Open Access Article
Open Access ArticleSecondary product creation potential (SPCP): a metric for assessing the potential impact of indoor air pollution on human health†
Nicola
Carslaw
 * and
David
Shaw
* and
David
Shaw

Department of Environment and Geography, University of York, York, UK. E-mail: nicola.carslaw@york.ac.uk
First published on 29th May 2019
Abstract
Indoor air is subject to emissions of chemicals from numerous sources. Many of these emissions contain volatile organic compounds (VOCs), which react to form a wide range of secondary products, some with adverse health effects. However, at present we lack a robust, standardised approach to rank the potential for different VOCs to cause harm, which prevents effective action to improve indoor air quality and reduce impacts on human health. This paper uses a detailed chemical model to quantify the impact of 63 VOCs on indoor air quality. We define a novel method for ranking the VOCs in terms of potentially harmful product formation through a new metric, the Secondary Product Creation Potential (SPCP). We established SPCPs for a range of ventilation rates, different proportions of transmitted outdoor light, as well as for varying outdoor concentrations of ozone and nitrogen oxides. The species having the largest SPCPs are the alkenes, terpenes and aromatic VOCs. trans-2-Butene has the largest individual SPCP owing to the ratio of its rate coefficient for reaction with the hydroxy radical relative to ozone. Increasing the proportion of outdoor transmitted light increased most SPCPs markedly. This is because oxidant levels increased under these conditions and promoted more chemical processing, suggesting that there may be more harmful products closer to a window than further from the attenuated outdoor light. The SPCP is the first metric for assessing the impact of different VOCs on human health and will be an essential tool for guiding the composition of products commonly used indoors.
Environmental significanceIndoor environments are subject to numerous chemical emissions, both direct and following chemical reactions. Volatile organic compounds (VOCs) are a common component in many products used indoors (e.g. cleaning fluids) and their reactions can lead to products that are harmful to health. However, the ability for an individual VOC to form such products depends on its reactivity. For the first time, this paper presents a robust, modelling-based methodology for ranking VOCs according to their ability to form potentially harmful products, for a range of typical indoor conditions. The ranking shows that double-bonded and aromatic VOCs are most efficient at forming potentially harmful products, particularly as indoor light levels increase. This ranking system can inform future design of product formulations used indoors. |
Introduction
Over recent decades, the quality of the air inside the buildings that we live, work and play in, has become increasingly important. Compared to previous generations, we spend much more of our time indoors, we live and work in buildings that are typically more airtight owing to energy efficiency measures and use chemicals indoors in far greater quantities, for a range of activities such as cleaning, cooking and air freshener use.Volatile organic compounds (VOCs) are ubiquitous in many of the products we use indoors, particularly cleaning products and fragrances.1,2 Furthermore, when we use products that contain VOCs indoors, they can undergo a series of oxidation reactions to produce a further swathe of secondary products. These products can then undergo further chemical reactions, as well as interact with surfaces, or be removed through exchange with outdoors.3 In fact, the use of personal care products indoors has recently been identified as a significant source of VOCs outdoors in urban areas.4
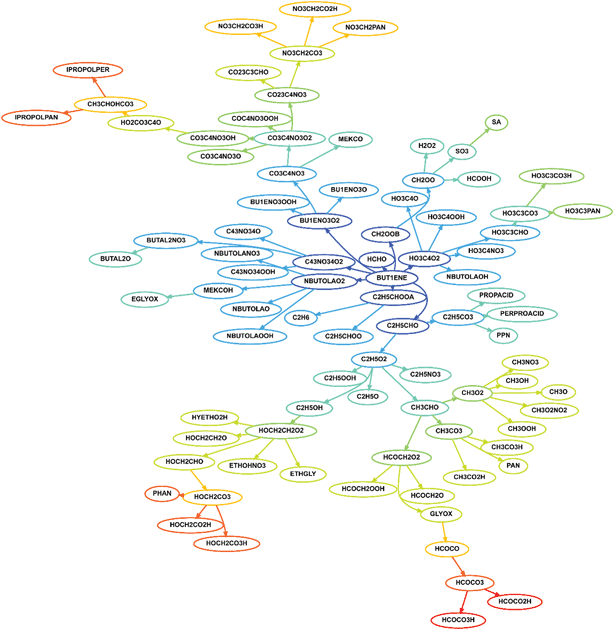
The degradation pathways for VOCs become increasingly complex as the VOC increases in size. For instance, using chemical mechanistic information from the Master Chemical Mechanism5 (see the website http://mcm.leeds.ac.uk/MCM), the degradation of methane (CH4) from its preliminary oxidation in the atmosphere to the formation of oxidation end-products carbon dioxide and water, can be represented by 23 reactions and 17 species. For the next simplest alkane ethane (C2H6), we need 46 species in 120 reactions. However, by the time we approach the degradation of limonene (C10H16), 713 species in 1241 reactions are needed to represent the degradation to the final products. Limonene is one of many of the fragrant terpenoid species that are ubiquitous indoors1,2 and its oxidation produces a range of polyfunctional species including alcohol, carbonyl and carboxylic acid groups, with each individual oxidation product containing 3–4 functional groups on average.6Fig. 1 shows a representation of the degradation scheme of 1-butene, an alkene containing four carbon atoms. Clearly, even for this relatively simple species, the complexity is clear if all potential reaction pathways are considered.
One of the main concerns with the vast number of reactions that can occur indoors following VOC release is the potential for forming harmful secondary products through oxidation.7–11 Some of the secondary products from indoor chemistry have well known adverse health effects such as formaldehyde, a known carcinogen.12 Its formation indoors has been documented following the use of limonene containing cleaners.13 However, for many of the secondary products formed indoors, there is very little information about their typical indoor concentrations and at what concentration they become a cause for concern.
One method that has been used to rank the potential for VOCs to form secondary pollutants (ozone in this case) outdoors is Photochemical Ozone Creation Potentials, or POCPs.14 A photochemical trajectory model for the ambient atmosphere was used to investigate the impact of increasing the concentration of one VOC concentration at a time. The change in ozone (O3) concentration formed through the resulting chemistry was then used to rank each VOC relative to the increase from addition of ethene. The resulting POCP values provide a ranking of the relative reactivity of the VOCs and their propensity to form ozone. The Maximum Incremental Reactivity (MIR) scale15 is an alternative method for investigating the chemical impact of different VOCs in the atmosphere.
However, the POCP ranking is not a health metric, nor is it suitable for use indoors. The reactivity of the VOCs outdoors is largely driven by photolysis, a process which is much less important (though not negligible) indoors. In addition, the POCP metric focuses on conditions which lead to high ozone concentrations, and ozone is rapidly removed indoors e.g. by deposition onto surfaces.16
The aim of this paper, therefore, is to use a detailed chemical model for the indoor environment to develop the first ever metric for assessing the relative impact of different VOCs on indoor air chemistry and to rank them according to potential adverse health effects. This new metric will provide a robust, consistent and simple-to-understand system that summarises the relative potential for harm from VOCs commonly used indoors, and how this varies across a range of conditions. Such information could be valuable for manufacturers of commonly used indoor products, particularly if we aim to prioritise VOCs to remove from such products according to potential adverse health effects.
Experimental
a. Model
The INdoor Detailed Chemical Model (INDCM) has been described in detail before.17–19 Briefly, the chemical mechanism in the INDCM is based on the Master Chemical Mechanism (version 3.2) and considers the near-explicit chemical degradation of ∼140 VOCs.5,20–22 Each VOC undergoes oxidation with the OH (hydroxyl) radical, the NO3 (nitrate radical), O3 and photolysis where relevant, to form a range of radical species (such as peroxy and oxy radicals) as well as more stable intermediates (aldehydes, ketones etc.). These products then undergo further reactions until they form the final oxidation products of carbon dioxide and water.As well as the chemical mechanism, the model contains terms for exchange with outdoors, deposition, photolysis, internal emissions and gas-to-particle partitioning. In total, the model contains around ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 reactions and 5000 species. The model environment is assumed to be well mixed and can be initialised to be a house, office, bedroom, classroom etc. as required. External pollutant concentrations and internal emissions can also be varied. The focus is on the chemical detail and the insight that the mechanism provides into the indoor air chemistry.
000 reactions and 5000 species. The model environment is assumed to be well mixed and can be initialised to be a house, office, bedroom, classroom etc. as required. External pollutant concentrations and internal emissions can also be varied. The focus is on the chemical detail and the insight that the mechanism provides into the indoor air chemistry.
For this work, the INDCM model has been initialised to represent a typical residence in a polluted European city during a typical summer as described in detail elsewhere.19 The mixing ratios of O3, nitrogen dioxide (NO2) and nitric oxide (NO) outdoors were 49, 19 and 16 ppb respectively from 09:00–17:00 h based on measurements from Milan in August 2009. These led to indoor modelled values of ∼8, 8 and 2 ppb respectively. There were no indoor emissions of VOCs, but VOC mixing ratios outdoors were typical for a polluted urban area.23 In the absence of indoor sources, indoor VOC mixing ratios were determined by the outdoor values and the air exchange rate (AER), which was set to 0.76 h−1 for the standard scenario.17,24 It was assumed that there were indoor lights, as well as attenuated outdoor light, with 3% of ultraviolet (UV) and 10% of visible light from outdoors assumed to be transmitted through windows.17 We assumed an indoor temperature of 300 K and relative humidity of 45%. The model was run for three days to ensure steady-state conditions and results are reported for day 3.
b. SPCP definition
A range of VOCs was chosen based on the reported key components of cleaning products and air fresheners,1 measurements from three studies from Canadian residences between 2009–2013 (ref. 25–27) and from 600 homes in Japan from 2011–2014.28 This identified 53 VOC species. Two older articles identified a further 6 species that could be present indoors29,30 and three species were included as potential degradation products from VOCs already in the mechanism: 1-butene (from hexanal), methacrolein (MACR from isoprene) and 4-hydroxy-4-methylpentan-2-one (MIBKAOH from methyl-isobutylketone, MIBK). Finally, MBO (2-methly-2-buten-2-ol) was included for interest given it is derived from biogenic processes31 like many of the terpene species in cleaning products. This process identified 63 unique species. The mixing ratio of one VOC at a time was then set to 10 ppb for the entirety of a model run.We then explored the change in concentration of the following species between 08:00–18:00 h relative to a run with no VOCs added indoors (the baseline for each set of conditions): hydroxyl (OH), hydroperoxy (HO2) and organic peroxy (RO2) radicals, the sum of organic nitrates (∼375) and the sum of all PAN (peroxyacetylnitrate) species (∼280), formaldehyde (HCHO), ozone, glyoxal and acetaldehyde. The changes in radical species for each VOC were explored to provide insight into the chemistry, which is presented in the next section. However, the other species were used to define a new VOC ranking mechanism for indoors, a Secondary Product Creation Potential (SPCP). The SPCP is assumed to be the sum of a range of products that are considered potentially harmful and is in units of the secondary products produced in pbb, per ppb of VOC added:
| SPCP = ∑(organic nitrates + PANs + HCHO + O3 + glyoxal + acetaldehyde)/β |
Ozone has a range of mainly respiratory and cardiovascular health effects.34 Although the health effects of this pollutant are well documented outdoors,34 ozone is deposited rapidly to indoor surfaces and typically exists at only 20–70% of outdoor concentrations indoors in the absence of an indoor source such as a photocopier or laser printer.16 There are also numerous health effects associated with exposure to PAN type species, given they behave as mutagens, phytotoxins and lachrymators.35 Finally, deposition of organic nitrates into the lung lining fluid may produce nitric acid following hydrolysis, leading to reductions in pulmonary function.36
For the model runs described here, the sum of the mixing ratios of the secondary products is divided by 10 (β = 10 as we added 10 ppb of each VOC for the model runs), so that the SPCP is a measure of the mixing ratio in ppb of ‘potentially harmful secondary products (SP)’ formed per ppb of VOC added to an indoor environment (or ppb SP/ppb VOC). The SPCP will of course under-estimate the total mixing ratios of potentially harmful products indoors as it only considers a few representative compounds. For instance, the formation of particles through some of these species is not considered in the SPCP at the moment. The INDCM only considers particle formation for terpenes at present and not the other chemical classes, so particles have been omitted from the SPCP. There are also other products formed through limonene oxidation chemistry that have known health effects such as 4-oxopentanal (4-OPA) and limonaldehyde.37 However, these species are unlikely to attain mixing rations indoors under typical conditions that impact on health. Another limitation is that owing to the absence of specific health data for many of the species included in the SPCP calculation, they are given parity in the calculation in terms of health effects. In other words, 1 ppb of any of these species is assumed to have the same health effect as 1 ppb of any of the others.
However, the SPCP considers a range of secondary pollutants from different chemical classes and will provide an indicative picture of which species have the biggest impact on the ongoing gas-phase chemistry and consequent formation of secondary pollutants. If future information becomes available on more specific health effects, or mechanism development leads to the consideration of new chemical pathways/species within VOC degradation mechanisms (e.g. particle formation for other VOCs), it would be trivial to add more species to the SPCP definition, or to weight species according to their toxicity.
c. Sensitivity studies
To test the impact of different indoor conditions on the calculated SPCP values, the model runs were repeated with: a higher (2 h−1) and lower (0.2 h−1) AER; a higher (25% of the UV and 75% of the visible light) and lower (1% of the UV and 3% of the visible light) transmission of outdoor light; halved outdoor ozone and nitrogen oxide mixing ratios to simulate a cleaner outdoor environment; doubled mixing ratios for NO outdoors to represent more polluted conditions, but also to provide a different NO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NO2 ratio. Outdoor VOC mixing ratios were left unaltered for all model runs. These additional runs were carried out for a subset of 14 of the original 63 VOCs: the top 8 from the original run, plus 6 additional species to represent different chemical classes and behaviours.
NO2 ratio. Outdoor VOC mixing ratios were left unaltered for all model runs. These additional runs were carried out for a subset of 14 of the original 63 VOCs: the top 8 from the original run, plus 6 additional species to represent different chemical classes and behaviours.
Results and discussion
a. Impacts on indoor air chemistry
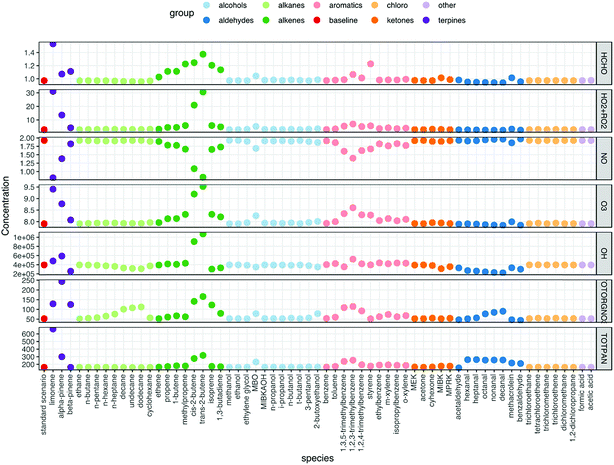
Fig. 2 shows the resulting mixing ratios of formaldehyde, ozone, nitric oxide, the sum of HO2 + RO2 radicals, total organic nitrates, total PANs and the concentration of OH radicals, when 10 ppb of each of the VOCs is independently added to the model run. The run with no VOCs indoors (‘standard scenario’) shown for comparison. For many of the VOCs, the basic pattern is the same. Addition of VOCs to the system generally causes an increase in ozone, radical, formaldehyde, organic nitrate and PAN mixing ratios and a decrease in NO mixing ratios. The biggest deviations from the baseline scenario are seen for the terpenes, alkenes and aromatic species. Note that aldehydes tend to remove OH radicals from the system relative to the baseline scenario.As VOCs are added to the system, they can react with OH and if they have a double bond, also with O3 to form radicals. The impact on the chemistry depends on the relative rates of reaction for each VOC with O3 and OH, as well as the efficiency of OH radical formation through ozonolysis of the VOC (blue arrow in Fig. 3) versus removal of the VOC by OH (red arrow in Fig. 3). It should be noted that ozonolysis of VOCs also produces HO2 and RO2 radicals, as well as OH radicals.5 Given the rapid cycling between these radicals (Fig. 3), even indoors, the formation of HO2 and RO2 radicals in this manner means that OH is produced indirectly as well as directly. The peroxy radicals then react with NO to form NO2. The NO2 can be photolysed to form NO and O atoms, the latter of which react with O2 molecules to form O3. So, in an analogous process to outdoors, adding VOCs can make O3 indoors, albeit at a reduced, but still appreciable rate. The NO is suppressed by additional VOCs, as it reacts with the enhanced number of peroxy radicals present (Fig. 3).
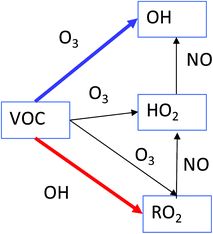 | ||
| Fig. 3 A simplified overview of radical production and loss when carbon–carbon double-bonded VOCs react with OH and O3. | ||
trans-2-Butene has a marked effect on the indoor chemistry when added to the indoor environment, especially in terms of OH formation. Table 1 shows reaction rate and radical formation information for trans-2-butene, along with three other butenes and limonene for comparison. The rate coefficient for reaction with ozone is similar for limonene and trans-2-butene, but the rate coefficient for reaction with OH is approximately twice as fast for limonene as for trans-2-butene. When it comes to radical yields through reaction with ozone, trans-2-butene is much more efficient at producing radicals than limonene. trans-2-Butene is clearly more efficient at producing OH than limonene through ozonolysis and gets destroyed less quickly through reaction with OH. Therefore, adding trans-2-butene to the indoor environment compared to limonene means that radical production is more efficient overall than adding limonene.
| VOC | Rate coefficientsa (cm3 per molecule per s) | Yields following reaction with O3a | Radical production: loss ratio × 106b | ||||
|---|---|---|---|---|---|---|---|
| VOC + OH | VOC + O3 | OH | HO2 | RO2 | OH only | All radicals | |
| a Yields and rate coefficients taken from MCM v3.2 (http://mcm.leeds.ac.uk/MCMv3.2/). b k(VOC + O3) × yield/(k(VOC + OH)). | |||||||
| Methylpropene | 5.12 × 10−11 | 1.14 × 10−17 | 0.82 | 0.41 | 0.41 | 0.2 | 0.4 |
| 1-Butene | 3.14 × 10−11 | 1.02 × 10−17 | 0.36 | 0.28 | 0.28 | 0.1 | 0.3 |
| trans-2-Butene | 6.40 × 10−11 | 1.90 × 10−16 | 0.57 | 0.125 | 0.695 | 1.7 | 4.1 |
| cis-2-Butene | 5.64 × 10−11 | 1.25 × 10−16 | 0.57 | 0.125 | 0.695 | 1.3 | 3.1 |
| Limonene | 1.64 × 10−10 | 2.13 × 10−16 | 0.865 | 0 | 0.865 | 1.1 | 2.2 |
The aromatic species do not react with O3 as the alkenes and terpenes do, but they are very efficient at making radicals (both RO2 and HO2) following fast reaction with OH (e.g. 3.3 × 10−11 cm3 per molecule per s for 1,2,3-trimethylbenzene). The peroxy radicals are recycled to OH as shown in Fig. 3. Addition of aldehydes generally removes radicals from the indoor environment. This is because reaction of aldehydes with OH leads to the formation of acetyl peroxy radicals which then go on to form PAN species, e.g. for acetaldehyde:
| OH + CH3CHO (+O2) → CH3C(O)OO + H2O | (R1) |
| CH3C(O)OO + NO2 ↔ CH3C(O)OONO2 | (R2) |
The radicals (and NO2) become locked up in the PAN species and so the feedback loop shown in Fig. 3 does not occur.
b. SPCP values
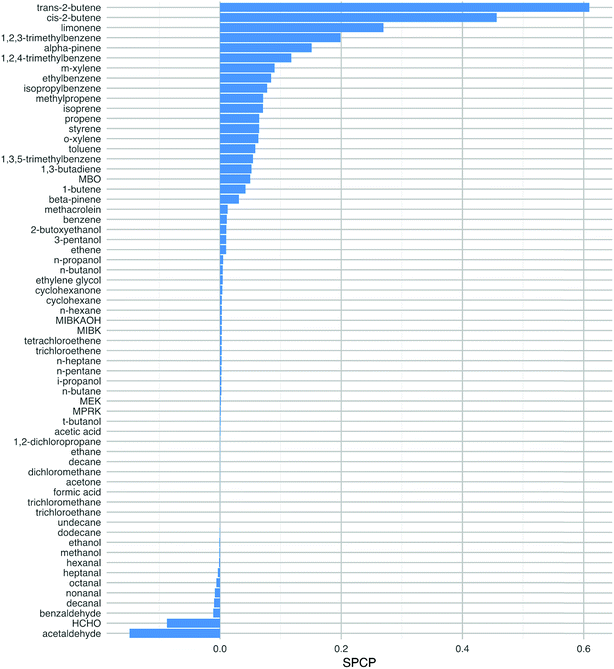
Fig. 4 shows the SPCP values for all 63 VOC species that were tested in the model. The chemical classes that displayed the highest reactivity, as described in the last few paragraphs, have the highest SPCP values, notably the terpenes, alkenes and aromatics. The SPCP values for the aldehyde species are slightly negative, owing to the removal of radicals from the system as described above through reactions like (R1) and (R2).The VOCs we should worry about most are the ones with a high SPCP as well as a high abundance indoors. In order to account for this, the 63 species have also been ranked according to the product of their SPCP and a ‘typical’ (mostly geometric means as described in Table 1, ESI†) mixing ratio based on indoor measurements. For four species MACR, 1-butene, MBO and MIBKAOH, no measured data were available. Therefore, based on their chemical structures, 1-butene was assumed to be the average of the measured mixing ratios of cis- and trans-2-butene, MACR was the average of the C7–C10 aldehydes, MIBKAOH the average of C3–C5 alcohols and MBO, the same as isoprene. Clearly, measurements of these species indoors would be beneficial.
This analysis changes the ranking of the VOCs so that limonene is of most concern by this measure (Fig. 5). Other species that are high in the ranking are α-pinene, toluene, isoprene, m-xylene and 1,2,4-trimethlybenzene. Limonene and α-pinene are used in many indoor products as detailed in the introduction, so we should be concerned about their potential to form harmful products following chemical reactions indoors. The aromatic species in this list are mostly likely to ingress from outdoors, although they can also be found in cleaners.1 Isoprene is a key component of breath emissions and is frequently found indoors.19 The mixing ratios used to estimate the impact of ethylene glycol, MBO, propene and methylpropene are either from older reviews, a low energy test-house (ethylene glycol) or estimated (MBO). Again, more measurements of these species indoors would be beneficial to be able to better assess their impact on health. Clearly, the ranking of the VOCs will depend on the VOC mixing ratios that exist in a particular environment and these will vary from building to building owing to the ubiquity of sources of these species indoors.
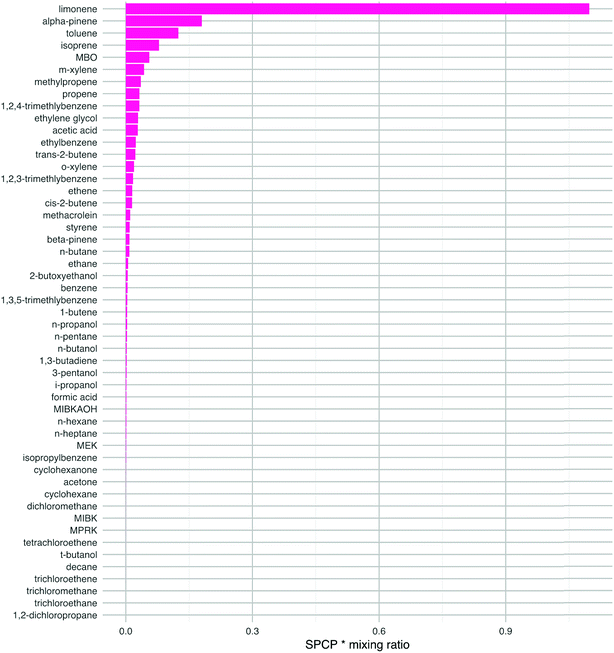 | ||
| Fig. 5 The product of the SPCP and the mixing ratio for each VOC, for species with a SPCP greater than zero. The species are ranked in order from highest to lowest. The mixing ratios are ‘typical’ values taken from the literature as described in the ESI.† | ||
Note that although the butenes have high SPCPs, their indoor mixing ratios are such that they become less important in terms of ranking for potentially harmful impacts (cf.Fig. 4vs.Fig. 5). The butenes are ubiquitous outdoors in urban areas and will ingress indoors through air exchange. Also, 1-butene is formed through photolysis of hexanal, the latter having numerous sources indoors, particularly following surface chemistry.19
c. Sensitivity runs
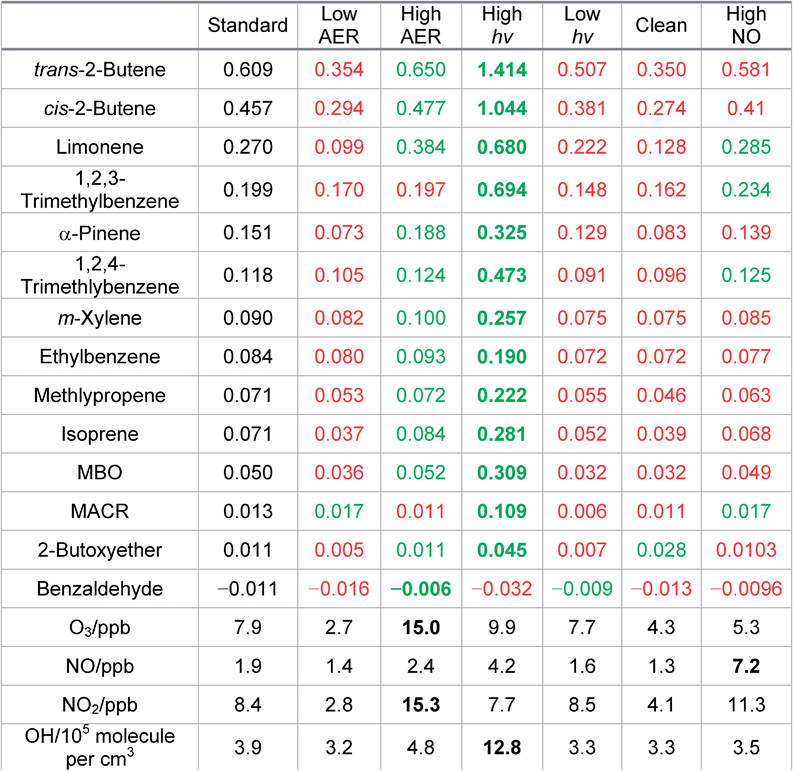
To test the impact of different indoor conditions on the calculated SPCP values, a range of sensitivity runs were carried out (Table 2). For the low AER case, most of the SPCPs are lower than the standard scenario. Indoor O3 under these conditions is reduced from 7.9 ppb to 2.7 ppb over the study period and this reduction affects the ability of VOCs that react with O3 to impact the chemistry summarised in Fig. 3. Consequently, the OH concentration and the mixing ratios of the secondary pollutants in the SPCP calculation are also suppressed. For the high AER case, the O3 mixing ratio increases to 15 ppb and the SPCPs for most of the VOCs increase as secondary chemistry is enhanced.When the transmission of light from outdoors is increased the SPCPs also increase (and generally decrease when outdoor transmission is decreased) showing that photolysis reactions play an important role in chemical processing, even indoors. NO2 mixing ratios are lower than the standard model run (Table 2), showing that under these conditions it is photolysed at a faster rate, which helps to enhance O3 as discussed above.
The SPCPs of the butene species show a pronounced increase under these conditions. When comparing the behaviour of limonene and trans-2-butene, it is interesting to note that they both form about the same amount of ozone (∼0.5 ppb/ppb VOC) relative to when no indoor VOCs are added for these conditions. Limonene produces around 0.13 ppb HCHO/ppb VOC, whilst trans-2-butene produces only 0.08 ppb HCHO/ppb VOC. However, the biggest difference is that the trans-2-butene chemistry produces ∼0.8 ppb CH3CHO/ppb VOC whereas limonene forms only ∼40 ppt CH3CHO/ppb VOC. In addition, whilst adding trans-2-butene leads to an additional OH concentration of 1.5 × 106 molecule per cm3/ppb VOC the addition of limonene leads to a loss of OH relative to the run with no VOCs added of 1.5 × 105 molecule per cm3/ppb VOC. There are clearly very different impacts on the chemistry and consequently the potential to form harmful products. This is because as well as the differing reaction rates discussed earlier, the reaction between trans-2-butene and O3 makes CH3CHO directly. The additional sunlight therefore enhances ozone mixing ratios and consequently CH3CHO formation through this reaction very efficiently.
Halving the mixing ratios of outdoor ozone and nitrogen oxides leads to most of the SPCPs decreasing. Under these conditions, indoor ozone is 4.3 ppb whilst indoor NO and NO2 mixing ratios are 1.3 and 4.1 ppb respectively. The OH concentration is much lower than for the baseline scenario at 1.1 × 105 molecule per cm3. The lower O3 mixing ratios clearly limit the chemical processing indoors and the ability of the VOCs to form secondary pollutants. When outdoor NO mixing ratios are doubled, the SPCPs are typically slightly lower than for the base case, although that for limonene increases slightly. The altered NO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NO2 ratio means that adding limonene leads to the production of more O3 indoors relative to adding it in the standard run, which increases its SPCP under these conditions.
NO2 ratio means that adding limonene leads to the production of more O3 indoors relative to adding it in the standard run, which increases its SPCP under these conditions.
For two of the species, limonene and trans-2-butene, a higher mixing ratio run was carried out where the VOC mixing ratio was set to 100 ppb. For these runs, the SPCP was 0.18 and 0.04 ppb SP/ppb VOC for trans-2-butene and limonene respectively. Under these conditions, the VOC mixing ratios are so high that the OH is removed from the system almost entirely and the chemistry is suppressed. Interestingly, this may imply that higher mixing ratios of VOCs are potentially less harmful than lower ones in terms of the secondary pollutants produced, assuming they do not have a direct effect (such as HCHO).
Finally, a run was carried out where 5 ppb each of limonene (SPCP = 0.270 ppb SP/ppb VOC) and trans-2-butene (SPCP = 0.609 ppb SP/ppb VOC) were added to the system to see if there were any additive effects in the chemistry. The resulting SPCP was 0.383 ppb SP/ppb VOC, in between the two single VOC values. If the SPCP were simply the average value of the two individual VOCs it would be 0.440 ppb SP/ppb VOC. The fact that the resulting SPCP is closer to the individual value of limonene is interesting and shows that there must be some compensation effects with the chemistry. Future work will focus in more detail on mixtures of VOCs and how these might affect ongoing chemistry.
Conclusions
This work proposes a robust methodology to quantify the relative impact of individual VOCs on indoor air chemistry and provide guidance as to when we might expect potentially harmful products to be formed. It is not intended to be an exhaustive measure of the potentially harmful species that can be formed under various indoor conditions. However, it can be used to identify the conditions when we might expect the formation of potentially harmful compounds to be enhanced following the use of individual VOCs, given the SPCP calculation considers a range of different chemical species that are formed through different chemical pathways following emissions. Coupled with measured concentrations for a specific indoor environment, it can provide guidance over prioritisation for removing VOCs most harmful to health. Further, as more detailed information relating to toxicity becomes available, the SPCP can be fine-tuned to apply extra weighting to the more harmful products, or to add new products into the calculation.The calculated SPCPs vary with the indoor conditions, including ventilation rates, the amount of light transmitted indoors through windows and the outdoor concentrations of ozone and nitrogen oxides. In particular, increasing the amount of light that is transmitted indoors from outdoors can enhance the production of potentially harmful species quite appreciably. Two buildings with different glass in their windows, or differing orientation of windows (and hence different transmission rates) could have quite different indoor concentrations even with the same indoor emissions. Further, the chemical composition of a parcel of air indoors may vary quite considerably depending on its location in a room and highlights the need for experimental studies indoors that aim to map concentrations across the entire space, rather than just in one location.
The SPCP metric provides a scientifically robust method for identifying VOCs that form potentially harmful products indoors through their reactive chemistry for the first time. It will permit manufacturers of products used indoors to consider exclusion of the VOCs that have the potential to form harmful products following their use. Future research will focus on further investigation of the modifying or enhancing effects of different mixtures of VOCs on the indoor air quality.
Conflicts of interest
The authors state that there are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Alfred P. Sloan Foundation for support under Grant Number: 2018-10083. They would also like to acknowledge the enthusiasm and support of Dr Paula Olsiewski as Program Director of the Chemistry of Indoor Environments Program.References
- W. W. Nazaroff and C. J. Weschler, Cleaning products and air fresheners: exposure to primary and secondary air pollutants, Atmos. Environ., 2004, 38, 2841–2865 CrossRef CAS.
- B. C. Singer, H. Destaillats, A. T. Hodgson and W. W. Nazaroff, Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids, Indoor Air, 2006, 16, 179–191 CrossRef CAS PubMed.
- C. J. Weschler and N. Carslaw, Indoor Chemistry, Environ. Sci. Technol., 2018, 52, 2419–2428 CrossRef CAS PubMed.
- B. C. McDonald, J. A. de Gouw, J. B. Gilman, S. H. Jathar, A. Akherati, C. D. Cappa, J. L. Jimeneze, J. Lee-Taylor, P. L. Hayes, S. A. McKeen, Y. Y. Cui, S.-W. Kim, D. R. Gentner, G. Isaacman-VanWertz, A. H. Goldstein, R. A. Harley, G. J. Frost, J. M. Roberts, T. B. Ryerson and M. Trainer, Volatile chemical products emerging as largest petrochemical source of urban organic emissions, Science, 2018, 359, 760–764 CrossRef CAS PubMed.
- M. E. Jenkin, S. M. Saunders and M. J. Pilling, The tropospheric degradation of volatile organic compounds: a protocol for mechanism development, Atmos. Environ., 1997, 31, 81–104 CrossRef CAS.
- M. L. Walser, Y. Desyaterik, J. Laskin, A. Laskin and S. A. Nizkorodov, High-resolution mass spectrometric analysis of secondary organic aerosol produced by ozonation of limonene, Phys. Chem. Chem. Phys., 2008, 10, 1009–1022 RSC.
- I. S. H. Buchanan, M. J. Mendell, A. G. Mirer and M. G. Apte, Air filter materials, outdoor ozone and building-related symptoms in the BASE study, Indoor Air, 2008, 18, 144–155 CrossRef CAS PubMed.
- P. Wolkoff, P. A. Clausen, C. K. Wilkins and G. D. Nielsen, Formation of strong airway irritants in terpene/ozone mixtures, Indoor Air, 2000, 10, 82–91 CrossRef CAS PubMed.
- P. Wolkoff, P. A. Clausen, B. Jensen, G. D. Nielsen and C. K. Wilkins, Are We Measuring the Relevant Indoor Pollutants?, Indoor Air, 1997, 7, 92–106 CrossRef CAS.
- C. J. Weschler, Ozone-initiated reaction products indoors may be more harmful than ozone itself, Atmos. Environ., 2004, 38, 5715–5716 CrossRef CAS.
- M. S. Waring and J. R. Wells, Volatile organic compound conversion by ozone, hydroxyl radicals, and nitrate radicals in residential indoor air: magnitudes and impacts of oxidant sources, Atmos. Environ., 2015, 106, 382–391 CrossRef CAS PubMed.
- G. D. Nielsen, S. T. Larsen and P. Wolkoff, Recent trend in risk assessment of formaldehyde exposures from indoor air, Arch. Toxicol., 2013, 87, 73–98 CrossRef CAS PubMed.
- C. M. Wang, B. Barratt, N. Carslaw, A. Doutsi, R. E. Dunmore, M. W. Ward and A. C. Lewis, Unexpectedly high concentrations of monoterpenes in a study of UK homes, Environ. Sci.: Processes Impacts, 2017, 19, 528–537 RSC.
- R. G. Derwent, M. E. Jenkin and S. M. Saunders, Photochemical Ozone Creation Potentials for Organic Compounds in Northwest Europe calculated with a Master Chemical Mechanism, Atmos. Environ., 1996, 30, 181–199 CrossRef CAS.
- W. P. L. Carter, Development of Ozone Reactivity Scales for Volatile Organic Compounds, Air Waste, 1994, 44, 881–889 CrossRef CAS.
- C. J. Weschler, Ozone in indoor environments: concentration and chemistry, Indoor Air, 2000, 10, 269–288 CrossRef CAS PubMed.
- N. Carslaw, A new detailed chemical model for indoor air pollution, Atmos. Environ., 2007, 41, 1164–1179 CrossRef CAS.
- N. Carslaw, T. Mota, M. E. Jenkin, M. H. Barley and G. McFiggans, A significant role for nitrate and peroxide groups on indoor secondary organic aerosol, Environ. Sci. Technol., 2012, 46, 9290–9298 CrossRef CAS PubMed.
- M. Kruza, A. C. Lewis, G. C. Morrison and N. Carslaw, Impact of surface ozone interactions on indoor air chemistry: a modelling study, Indoor Air, 2017, 27, 1001–1011 CrossRef CAS PubMed.
- M. E. Jenkin, S. M. Saunders, V. Wagner and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3, 181–193 CrossRef CAS.
- S. M. Saunders, M. E. Jenkin, R. G. Derwent and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3, 161–180 CrossRef CAS.
- C. Bloss, V. Wagner, M. E. Jenkin, R. Volkamer, W. J. Bloss, J. D. Lee, D. E. Heard, K. Wirtz, M. Martin-Reviejo, G. Rea, J. C. Wenger and M. J. Pilling, Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric oxidation of aromatic hydrocarbons, Atmos. Chem. Phys., 2005, 5, 641–664 CrossRef CAS.
- M. Kruza and N. Carslaw, How do breath and skin emissions impact indoor air chemistry?, Indoor Air, 2019, 29, 369–379 CrossRef CAS PubMed.
- D. M. Murray and D. E. Burmaster, Residential air exchange rates in the United States: empirical and estimated parametric distributions by season and climatic region, Risk Anal., 1995, 15, 459–465 CrossRef.
- J. Zhu, S. L. Wong and S. Cakmak, Nationally Representative Levels of Selected Volatile Organic Compounds in Canadian Residential Indoor Air: Population-Based Survey, Environ. Sci. Technol., 2013, 47, 13276–13283 CrossRef CAS PubMed.
- M. A. Bari, W. B. Kindzierski, A. J. Wheeler, M.-E. Herous and L. A. Wallace, Source apportionment of indoor and outdoor volatile organic compounds at homes in Edmonton, Canada, Build. Environ., 2015, 90, 114–124 CrossRef.
- Y. Li, S. Cakmak and J. Zhu, Profiles and monthly variations of selected volatile organic compounds in indoor air in Canadian homes: Results of Canadian national indoor air survey 2012–2013, Environ. Int., 2019, 126, 134–144 CrossRef CAS PubMed.
- S. Uchiyama, T. Tomizawa, A. Tokoro, M. Aoki, M. Hishiki, T. Yamada, R. Tanaka, H. Sakamoto, T. Yoshida, K. Bekki, Y. Inaba, H. Nakagome and N. Kunugita, Gaseous chemical compounds in indoor and outdoor air of 602 houses throughout Japan in winter and summer, Environ. Res., 2015, 137, 364–372 CrossRef CAS PubMed.
- G. Sarwar, R. Corsi, Y. Kimura, D. Allen and C. Weschler, Hydroxyl radicals in indoor environments, Atmos. Environ., 2002, 36, 3973–3988 CrossRef CAS.
- A. T. Hodgson and H. Levin, Volatile organic compounds in indoor air: a review of concentrations measured in North America since 1990, Lawrence Berkeley National Laboratory, LBNL Report #: LBNL-51715, 2003, Retrieved from https://escholarship.org/uc/item/0hj3n87n, on March 28th, 2019 Search PubMed.
- A. S. D. Eller, L. L. Young, A. M. Trowbridge and R. K. Monson, Differential controls by climate and physiology over the emission rates of biogenic volatile organic compounds from mature trees in a semi-arid pine forest, Oecologia, 2016, 180, 345–358 CrossRef PubMed.
- K.-H. Lui, W.-T. Dai, C.-S. Chan, L. Tian, B.-F. Ning, Y. Zhou, X. Song, B. Wang, J. Li, J.-J. Cao, S.-C. Lee and K.-F. Ho, Cancer risk from gaseous carbonyl compounds in indoor environment generated from household coal combustion in Xuanwei, China, Environ. Sci. Pollut. Res., 2017, 24, 17500–17510 CrossRef CAS PubMed.
- R. Olsen, P. Molander, S. Øvrebø, D. G. Ellingsen, S. Thorud, Y. Thomassen, E. Lundanes, T. Greibrokk, J. Backman, R. Sjöholm and L. Kronberg, Reaction of glyoxal with 2′-deoxyguanosine, 2′-deoxyadenosine, 2′-deoxycytidine, cytidine, thymidine, and calf thymus DNA: identification of DNA adducts, Chem. Res. Toxicol., 2005, 18, 730–739 Search PubMed.
- D. Nuvolone, D. Petril and F. Voller, The effects of ozone on human health, Environ. Sci. Pollut. Res., 2018, 25, 8074–8088 CrossRef CAS PubMed.
- G. Zhang, Y. Mu, L. Zhou, C. Zhang, Y. Zhang, J. Liu, S. Fang and B. Yao, Summertime distributions of peroxyacetyl nitrate (PAN) and peroxypropionyl nitrate (PPN) in Beijing: Understanding the sources and major sink of PAN, Atmos. Environ., 2015, 103, 289–296 CrossRef CAS.
- T. Berkemeier, M. Ammann, T. F. Mentel, U. Pöschl and M. Shiraiwa, Organic nitrate contribution to new particle formation and growth in secondary organic aerosols from α-pinene ozonolysis, Environ. Sci. Technol., 2016, 50, 6334–6342 CrossRef CAS PubMed.
- P. Wolkoff, S. T. Larsen, M. Hammer, V. Kofoed-Sorensen, P. A. Clausen and G. D. Nielsen, Human reference values for acute airway effects of five common ozone-initiated terpene reaction products in indoor air, Toxicol. Lett., 2013, 216, 54–64 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00140a |
| This journal is © The Royal Society of Chemistry 2019 |