Editorial Perspectives: the value proposition of resource recovery
Zhiyong
Jason Ren
 ab
ab
aDepartment of Civil and Environmental Engineering and Andlinger Center for Energy and the Environment, Princeton University, Princeton, NJ 08544, USA. E-mail: zjren@princeton.edu; Fax: +609 258 4899; Tel: +609 258 7580
bDepartment of Civil, Environmental, and Architectural Engineering, University of Colorado Boulder, Boulder, Colorado 80309, USA
However, despite all the efforts, the technology-to-market process has been slow, even compared with other public service sectors such as energy and transportation. While there are many non-technical reasons such as the risk-averse nature of the industry, the low margin in water sector investment, and the complexity of environmental regulations, identifying the market niche to maximize value proposition is one thing that all researchers and innovators can keep in mind when pursuing a new process or product. So, beyond our hypothesis-driven research questions, maybe we can also ask some market-driven questions such as: can this product make an impact? Can this process scale? Where can I sell this or who will buy this?
There is no good or bad product, but there can be a right or wrong product for a specific WRRF. What should a WRRF recover to ensure full-scale viability? Well, it depends. The “right” product depends on WRRF variables including size, location, treatment train, local regulations, and product market potential. The resources recovered should have a promising market, preferably locally, and with a high margin. Here are several examples we can consider:
- Biogas or upgrades: biogas is probably the easiest product to generate because methanogenesis is a spontaneous process in anaerobic digestion with the lowest oxidation state. However, because of the low value and clean-up needs before it can be used as a clean fuel, the economics cannot justify investment in biogas production unless there are significant tax or other incentives. Therefore, even new technologies like anaerobic membrane bioreactors (AnMBRs) that can produce biogas at a higher rate may not be viable.4 Such processes should consider upgraded products such carboxylic acids, alcohols, polymers, or food proteins, accomplished by arrested methanogenesis via electro-fermentation or methane oxidation.5,6 The unit value differences of such changes can be increased by orders of magnitude.
- Electricity or reducing power: another example is electricity from microbial fuel cells (MFCs), which is struggling in the scaleup phase because electricity can be obtained in a cheaper way, and the low current cannot justify the expensive upgrades needed to make this type of system a reality. However, MFCs can be a great technology when used for lighting, phone charging, or sensing in decentralized communities or even refugee camps that lack energy infrastructure. This principle also applies if the electrons are not used directly but are used as reducing power to generate higher value products from CO2 or other feedstocks.7
- Fertilizer or not: similar stories can be told in nutrient recovery as well, with the resources currently recovered, such as ammonia or struvite products, often too cheap to justify. Higher value products such as food additives can be more desirable.8 Another emerging approach for nutrient “recovery” avoids the energy-intensive nutrient removal/recovery process altogether, opting instead to keep nutrients in the effluent for fertigation in agriculture.9
Despite a diversity in products, there are common research endeavors needed to move this field forward. For example, synthetic biology and (bio)catalysts have been effective for valorizing low-value feedstocks such as sugar and CO2 to value-added chemicals and products, but this has been primarily done in pure culture systems with defined substrates. As a highly diverse substrate in the open environment, wastewater poses significant challenges to this bottom-up approach, but the challenges are surmountable if additional research is focused on areas such as microbial consortia development and process engineering. Another tool we environmental engineers can use more is technoeconomic analysis (TEA), which is popular in chemical engineering and has become a standard requirement in many DOE projects.
Resource recovery is not only an environmental case but also a business case. A successful business not only kills the pain (treats waste) but also creates a gain (makes revenue) for their clients. Imagine that one day wastewater becomes a commodity and utilities need to pay back customers for receiving their wastewater. Then the utilities will be redefined as waste refineries with a unique supply chain and profit generation and sharing (Fig. 1). This business model can be similar to those of social media companies that pay you for your data, or power utilities that pay back customers for their rooftop solar panels. We need the Tesla and Facebook of the water industry to disrupt the current business model, and such efforts will need close collaboration among different stakeholders.10 Of course, we didn't learn this when we were in graduate school, but this is something current scientists- or engineers-in-training should consider when picking courses next semester.
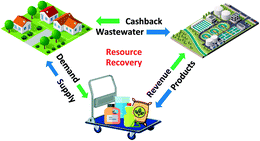 | ||
| Fig. 1 Resource recovery enabled circular economy. Icons from Shutterstock and Freepik viahttp://www.flaticon.com. | ||
Acknowledgements
The author is grateful to the insights and edits provided by Prof. Paige Novak and Prof. David Cwiertny.References
- J. Mateo-Sagasta, L. Raschid-Sally and A. Thebo, Global Wastewater and Sludge Production, Treatment and Use, Wastewater: Economic asset in an urbanizing world. Springer, 2015 Search PubMed.
- L. Lu, J. S. Guest, C. A. Peters, X. Zhu, G. H. Rau and Z. J. Ren, Wastewater treatment for carbon capture and utilization, Nat. Sustain., 2018, 1, 750–758 CrossRef.
- W. W. Li, H. Q. Yu and B. E. Rittmann, Chemistry: reuse water pollutants, Nature, 2015, 528(7580), 29 CrossRef CAS PubMed.
- A. L. Smith, L. B. Stadler, N. G. Love, S. J. Skerlos and L. Raskin, Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: a critical review, Bioresour. Technol., 2012, 122, 149–159 CrossRef CAS PubMed.
- A. Alloul, R. Ganigué, M. Spiller, F. Meerburg, C. Cagnetta, K. Rabaey and S. E. Vlaeminck, Capture–Ferment–Upgrade: A Three-Step Approach for the Valorization of Sewage Organics as Commodities, Environ. Sci. Technol., 2018, 52(12), 6729–6742 CrossRef CAS PubMed.
- Y. Jiang, D. H. May, L. Lu, P. Liang, X. Huang and Z. J. Ren, Carbon Dioxide and Organic Waste Valorization by Microbial Electrosynthesis and Electro-fermentation, Water Res., 2019, 149, 42–55 CrossRef CAS PubMed.
- O. Modin and F. Aulenta, Three promising applications of microbial electrochemistry for the water sector, Environ. Sci.: Water Res. Technol., 2017, 3(3), 391–402 RSC.
- I. Pikaar, S. Matassa, K. Rabaey, B. L. Bodirsky, A. Popp, M. Herrero and W. Verstraete, Microbes and the next Nitrogen revolution, Environ. Sci. Technol., 2017, 51(13), 7297–7303 CrossRef CAS PubMed.
- P. L. McCarty, What is the Best Biological Process for Nitrogen Removal: When and Why?, Environ. Sci. Technol., 2018, 52(7), 3835–3841 CrossRef CAS PubMed.
- J. R. Mihelcic, Z. J. Ren, P. K. Cornejo, A. Fisher, A. J. Simon, S. W. Snyder, Q. Zhang, D. Rosso, T. M. Huggins, W. Cooper, J. Moeller, B. Rose, B. L. Schottel and J. Turgeon, Accelerating Innovation that Enhances Resource Recovery in the Wastewater Sector: Advancing a National Testbed Network, Environ. Sci. Technol., 2017, 51(14), 7749–7758 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |
