 | 156 |
| MWCNTs–g-C3N4 | MB | 10 | 50 mg | 300 W Xe lamp | 180 | 100 | 157 |
| RhB | 180 | 89.7 | |||||
| MO | 180 | 84.5 | |||||
| TiO2/g-C3N4 | MB | 20 | 100 mg | 400 W Xe lamp | 180 | 90 | 158 |
| BN–g-C3N4 | RhB | 20 | 50 mg | 300 W Xe lamp | 120 | 98.0 | 159 |
| TC | 60 | 79.7 | |||||
| CQDs/g-C3N4 | TC | 10 | 500 mg | 300 W XL | 120 | 65 | 160 |
| MoS2/g-C3N4 | MB | 5 | NA | UV light | 80 | 98.7 | 161 |
| U doped C3N4 | RhB | 5 | 300 W XL | 50 | 100 | 162 | |
| g-C3N4/ZnO | MB | 10 | 50 | Solar simulator | 16 | 100 | 163 |
| S-doped g-C3N4 | MB | 10 | NA | 100 W lamp | 180 | 90 | 164 |
| g-C3N4/CdWO4 | TC | 10 | 50 | 250 W Xe lamp | 80 | 300 | 165 |
| Ag-g-C3N4 | MB | 10 | NA | 200 W Xe | 96 | 120 | 166 |
| CV | 80 | ||||||
| RhB | 78 | ||||||
| Sm-g-C3N4 | MY | 20 mM | 100 | LED light | 80 | 360 | 167 |
| P-doped g-C3N4 | RhB | 20 | 20 | Xe lamp | 70 | 99.5 | 168 |
| Fe-g-C3N4 | RhB | 10 | 20 | 300 W Xe lamp | 45 | 90 | 169 |
| BiOI exfoliated g-C3N4 | TC | 20 | 50 | 500 W xenon lamp | 30 | 86 | 170 |
| Ti0.7Sn0.3O2/g-C3N4 | TC | 20 | 25 | 1.5 W LED lamp | 40 | 83 | 171 |
| TiO2/g-C3N4 | APAP | 10 | 25 | PLS-SXE300 Xe lamp (300 W) | 45 | 96.7 | 172 |
| C3N4–Ce2S3 | ATZ | 100 | 30 | Xe lamp of 300 W (6280 lumens) | 90 | 95 | 173 |
| CN/MoO3−x | Phenol | 50 | NA | Full light, 300 W | 60 | 98 | 174 |
| CoOx/g-C3N4 | MO | 10 | 35 | 500 W Xenon lamp | 180 | 92.0 | 175 |
| Phenol | 10 | 49 | |||||
| norfloxacin | 10 | 80 | |||||
| Zn3V2O8/g-C3N4 Z | DZN | 5 | 350 | Visible light (180 mW cm−2) | 60 | 95.2 | 176 |
| BiVO4/g-C3N4 | IMD | 50 | 60 | UV-C light (15 W m−2) | 30 | 94.2% | 177 |
| Bi2WO6/g-C3N4 | ATZ | 800 | 200 | 500 W long-arc xenon lamp | 180 | 99.9 | 178 |
| Photocatalyst composite | Pollutant | Initial concentration (mg L−1) | Catalyst dose (mg) | Light source | Irradiation time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| K-doped g-C3N4/BiOBr | RhB | 20 | 50 | 500 W Xe | 90 | 90 | 179 |
| g-C3N4/CuO/ZnO | MB | 10−5 mol L−1 | 50 | Visible light | 75 | 99 | 180 |
| Ag/ZnO/S-g-C3N4 | MB | 10 | 10 | Visible light (57–63 Klux) | 60 | 98 | 181 |
| Ag10-C3N4-NA2SO4 | RhB | 10 | 25 | Visible light | 50 | 96.5 | 182 |
| g-C3N4/TiO2/carbon fiber | TC | 10 | 25 | 350 W xenon lamp | 90 | 99.9 | 183 |
| Bi2O2CO/g-C3N4/Bi2O3 | TC | 10 | 10 | Visible light (490–540) mW cm−2 | 60 | 80 | 184 |
| WO@g-C3N4@MWCNTs | TC | 20 | 20 | Halogen lamp 500 W, 420 nm | 120 | 79.5 | 185 |
| AgPO4/g-C3N4/ZnO | TC | 30 | NA | 45 W visible lamp | 120 | 88.4 | 186 |
| Bi7O9I3/g-C3N4/Bi3O4Cl | Phenol | 10 | 50 | NA | 100 | 100 | 187 |
| Ag@SrTiO3/g-C3N4 | Dicofol | 5 | 50 | 300 W Xe lamp | 60 | 92.2% | 188 |
| BC-g-C3N4-MgO | Dinotefuran | 10 | 100 | (CEL-HXF300) | 260 | 80.1 | 189 |
| CDs@BiOI/g-C3N4 | TC | 20 | NA | 30 W LED | 60 | 82.7% | 190 |
| MIL125(Ti)/g-C3N4/rGO | RhB | 10 | 25 | Fluorescent lamp (32 W) | 120 | 98 | 191 |
3 Applications of g-C3N4 based nanocomposites
3.1. Applications in water treatment
For instance, heterojunctions of Bi2S3/g-C3N4 with varying concentrations of Bi2S3 have been developed for the Rhodamine B (RhB) degradation under sunlight.196 The photocatalytic response is moved to the deep visible spectrum by depositing Bi2S3 on g-C3N4. When exposed to natural solar radiation, the rate of RhB dye breakdown on 10% Bi2S3/g-C3N4 is four times higher compared to bare g-C3N4 and Bi2S3 alone. This is explained by the fact that Bi2S3 nanoparticles extend optical reactivity under the whole range of natural sunlight, which lowers the rate at which hole–electron pairs recombine, promotes large charge-carrier movement, and ultimately raises photocatalytic efficiency. The decomposition of RhB is primarily impacted by positive holes, radical species, and superoxide radicals. The S-scheme mechanism described the movement of charge carriers (Fig. 8a), as revealed by terephthalic acid PL examinations and radical scavenging tests (Fig. 8b and c).196
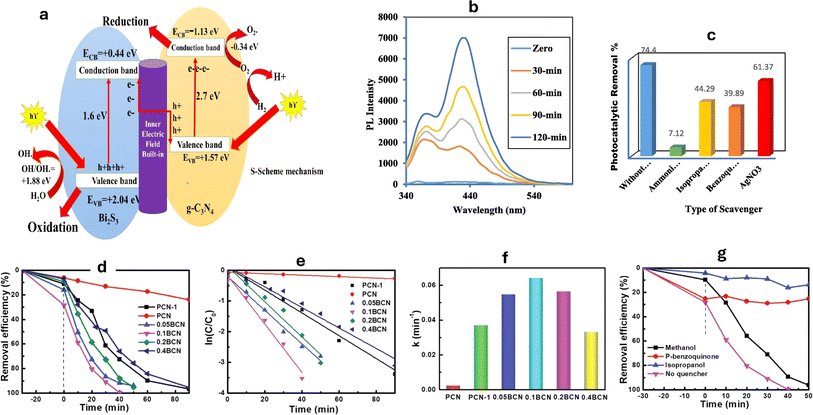 | ||
| Fig. 8 (a) An S-scheme for charge transfer between g-C3N4 and Bi2S3 in CNBiS10 catalyst, (b) PL of terephthalic acid over CNBiS10, and (c) effect of various scavengers on photocatalytic removal of terephthalic acid over CNBiS10 reprinted with the permission of ref. 196, copyright 2024, Elsevier; (d) degradation profiles of RhB catalyzed by polymeric carbon nitride (PCN) and Bi doped g-C3N4 at various ratios (BCN), (e) Pseudo first-order kinetics curves of RhB degradation, (f) apparent rate constant histogram of RhB, and (g) degradation of RhB with various radical quenchers reprinted with the permission of ref. 197, copyright 2024, RSC. | ||
Further, the degradation of methylene blue dye (MB) was carried out using MoO3/g-C3N4 heterojunction enhanced with biomass carbon dots. In comparison to bulk g-C3N4, pure MoO3, and pure carbon dots, the heterojunction demonstrated a better degradation rate of 67% throughout one hour of simulated sunlight irradiation.198 Under ideal compounding circumstances, the heterojunction between MoO3 and g-C3N4 was verified, resulting in an enhanced charge transfer rate at the interface.198
To enhance the photocatalytic activity of g-C3N4, the researchers loaded g-C3N4 with different magnesium salts.199 Among the various samples tested, the MgSO4-g-C3N4 composite exhibited the highest efficiency for photocatalytic degradation, achieving a photodynamic parameter of 26.36 × 10−3 min−1. Reactive substances including O2˙−, h+, and ˙OH that oxidized MB during the photocatalytic degradation process, where the ˙OH was the most contributing species.199 In another investigation, g-C3N4 was loaded with potassium salts such as KF, KCl, and KBr, resulting in the formation of KX-g-C3N4 (X = F, Cl, and Br).200 Remarkably, KF-g-C3N4 exhibited exceptional performance in the degradation of MB when exposed to visible light. Notably, KF-g-C3N4 effectively suppressed the recombination of holes and electrons, surpassing the photocatalytic activity of KCl-g-C3N4, KBr-g-C3N4, and pure g-C3N4 materials.200 On the other hand, bismuth/g-C3N4 nanotubes (BCN) with a porous structure having various bismuth fractions (0.05–0.40 g) were utilized for the RhB degradation of.197 The highest degradation efficiency was observed with the 0.1 BCN sample, which completely degraded RhB within 40 minutes (Fig. 8d). The degradation kinetics followed pseudo-first-order behavior (Fig. 8e), and the rate constant (k for 0.1 BCN was 0.0644 min−1), which was 26.8 times higher than that of pure g-C3N4 (PCN) (Fig. 8f), where the degradation was inhibited in the presence of isopropanol and p-benzoquinone (Fig. 8g).197 Furthermore, by adopting a straightforward impregnation technique, g-C3N4-TiO2 nanocomposites with varying weight proportions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 2
3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 3
2, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were produced. Under UV-visible illumination, the effectiveness of these nanocomposites in MB dye photocatalytic degradation was examined.201 When contrasted with virgin g-C3N4 and different weight percentages of g-C3N4/TiO2, the nanocomposite with a 3
1) were produced. Under UV-visible illumination, the effectiveness of these nanocomposites in MB dye photocatalytic degradation was examined.201 When contrasted with virgin g-C3N4 and different weight percentages of g-C3N4/TiO2, the nanocomposite with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio had the highest photocatalytic activity. Because there were fewer TiO2 nanoparticles deposited on the g-C3N4 nanosheets, the electron–hole pair transport features were improved, which increased the catalytic efficiency. The creation of a Z-scheme system between TiO2 and g-C3N4 explains the improved photocatalytic behavior.201
1 weight ratio had the highest photocatalytic activity. Because there were fewer TiO2 nanoparticles deposited on the g-C3N4 nanosheets, the electron–hole pair transport features were improved, which increased the catalytic efficiency. The creation of a Z-scheme system between TiO2 and g-C3N4 explains the improved photocatalytic behavior.201
In order to create the TiO2@g-C3N4 (TCN) core–shell quantum heterojunction, an effective way of polymerizing the quantum-thick g-C3N4 onto the surface of TiO2 with exposed facets was adopted and applied the obtained nanocomposite to the photocatalytic degradation of tetracycline (TC), as shown in Fig. 9a.202,205 The maximum rate of tetracycline degradation, exhibited using 100 mg TCN nanocomposite photocatalyst, was 2.2 mg min−1; that is 36% more than the rate observed in the TiO2/g-C3N4 random mixture (TCN(mix)), twice as high as TiO2, and 2.3 times higher than pure g-C3N4. The distinct advantages of the structure of the quantum-thick g-C3N4 shell, the abundance of readily accessible reaction sites, and the compact and consistent contact interface, are what make TCN more photocatalytically active. The notable improvement in the photocurrent responsiveness of TCN electrodes further supports efficient mobility of electrons among TiO2 and g-C3N4. The catalyst's stability was verified by TEM analysis and XRD, as shown in Fig. 9b. The principal oxidant species for the successful photocatalytic process, according to the results, are h+ and ˙O2−, as shown in Fig. 9c.202 Furthermore, the researchers found that the improved catalytic activity of CuAl2O4/g-C3N4 for TC photodegradation is mainly due to the significant separation of charge carriers, as shown by the transient photocurrent response.110 Moreover, the use of g-C3N4 loaded various metals (Na, K, Ca, Mg) has been studied for the degradation of enrofloxacin (ENR).203 The presence of oxygen atoms in the g-C3N4 nanocomposites has been confirmed through XPS, TEM, and FTIR analysis. These added metals, combined with the oxygen atoms, have altered the electronic structures and morphology of the g-C3N4, resulting in reduced charge recombination and improved light absorption. As a result, g-C3N4–Na and g-C3N4–K produced both hydroxyl radicals and superoxide, while g-C3N4, g-C3N4–Ca, and g-C3N4–Mg only produced superoxide radicals (Fig. 9d). In another study, the integration of graphene onto the edges of g-C3N4 enhanced the absorption of photons with energies below the intrinsic bandgap.206 This integration resulted in a broad-spectrum-driven response and facilitated near-field electron transfer. The strong π-conjugated bond-stitched nanostructures between graphene and g-C3N4 were found to effectively capture adsorbed oxygen molecules, leading to the production of ˙O2−, promoting the interaction between pollutant molecules and the photocatalyst NPs.206 Additionally, the incorporation of reduced graphene oxide (rGO) into g-C3N4 greatly enhanced the photocatalytic activity of bisphenol A (BPA) approximately three times at neutral pH to give 99% removal within 60 minutes.204 The synthesized rGO/g-C3N4 nanocomposite exhibited increased electrical conductivity and improved surface area, leading to enhanced separation of electron–hole pairs, as shown in Fig. 9e and g. The positioning of heterocyclic nitrogen pz orbitals in g-C3N4 was shifted after decorating with rGO, facilitating the polarization of charge distribution, and resulting in the formation of active holes that boosted the BPA degradation.204
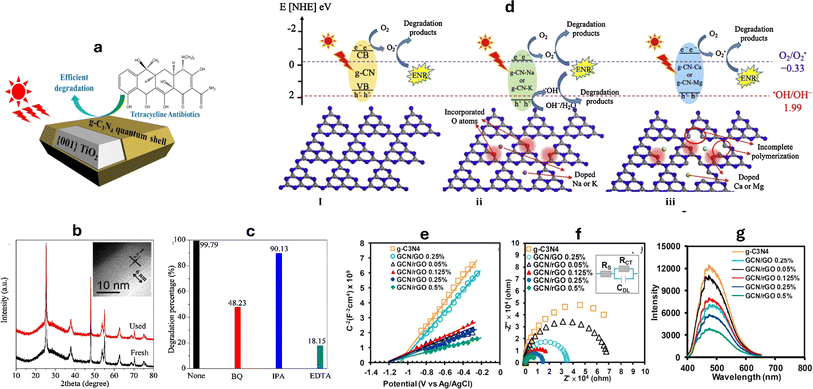 | ||
| Fig. 9 (a) Degradation of tetracycline by TiO2/g-C3N4 (TCN),202 (b) XRD patterns of TCN before and after TC degradation process, TEM image (inset) of the used TCN, (c) effect of different scavenger on TCN photocatalytic activity reprinted with the permission of ref. 202, copyright 2024, Elsevier; (d) degradation mechanism of enrofloxacin (ENR) by g-C3N4, in the absence and the presence of Na, K, Ca, and Mg dopants reprinted with the permission of ref. 203, copyright 2024, Elsevier; (e) EIS measurements presented as the Mott–Schottky plot,204 (f) the Nyquist plot, and (g) the photoluminescence spectra of bisphenol A photodegradation in the presence of rGO/g-C3N4 nanocomposites with different rGO ratios reprinted with the permission of ref. 204, copyright 2024, Elsevier. | ||
One of the key parameters to consider is the pH of the reaction medium. pH influences not only the adsorption capacity of the catalyst but also the protolytic equilibria involving the catalyst and the pollutant, as well as the pollutant's solubility.207,208 These factors can significantly affect the surface charge of the catalyst and the pollutant molecules, thereby impacting their interaction and subsequent degradation efficiency.209 Therefore, determining the optimum pH range is essential to maximize the photocatalytic performance. However, it should be noted that pH optimization is highly dependent on the specific pollutant and composite being used, as different materials may exhibit different pH sensitivities. The photocatalyst shows positive/negative zeta potentials depending on pH, demonstrating that its surface charge varies significantly with the solution's pH.210 For instance, the researchers investigated the pH impact on the degradation of RhB and MO dyes, using g-C3N4@NiAl LDH catalyst.211 They found that the catalyst had a point of zero charge (PZC) value of 6.6 where the highest efficiency for degrading MO occurred at a pH of 3, while RhB degradation was most effective at a pH of 10. Since RhB is a positively charged dye, it experiences repulsion when it approaches the positive surface of the catalyst in the presence of free H+ ions, leading to lower degradation at pH 3 compared to neutral or basic conditions. Similarly, MO degradation was reduced under basic circumstances by competition and repulsion among the OH− anions and the anionic MO moieties for adsorption on the photocatalyst.211 Additionally, in the photodegradation of trimethoprim (TMP), peroxymonosulfate (PMS) can be activated by Fe-g-C3N4 with various compositions.212 Thus, it was shown that 0.2% Fe-g-C3N4/2 wt% rGO/PMS greatly increased the TMP degradation rate in the acidic environment (pH = 3), from 61.4% at pH = 6 to almost 100%. On the other hand, at basic pH levels, where TMP existed primarily as an anionic species, the repulsion among the Fe-doped g-C3N4/rGO composites and TMP hindered its degradation, leading to lower performance.212 Furthermore, g-C3N4/TiO2 (PZC = 6.0) exhibited the highest effectiveness in basic and neutral pH conditions, which promoted the interaction between the cationic RhB molecules and the catalyst's negatively charged surface functional moieties at pH > 6, leading to improved photodegradation of RhB, as shown in Fig. 10a.213 Conversely, g-C3N4/rGO exhibited pH-sensitive photocatalytic performance toward the photocatalytic degradation of the Rh Cationic dye, with a significantly greater rate of photodegradation at low acidity levels (pH = 1.98).217 The rate of RhB photodegradation dropped markedly as pH increased and reached almost zero at pH ≥ 7. This pH sensitive behavior was attributed to the promoted electron-transfer, at lower pH, between RhB, H+, and rGO that acted as a good platform for transferring e− through its atomic sheets.217
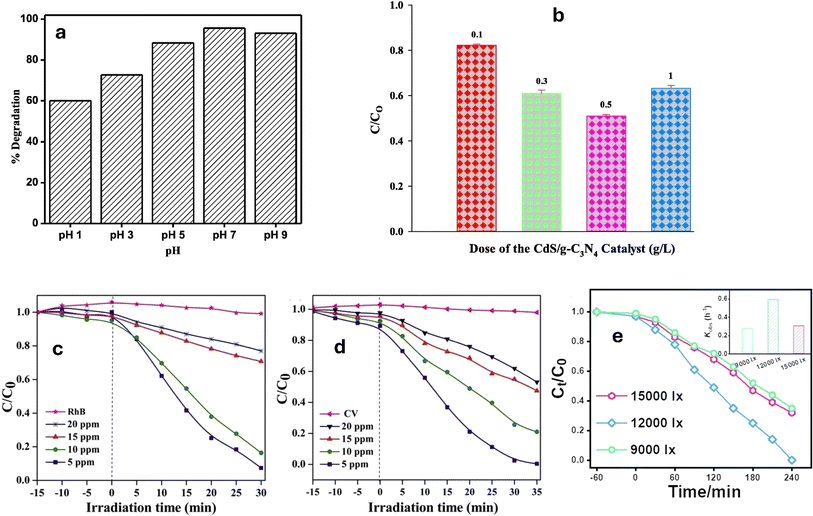 | ||
| Fig. 10 (a) Effect of pH on degradation of RhB dye reprinted with the permission of ref. 213, copyright 2024, Elsevier, (b) the effect of catalyst mass on degradation of MO dye, reprinted with the permission of ref. 214, copyright 2024, Elsevier; (c and d) effect of initial RhB and CV dye concertation on the degradation performance reprinted with the permission of ref. 215, copyright 2024, Elsevier; (e) effect of light intensity on sulfamethoxazole removal, reprinted with the permission of ref. 216, copyright 2024, Elsevier. | ||
The weight or loading amount of the catalyst material can impact various aspects of the photocatalytic process, ultimately affecting the degradation efficiency. One of the key aspects influenced by the weight of the catalyst is surface area.218 Increasing the weight of the catalyst generally leads to an increase in the available active surface area for pollutant adsorption and subsequent reaction.209,219,220 This can be beneficial as it provides more sites for catalytic activity, allowing for a higher number of reactive species to be generated. Consequently, the degradation rate of the organic pollutant may increase with increasing catalyst dose. However, it is important to note that there is an optimum weight or loading amount beyond which further increases may not result in proportional enhancements in degradation efficiency.221 This is because excessively high loadings can lead to aggregation or agglomeration of the catalyst particles, reducing the accessible surface area and hindering the photocatalytic process.209 Moreover, high-weight loadings can also cause light scattering or absorption, limiting the penetration of photons and reducing the overall photocatalytic activity. For instance, the photocatalytic performance of TiO2/g-C3N4 improved with increasing catalyst doses until the optimal dose was reached due to the enhancement in the available active sites.222 Moreover, the photocatalytic degradation efficiency of MO dye increased with the CdS/g-C3N4 mass; however, beyond the optimal mass the catalyst particles tended to aggregate, resulting in increased light scattering and lowered overall effective surface area, as well as reduced catalytic activity.214 The results presented in Fig. 10b indicate that the optimal dose of CdS/g-C3N4 for achieving the highest photodegradation of MO is 0.5 g L−1. A similar trend was also observed for the degradation RB in the presence of g-C3N4/CdO photocatalyst.223
Increasing the initial pollutant's concentration can lead to a greater number of pollutant molecules available for adsorption onto the catalyst surface.195,224 This can result in improved initial degradation rates, as more pollutant molecules can interact with the generated reactive radicals. Higher pollutant concentrations can also lead to an increased chance of collisions between the target molecules and the photocatalyst, enhancing the overall degradation efficiency. However, it is important to note that there is an optimum concentration beyond which increasing the initial pollutant concentration may not lead to further enhancements in photocatalytic activity. This is primarily due to two factors. Firstly, at high concentrations, the adsorption sites on the catalyst surface may become saturated, hindering further adsorption of the pollutant molecules. This can limit the availability of reactive radicals and decrease the overall degradation efficiency. Secondly, high concentrations of pollutant molecules in the reaction medium can absorb or even scatter the incident light, preventing it from reaching the photocatalyst surfaces effectively.225 Consequently, the generation of electron–hole pairs and the subsequent reactions may be limited, resulting in reduced photocatalytic activity. For instance, the rate of degradation of the rhodamine B and crystal violet (CV) dyes by the zeolite nanorods decorated g-C3N4 nanosheets (H-ZSM-5/g-C3N4) was demonstrated in Fig. 10c and d, illustrating the impact of varying starting dye concentrations.215 In this case, a pseudo-first-order (PFO) kinetic model explained the dye elimination process. The degradation rate was low at a high concentration (20 ppm) owing to light being impeded from reaching the active sites by the high chromaticity dye molecules present in considerable quantities. Other researchers reported a reduction in the dye degradation at higher concentrations owing to competition among hydroxyl ions and organic substances on active sites as well as the distracted light before reaching the catalyst surface.223 Similarly, in studying the effect of loading ZnO/g-C3N4 nanocomposites with aluminum, magnesium, nickel, copper, and silver, on the degradation rate of 50–300 mg L−1 Eriochrome Black T dye (EBT), the results showed a decreased dye degradation efficiency at higher concentrations of the EBT dye.226
The light intensity plays a significant role in photocatalytic degradation processes as it directly affects the absorption of photons by the catalyst. Higher light intensities provide a greater number of photons, leading to increased electron–hole pair generation and subsequent formation of reactive species, resulting in improved degradation rates.68 However, it is important to note that once a certain light intensity threshold is reached, further increases may not proportionally enhance photocatalytic activity. In fact, excessive light intensities can lead to increased energy consumption without providing substantial benefits. Thus, optimizing light intensity is crucial to achieve the optimal photocatalytic performance. Factors such as the source of light, the wavelength, and the type of catalyst used should all be considered when determining the ideal light intensity for a specific photocatalytic system. For instance, the photocatalytic degradation of sulfamethoxazole (SMX) using Fe-UCN's catalyst was greatly affected by the used light intensity. Under 9000, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 15
000, and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lx of LED light intensity, the SMX % removals were 48%, 75%, and 53%, respectively, as shown in Fig. 10e.216 Therefore, while more intense light may provide the catalysts with photons for creating ˙OH and lower the pollutant's concentration, too much light may actually inhibit photocatalytic activity due to excessive electron consumption, resulting in the accumulation of extra holes on the catalysts, which hinders the photodegradation process.216
000 lx of LED light intensity, the SMX % removals were 48%, 75%, and 53%, respectively, as shown in Fig. 10e.216 Therefore, while more intense light may provide the catalysts with photons for creating ˙OH and lower the pollutant's concentration, too much light may actually inhibit photocatalytic activity due to excessive electron consumption, resulting in the accumulation of extra holes on the catalysts, which hinders the photodegradation process.216
The presence of multiple pollutants can lead to either synergistic or inhibitory effects on the degradation process. Synergistic effects occur when the presence of one pollutant enhances the degradation of another pollutant, due to the formation of reactive species or the modification of the degradation pathway. On the other hand, inhibitory effects can occur when the presence of one pollutant hinders the degradation of another pollutant, due to interactions between the pollutants that can compete for reactive species or affect the availability of active sites, thereby reducing the overall degradation efficiency. Therefore, it is crucial to consider the interactions between pollutants in a mixed system when evaluating degradation efficiency. For instance, pCN-N/ZIS Z-scheme heterojunction was evaluated for the synergistic photodegradation of metronidazole (MNZ) and methyl orange (MO).227 The combination of electron-donating groups on MO and MNZ molecules and electron traps on catalyst surfaces, which improves the catalyst's capacity to contact and adsorb pollutants and ultimately improves the catalytic degradation performance.227 Moreover, the degradation of a mixed MB and RhB dye solution was investigated using ZnFe2O4-g-C3N4 as the photocatalyst with the addition of H2O2 under sunlight illumination. The MB degradation rate was found to be much greater than that of RhB. As shown in Fig. 11a, after 35 minutes of exposure to sunlight, the maximum removal of MB was 100%, and in the presence of H2O2, the maximum removal of RhB was 92%.228 Similarly, the synergistic degradation efficiency of g-C3N4/α-Fe2O3 for the mixed RhB and MB solution was reported.229 At five cycles, the fabricated catalyst exhibits a high-performance, as shown in Fig. 11b. Conversely, Co3O4/g-C3N4 nano-heterojunctions were fabricated to degrade a mixture of TC antibiotic and MB dye pollutants, under solar irradiation. The researchers noticed that MB in the mixed solution showed an improved degradation rate (nearly 100% in 120 minutes) than when it was eliminated individually (90% in 120 minutes).230 When compared to the TC antibiotic's solo activity (97% in 180 minutes), the mixture's antibiotic degradation efficiency was slightly lower (78% in 180 minutes). The formation of intimate interfaces with enhanced photophysical properties was attributed to the band bending induced by the p–n nano-heterojunctions, as shown in Fig. 11c and d.230 The degradation efficiency of a g-C3N4-based Ce2O3/CuO (GCC) ternary nanocomposite was studied for mixed anionic metanil yellow (MY) and cationic MB dyes, under visible light exposure.231 Notably, the ternary GCC nanocomposite exhibited excellent performance, achieving high removal efficiencies for both MY and MB aqueous dyes (94.5% and 90.3%, respectively). This superior performance can be attributed to the optimal amounts of Ce2O3 and CuO present on the g-C3N4 surface, which facilitated the creation of heterojunction surfaces, thereby efficiently reducing the recombination rates of photo-excited charges.231
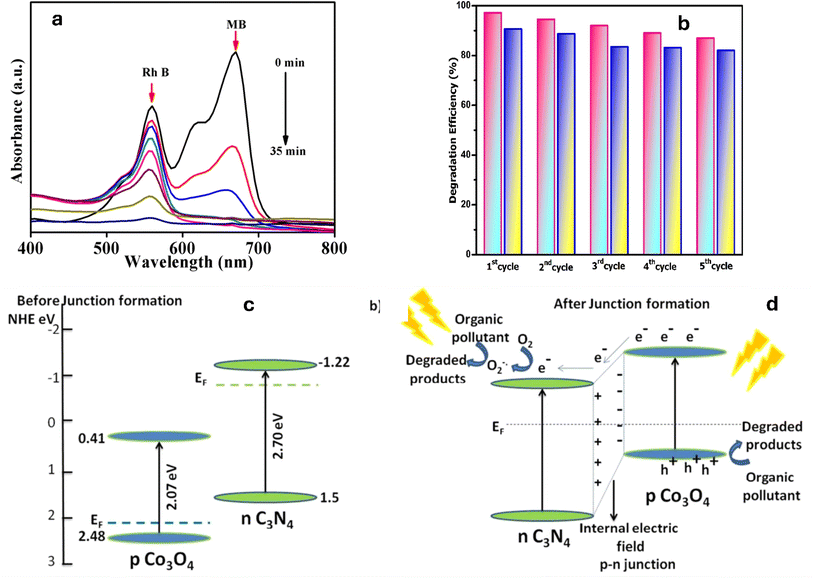 | ||
| Fig. 11 (a) UV absorption spectrum of Mixed dye (MB + RhB) by ZnFe2O4–CN,228 (b), recycling catalytic activity measurement for mixed pollutants,229 (c) band alignment of p-type Co3O4 and n-type C3N4 before junction formation and (d) band alignment and the photocatalytic mechanism of Co3O4–C3N4 p–n nano-heterojunctions, reprinted with the permission of ref. 230, copyright 2024, RSC. | ||
3.2. Hydrogen production
The production of hydrogen as a clean and sustainable energy source has gained significant attention in recent years. Several strategies were employed to enhance the photocatalytic activity of g-C3N4, which is an efficient photocatalyst that utilizes solar energy to split water and produce hydrogen. When excited by photons with energy equal to or higher than its bandgap, g-C3N4 can generate electron–hole pairs that can be involved in a series of reactions to produce hydrogen. The key steps involved in photocatalytic hydrogen production include light absorption, charge, separation, surface reactions and mass transfer.232,233One common approach is the modification of g-C3N4 through metal co-catalyst decoration. For instance, platinum (Pt) nanoparticles can be loaded onto g-C3N4 to enhance the hydrogen evolution reaction (HER) kinetics by providing active sites for hydrogen formation. Other transition metals, such as nickel (Ni) and cobalt (Co), have also been utilized as co-catalysts due to their cost-effectiveness and abundance. Thus, the researchers created a combination of sulfidized bimetallic nickel and platinum decorated g-C3N4 with various Pt masses for the production of H2 using visible light. They found that the addition of the NiSx electron acceptor in the S-PtNiX/g-C3N4 catalyst resulted in improved performance compared to catalysts without it, such as PtSx or Sulfidized-g-C3N4.234 The existence of PtNiX assisted in the correct transmission of charges. The impressive photocatalytic activity of the S-PtNiX/g-C3N4 catalyst, which achieved a rate of 4966 μmol g−1 h−1, can be attributed to the collaborative effect of NiSX ability to accept electrons and PtNiX superior charge transfer capabilities.234
Another study described single Pt atom co-catalysts embedded on g-C3N4 via a procedure that involves two stages including incipient wetness impregnation and copolymerization.235 During a 4 hours period, the studies conducted with pure g-C3N4 exhibited minimal activity, generating around 12.7 μmol h−1 g−1. This suggests that subjecting g-C3N4 to visible light resulted in minimal photocatalytic efficiency. In contrast, the photocatalytic hydrogen evolution of g-C3N4 dramatically improved upon the adoption of 0.1–0.3 wt% of single Pt atoms as co-catalysts. The photocatalytic H2 evolution for Pt0.1-g-C3N4, Pt0.2-g-C3N4 and Pt0.3-g-C3N4 were about 1054.3, 4875.0 and 2932.8 μmol g−1 in 4 h, respectively. The highest rate of hydrogen generation was obtained with 0.2% Pt-based catalyst, due to its highest negative CB location and remarkable capacity to separate and transmit photogenerated charge carriers.235
Moreover, a heterojunction consisting of NiS grown on a 2D ultrathin g-C3N4 matrix was constructed for visible light-induced H2 generation.236 The presence of the NiS/g-C3N4 resulted in a synergistic impact, effectively enhancing the separation of photo-generated carriers and promoting interfacial charge transfer performance. The rate of H2 generation using the exfoliated NiS/g-C3N4 catalyst reached 4.2 μmol h−1 g−1, which is approximately 2.6 times higher compared to bulk C3N4/NiS.236 The creation of 0-D/2-D heterojunctions using g-C3N4 nanosheets and polyfluorene dots (Pdots) (Pdots/g-C3N4) was investigated and showed a substantial rise in photocatalytic HER, reaching 929.3 μmol g−1 h−1 with an apparent quantum efficiency of 5.7% at 420 nm.237
The photocatalytic water-splitting capability of Ag3PO4/g-C3N4 has been studied.238 The nanocomposite band gap energy value of 2.90 eV, suggest that it may be a successful visible light-harvesting composite. According to the research, compared to the electrons in the CB (0.21 eV) of g-C3N4, the Ag3PO4 electrons in CB (−1.08 eV) showed more potential for reducing water and protons to form H2. Similarly, VB holes of g-C3N4 exhibited stronger oxidizing capabilities than those of Ag3PO4, resulting in the production of ·OH radicals. Ag3PO4/g-C3N4 composite showed an electron transformation mechanism that resulted in the production of a Z-scheme process, which is beneficial for water splitting to produce H2.238
Using Ti3C2 MXene as a precursor, carbon-doped TiO2 (C–TiO2) linked with g-C3N4 was synthesized.239 In comparison to pure TiO2 with average particle size of 25 nm (P25), the C–TiO2 exhibited a lowered bandgap of 2.94 eV, implying boosted visible light absorption with a redshifted absorption edge at 425 nm. As a result, the 10% C–TiO2/g-C3N4 catalyst produced hydrogen at a rate of 1409 μmol g−1 h−1 (λ > 420 nm) with enhanced activity ascribed to the creation of a Type II heterojunction, which enables optimum charge separation and increased accessorial surface area, offering extra reaction sites upon coupling with C–TiO2.239
Moreover, the researchers applied FeOx/g-C3N4 for the improved efficiency H2 evolution through water splitting.240 The optimized amount of FeOx led to an impressive H2 evolution rate of 108 μmol h−1 that is 4.2 times higher than that of pristine g-C3N4. Numerous reasons, such as increased surface area, greater electron transfer ability, better visible light absorption, and superior charge carrier separation, are responsible for this improvement.239
Other researchers conducted a study on the fabrication of g-C3N4/CNTs for achieving high-efficiency H2 production.241 They incorporated different types of CNTs, including single-walled (SW), double-walled (D), and multi-walled (MW), to enhance the activity of g-C3N4-based photocatalysts. Enhanced production of photocatalytic hydrogen was seen when the amount of CNTs is low, leading to a boost in the stability and quantity of photogenerated charges. The improved electron transport from g-C3N4 to CNTs, which was particularly apparent in the case of SWCNTs, accounts for this improvement.241
Additionally, the S-scheme heterojunction of N-doped MoS2/S-doped g-C3N4 was successfully constructed using a straightforward one-step thermal polymerization approach.242 Following material optimization, the catalyst's photocatalytic hydrogen generation rate reached 658.5 μmol g−1 h−1. This was made possible by the boost in visible light absorption and photogenerated carrier separation yield caused by the S-scheme's design.
Further, a comprehensive investigation on the impact of three common transition metal phosphides (M2P, M = Fe, Co, and Ni) as cocatalysts in sulfur-doped g-C3N4 (S–CN) was investigated.243 The researchers utilized an ultrasound-assisted approach to create M2P/S–CN with similar load ratios, ensuring comparable crystallization levels and particle sizes. Ni2P/S–CN demonstrated the most rapid charge transfer and separation among the three phosphides, resulting in smaller photocatalytic overpotential. This remarkable performance yielded a rate of hydrogen generation that was comparable to that of Pt/S–CN catalysts and 22.7 times higher than that of bare S–CN.243
Otherwise, a simple wet-chemical fabrication approach was used to successfully produce a dual Z-scheme heterostructure of g-C3N4, PrFeO3, and Fe2O3.244 This cascade dual Z-scheme exhibited impressive production, generating 379.29 μmol g−1 h−1 under visible-light exposure. The inclusion of magnetic components in the heterostructure facilitated the easy separation of the catalyst and enabled its reusability. Additionally, RuNi/g-C3N4 catalysts doped with 2D bimetallic RuNi alloys were created using the solvothermal deposition approach involving various Ru ratios. The catalyst sample having 2.3 wt% Ru revealed the greatest hydrogen evolution, reaching 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 μmol g−1 h−1, surpassing the performance of the Pt/g-C3N4 photo-catalyst.245 Table 3 shows the photocatalytic H2-evolution performance characteristics of representative g-C3N4-based photocatalysts.
100 μmol g−1 h−1, surpassing the performance of the Pt/g-C3N4 photo-catalyst.245 Table 3 shows the photocatalytic H2-evolution performance characteristics of representative g-C3N4-based photocatalysts.
| Photocatalyst composite | Sacrificial agent | Catalyst dose (mg) | Light source | H2 evolution rate | Ref. |
|---|---|---|---|---|---|
| Graphene with 1% wt. and g-C3N4 | Methanol | 80 mg | 350 W Xe arc lamp | 451μmol h−1 g−1 | 246 |
| AgIO4/g-C3N4 | Methanol | 0.1 g | Solar simulator | 23 mmol h−1 g−1 | 247 |
| BiVO3/g-C3N4 | Methanol | 0.05 g | 350 W Xenon | 6.8 mmol g−1 h−1 | 248 |
| C60/g-C3N4/graphene | TEOA | 100 mg | 5 W light-emitting diode (LED) irradiation | 545 μmol h−1 g−1 | 249 |
| Graphene/ZnIn2S4/g-C3N4 | — | 5 mg | Solar light | 545 μmol h−1 g−1 | 250 |
| TiO2/g-C3N4 | Methanol | 0.1 g | Xenon lamp of 350 W | 4.9 mmol g−1 h−1 | 222 |
| SnO2/g-C3N4 | Methanol | 0.1 g | Xenon lamp of 350 W | 6.56 mmol g−1 h−1 | 251 |
| g-C3N4/0.25% RGO/3% NiS | TEOA | 50 mg | 300 W Xe arc lamp | 393 μmol h−1 g−1 | 252 |
| Cu2O@g-C3N4 | TEOA | 0.3 g | 300 W xenon lamp | 265 μmol h−1 g−1 | 253 |
| TiO2–g-C3N4 | Methanol | 0.1 g | Xenon lamp | 35.44 μmol h−1 g−1 | 254 |
| g-C3N4/WO3 | — | 50 mg | 300 W Xe lamp | 982 μmol h−1 g−1 | 255 |
3.3. Carbon dioxide reduction
Applying g-C3N4-based nanocomposites for CO2 reduction holds significant promise for addressing the global challenge of climate change by transforming CO2 emissions into valuable products, such as methane, methanol, and hydrocarbons. The photocatalytic activity of g-C3N4-based composites is attributed to their unique structure and composition, which facilitate the absorption of light and generation of reactive species for CO2 sequestration, contributing to the reduction of greenhouse gas emissions and the development of a circular carbon economy.256 Fig. 12 depicts the basic steps involved in CO2 photoreduction involving surface and optoelectronic properties.257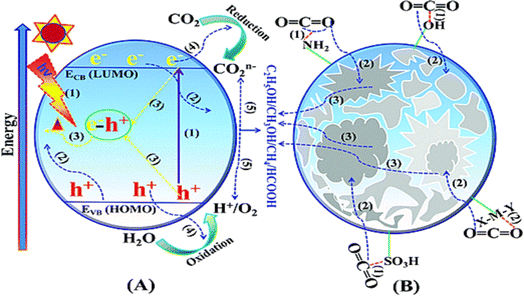 | ||
| Fig. 12 General steps involved in CO2 photoreduction coupled with water oxidation: (A) optoelectronic: (1) e−–h+ generation, (2) charge migration to the surface, (3) e−–h+ recombination, (4) CO2 photoreduction, (B) physicochemical: (1) CO2 adsorption, (2) CO2 activation, and (3) product desorption.257 | ||
g-C3N4 based photocatalysts play a robust role in the process of CO2 photoreduction through their optoelectronic and physicochemical features. These catalysts expose active sites on their surfaces where CO2 adsorption and activation take place. Therefore, when designing C3N4-based photocatalysts, it is essential to prioritize factors such as efficient visible light absorption, promote surface area, quick electron transfer to the catalyst surface, exposure of functional groups, minimized recombination rate, and a robust redox potential value. Fig. 13 shows how CO2 is transformed into methane and methanol on the surfaces of g-C3N4.258 The process starts by capturing and activating CO2 when two electrons are generated.257 Then, an intermediate called COOH* is produced, which eventually converts into CO. The hydrogenation of CO* into COH* or CHO* is a significant step in CO2 reduction.258 For instance, the Ag3PO4@g-C3N4 hybrid promoted the photocatalytic reduction of CO2.259 This was achieved by forming a heterojunction structure between Ag3PO4 and g-C3N4, which promoted the CO2 reduction activity through a Z-scheme mechanism that facilitated the charge separation phenomena. When exposed to simulated sunlight, the optimized Ag3PO4@g-C3N4 hybrid demonstrated a robust CO2 conversion rate of 57.5 μmol h−1 gcat−1, surpassing the rates of pure g-C3N4 and P25 catalysts by 6.1 and 10.4 times, respectively. Further, graphene-supported 1D nano-arrays of crystalline carbon nitride (1D-CCN) heterojunction was developed and demonstrated promoted interface charge transfer, facilitated light absorption, and promoted CO2 capture capabilities.260 Furthermore, the 1D-CCN demonstrated a 44% selectivity for CO2 over N2, with isosteric heat adsorption of 55.2 kJ mol−1 for CO.
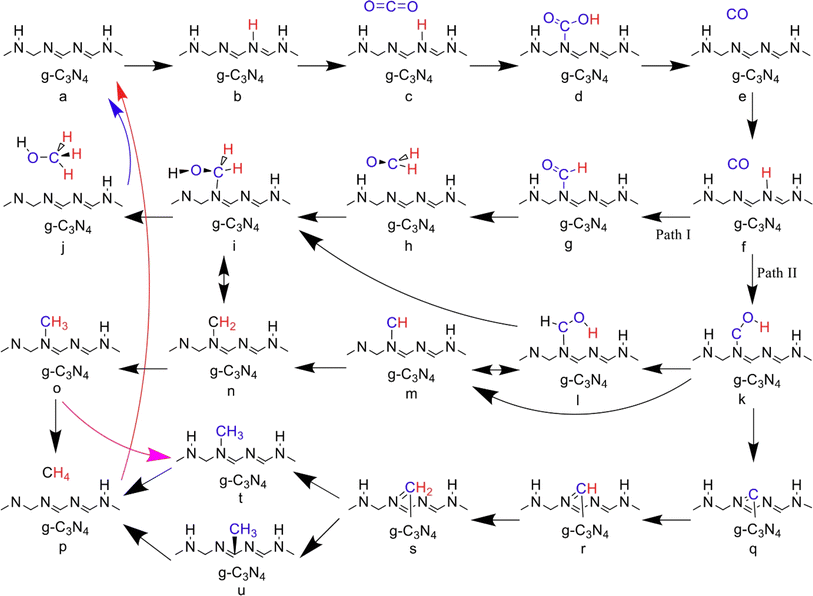 | ||
| Fig. 13 Proposed reaction pathway for CO2 reduction to methanol and methane on the surface of g-C3N4, reprinted with the permission of ref. 258, copyright 2024, Elsevier. | ||
Researchers have introduced B, S, Mo, O, and P heteroatoms into g-C3N4 to promote its performance in CO2 photoreduction.79,260–263 Among these dopants, O- and P-doped g-C3N4 demonstrated robust conversion capabilities compared to pure g-C3N4. Additionally, S-doping and creating N-vacancies can introduce impurities in the conduction band position of g-C3N4, expanding light absorption to longer wavelengths and minimizing recombination rates. Moreover, the researchers created ternary hybrids (ACNNG-x) by combining AgBr with g-C3N4-modified nitrogen-doped graphene (NG) in various ratios.264 These catalysts were employed for reducing CO2 using visible light. The process of making the composite and SEM image of the optimized ternary hybrid are displayed in Fig. 14a and b, respectively. The optimized ternary composite demonstrated promising CO2 reduction rates of 105.89 μmol g−1 for methanol and 256.45 μmol g−1 for ethanol. A proposed mechanism for the process is presented in Fig. 14c. Similarly, g-C3N4/NaNbO3 nanowires were synthesized for CO2 reduction.265
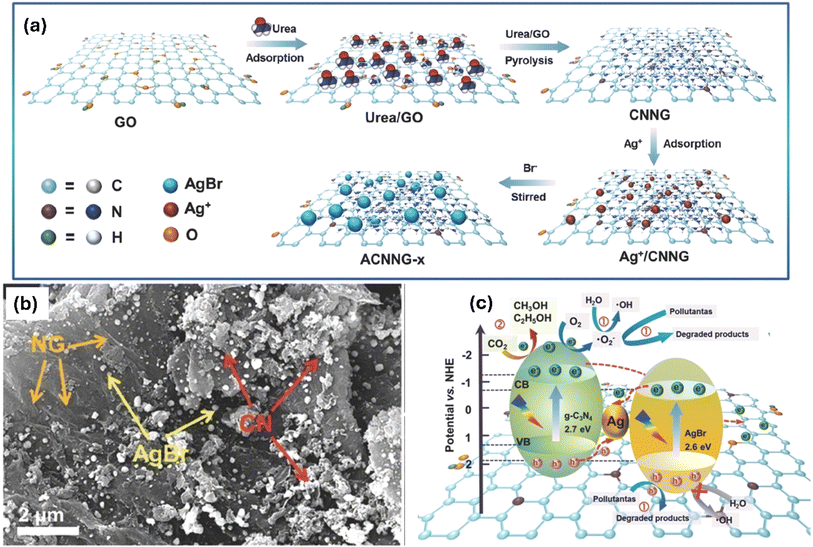 | ||
| Fig. 14 (a) Synthesis process of ACNNG-x hybrid, and (b) its SEM image, and (c) a mechanism for CO2 reduction by the hybrid nanocomposites, reprinted with the permission of ref. 264, copyright 2024, Elsevier. | ||
Enhancing the overall system performance by modifying g-C3N4 with a component for CO2 adsorption has proven effective. For instance, g-C3N4 combined with a cobalt-containing zeolitic imidazole framework (Co-ZIF-9), demonstrated high CO2 adsorption capacity (2.7 mmol g−1) and a significant microporous surface area (1607 m2 g−1), facilitating CO2 capture and concentration in its pores.266 The addition of bipyridine as an electron mediator allowed photoexcited electrons to transfer from g-C3N4 to Co-ZIF-9 for CO2 reduction, as shown in a PL quenching study. In this system, CO was the primary product, achieving a quantum efficiency of 0.9% without the need for a cocatalyst.266 Moreover, g-C3N4/Bi2WO6 hybrid was hydrothermally fabricated to selectively convert CO2 to CO through photoreduction.267 The hybrid demonstrated a visible-light CO generation rate of 5.19 mmol g−1 h−1, surpassing that of g-C3N4 alone. The hybrid's improved photocatalytic activity was attributed to effective charge separation and transfer following a Z-scheme mechanism.267
3.4. Hydrogen peroxide production
Hydrogen peroxide (H2O2) production using g-C3N4-based photocatalysts is a promising approach that has gained significant attention in recent years. The photocatalytic strategy for generation H2O2 typically involves two major approaches: the reduction of O2 and the oxidation of H2O. The reduction of O2 can occur through a one-step, two-electron process or a two-step, one-electron process. The oxidation of H2O, on the other hand, occurs in a single step, with photogenerated holes driving the reaction. However, the direct oxidation of H2O for H2O2 production is challenging due to the robust thermodynamics involved and the tendency of H2O2 to act as a scavenger for the photogenerated holes at high oxidation potentials.268–271The photocatalytic production of H2O2 using g-C3N4-based composite photocatalysts typically involves the reduction of oxygen (O2) to H2O2 using the photogenerated electrons in the conduction band of the composite. The key to efficient H2O2 production lies in the ability of the composite to efficiently absorb visible light and facilitate the separation of photogenerated electron–hole pairs. Recent progress of g-C3N4-based catalyst for H2O2 production is shown in Fig. 15.269
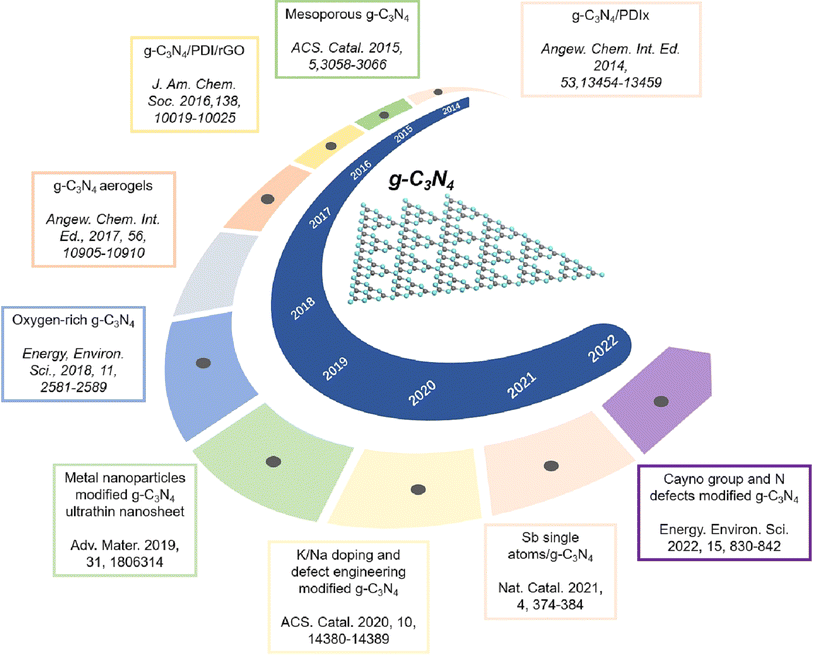 | ||
| Fig. 15 A summary of recent H2O2-generation methods based on g-C3N4 photocatalysts.269 | ||
One of the key factors that influence the H2O2 production efficiency of g-C3N4-based composite photocatalysts is the design of the heterojunction interface. A widely studied g-C3N4-based composite photocatalyst for H2O2 production is the g-C3N4/TiO2 system.272 Where n S-scheme heterojunction promoted the light absorption and the separation of photogenerated charges, resulting in enhanced photocatalytic performance and improved H2O2 production.273 Additionally, pairing of g-C3N4 with other materials, such as GO and metal–organic frameworks (MOFs) further improved the H2O2 production efficiency.274,275
In the case of g-C3N4/GO composites, the GO acts as an efficient electron acceptor, facilitating the separation of photogenerated electron–hole pairs in the composite.191 The high specific surface area and excellent electrical conductivity of GO contribute to the improved H2O2 production efficiency. Similarly, the integration of g-C3N4 with MOFs can provide a high surface area and tunable pore structure, enhancing the adsorption of reactants and the photocatalytic H2O2 production.276
The choice of materials and their relative positioning within the composite can synergistically impact the light absorption, charge separation and transfer processes and the overall catalytic activity. Computational studies using density functional theory (DFT) calculations have provided valuable insights into the electronic structure, band alignment, and charge carrier dynamics at the g-heterostructure interfaces.276,277 DFT calculations and experimental data attributed the boosted photocatalytic activity of the modified heterostructure to the positively charged MOF sheet interlayer, and the coupling between MOF nanosheet, g-C3N4, and CuO that can enrich ions, electrons, and molecules and obstruct holes to greatly boost the rapid separation of photogenerated carriers from g-C3N4 and/or CuO, and the reactants' adsorption.276 Thus, incorporating suitable metal active centers into the g-C3N4 framework is an effective approach to promote the activity and selectivity of the oxygen reduction reaction (O2RR).278 The adsorption of O2 on the metal surface can occur in three different configurations: Griffiths-type (side-on), Pauling-type (end-on), and Yeager-type (side-on).269 For instance, the researchers developed a novel Sb-single-atom photocatalyst (Sb-SAPC) doped g-C3N4 that exhibited exceptional performance, generating H2O2 at 12.4 mg L−1, 248 times higher than pristine g-C3N4.279 The enhanced activity of Sb-SAPC-g-C3N4 is attributed to the Sb-SAPC sites that facilitated O2 adsorption and activation, where the accumulation of photogenerated holes at neighboring N-atoms near Sb sites promotes the oxygen evolution reaction (OER) for O2 production. The Sb–OOH intermediates suggest a direct one-step, two-electron reduction pathway for H2O2 generation.279
Forming a heterojunction structure is a successful approach to addressing the challenge of charge carrier recombination in pristine g-C3N4. This is because the difference in Fermi level between g-C3N4 and the coupled co-catalysts drives the photogenerated charge carriers to migrate between the two components. For instance, a 2D/2D heterojunction composed of ZnIn2S4 and g-C3N4 (ZIS/CN) was prepared employing a simple oil bath heating approach.280 The obtained data demonstrated that the H2O2 production proceeded through a 2-electron oxygen reduction (2e− O2RR), reflecting a robust selectivity towards H2O2 generation. The promoted photocatalytic performance was attributed to the synergistic impact of intimate interfacial contact. In another study, an oxygen-doped g-C3N4 modified g-C3N4/TiO2 (OCN@CNT-2) hybrid system was constructed through an electrostatic self-assembly approach, where a double Z-scheme architecture was formed within the target OCN@CNT-2 composite.272 This unique heterojunction promotes the charge separation under the influence of an internal electric field. As a result, after 60 minutes, the system was able to achieve a remarkably high H2O2 yield of up to 133.04 μmol L−1.
The produced H2O2 can find various applications, including water purification, disinfection, and oxidation processes. Ongoing research aims to further improve the H2O2 production efficiency, stability, and scalability of g-C3N4-based composite photocatalysts, paving the way for their practical implementation in large-scale H2O2 production systems.
4 Conclusion and prospective
In conclusion, this comprehensive review article has covered various aspects of g-C3N4 based nanocomposites, including their synthesis and characterization methods, their application in the removal of organic pollutants and hydrogen production, and the factors influencing their photocatalytic activities. Through the incorporation of dopants, metal deposition, metal chalcogenide semiconductors, and carbon materials, these nanocomposites have exhibited remarkable photocatalytic capabilities with potential for real-world environmental remediation and energy production. The synthesis and characterization techniques discussed in this article have provided valuable insights into enhancing the performance and stability of g-C3N4-based composites. The introduction of dopants and metal deposition, as well as metal chalcogenide semiconductors have enabled the modification of the band structure and surface properties, thereby improving the separation and transfer of photogenerated charge carriers. The incorporation of carbon materials, such as graphene or carbon nanotubes, has contributed to the enhancement of photocatalytic activity by increasing the surface area and facilitating electron transfer. The photocatalytic degradation of various organic pollutants, including dyes, pesticides, and pharmaceutical compounds, has been effectively achieved using g-C3N4 based composites. Additionally, the production of hydrogen as a clean and sustainable energy source has been successfully demonstrated through photocatalytic water splitting. The investigation of factors affecting the photocatalytic process has deepened our understanding of the mechanisms involved and has highlighted the important working factors such as catalyst dose, pH, and light intensity. This knowledge can be utilized to optimize the design of g-C3N4 based nanocomposites, tailoring them for specific applications and improving their overall performance and efficiency.Looking to the future, there are several exciting prospectives for further development in the field.
1. The scale-up of synthesis methods and the development of cost-effective production techniques are essential for the practical application of g-C3N4-based composites. Efforts should also be made to evaluate their long-term stability and recyclability to ensure their viability for laboratory, pilot-plant and large-scale implementation with the involvement of engineering and chemistry disciplines.
2. In the pursuit of constructing novel g-C3N4-based photocatalysts, there is a need for template-free and environmentally friendly synthetic approaches that can yield unique structures and exceptional intrinsic properties. However, the current methods of modifying these photocatalysts have certain limitations. Some of the selected composite materials contain expensive and environmentally detrimental elements. Achieving precise chemical doping of g-C3N4 is a difficult task that often results in the introduction of impurities. Furthermore, the available techniques for controlling the structure of g-C3N4 are relatively limited and have only minimal effects. Additionally, achieving precise control over the microstructure of these photocatalysts remains a challenging endeavor.
3. More detailed and specific reporting is needed to elucidate the synergistic effects that occur among the individual materials in complex heterostructures.
4. While there is a theoretical understanding of the charge transfer and separation pathways, further experimental evidence is necessary to validate these photochemical mechanisms and establish effective photocatalytic systems on a larger scale.
5. In the realm of photocatalytic degradation, it is crucial to address the simultaneous degradation several pollutants present in real wastewater using g-C3N4-based materials. Furthermore, g-C3N4-based photocatalysts hold significant potential for bifunctional catalysis, considering their catalytic economy and efficiency.
6. Furthermore, it is crucial to preserve and enhance the biocompatibility and eco-friendly properties of future g-C3N4-based nanomaterials.
7. To meet the industrial aim of photocatalytic hydrogen production, the solar to hydrogen (STH) efficiency must be at least 10%. Currently, the maximum efficiency attained in laboratory research is 9.2%, while the STH efficiency for g-C3N4 is less than 3%, indicating that much more work remains to be done. The most significant job for the g-C3N4 photocatalyst is to construct more efficient electron transport systems.
8. Gaining a comprehensive understanding of the underlying mechanisms driving photocatalytic H2O2 production is essential. Researchers should direct their efforts towards meticulously analyzing the various factors influencing this process, such as the adsorption dynamics of O2, the impact of the catalyst's surface properties on the adsorption and activation of O2, the intermediate stages involved in H2O2 generation, and the role of active species in modulating H2O2 production.
Despite the challenges mentioned, with continued efforts, g-C3N4-based materials still can hold great potential and limitless opportunities for large-scale environmental applications.
Data availability
The data analyzed in this review article are from previously published studies. The specific datasets and sources are cited throughout the manuscript and listed in the reference section. Readers can access the underlying data from the original published sources as cited. The authors confirm that they did not have any special access privileges to these datasets. The data analyzed in this review article are from previously published studies. The specific datasets and sources are cited throughout the manuscript and listed in the reference section. Readers can access the underlying data from the original published sources as cited. The authors confirm that they did not have any special access privileges to these datasets.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number “NBU-FFR-2024-2292-05”.References
- J. Xu, et al., Mg-induced g-C3N4 synthesis of nitrogen-doped graphitic carbon for effective activation of peroxymonosulfate to degrade organic contaminants, Chin. Chem. Lett., 2022, 33(6), 3113–3118 CrossRef CAS.
- M. A. Ahmed, S. A. Mahmoud and A. A. Mohamed, Unveiling the complexities of microbiologically induced corrosion: mechanisms, detection techniques, and mitigation strategies, Front. Environ. Sci. Eng., 2024, 18(10), 120 CrossRef CAS.
- T. Z. Maung, et al., Indoor air pollution and the health of vulnerable groups: a systematic review focused on particulate matter (PM), volatile organic compounds (VOCs) and their effects on children and people with pre-existing lung disease, Int. J. Environ. Res. Publ. Health, 2022, 19(14), 8752 CrossRef CAS PubMed.
- M. A. Bhat, et al., Impact of textile product emissions: toxicological considerations in assessing indoor air quality and human health, Ecological and Health Effects of Building Materials, 2022, pp. 505–541 Search PubMed.
- H. Samudro, G. Samudro and S. Mangkoedihardjo, Prevention of indoor air pollution through design and construction certification: A review of the sick building syndrome conditions, J. Air Pollut. Health, 2022, 7(1), 81–94 Search PubMed.
- M. A. Ahmed and A. A. Mohamed, The use of chitosan-based composites for environmental remediation: A review, Int. J. Biol. Macromol., 2023, 242, 124787 CrossRef CAS PubMed.
- Z. H. Jabbar and B. H. Graimed, Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study, J. Water Proc. Eng., 2022, 47, 102671 CrossRef.
- F. Alston and O. Okorie, Occupational Exposures: Chemical Carcinogens and Mutagens, CRC Press, 2023 Search PubMed.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Facile adsorptive removal of dyes and heavy metals from wastewaters using magnetic nanocomposite of zinc ferrite@ reduced graphene oxide, Inorg. Chem. Commun., 2022, 144, 109912 CrossRef CAS.
- H. Ishaq, I. Dincer and C. Crawford, A review on hydrogen production and utilization: Challenges and opportunities, Int. J. Hydrogen Energy, 2022, 47(62), 26238–26264 CrossRef CAS.
- N. S. Lewis and D. G. Nocera, Powering the planet: Chemical challenges in solar energy utilization, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(43), 15729–15735 CrossRef CAS PubMed.
- T. P. Quach and L. Doan, Surface Modifications of Superparamagnetic Iron Oxide Nanoparticles with Polyvinyl Alcohol, Chitosan, and Graphene Oxide as Methylene Blue Adsorbents, Coatings, 2023, 13(8), 1333 CrossRef CAS.
- M. A. Ahmed, S. Amin and A. A. Mohamed, Fouling in reverse osmosis membranes: monitoring, characterization, mitigation strategies and future directions, Heliyon, 2023, 9, e14908 CrossRef PubMed.
- E. Karbassiyazdi, et al., A juxtaposed review on adsorptive removal of PFAS by metal-organic frameworks (MOFs) with carbon-based materials, ion exchange resins, and polymer adsorbents, Chemosphere, 2023, 311, 136933 CrossRef CAS PubMed.
- H. Zhang, et al., A novel BC/g-C3N4 porous hydrogel carrier used in intimately coupled photocatalysis and biodegradation system for efficient removal of tetracycline hydrochloride in water, Chemosphere, 2023, 317, 137888 CrossRef CAS PubMed.
- C. U. Montaño-Medina, et al., New pyridyl and aniline-functionalized carbamoylcarboxylic acids for removal of metal ions from water by coagulation-flocculation process, Chem. Eng. J., 2023, 451, 138396 CrossRef.
- M. Adel, et al., Characterization of fouling for a full-scale seawater reverse osmosis plant on the Mediterranean sea: membrane autopsy and chemical cleaning efficiency, Groundw. Sustain. Dev., 2022, 16, 100704 CrossRef.
- M. Adel, et al., Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review, Environ. Nanotechnol. Monit. Manag., 2022, 18, 100719 CAS.
- S. Kumaravel, et al., Highly efficient solar-light-active Ag-decorated g-C3N4 composite photocatalysts for the degradation of methyl orange dye, Micromachines, 2023, 14(7), 1454 CrossRef PubMed.
- D. Bhanderi, P. Lakhani and C. K. Modi, Graphitic carbon nitride (gC3N4) as an emerging photocatalyst for sustainable environmental applications: a comprehensive review, RSC Sustainability, 2024, 2, 265–287 RSC.
- U. Hübner, et al., Advanced oxidation processes for water and wastewater treatment–Guidance for systematic future research, Heliyon, 2024, 10, e30402 CrossRef PubMed.
- M. Ismael, A review on graphitic carbon nitride (g-C3N4) based nanocomposites: synthesis, categories, and their application in photocatalysis, J. Alloys Compd., 2020, 846, 156446 CrossRef CAS.
- Q. Ding, et al., S-scheme 3D/2D NiCo2O4@ g-C3N4 hybridized system for boosting hydrogen production from water splitting, Renew. Energy, 2023, 203, 677–685 CrossRef CAS.
- D. Pradhan, et al., Sustainable and solar light assisted photocatalytic degradation of MB and MG dyes by Co3O4/g-C3N4 nanocomposite, Inorg. Chem. Commun., 2023, 156, 111259 CrossRef CAS.
- A. Hayat, et al., Different Dimensionalities, Morphological Advancements and Engineering of g-C3N4-Based Nanomaterials for Energy Conversion and Storage, Chem. Rec., 2023, 23(5), e202200171 CrossRef CAS PubMed.
- M. Joseph, et al., A review on the advancements of graphitic carbon nitride-based photoelectrodes for photoelectrochemical water splitting, Energy Adv., 2024, 3, 30–59 RSC.
- R.-H. Gao, et al., Graphitic carbon nitride (g-C3N4)-based photocatalytic materials for hydrogen evolution, Front. Chem., 2022, 10, 1048504 CrossRef CAS PubMed.
- J. Wang, et al., One-step fabrication of Cu-doped Bi2MoO6 microflower for enhancing performance in photocatalytic nitrogen fixation, J. Colloid Interface Sci., 2023, 638, 427–438 CrossRef CAS PubMed.
- S. Mathew, et al., Synthesis, mechanisms, challenges, and future prospects of Ti3C2 MXene and its heterojunctions for photocatalytic dye degradation efficiency: A comprehensive review, Mater. Today Sustain., 2023, 100568 Search PubMed.
- M. Ismael, Environmental remediation and sustainable energy generation via photocatalytic technology using rare earth metals modified g-C3N4: A review, J. Alloys Compd., 2023, 931, 167469 CrossRef CAS.
- M. Ismael and Y. Wu, A mini-review on the synthesis and structural modification of gC3N4-based materials, and their applications in solar energy conversion and environmental remediation, Sustain. Energy Fuels, 2019, 3(11), 2907–2925 RSC.
- S. Sankeetha, et al., Interaction of BiVO4 anchored 2D hexagonal boron nitride nanocomposite for photocatalytic water pollutants degradation and phytotoxicity assessment, Colloids Surf., A, 2023, 675, 132024 CrossRef CAS.
- S. Hussain, et al., WS2-embedded MXene/GO hybrid nanosheets as electrodes for asymmetric supercapacitors and hydrogen evolution reactions, Chem. Eng. J., 2023, 452, 139523 CrossRef CAS.
- R. Dutta, et al., Optical Enhancement of Indirect Bandgap Two-Dimensional Transition Metal Dichalcogenides for Multi-Functional Optoelectronic Sensors, Adv. Mater., 2023, 2303272 CrossRef CAS PubMed.
- Y.-H. Lai, et al., Solar-driven hydrogen evolution in alkaline seawater over earth-abundant g-C3N4/CuFeO2 heterojunction photocatalyst using microplastic as a feedstock, Chem. Eng. J., 2023, 475, 146413 CrossRef CAS.
- J. Joseph and S. Roopan, Synthesis of Graphitic Carbon Nitride Quantum Dots from Bulk Graphitic Carbon Nitride, Sustainable Approaches in Pharmaceutical Sciences, 2023, pp. 237–253 Search PubMed.
- W. Meng, et al., High-sensitivity resistive humidity sensor based on graphitic carbon nitride nanosheets and its application, Sens. Actuators, B, 2020, 315, 128058 CrossRef CAS.
- P. Thakur, et al., Exploring recent advances in silver halides and graphitic carbon nitride-based photocatalyst for energy and environmental applications, Arab. J. Chem., 2020, 13(11), 8271–8300 CrossRef CAS.
- Y. Zhang, et al., Near-infrared triggered Ti3C2/g-C3N4 heterostructure for mitochondria-targeting multimode photodynamic therapy combined photothermal therapy, Nano Today, 2020, 34, 100919 CrossRef CAS.
- Z. Deng, et al., Efficient Activation of Peroxymonosulfate by V-Doped Graphitic Carbon Nitride for Organic Contamination Remediation, Materials, 2022, 15(24), 8936 CrossRef CAS PubMed.
- M. Tan, et al., Engineering of g-C3N4-based photocatalysts to enhance hydrogen evolution, Adv. Colloid Interface Sci., 2021, 295, 102488 CrossRef CAS PubMed.
- B. He, et al., One-pot construction of chitin-derived carbon/g-C3N4 heterojunction for the improvement of visible-light photocatalysis, Appl. Surf. Sci., 2020, 527, 146737 CrossRef CAS.
- A. Sewnet, et al., Visible-light-driven g-C3N4/TiO2 based heterojunction nanocomposites for photocatalytic degradation of organic dyes in wastewater: a review, Air Soil. Water Res., 2022, 15, 11786221221117266 Search PubMed.
- L. H. Madkour, Graphitic Carbon Nitride Quantum Dots (g-C3N4): Fundamentals and Applications, International Research Journal of Basic and Clinical Studies, 2023, 48 Search PubMed.
- Q. Zhu, et al., Emerging cocatalysts on g-C3N4 for photocatalytic hydrogen evolution, Small, 2021, 17(40), 2101070 CrossRef CAS PubMed.
- M. Youshi, M. R. Farahpour and Z. G. Tabatabaei, Facile fabrication of carboxymethylcellulose/ZnO/g-C3N4 containing nutmeg extract with photocatalytic performance for infected wound healing, Sci. Rep., 2023, 13(1), 18704 CrossRef CAS PubMed.
- L. Wang, et al., Hydroxyl decorated g-C3N4 nanoparticles with narrowed bandgap for high efficient photocatalyst design, Appl. Catal., B, 2019, 244, 262–271 CrossRef CAS.
- S. Yang, et al., Carbon fibers derived from spent cigarette filters for supporting ZnIn2S4/g-C3N4 heterojunction toward enhanced photocatalytic hydrogen evolution, Mater. Sci. Eng. B, 2023, 288, 116214 CrossRef CAS.
- W. Quan, et al., 2D/2D Z-scheme photocatalyst of g-C3N4 and plasmonic Bi metal deposited Bi2WO6: Enhanced separation and migration of photoinduced charges, J. Alloys Compd., 2023, 946, 169396 CrossRef CAS.
- H. Dong, et al., Insight into the activity and stability of Rh x P Nano-species supported on g-C3N4 for photocatalytic H2 production, ACS Catal., 2019, 10(1), 458–462 CrossRef.
- H. Charles, et al., Synergistic effect of surface modification and effective interfacial charge transfer over faceted g-C3N4/ZnSe heterojunction to enhance CO2 photoreduction activity, J. Water Proc. Eng., 2023, 56, 104307 CrossRef.
- Y. Li, et al., Crystallinity-defect matching relationship of g-C3N4: Experimental and theoretical perspectives, Green Energy Environ., 2023, 9, 623–658 CrossRef.
- F. Tian, et al., Weak Interaction between Nickel Thiolate and g-C3N4 Improving Electron–Hole Separation for Photocatalysis, ACS Catal., 2023, 13(18), 12186–12196 CrossRef CAS.
- H. M. ul Hassan, et al., Reduce the recombination rate by facile synthesis of MoS2/g-C3N4 heterostructures as a solar light responsive catalyst for organic dye degradation, Diamond Relat. Mater., 2023, 140, 110420 CrossRef.
- J. Fu, et al., g-C3N4-Based heterostructured photocatalysts, Adv. Energy Mater., 2018, 8(3), 1701503 CrossRef.
- F. Qi, et al., A CuS@ g-C3N4 heterojunction endows scaffold with synergetic antibacterial effect, Colloids Surf., B, 2023, 230, 113512 CrossRef CAS PubMed.
- S. Mir, et al., Development of Self-Cleaning g-C3N4/Zn (OH) 2 Nanocomposite-Coated Mesh for Oil–Water Emulsion Separation, Ind. Eng. Chem. Res., 2023, 62(28), 11096–11108 CrossRef CAS.
- C. Chai, Photocatalytic degradation of polyethylene and polystyrene microplastics by α-Fe2O3/g-C3N4, Environ. Sci. Pollut. Res., 2023, 30, 121702–121712 CrossRef CAS PubMed.
- H. Qie, et al., High-efficiency control of pesticide and heavy metal combined pollution in paddy soil using biochar/g-C3N4 photoresponsive soil remediation agent, Chem. Eng. J., 2023, 452, 139579 CrossRef CAS.
- Q. Zhang, et al., Facile construction of CuO/g-C3N4 heterojunctions with promoted photocatalytic hydrogen generation behaviors, Fuel, 2023, 353, 129224 CrossRef CAS.
- M. Ismael, One-step ultrasonic-assisted synthesis of Ni-doped g-C3N4 photocatalyst for enhanced photocatalytic hydrogen evolution, Inorg. Chem. Commun., 2023, 151, 110607 CrossRef CAS.
- S. Mishra and R. Acharya, Recent updates in modification strategies for escalated performance of Graphene/MFe2O4 heterostructured photocatalysts towards energy and environmental applications, J. Alloys Compd., 2023, 170576 CrossRef CAS.
- S. Chowdhury and R. Balasubramanian, Graphene/semiconductor nanocomposites (GSNs) for heterogeneous photocatalytic decolorization of wastewaters contaminated with synthetic dyes: a review, Appl. Catal., B, 2014, 160, 307–324 CrossRef.
- J. Zou, et al., In-situ construction of sulfur-doped g-C3N4/defective g-C3N4 isotype step-scheme heterojunction for boosting photocatalytic H2 evolution, Chin. J. Struct. Chem., 2022, 41(1), 2201025–2201033 CAS.
- F. Lin, et al., Electrostatic self-assembly combined with microwave hydrothermal strategy: construction of 1D/1D carbon nanofibers/crystalline g-C3N4 heterojunction for boosting photocatalytic hydrogen production, Nano Energy, 2022, 99, 107432 CrossRef CAS.
- K. Gayathri, et al., In situ-grown ZnO particles on g-C3N4 layers: a direct Z-scheme-driven photocatalyst for the degradation of dye and pharmaceutical pollutants under solar irradiation, J. Mater. Sci.: Mater. Electron., 2022, 33(12), 9774–9784 CrossRef CAS.
- D. D. Pathak, et al., An insight into the effect of g-C3N4 support on the enhanced performance of ZnS nanoparticles as anode material for lithium-ion and sodium-ion batteries, Electrochim. Acta, 2021, 370, 137715 CrossRef CAS.
- M. A. Ahmed and A. A. Mohamed, Recent progress in semiconductor/graphene photocatalysts: synthesis, photocatalytic applications, and challenges, RSC Adv., 2023, 13(1), 421–439 RSC.
- M. A. Ahmed, S. A. Mahmoud and A. A. Mohamed, Nanomaterials-modified reverse osmosis membranes: a comprehensive review, RSC Adv., 2024, 14(27), 18879–18906 RSC.
- K. Saravanakumar, et al., Noble metal nanoparticles (Mx = Ag, Au, Pd) decorated graphitic carbon nitride nanosheets for ultrafast catalytic reduction of anthropogenic pollutant, 4-nitrophenol, Environ. Res., 2022, 212, 113185 CrossRef CAS PubMed.
- L. Ge, et al., Enhanced visible light photocatalytic activity of novel polymeric g-C3N4 loaded with Ag nanoparticles, Appl. Catal., A, 2011, 409, 215–222 CrossRef.
- N. Cheng, et al., Au-nanoparticle-loaded graphitic carbon nitride nanosheets: green photocatalytic synthesis and application toward the degradation of organic pollutants, ACS Appl. Mater. Interfaces, 2013, 5(15), 6815–6819 CrossRef CAS PubMed.
- P. C. Nagajyothi, et al., Enhanced photocatalytic activity of Ag/g-C3N4 composite, Separ. Purif. Technol., 2017, 188, 228–237 CrossRef CAS.
- S. Tonda, et al., Fe-doped and-mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight, J. Mater. Chem. A, 2014, 2(19), 6772–6780 RSC.
- L. Hu, et al., Single Pd atoms anchored graphitic carbon nitride for highly selective and stable photocatalysis of nitric oxide, Carbon, 2022, 200, 187–198 CrossRef CAS.
- M. Z. Asghar, et al., A new Y-Zr/g-C3N4 nanoflakes anchored mesoporous silica composite for efficient environmental remediation applications, Diamond Relat. Mater., 2023, 135, 109850 CrossRef CAS.
- Y. Liu, et al., Phenanthroline bridging graphitic carbon nitride framework and Fe (II) ions to promote transfer of photogenerated electrons for selective photocatalytic reduction of Nitrophenols, J. Colloid Interface Sci., 2022, 608, 2088–2099 CrossRef CAS PubMed.
- X.-W. Guo, et al., Single-atom molybdenum immobilized on photoactive carbon nitride as efficient photocatalysts for ambient nitrogen fixation in pure water, J. Mater. Chem. A, 2019, 7(34), 19831–19837 RSC.
- Y. Wang, et al., Synthesis of Mo-doped graphitic carbon nitride catalysts and their photocatalytic activity in the reduction of CO2 with H2O, Catal. Commun., 2016, 74, 75–79 CrossRef CAS.
- S. Le, et al., Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis, RSC Adv., 2016, 6(45), 38811–38819 RSC.
- P.-W. Chen, et al., Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution, Appl. Surf. Sci., 2017, 392, 608–615 CrossRef CAS.
- T. H. Pham, J.-W. Park and T. Kim, Enhanced photodegradation of paracetamol from water by cobalt doped graphitic carbon nitride, Sol. Energy, 2021, 215, 151–156 CrossRef CAS.
- N. Paramasivam, A. Sambandam and B. Nastesan, Metalloids (B, Si) and non-metal (N, P, S) doped graphene nanosheet as a supercapacitor electrode: A density functional theory study, Mater. Today Commun., 2023, 35, 105905 CrossRef CAS.
- L. Chen, et al., Phosphorus Doping Strategy-Induced Synergistic Modification of Interlayer Structure and Chemical State in Ti3C2Tx toward Enhancing Capacitance, Molecules, 2023, 28(13), 4892 CrossRef CAS PubMed.
- D. Masih, Y. Ma and S. Rohani, Graphitic C3N4 based noble-metal-free photocatalyst systems: a review, Appl. Catal., B, 2017, 206, 556–588 CrossRef CAS.
- S. Zhang, et al., Which kind of nitrogen chemical states doped carbon dots loaded by g-C3N4 is the best for photocatalytic hydrogen production, J. Colloid Interface Sci., 2022, 622, 662–674 CrossRef CAS PubMed.
- S. Kumar, V. R. Battula and K. Kailasam, Single molecular precursors for CxNy materials-Blending of carbon and nitrogen beyond g-C3N4, Carbon, 2021, 183, 332–354 CrossRef CAS.
- J. Jiang, et al., Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution, J. Mater. Sci. Technol., 2022, 118, 15–24 CrossRef CAS.
- K. Chen, et al., 2D/2D Boron/g-C3N4 Nanosheet Heterojunction Boosts Photocatalytic Hydrogen Evolution Performance, ACS Appl. Energy Mater., 2022, 5(9), 10657–10666 CrossRef CAS.
- K. S. Pasupuleti, et al., Boron doped g-C3N4 quantum dots based highly sensitive surface acoustic wave NO2 sensor with faster gas kinetics under UV light illumination, Sens. Actuators, B, 2023, 378, 133140 CrossRef CAS.
- Y. Zhang, et al., Phosphorus-doped carbon nitride solid: enhanced electrical conductivity and photocurrent generation, J. Am. Chem. Soc., 2010, 132(18), 6294–6295 CrossRef CAS PubMed.
- Y. Zhou, et al., Brand new P-doped gC3N4: enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light, J. Mater. Chem. A, 2015, 3(7), 3862–3867 RSC.
- Y.-P. Zhu, T.-Z. Ren and Z.-Y. Yuan, Mesoporous phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance, ACS Appl. Mater. Interfaces, 2015, 7(30), 16850–16856 CrossRef CAS PubMed.
- Q. Fan, et al., A simple fabrication for sulfur doped graphitic carbon nitride porous rods with excellent photocatalytic activity degrading RhB dye, Appl. Surf. Sci., 2017, 391, 360–368 CrossRef CAS.
- J. Li, et al., A facile approach to synthesize novel oxygen-doped g-C 3 N 4 with superior visible-light photoreactivity, Chem. Commun., 2012, 48(98), 12017–12019 RSC.
- X. Yang, et al., Simple hydrothermal preparation of sulfur fluoride-doped g-C3N4 and its photocatalytic degradation of methyl orange, Mater. Sci. Eng. B, 2023, 288, 116216 CrossRef CAS.
- Z. Ding, et al., Synthesis of transition metal-modified carbon nitride polymers for selective hydrocarbon oxidation, ChemSusChem, 2011, 4(2), 274–281 CrossRef CAS PubMed.
- M. Wang, et al., Synthesis of hollow lantern-like Eu (III)-doped g-C3N4 with enhanced visible light photocatalytic perfomance for organic degradation, J. Hazard Mater., 2018, 349, 224–233 CrossRef CAS PubMed.
- G. Li, et al., Er-doped g-C3N4 for photodegradation of tetracycline and tylosin: high photocatalytic activity and low leaching toxicity, Chem. Eng. J., 2020, 391, 123500 CrossRef CAS.
- Y. Wang, et al., Bio-template synthesis of Mo-doped polymer carbon nitride for photocatalytic hydrogen evolution, Appl. Catal., B, 2019, 248, 44–53 CrossRef CAS.
- A. Ahmed, et al., Zinc-doped mesoporous graphitic carbon nitride for colorimetric detection of hydrogen peroxide, ACS Appl. Nano Mater., 2019, 2(8), 5156–5168 CrossRef CAS.
- F. Dong, et al., A general method for type I and type II gC3N4/gC3N4 metal-free isotype heterostructures with enhanced visible light photocatalysis, New J. Chem., 2015, 39(6), 4737–4744 RSC.
- Y. Zhu, et al., Tunable Type I and II heterojunction of CoOx nanoparticles confined in g-C3N4 nanotubes for photocatalytic hydrogen production, Appl. Catal., B, 2019, 244, 814–822 CrossRef CAS.
- Y. Shi, et al., Engineering of 2D/3D architectures type II heterojunction with high-crystalline g-C3N4 nanosheets on yolk-shell ZnFe2O4 for enhanced photocatalytic tetracycline degradation, Mater. Res. Bull., 2022, 150, 111789 CrossRef CAS.
- W. Shi, et al., Fabrication of ternary Ag3PO4/Co3 (PO4)2/g-C3N4 heterostructure with following Type II and Z-Scheme dual pathways for enhanced visible-light photocatalytic activity, J. Hazard Mater., 2020, 389, 121907 CrossRef CAS PubMed.
- M. Que, et al., Recent advances in gC3N4 composites within four types of heterojunctions for photocatalytic CO 2 reduction, Nanoscale, 2021, 13(14), 6692–6712 RSC.
- S. Obregón, et al., A novel type-II Bi2W2O9/g-C3N4 heterojunction with enhanced photocatalytic performance under simulated solar irradiation, Mater. Sci. Semicond. Process., 2020, 113, 105056 CrossRef.
- H. Huang, et al., Self-sacrifice transformation for fabrication of type-I and type-II heterojunctions in hierarchical BixOyIz/g-C3N4 for efficient visible-light photocatalysis, Appl. Surf. Sci., 2019, 470, 1101–1110 CrossRef CAS.
- W. He, et al., Controllable morphology CoFe2O4/g-C3N4 pn heterojunction photocatalysts with built-in electric field enhance photocatalytic performance, Appl. Catal., B, 2022, 306, 121107 CrossRef CAS.
- W. Chen, et al., Accelerated photocatalytic degradation of tetracycline hydrochloride over CuAl2O4/g-C3N4 pn heterojunctions under visible light irradiation, Sep. Purif. Technol., 2021, 277, 119461 CrossRef CAS.
- S. Yin, et al., Ionic liquid-assisted synthesis and improved photocatalytic activity of pn junction gC3N4/BiOCl, J. Mater. Sci., 2016, 51, 4769–4777 CrossRef CAS.
- L. Acharya, et al., Development of MgIn2S4 microflower-embedded exfoliated B-doped g-C3N4 nanosheets: p–n heterojunction photocatalysts toward photocatalytic water reduction and H2O2 production under visible-light irradiation, ACS Appl. Energy Mater., 2022, 5(3), 2838–2852 CrossRef CAS.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Fabrication of NiO/g-C3N4 Z-scheme heterojunction for enhanced photocatalytic degradation of methylene blue dye, Opt. Mater., 2024, 151, 115339 CrossRef CAS.
- W. Zhao, W. Wang and H. Shi, 2D/2D Z-scheme BiO1-XBr/g-C3N4 heterojunction with rich oxygen vacancies as electron mediator for enhanced visible-light degradation activity, Appl. Surf. Sci., 2020, 528, 146925 CrossRef CAS.
- A. Behera, A. K. Kar and R. Srivastava, Oxygen vacancy-mediated Z-scheme charge transfer in a 2D/1D B-doped g-C3N4/rGO/TiO2 heterojunction visible light-driven photocatalyst for simultaneous/efficient oxygen reduction reaction and alcohol oxidation, Inorg. Chem., 2022, 61(32), 12781–12796 CrossRef CAS PubMed.
- M. Chandra, U. Guharoy and D. Pradhan, Boosting the Photocatalytic H2 Evolution and Benzylamine Oxidation using 2D/1D g-C3N4/TiO2 Nanoheterojunction, ACS Appl. Mater. Interfaces, 2022, 14, 22122–22137 CrossRef CAS PubMed.
- Y. R. Girish, et al., Facile and rapid synthesis of solar-driven TiO2/g-C3N4 heterostructure photocatalysts for enhanced photocatalytic activity, J. Sci.: Adv. Mater. Devices, 2022, 7(2), 100419 CAS.
- J. Yang, et al., CVD Assisted Synthesis of Macro/Mesoporous TiO2/g-C3N4 S-Scheme Heterojunction for Enhanced Photocatalytic Hydrogen Evolution, Adv. Sustainable Syst., 2022, 6(8), 2200056 CrossRef CAS.
- T. H. Pham, et al., Enhanced photodegradation of tetracycline in wastewater and conversion of CO2 by solar light assisted ZnO/g-C3N4, Environ. Res., 2023, 217, 114825 CrossRef CAS PubMed.
- T. Kobkeatthawin, et al., Photocatalytic activity of TiO2/g-C3N4 nanocomposites for removal of monochlorophenols from water, Nanomaterials, 2022, 12(16), 2852 CrossRef CAS PubMed.
- J. Singh, A. Arora and S. Basu, Synthesis of coral like WO3/g-C3N4 nanocomposites for the removal of hazardous dyes under visible light, J. Alloys Compd., 2019, 808, 151734 CrossRef CAS.
- H. Wang, et al., Constructing defect engineered 2D/2D MoO3/g-C3N4 Z-scheme heterojunction for enhanced photocatalytic activity, J. Alloys Compd., 2022, 926, 166964 CrossRef CAS.
- S. Bellamkonda and G. R. Rao, Nanojunction-mediated visible light photocatalytic enhancement in heterostructured ternary BiOCl/CdS/g-C3N4 nanocomposites, Catal. Today, 2019, 321, 18–25 CrossRef.
- H. A. Omr, et al., Design of sculptured SnS/g-C3N4 photocatalytic nanostructure for highly efficient and selective CO2 conversion to methane, Appl. Catal., B, 2023, 324, 122231 CrossRef CAS.
- J. Song, et al., Exploration of the g-C3N4 Heterostructure with Ag–In sulfide quantum dots for enhanced photocatalytic activity, ACS Appl. Electron. Mater., 2023, 5(8), 4134–4144 CrossRef CAS.
- Q. Wu, et al., In-situ synthesis of ternary heterojunctions via g-C3N4 coupling with noble-metal-free NiS and CdS with efficient visible-light-induced photocatalytic H2 evolution and mechanism insight, Int. J. Hydrogen Energy, 2022, 47(30), 14063–14076 CrossRef CAS.
- Y. Ren, D. Zeng and W.-J. Ong, Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: a review, Chin. J. Catal., 2019, 40(3), 289–319 CrossRef CAS.
- D. Wei, et al., Cooperative effects of zinc–nickel sulfides as a dual cocatalyst for the enhanced photocatalytic hydrogen evolution activity of g-C3N4, J. Environ. Chem. Eng., 2022, 10(2), 107216 CrossRef CAS.
- X.-y. Ji, et al., Fabrication of a ternary NiS/ZnIn2S4/g-C3N4 photocatalyst with dual charge transfer channels towards efficient H2 evolution, J. Colloid Interface Sci., 2022, 618, 300–310 CrossRef CAS PubMed.
- S. Yang, et al., Size effect of CoS2 cocatalyst on photocatalytic hydrogen evolution performance of g-C3N4, J. Colloid Interface Sci., 2023, 635, 305–315 CrossRef CAS PubMed.
- D. C. Onwudiwe, et al., Dual S-scheme heterojunction g-C3N4/Bi2S3/CuS composite with enhanced photocatalytic activity for methyl orange degradation, Inorg. Chem. Commun., 2023, 155, 111075 CrossRef CAS.
- C. Wang, et al., S-scheme bimetallic sulfide ZnCo2S4/g-C3N4 heterojunction for photocatalytic H2 evolution, Ceram. Int., 2021, 47(21), 30194–30202 CrossRef CAS.
- Z. Chen, et al., Single-sites Rh-phosphide modified carbon nitride photocatalyst for boosting hydrogen evolution under visible light, Appl. Catal., B, 2020, 274, 119117 CrossRef CAS.
- Y. Lin, et al., LaOCl-coupled polymeric carbon nitride for overall water splitting through a one-photon excitation pathway, Angew. Chem., Int. Ed., 2020, 59(47), 20919–20923 CrossRef CAS PubMed.
- A. Alsulmi, et al., Engineering S-scheme Ag2 CO3/gc3 N4 heterojunctions sonochemically to eradicate Rhodamine B dye under solar irradiation, RSC Adv., 2023, 13(18), 12229–12243 RSC.
- N. Tian, et al., Facet-charge-induced coupling dependent interfacial photocharge separation: a case of BiOI/g-C3N4 pn junction, Appl. Catal., B, 2020, 267, 118697 CrossRef CAS.
- A. Alsulmi, et al., Sonochemical Fabrication of S-Scheme AgI/g-C3N4 Heterojunction for Efficient Photocatalytic Degradation of RhB Dye, J. Inorg. Organomet. Polym. Mater., 2023, 1–15 Search PubMed.
- R. Manjupriya and S. M. Roopan, Unveiling the Photocatalytic Activity of Carbon Dots/g-C3N4 Nanocomposite for the O-Arylation of 2-Chloroquinoline-3-carbaldehydes, Catalysts, 2023, 13(2), 308 CrossRef CAS.
- X. Zhou, et al., Superior uniform carbon nanofibers@ g-C3N4 core-shell nanostructures embedded by Au nanoparticles for high-efficiency photocatalyst, J. Hazard Mater., 2020, 388, 121759 CrossRef CAS PubMed.
- Y. Shan, et al., Nanocellulose-derived carbon/g-C3N4 heterojunction with a hybrid electron transfer pathway for highly photocatalytic hydrogen peroxide production, J. Colloid Interface Sci., 2021, 599, 507–518 CrossRef CAS PubMed.
- M. Inagaki, et al., Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials, Carbon, 2019, 141, 580–607 CrossRef CAS.
- G. Bottari, et al., Covalent and noncovalent phthalocyanine− carbon nanostructure systems: synthesis, photoinduced electron transfer, and application to molecular photovoltaics, Chem. Rev., 2010, 110(11), 6768–6816 CrossRef CAS PubMed.
- Z. Li, et al., Carbon-based functional nanomaterials: Preparation, properties and applications, Compos. Sci. Technol., 2019, 179, 10–40 CrossRef CAS.
- Y. Fu, et al., Photocatalytic H2O2 and H2 Generation from Living Chlorella vulgaris and Carbon Micro Particle Comodified g-C3N4, Adv. Energy Mater., 2018, 8(34), 1802525 CrossRef.
- S. Zhao, et al., Carbon-based metal-free catalysts for key reactions involved in energy conversion and storage, Adv. Mater., 2019, 31(9), 1801526 CrossRef PubMed.
- T. Su, et al., 2D/2D heterojunction of Ti3C2/gC3N4 nanosheets for enhanced photocatalytic hydrogen evolution, Nanoscale, 2019, 11(17), 8138–8149 RSC.
- L. Cheng, et al., Carbon–graphitic carbon nitride hybrids for heterogeneous photocatalysis, Small, 2021, 17(1), 2005231 CrossRef CAS PubMed.
- Y. Xu, et al., Carbon-based nanostructures for emerging photocatalysis: CO2 reduction, N2 fixation, and organic conversion, Trends Chem., 2022, 4, 984–1004 CrossRef CAS.
- Y. Li, et al., ZIF-67 derived Co@ NC/g-C3N4 as a photocatalyst for enhanced water splitting H2 evolution, Environ. Res., 2021, 197, 111002 CrossRef CAS PubMed.
- R. Zhang, et al., Multifunctional g-C3N4/graphene oxide wrapped sponge monoliths as highly efficient adsorbent and photocatalyst, Appl. Catal., B, 2018, 235, 17–25 CrossRef CAS.
- H. Wang, et al., Preparation of nanoscale-dispersed g-C3N4/graphene oxide composite photocatalyst with enhanced visible-light photocatalytic activity, Mater. Lett., 2018, 217, 143–145 CrossRef CAS.
- R. Zhang, et al., Surface modification to control the secondary pollution of photocatalytic nitric oxide removal over monolithic protonated g-C3N4/graphene oxide aerogel, J. Hazard. Mater., 2020, 397, 122822 CrossRef CAS PubMed.
- Z. Shi, et al., The photocatalytic activity and purification performance of g-C3N4/carbon nanotubes composite photocatalyst in underwater environment, Environ. Sci. Pollut. Res., 2022, 29(55), 83981–83992 CrossRef CAS PubMed.
- G. Liu, et al., Enhanced photodegradation performance of Rhodamine B with g-C3N4 modified by carbon nanotubes, Sep. Purif. Technol., 2020, 244, 116618 CrossRef CAS.
- Z. Shi, et al., Influences of different carbon substrates on the morphologies of carbon/g-C3N4 photocatalytic composites and the purification capacities of different composites in the weak UV underwater environment, Chemosphere, 2022, 308, 136257 CrossRef CAS PubMed.
- Y. Li, et al., Cross-linked g-C3N4/rGO nanocomposites with tunable band structure and enhanced visible light photocatalytic activity, Small, 2013, 9(19), 3336–3344 CrossRef CAS PubMed.
- Y. Xu, et al., The CNT modified white C3N4 composite photocatalyst with enhanced visible-light response photoactivity, Dalton Trans., 2013, 42(21), 7604–7613 RSC.
- Y. Wu, et al., TiO 2/gC3N4 nanosheets hybrid photocatalyst with enhanced photocatalytic activity under visible light irradiation, Res. Chem. Intermed., 2016, 42, 3609–3624 CrossRef CAS.
- L. Jiang, et al., Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant, Appl. Catal., B, 2018, 221, 715–725 CrossRef CAS.
- J. Liu, et al., Graphene quantum dots modified mesoporous graphite carbon nitride with significant enhancement of photocatalytic activity, Appl. Catal., B, 2017, 207, 429–437 CrossRef CAS.
- D. Monga, et al., 2D/2d heterojunction of MoS2/g-C3N4 nanoflowers for enhanced visible-light-driven photocatalytic and electrochemical degradation of organic pollutants, J. Environ. Manage., 2020, 274, 111208 CrossRef CAS PubMed.
- X. Zhan, et al., Uracil-doped graphitic carbon nitride for enhanced photocatalytic performance, ACS Appl. Mater. Interfaces, 2021, 13(10), 12118–12130 CrossRef CAS PubMed.
- P. Yang, et al., Constructing mesoporous g-C3N4/ZnO nanosheets catalyst for enhanced visible-light driven photocatalytic activity, J. Photochem. Photobiol., A, 2020, 388, 112169 CrossRef.
- A. N. Kadam, M. Moniruzzaman and S.-W. Lee, Dual functional S-doped g-C3N4 pinhole porous nanosheets for selective fluorescence sensing of Ag+ and visible-light photocatalysis of dyes, Molecules, 2019, 24(3), 450 CrossRef PubMed.
- K. Huang, et al., Hydrothermal synthesis of gC3N4/CdWO4 nanocomposite and enhanced photocatalytic activity for tetracycline degradation under visible light, CrystEngComm, 2016, 18(34), 6453–6463 RSC.
- D. R. Paul, et al., Silver doped graphitic carbon nitride for the enhanced photocatalytic activity towards organic dyes, J. Nanosci. Nanotechnol., 2019, 19(8), 5241–5248 CrossRef CAS PubMed.
- N. Masunga, B. B. Mamba and K. K. Kefeni, Trace samarium doped graphitic carbon nitride photocatalytic activity toward metanil yellow dye degradation under visible light irradiation, Colloids Surf., A, 2020, 602, 125107 CrossRef CAS.
- Z. Li, et al., Three-dimensional P-doped porous g-C3N4 nanosheets as an efficient metal-free photocatalyst for visible-light photocatalytic degradation of Rhodamine B model pollutant, J. Taiwan Inst. Chem. Eng., 2020, 114, 249–262 CrossRef CAS.
- S. Ji, et al., Photocatalysis-Fenton of Fe-doped g-C3N4 catalyst and its excellent degradation performance towards RhB, J. Water Proc. Eng., 2021, 40, 101804 CrossRef.
- H. Liu, et al., Photocatalytic removal of tetracycline by a Z-scheme heterojunction of bismuth oxyiodide/exfoliated g-C3N4: performance, mechanism, and degradation pathway, Mater. Today Chem., 2022, 23, 100729 CrossRef CAS.
- B. Guo, et al., S-scheme Ti0. 7Sn0. 3O2/g-C3N4 heterojunction composite for enhanced photocatalytic pollutants degradation, J. Environ. Chem. Eng., 2022, 10(2), 107118 CrossRef CAS.
- Y. Liu, et al., Supramolecule self-assembly approach to direct Z-scheme TiO2/g-C3N4 heterojunctions for efficient photocatalytic degradation of emerging phenolic pollutants, Appl. Surf. Sci., 2022, 593, 153401 CrossRef CAS.
- M. Ubaidullah, et al., Photocatalytic CO2 reduction and pesticide degradation over g-C3N4/Ce2S3 heterojunction, J. Environ. Chem. Eng., 2023, 11(3), 109675 CrossRef CAS.
- T. Song, et al., Enhanced carrier separation in g-C3N4/MoO3-x heterostructures towards efficient phenol removal, J. Ind. Eng. Chem., 2023, 122, 415–425 CrossRef CAS.
- L. Chen, et al., In situ embedment of CoOx on g-C3N4 as Z scheme heterojunction for efficient photocatalytic degradation of methyl orange and phenol under visible light, J. Alloys Compd., 2022, 927, 167047 CrossRef CAS.
- P. R. Thakur, et al., Fabrication of a Z-scheme Zn3V2O8/g-C3N4 nano-heterojunction with high interfacial charge transfer for superior photocatalytic removal of diazinon pesticide under visible light, Appl. Nanosci., 2023, 13(6), 3643–3658 CrossRef CAS.
- B. Patial, et al., Hydrothermal synthesis of (mt) BiVO4/g-C3N4 heterojunction for enhancement in photocatalytic degradation of imidacloprid, J. Environ. Chem. Eng., 2023, 11(5), 111138 CrossRef CAS.
- C. Yang, et al., One-step synthesis of a 3D/2D Bi2WO6/g-C3N4 heterojunction for effective photocatalytic degradation of atrazine: Kinetics, degradation mechanisms and ecotoxicity, Sep. Purif. Technol., 2022, 288, 120609 CrossRef CAS.
- J. Qu, et al., Visible-light-responsive K-doped g-C3N4/BiOBr hybrid photocatalyst with highly efficient degradation of Rhodamine B and tetracycline, Mater. Sci. Semicond. Process., 2020, 112, 105023 CrossRef CAS.
- S. Sivasakthi and K. Gurunathan, Graphitic carbon nitride bedecked with CuO/ZnO hetero-interface microflower towards high photocatalytic performance, Renew. Energy, 2020, 159, 786–800 CrossRef CAS.
- S. Iqbal, et al., Critical role of the heterojunction interface of silver decorated ZnO nanocomposite with sulfurized graphitic carbon nitride heterostructure materials for photocatalytic applications, J. Alloys Compd., 2021, 858, 158338 CrossRef CAS.
- K.-L. Wang, et al., Ultrafine silver nanoparticles deposited on sodium-doped graphitic carbon nitride towards enhanced photocatalytic degradation of dyes and antibiotics under visible light irradiation, Appl. Surf. Sci., 2019, 476, 741–748 CrossRef CAS.
- Y. Guo, et al., S-scheme g-C3N4/TiO2/CFs heterojunction composites with multi-dimensional through-holes and enhanced visible-light photocatalytic activity, Ceram. Int., 2022, 48(6), 8196–8208 CrossRef CAS.
- C. Wu, et al., Construction of layered embedding dual Z-Scheme Bi2O2CO3/g-C3N4/Bi2O3: Tetracycline degradation pathway, toxicity analysis and mechanism insight, Sep. Purif. Technol., 2022, 282, 120096 CrossRef CAS.
- V. Manikandan, et al., Fabrication of novel hybrid Z-Scheme WO3@ g-C3N4@ MWCNT nanostructure for photocatalytic degradation of tetracycline and the evaluation of antimicrobial activity, Chemosphere, 2022, 287, 132050 CrossRef CAS PubMed.
- P. Zhu, et al., High visible light response Z-scheme Ag3PO4/g-C3N4/ZnO composite photocatalyst for efficient degradation of tetracycline hydrochloride: preparation, properties and mechanism, J. Alloys Compd., 2020, 840, 155714 CrossRef CAS.
- Y. Yuan, et al., Fabrication of a dual S-scheme Bi7O9I3/g-C3N4/Bi3O4Cl heterojunction with enhanced visible-light-driven performance for phenol degradation, Chemosphere, 2022, 287, 132241 CrossRef CAS PubMed.
- T. Ahamad and S. M. Alshehri, Fabrication of Ag@ SrTiO3/g-C3N4 heterojunctions for H2 production and the degradation of pesticides under visible light, Sep. Purif. Technol., 2022, 297, 121431 CrossRef CAS.
- X. An, et al., Bi-functional biochar-g-C3N4-MgO composites for simultaneously minimizing pollution: Photocatalytic degradation of pesticide and phosphorus recovery as slow-release fertilizer, J. Environ. Manage., 2023, 344, 118489 CrossRef CAS PubMed.
- C. Yin, et al., Carbon dots as heterojunction transport mediators effectively enhance BiOI/g-C3N4 synergistic persulfate degradation of antibiotics, Appl. Surf. Sci., 2022, 601, 154249 CrossRef CAS.
- R. Fatima and J.-O. Kim, De novo synthesis of photocatalytic bifunctional MIL-125 (Ti)/gC3N4/RGO through sequential self-assembly and solvothermal route, Environ. Res., 2022, 205, 112422 CrossRef CAS PubMed.
- C. Li, et al., Recent advances on g-C3N4-based Z-scheme photocatalysts for organic pollutants removal, Catal. Sci. Technol., 2023, 13, 196–231 Search PubMed.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Removal of 4-nitrophenol and indigo carmine dye from wastewaters by magnetic copper ferrite nanoparticles: Kinetic, thermodynamic and mechanistic insights, J. Saudi Chem. Soc., 2023, 27(6), 101748 CrossRef CAS.
- M. A. Ahmed and A. A. Mohamed, Advances in ultrasound-assisted synthesis of photocatalysts and sonophotocatalytic processes: A review, iScience, 2023, 27, 108583 CrossRef PubMed.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Adsorptive removal of tetracycline antibiotic onto magnetic graphene oxide nanocomposite modified with polyvinylpyrroilidone, React. Funct. Polym., 2023, 191, 105701 CrossRef CAS.
- A. M. Basely, et al., Construction of Bi2S3/g-C3N4 step S-scheme heterojunctions for photothermal decomposition of rhodamine B dye under natural sunlight radiations, Inorg. Chem. Commun., 2023, 148, 110300 CrossRef CAS.
- Q. Gao, et al., Bi-doped graphitic carbon nitride nanotubes boost the photocatalytic degradation of Rhodamine B, New J. Chem., 2022, 46(8), 3588–3594 RSC.
- F. Tang, et al., Synthesis of molybdenum trioxide/graphite carbon nitride heterojunction modified by biomass carbon dots and its application in photocatalytic degradation of methylene blue, Diamond Relat. Mater., 2023, 110078 CrossRef CAS.
- G. Zeng, et al., Sulfate doped graphitic carbon nitride with enhanced photocatalytic activity towards degradation of methylene blue, Mater. Lett., 2022, 309, 131310 CrossRef CAS.
- E. Prabakaran, et al., Comparative study of KF, KCl and KBr doped with graphitic carbon nitride for superior photocatalytic degradation of methylene blue under visible light, J. Mater. Res. Technol., 2021, 15, 6340–6355 CrossRef CAS.
- M. Vijayan, et al., Constructing Z-scheme g-C3N4/TiO2 heterostructure for promoting degradation of the hazardous dye pollutants, Chemosphere, 2023, 311, 136928 CrossRef CAS PubMed.
- W. Wang, et al., Compact and uniform TiO2@ g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics, Appl. Catal., B, 2017, 217, 57–64 CrossRef CAS.
- W. Yan, L. Yan and C. Jing, Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms, Appl. Catal., B, 2019, 244, 475–485 CrossRef CAS.
- Y.-H. Chen, B.-K. Wang and W.-C. Hou, Graphitic carbon nitride embedded with graphene materials towards photocatalysis of bisphenol A: The role of graphene and mediation of superoxide and singlet oxygen, Chemosphere, 2021, 278, 130334 CrossRef CAS PubMed.
- K. Zhang, et al., Iron phthalocyanine nanodots decorated ultra-thin porous carbon nitride: a combination of photocatalysis and Fenton reaction to achieve two-channel efficient tetracycline degradation, J. Alloys Compd., 2023, 966, 171580 CrossRef CAS.
- F. Li, et al., Two-dimensional graphene/g-C3N4 in-plane hybrid heterostructure for enhanced photocatalytic activity with surface-adsorbed pollutants assistant, Appl. Catal., B, 2020, 268, 118397 CrossRef CAS.
- M. A. Ahmed and A. A. Mohamed, A systematic review of layered double hydroxide-based materials for environmental remediation of heavy metals and dye pollutants, Inorg. Chem. Commun., 2022, 110325 Search PubMed.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Synthesis, characterization and application of chitosan/graphene oxide/copper ferrite nanocomposite for the adsorptive removal of anionic and cationic dyes from wastewater, RSC Adv., 2023, 13(8), 5337–5352 RSC.
- M. A. Ahmed and A. A. Mohamed, The use of chitosan-based composites for environmental remediation: A review, Int. J. Biol. Macromol., 2023, 124787 CrossRef CAS PubMed.
- R. He, et al., Room-temperature in situ fabrication and enhanced photocatalytic activity of direct Z-scheme BiOI/g-C3N4 photocatalyst, Appl. Surf. Sci., 2019, 465, 964–972 CrossRef CAS.
- G. Salehi, R. Abazari and A. R. Mahjoub, Visible-light-induced graphitic–C3N4@ nickel–aluminum layered double hydroxide nanocomposites with enhanced photocatalytic activity for removal of dyes in water, Inorg. Chem., 2018, 57(14), 8681–8691 CrossRef CAS PubMed.
- R. Li, et al., Activation of peroxymonosulfate by Fe doped g-C3N4/graphene under visible light irradiation for Trimethoprim degradation, J. Hazard Mater., 2020, 384, 121435 CrossRef CAS PubMed.
- D. Monga and S. Basu, Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: Role of shape of TiO2, Adv. Powder Technol., 2019, 30(5), 1089–1098 CrossRef CAS.
- N. Pourshirband and A. Nezamzadeh-Ejhieh, An efficient Z-scheme CdS/g-C3N4 nano catalyst in methyl orange photodegradation: focus on the scavenging agent and mechanism, J. Mol. Liq., 2021, 335, 116543 CrossRef CAS.
- K. Prakash, S. Karuthapandian and S. Senthilkumar, Zeolite nanorods decorated g-C3N4 nanosheets: a novel platform for the photodegradation of hazardous water contaminants, Mater. Chem. Phys., 2019, 221, 34–46 CrossRef CAS.
- Y. Jiang, et al., Insights into mechanisms, kinetics and pathway of continuous visible-light photodegradation of PPCPs via porous g-C3N4 with highly dispersed Fe (III) active sites, Chem. Eng. J., 2021, 423, 130095 CrossRef CAS.
- B. Yuan, et al., Simple synthesis of g-C3N4/rGO hybrid catalyst for the photocatalytic degradation of rhodamine B, Chin. J. Catal., 2015, 36(7), 1009–1016 CrossRef CAS.
- M. Adel, M. A. Ahmed and A. A. Mohamed, Effective removal of cationic dyes from aqueous solutions using reduced graphene oxide functionalized with manganese ferrite nanoparticles, Compos. Commun., 2020, 22, 100450 CrossRef.
- M. Adel, M. A. Ahmed and A. A. Mohamed, Removal of 4-Nitrophenol and Indigo Carmine Dye from Wastewaters by Magnetic Copper Ferrite Nanoparticles: Kinetic, Thermodynamic and Mechanistic Insights, J. Saudi Chem. Soc., 2023, 101748 Search PubMed.
- M. Adel, M. A. Ahmed and A. A. Mohamed, A facile and rapid removal of cationic dyes using hierarchically porous reduced graphene oxide decorated with manganese ferrite, FlatChem, 2021, 26, 100233 CrossRef CAS.
- M. Adel, M. A. Ahmed and A. A. Mohamed, Effective removal of indigo carmine dye from wastewaters by adsorption onto mesoporous magnesium ferrite nanoparticles, Environ. Nanotechnol. Monit. Manag., 2021, 16, 100550 CAS.
- A. Alsalme, et al., Fabrication of S-scheme TiO2/g-C3N4 nanocomposites for generation of hydrogen gas and removal of fluorescein dye, Diamond Relat. Mater., 2022, 122, 108819 CrossRef CAS.
- T. D. Munusamy, C. S. Yee and M. M. R. Khan, Construction of hybrid g-C3N4/CdO nanocomposite with improved photodegradation activity of RhB dye under visible light irradiation, Adv. Powder Technol., 2020, 31(7), 2921–2931 CrossRef CAS.
- M. Adel, M. A. Ahmed and A. A. Mohamed, Synthesis and characterization of magnetically separable and recyclable crumbled MgFe2O4/reduced graphene oxide nanoparticles for removal of methylene blue dye from aqueous solutions, J. Phys. Chem. Solids, 2021, 149, 109760 CrossRef CAS.
- K. M. Reza, A. Kurny and F. Gulshan, Parameters affecting the photocatalytic degradation of dyes using TiO 2: a review, Appl. Water Sci., 2017, 7, 1569–1578 CrossRef CAS.
- I. Ahmad, Comparative study of metal (Al, Mg, Ni, Cu and Ag) doped ZnO/g-C3N4 composites: Efficient photocatalysts for the degradation of organic pollutants, Sep. Purif. Technol., 2020, 251, 117372 CrossRef CAS.
- L. Hou, et al., Flower-like dual-defective z-scheme heterojunction g-C3N4/Znin2s4 high-efficiency photocatalytic hydrogen evolution and degradation of mixed pollutants, Nanomaterials, 2021, 11(10), 2483 CrossRef CAS PubMed.
- B. Palanivel, et al., Magnetic binary metal oxide intercalated g-C3N4: Energy band tuned pn heterojunction towards Z-scheme photo-Fenton phenol reduction and mixed dye degradation, J. Water Proc. Eng., 2019, 32, 100968 CrossRef.
- S. Vignesh and H. Kim, Interfacial coupling effects in α-Fe2O3/g-C3N4 composite magnetically separable heterojunction with upgraded Z-scheme photocatalytic performance of mixed organic pollutant degradation, J. Phys. Chem. Solids, 2022, 169, 110869 CrossRef CAS.
- P. Suyana, et al., Co3 O4–C3N4 p–n nano-heterojunctions for the simultaneous degradation of a mixture of pollutants under solar irradiation, Environ. Sci.: Nano, 2017, 4(1), 212–221 RSC.
- S. Vignesh, et al., Design a novel g-C3N4 based Ce2O3/CuO ternary photocatalysts for superior photo-degradation performance of organic mixed pollutants: insights of Z-scheme charge transfer mechanism, J. Phys. Chem. Solids, 2022, 162, 110514 CrossRef CAS.
- R. Acharya and K. Parida, A review on TiO2/g-C3N4 visible-light-responsive photocatalysts for sustainable energy generation and environmental remediation, J. Environ. Chem. Eng., 2020, 8(4), 103896 CrossRef CAS.
- S. Pati and R. Acharya, An overview on g-C3N4 as a robust photocatalyst towards the sustainable generation of H2 energy, Mater. Today: Proc., 2021, 35, 175–178 CAS.
- L. Bi, et al., Sulfidization of platinum nickel bimetal-decorated g-C3N4 for photocatalytic hydrogen production: photogenerated charge behavior study, ACS Sustain. Chem. Eng., 2019, 7(17), 15137–15145 CrossRef CAS.
- M. Ou, et al., Single Pt atoms deposition on g-C3N4 nanosheets for photocatalytic H2 evolution or NO oxidation under visible light, Int. J. Hydrogen Energy, 2017, 42(44), 27043–27054 CrossRef CAS.
- Y. Lu, et al., Exfoliated carbon nitride nanosheets decorated with NiS as an efficient noble-metal-free visible-light-driven photocatalyst for hydrogen evolution, Phys. Chem. Chem. Phys., 2015, 17(26), 17355–17361 RSC.
- W. Zhou, et al., Conjugated polymer dots/graphitic carbon nitride nanosheet heterojunctions for metal-free hydrogen evolution photocatalysis, J. Mater. Chem. A, 2019, 7(1), 303–311 RSC.
- N. Raeisi-Kheirabadi and A. Nezamzadeh-Ejhieh, A Z-scheme g-C3N4/Ag3PO4 nanocomposite: its photocatalytic activity and capability for water splitting, Int. J. Hydrogen Energy, 2020, 45(58), 33381–33395 CrossRef CAS.
- X. Han, et al., Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation, Appl. Catal., B, 2020, 265, 118539 CrossRef CAS.
- R. Cheng, et al., One-step construction of FeOx modified g-C3N4 for largely enhanced visible-light photocatalytic hydrogen evolution, Carbon, 2016, 101, 62–70 CrossRef CAS.
- K. C. Christoforidis, et al., Metal-free dual-phase full organic carbon nanotubes/g-C3N4 heteroarchitectures for photocatalytic hydrogen production, Nano Energy, 2018, 50, 468–478 CrossRef CAS.
- Y. Chen, et al., One-step construction of S-scheme heterojunctions of N-doped MoS2 and S-doped g-C3N4 for enhanced photocatalytic hydrogen evolution, Chem. Eng. J., 2021, 404, 126498 CrossRef CAS.
- Z. Sun, et al., Insight into iron group transition metal phosphides (Fe2P, Co2P, Ni2P) for improving photocatalytic hydrogen generation, Appl. Catal., B, 2019, 246, 330–336 CrossRef CAS.
- A. G. Shende, et al., Exciton Dissociation on Double Z-scheme Heterojunction for Photocatalytic Application, ChemistrySelect, 2021, 6(26), 6707–6713 CrossRef CAS.
- X. Han, et al., 2D bimetallic RuNi alloy Co-catalysts remarkably enhanced the photocatalytic H2 evolution performance of g-C3N4 nanosheets, Chem. Eng. J., 2021, 426, 130824 CrossRef CAS.
- Q. Xiang, J. Yu and M. Jaroniec, Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites, J. Phys. Chem. C, 2011, 115(15), 7355–7363 CrossRef CAS.
- N. Al-Zaqri, et al., Construction of novel direct Z-scheme AgIO4-g-C3N4 heterojunction for photocatalytic hydrogen production and photodegradation of fluorescein dye, Diamond Relat. Mater., 2020, 109, 108071 CrossRef CAS.
- M. Benaissa, et al., BiVO3/g-C3N4 S-scheme heterojunction nanocomposite photocatalyst for hydrogen production and amaranth dye removal, Opt. Mater., 2021, 118, 111237 CrossRef CAS.
- L. Song, et al., C60/graphene/g-C3N4 composite photocatalyst and mutually-reinforcing synergy to improve hydrogen production in splitting water under visible light radiation, Ceram. Int., 2017, 43(10), 7901–7907 CrossRef CAS.
- S. Manchala, et al., Fabrication of a novel ZnIn2S4/gC3N4/graphene ternary nanocomposite with enhanced charge separation for efficient photocatalytic H 2 evolution under solar light illumination, Photochem. Photobiol. Sci., 2019, 18, 2952–2964 CrossRef CAS PubMed.
- H. S. Abd-Rabboh, Boosting hydrogen gas production and mitigation of fluorescein dye on the surface of S-scheme g-C3N4/SnO2 heterojunction, Desalin. Water Treat., 2022, 268, 113–125 CrossRef CAS.
- Z. Chen, et al., NiS and graphene as dual cocatalysts for the enhanced photocatalytic H2 production activity of g-C3N4, Appl. Surf. Sci., 2019, 469, 657–665 CrossRef CAS.
- L. Liu, et al., Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core@ shell Cu2O@ g-C3N4 octahedra, Appl. Surf. Sci., 2015, 351, 1146–1154 CrossRef CAS.
- A. Qu, et al., Effects of calcining temperature on photocatalysis of g-C3N4/TiO2 composites for hydrogen evolution from water, Mater. Res. Bull., 2016, 80, 167–176 CrossRef CAS.
- J. Fu, et al., Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst, Appl. Catal., B, 2019, 243, 556–565 CrossRef CAS.
- J. Khan, Y. Sun and L. Han, A comprehensive review on graphitic carbon nitride for carbon dioxide photoreduction, Small Methods, 2022, 6(12), 2201013 CrossRef CAS PubMed.
- Q. Zhang, et al., Structural reorganization of ultrathin g-C3N4 nanosheets for significantly boosting wide-spectrum-driven CO2 photoreduction, Appl. Surf. Sci., 2023, 638, 157989 CrossRef CAS.
- H.-Z. Wu, et al., Mechanism of CO2 conversion into methanol and methane at the edge of graphitic carbon nitride sheet: A first-principle study, Carbon, 2020, 169, 73–81 CrossRef CAS.
- Y. He, et al., New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel, Environ. Sci. Technol., 2015, 49(1), 649–656 CrossRef CAS PubMed.
- Y. Xia, et al., Highly selective CO2 capture and its direct photochemical conversion on ordered 2D/1D heterojunctions, Joule, 2019, 3(11), 2792–2805 CrossRef CAS.
- M. Arumugam, M. Tahir and P. Praserthdam, Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4, Chemosphere, 2022, 286, 131765 CrossRef CAS PubMed.
- T. Mahvelati-Shamsabadi and B.-K. Lee, Photocatalytic H2 evolution and CO2 reduction over phosphorus-doped g-C3N4 nanostructures: electronic, Optical, and Surface properties, Renew. Sustain. Energy Rev., 2020, 130, 109957 CrossRef CAS.
- F. A. Qaraah, et al., Synergistic effect of hierarchical structure and S-scheme heterojunction over O-doped g-C3N4/N-doped Nb2O5 for highly efficient photocatalytic CO2 reduction, Appl. Catal., B, 2022, 315, 121585 CrossRef CAS.
- H. Li, et al., Intercorrelated Superhybrid of AgBr Supported on Graphitic-C3N4-Decorated Nitrogen-Doped Graphene: High Engineering Photocatalytic Activities for Water Purification and CO2 Reduction, Adv. Mater., 2015, 27(43), 6906–6913 CrossRef CAS PubMed.
- H. Shi, et al., Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel, ACS Catal., 2014, 4(10), 3637–3643 CrossRef CAS.
- S. Wang, J. Lin and X. Wang, Semiconductor–redox catalysis promoted by metal–organic frameworks for CO 2 reduction, Phys. Chem. Chem. Phys., 2014, 16(28), 14656–14660 RSC.
- M. Li, et al., Highly selective CO2 photoreduction to CO over gC3N4/Bi2WO6 composites under visible light, J. Mater. Chem. A, 2015, 3(9), 5189–5196 RSC.
- Z. Teng, et al., Photoexcited single metal atom catalysts for heterogeneous photocatalytic H2O2 production: pragmatic guidelines for predicting charge separation, Appl. Catal., B, 2021, 282, 119589 CrossRef CAS.
- C. Liang, et al., Functionalized graphitic carbon nitride based catalysts in solar-to-chemical conversion for hydrogen peroxide production, Chem. Eng. J., 2023, 466, 142931 CrossRef CAS.
- S. Sahoo and R. Acharya, An overview on recent developments in synthesis and molecular level structure of visible-light responsive g-C3N4 photocatalyst towards environmental remediation, Mater. Today: Proc., 2021, 35, 150–155 CAS.
- S. Mishra, R. Acharya and K. Parida, Spinel-ferrite-decorated graphene-based nanocomposites for enhanced photocatalytic detoxification of organic dyes in aqueous medium: a review, Water, 2023, 15(1), 81 CrossRef CAS.
- W. Gan, et al., Introducing oxygen-doped g-C3N4 onto g-C3N4/TiO2 heterojunction for efficient catalytic gatifloxacin degradation and H2O2 production, Sep. Purif. Technol., 2023, 317, 123791 CrossRef CAS.
- Z. Jiang, et al., 3D ordered macroporous sulfur-doped g-C3N4/TiO2 S-scheme photocatalysts for efficient H2O2 production in pure water, J. Mater. Sci. Technol., 2023, 162, 1–10 CrossRef CAS.
- G. Zhang, et al., Megamerger of MOFs and gC3N4 for energy and environment applications: upgrading the framework stability and performance, J. Mater. Chem. A, 2020, 8(35), 17883–17906 RSC.
- S. R. Gujjula, et al., Versatile bifunctional Ag@ g-C3N4/r-GO catalyst for efficient photo-and electrocatalytic H2 production, Energy Fuels, 2023, 37(13), 9722–9735 CrossRef CAS.
- X. Li, S. Wang, D. Ye, W. Wen, H. Li, Z. Ma, G. Li, W. Fu and M. Fu, g-C3N4/Metal–Organic Framework Nanosheet/CuO Heterostructure for the Visible Photocatalytic Degradation of Tetracycline, ACS Appl. Nano Mater., 2024, 7(2), 1586–1597 CrossRef CAS.
- X. Li, et al., g-C3N4/Metal–Organic Framework Nanosheet/CuO Heterostructure for the Visible Photocatalytic Degradation of Tetracycline, ACS Appl. Nano Mater., 2024, 7(2), 1586–1597 CrossRef CAS.
- S. Yao, et al., Atomically dispersed scandium lewis acid sites on carbon nitride for efficient photocatalytic hydrogen peroxide production, Sci. China Mater., 2023, 66(2), 672–678 CrossRef CAS.
- Z. Teng, et al., Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide, Nat. Catal., 2021, 4(5), 374–384 CrossRef CAS.
- Y. Shao, et al., Significantly enhanced photocatalytic in-situ H2O2 production and consumption activities for efficient sterilization by ZnIn2S4/g-C3N4 heterojunction, Carbon, 2022, 190, 337–347 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |