Open Access Article
Open Access ArticleDistribution of five SVOCs in a model room: effect of vacuuming and air cleaning measures†
Erik
Uhde
 ,
Deniz
Varol
,
Birte
Mull
and
Tunga
Salthammer
,
Deniz
Varol
,
Birte
Mull
and
Tunga
Salthammer
 *
*
Fraunhofer WKI, Department of Material Analysis and Indoor Chemistry, Bienroder Weg 54E, 38108 Braunschweig, Germany. E-mail: tunga.salthammer@wki.fraunhofer.de; Tel: +49-531-2155-213
First published on 1st May 2019
Abstract
With regard to the application of semi-volatile organic compounds (SVOCs) in products for indoor use, a distinct trend towards substitutions can currently be observed. Among the possible phthalate alternatives, in particular the adipic acid esters have gained in market importance. The chemical–physical and thermodynamic properties of the phthalates and adipates allow the conclusion to be drawn that they are distributed between different compartments (gas phase, particle phase, dust, material surfaces) of the indoor space. There are, however, hardly any data in existence which were collected in a real environment over six months and longer. Diisobutyl adipate (DiBA), di-n-butyl adipate (DnBA), dipentyl phthalate (DPP), butyl benzyl phthalate (BBzP) and di-2-ethylhexyl adipate (DEHA) were selected as model substances. By means of spiked latex paint and spiked house dust, these SVOCs were introduced into two identically equipped test rooms. One room was cleaned regularly, whilst the reference room was not entered for a 133 day experimental period. The concentrations of the five target substances were determined in the air and in material samples (carpet, vacuum-cleaner bags, filters). During the operation of an air purifier, the air concentration of the target substances in a room could be reduced by more than 50%. In the reference room, a correlation between the logarithmic air concentration and the reciprocal room temperature was found. The results show with great clarity the complexity of the conditions in an indoor room. Models can therefore depict the exposure as a statistical average but not, however, describe the individual case.
Environmental significanceIn indoor areas, humans are exposed to widely differing noxious agents via the various uptake pathways. Particular attention is thereby directed at semi-volatile organic compounds (SVOCs) which, due to their chemical–physical properties, are distributed between diverse compartments. Model calculations which describe the distribution of a substance between gas phase, airborne particle phase and settled dust phase often presuppose equilibrium conditions which do not occur in a real indoor area. Within the course of this work, the concentration profiles of five SVOCs in the air and on surfaces in the rooms of a test house were recorded over a period of six months. The results provide an insight into the dynamics of these SVOCs under the influence of the outdoor climate and taking into consideration cleaning processes, both with and without human activity. |
1 Introduction
Semi-volatile organic compounds, the so-called SVOCs, have the characteristic of distributing themselves in differing ways within the diverse compartments of the indoor area (gas phase, airborne particle phase, sedimented house dust). In 2003 systematic investigations into the occurrence of endocrine-disrupting compounds in indoor air and house dust were published by Rudel et al.1 Indoor measurements by Fromme et al.2 on phthalates revealed high concentrations in the air and low concentrations in dust for substances with high vapor pressure (Ps) and low octanol/air distribution coefficients (KOA). For substances with low vapor pressure and high KOA, the situation was reversed. Following this, further investigations into diverse substance groups such as perfluorinated sulfonamides,3 organophosphate esters,4 pyrethroids,5 polybrominated diphenyl ethers6 and many others7 were published within a short period of time. A paper by Weschler and Nazaroff8 summarizes the significant literature up to 2008 and offers theoretical approaches for assessing the distribution of SVOCs between gas phase and particles and between gas phase and sedimented dust. For various phthalates, Weschler et al.9 found, based on the theories of Pankow10 and Finizio et al.,11 correlations in the data of Fromme et al.2 between the concentrations in airborne particles and in sedimented house dust. A detailed treatise on the distribution of SVOCs between gas phase and sedimented dust was presented by Weschler and Nazaroff12 in 2010.The dynamics of SVOCs between gas phase, particle phase, dust phase, and other surfaces remain the subject of theoretical and practical studies.13–19 The majority of the investigations have been carried out in test chambers19–22 or emission cells.23 In most cases, it is only possible to approximate the actualities of the situation in indoor areas through the use of models. There are diverse reasons for this. As a rule, the models presuppose equilibrium conditions which, however, are scarcely adhered to in reality. In addition to this, the establishment of equilibrium between gas phase and dust phase for SVOCs such as di-2-ethylhexyl phthalate (DEHP) is a process lasting several weeks, which was theoretically predicted by Weschler and Nazaroff8 and experimentally demonstrated by Schripp et al.24 The parameters necessary for modelling in accordance with Pankow10 or Finizio et al.,11 such as vapor pressure and KOA value, are often only very inaccurately known; this leads to corresponding uncertainties in the results.25,26 An alternative approach by Goss and Schwarzenbach27 uses experimentally determined hexadecane/air distribution coefficients; calculated molecular values are, however, also utilized in this model.28
Nevertheless, various studies exist in which investigations were carried out on the distribution of SVOCs in residential buildings or test houses. On the basis of a literature study, Wei et al.29 determined the distribution of gas/particle and gas/dust coefficients for a total of 72 organic compounds. Kolarik et al.30 investigated phthalates in the house dust of Bulgarian homes in dependence of cleaning habits. Venier et al.31 compared the distribution of 20 brominated flame retardants between gas phase, dust phase and glass surfaces using measurements recorded in the USA, Canada and the Czech Republic. Sukiene et al.32,33 marked various SVOCs with deuterium and were thereby able to follow the migration of these compounds within a test house and, in particular, the transfer into house dust. Bi et al.34 investigated the temperature-dependent distribution of phthalates in a test house between 21 °C and 30 °C and found distinct effects. Wei et al.35 described the temperature-dependence of distribution coefficients of SVOCs in accordance with the known equations of Van't Hoff and Clausius–Clapeyron.
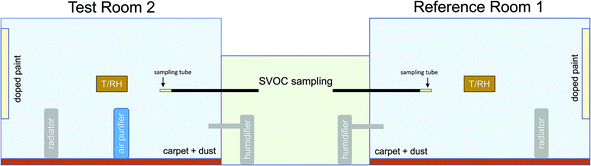
What has barely been investigated so far are questions concerning the establishment of equilibrium of SVOCs in an indoor area and how the equilibrium reacts to disturbances such as cleaning processes. For this purpose, SVOC-spiked latex paint and SVOC-spiked dust were introduced as emission sources into two identically equipped rooms of a test house. The five selected target SVOCs represent a broad range of well-known physical properties and are expected to be mainly found in the gas phase.28 Two of the SVOCs were only present in the paint and three were only present in the dust. This experimental design was chosen to study the transfer of the SVOCs between different compartments. In the reference Room 1, no further activities were carried out for a period of 184 days. In the test Room 2, an air purifier was operated in accordance with a defined protocol and parts of the floor were cleaned using a vacuum cleaner. At regular intervals, air and material samples were taken and analyzed. The results enable the description of the dynamics of the air concentration of SVOCs in indoor areas under conditions of use and equilibrium.
2 Material and methods
2.1 Chemical substances
All compounds are commercially available. The purity was checked by gas chromatography, the percentage purity refers to the area under the gas chromatographic signal. Dipentyl phthalate (DPP, CAS no. 131-18-0): ABCR GmbH (99%); butylbenzyl phthalate (BBzP, CAS no. 85-68-7): Follmann & Co. KG, 99.9%; di-n-butyl adipate (DnBA, CAS no. 105-99-7): Sigma Aldrich, 99.9%; di-iso-butyl adipate (DiBA, CAS no. 141-04-8): ABCR GmbH, 99.9%; di-2-ethylhexyl adipate (DEHA, CAS no. 103-23-1): Sigma-Aldrich, 99.0%.2.2 Design of the test house experiments
The test house is divided into three rooms (see Fig. 1). Rooms 1 and 2 (7.1 m × 4.7 m × 2.7 m = 90.1 m3) were used for the experiments. The room in the middle was used for the sampling equipment. Rooms 1 and 2 have three windows each (98.5 cm × 137.5 cm) with lacquered wooden frame and one door (honeycomb plate with decorative film) (109 cm × 204 cm). The walls are made of oriented strand board (OSB) and covered with a low-emitting water-based paint. The floor is made of particleboard and covered with textile carpet (synthetic fiber). No adhesive was applied.The reference Room 1 and the test Room 2 were separately air conditioned by use of radiators (Renkforce Luma 2000 Eco) and humidifiers (Honeywell Ultrastar) (target values: T = 23 °C and RH = 50%). The radiators were set up in Room 1 and 2, the humidifiers were in the middle room. An air cleaning device (Philips 3000 Series AC3256/10 air purifier, Clean Air Delivery Rate (CADR) = 393 m3 h−1) was installed in the test Room 2. At certain times vacuum cleaning (Philips FC8726/09) was performed in Room 2. The air cleaner was equipped with a multilayer filter, consisting of prefilter, pollen filter, microfiber, active charcoal filter and HEPA filter. The vacuum cleaner was equipped with a HEPA 13 filter and an active charcoal filter. The reference Room 1 was not entered until day 133 of the experiment.
Latex paint, formulated with 10% BBzP and 10% DEHA (by weight), was applied on aluminum plates (2070 g per 20 m2) and used to deliver these SVOCs into the room air at a constant emission rate (see Schripp et al.36 for further details).
Sieved house dust from vacuum cleaner bags (<63 μm fraction, density ρ = 0.75 g cm−3, fraction of organic carbon fOC = 0.44, fraction of elemental carbon fEC = 0.03) was Soxhlet extracted using acetone/n-hexane to reduce the background concentration of VOCs and SVOCs and the solvents were blown-off in a vacuum. The dust was mixed with 10% DiBA, 10% DnBA, 10% DPP (by weight) and praseodymium (Pr) as internal marker in acetone solution. Then the acetone was blown-off in a vacuum. For dispersing the dried dust, approximately 2 g were placed in a polished stainless steel tube (1000 mm × 10 mm) and blown into the respective room from the middle room using compressed air. This procedure was repeated at different angles and alignments until the planned amount of dust was introduced (14.95 g in Room 1 and 15.05 g in Room 2). Praseodymium (141Pr) is a stable and comparatively rare lanthanoid (5 ppm in earth's crust), which makes the metal suitable as a marker compound.
The full experimental design is shown in Fig. 1 and the experimental protocol is provided in Table 1. The experiments started on Nov 29, 2017 (introducing paint and dust) and ended on Jun 1, 2018 (after 184 days). After 453 days carpet samples were taken in Rooms 1 and 2. These were analyzed for the five target compounds and praseodymium. Between day 481 and day 486 the reference Room 1 was vacuumed three times and the particle number concentration was measured. Both rooms were left unchanged between day 184 and day 485 (humidifier and radiator on, air cleaner off).
| Day | Room 1 (reference room) | Room 2 (test room) |
|---|---|---|
| 001 | Start of experiment (Nov 29, 2017) | Start of experiment (Nov 29, 2017) |
| 084 | Air purifier operated for 5 h | |
| 096 | Filter replaced | |
| Air purifier operated for 5 h | ||
| 097 | Air purifier operated for 5 h | |
| 098 | Air purifier operated for 5 h | |
| 119 | Filter replaced | |
| Air purifier operated for 5 h | ||
| 126–128 | Air purifier operated for 50 h | |
| 132 | Filter replaced | |
| Air purifier operated for 5 h | ||
| 133 | Carpet sample (uncleaned) | Air purifier operated for 5 h |
| Carpet sample (uncleaned) | ||
| 134 | Carpet sample (uncleaned) | Air purifier operated for 5 h |
| Filter replaced | ||
| Vacuuming (10 min) | ||
| Sampling bag, HEPA 13 filter, charcoal filter replaced | ||
| Carpet sample (cleaned + uncleaned) | ||
| 167 | Air purifier operated for 5 h | |
| 168 | Carpet sample (uncleaned) | Air purifier operated for 5 h |
| Carpet sample (uncleaned) | ||
| 169 | Carpet sample (uncleaned) | Air purifier operated for 5 h |
| Filter replaced | ||
| Vacuuming (10 min) | ||
| Sampling bag, HEPA 13 filter, charcoal filter replaced | ||
| Carpet sample (cleaned + uncleaned) | ||
| 176 | Carpet sample (uncleaned) | Carpet sample (cleaned + uncleaned) |
| Vacuuming (10 min) | ||
| Sampling bag, HEPA 13 filter, charcoal filter replaced | ||
| Carpet sample (freshly cleaned) | ||
| 184 | End of experiment (Jun 1, 2018) | End of experiment (Jun 1, 2018) |
| 453 | Carpet sample (uncleaned) | Carpet sample (cleaned) |
| 481–486 | Vacuuming and particles measured |
2.3 Sampling and analysis
All used materials (unspiked dust, paint, carpet, filters, vacuum cleaner bags, etc.) were measured for the five target compounds before the experiment started. In all cases the blank concentrations were below the respective LOQ. The concentrations of DiBA, DnBA, DPP, DEHA, and BBzP in the room air before spiking were also below LOQ.
3 Results and discussion
3.1 Air exchange and climatic parameters
The air exchange rates with all windows and doors closed were determined in Room 1 and Room 2 before and after the investigation period. In all cases the N2O time vs. concentration curves followed a single exponential law. Depending on the outdoor conditions, the AER ranged between 0.2 h−1 and 0.3 h−1 in both rooms. However, no information is available about air exchange rates during the experimental phase. The profiles for temperature and relative humidity are shown as average daily values in Fig. 2. Although both rooms were air-conditioned using controllable radiators and humidifiers, deviations from the target values (23 °C, 50% RH) were observed. Over the total period of 184 days, the temperature in the reference Room 1 varied between 16.9 °C and 28.7 °C, with an average value of 22.9 °C, and in the test Room 2 between 18.4 °C and 28.6 °C, with an average value of 23.3 °C. The extreme temperature minimum after 93 days was caused by a cold snap with outdoor temperatures of −12 °C. The rising temperatures after 170 days were due to a warm and fair-weather period with maximum values >30 °C and strong global radiation. The average relative humidity was 46% (Room 1) and 44% (Room 2). The short-term deviations from the target value result from the operation of the humidifier. The dosage of the quantity of water discharged into the room depends on the fill level of the reservoir.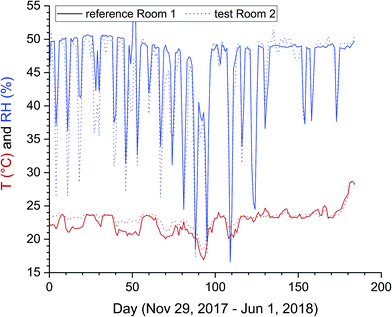 | ||
| Fig. 2 Temperature and humidity profiles in the reference Room 1 and the test Room 2 between Nov 29, 2017 and Jun 1, 2018 (184 days). The data represent daily averages from recorded 20 min intervals. Temperature, relative humidity and global irradiation in the outdoor air are provided in the ESI.† | ||
3.2 SVOCs in the test house air
The chemical properties of the target substances are summarized in Table 2. DiBA and DnBA have similar properties (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA ≈ 8, log
KOA ≈ 8, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps (Pa) ≈ −1.5). For the other three compounds, KOA and Ps are higher or lower by approx. three orders of magnitude, respectively. All compounds are, however, SVOCs pursuant to the definition in accordance with ISO 16000-6.38 The ratio of particle and gas phase concentration of the target compound is defined as distribution coefficient Kip. For DiBA, DnBA and DPP, the calculated distribution coefficients between gas phase and airborne particles are Kip < 10−3 m3 μg−1. For BBzP Kip is <10−2 m3 μg−1 and for DEHA Kip is <10−1 m3 μg−1.28 This means that solely for DEHA it can be expected that a significant proportion (>20%) will accumulate in airborne particles.28 Measurement of particles was not undertaken for practical reasons during the experimental phase (days 1–184). As the sample was taken from the middle room, sampling lines with a length in excess of 1 m were used, which would have led to major experimental errors due to the assumed organic nature of the particles.39 Furthermore, the air volumes and volume flows necessary for the determination of PM2.5 and PM10 would have seriously disrupted the equilibrium in the rooms. However, particle number concentrations were measured at days 481–486.
Ps (Pa) ≈ −1.5). For the other three compounds, KOA and Ps are higher or lower by approx. three orders of magnitude, respectively. All compounds are, however, SVOCs pursuant to the definition in accordance with ISO 16000-6.38 The ratio of particle and gas phase concentration of the target compound is defined as distribution coefficient Kip. For DiBA, DnBA and DPP, the calculated distribution coefficients between gas phase and airborne particles are Kip < 10−3 m3 μg−1. For BBzP Kip is <10−2 m3 μg−1 and for DEHA Kip is <10−1 m3 μg−1.28 This means that solely for DEHA it can be expected that a significant proportion (>20%) will accumulate in airborne particles.28 Measurement of particles was not undertaken for practical reasons during the experimental phase (days 1–184). As the sample was taken from the middle room, sampling lines with a length in excess of 1 m were used, which would have led to major experimental errors due to the assumed organic nature of the particles.39 Furthermore, the air volumes and volume flows necessary for the determination of PM2.5 and PM10 would have seriously disrupted the equilibrium in the rooms. However, particle number concentrations were measured at days 481–486.
| Compound | MW (g mol−1) | bpa (°C) | Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Psa (Pa) Psa (Pa) |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOAa KOAa |
K ip (m3 μg−1) |
|---|---|---|---|---|---|
| a Data from SPARC On-line Calculator (http://archemcalc.com). b From Salthammer and Goss.28 The fraction in the particle phase can be determined from Φ = Kip × [TSP]/(1 + Kip × [TSP]), where [TSP] is the concentration of total suspended particulate matter. | |||||
| DiBA | 258.4 | 327.2 | −1.35 | 7.93 | 1.1 × 10−5 |
| DnBA | 258.4 | 345.0 | −1.53 | 8.14 | 2.4 × 10−5 |
| DEHA | 370.6 | 393.5 | −5.07 | 11.29 | 2.8 × 10−2 |
| BBzP | 312.4 | 439.0 | −5.43 | 11.59 | 1.3 × 10−3 |
| DPP | 306.4 | 412.0 | −4.42 | 10.72 | 4.4 × 10−4 |
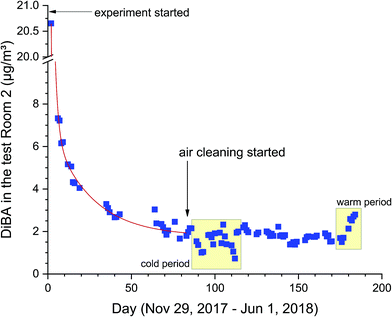
A typical concentration curve in the test Room 2 is shown in Fig. 3 for the substance DiBA. Following application of the spiked latex paint and the spiked house dust, the initial concentration (day 2) is 20.7 μg m−3. Up to day 83 the curve can be fitted to a double exponential function C(t) = A1 × exp(−k1 × t) + A2 × exp(−k2 × t) + B (OriginPro 2018G, non-linear least squares algorithm). The fast decay process at the beginning of the experiment is expressed by k1 = 0.02 h−1. However, k1 is significantly smaller than the air exchange, which is due to the constant release of DiBA from the dust. The much smaller kinetic constant k2 = 0.001 h−1 shows that the system is approaching a state of equilibrium, which is attained at day 80. The yellow boxes in Fig. 3 mark the episodes with cold and particularly warm days (see Section 3.1), which are also conspicuous in the concentration profile of DiBA.
Overall, the highest concentrations in the air were measured for DiBA, followed by its isomer DnBA, which has similar properties. The concentrations of the heavier compounds DPP, BBzP and DEHA were easily measurable but lay consistently below 1 μg m−3. The concentration vs. time behavior was similar but not identical in Rooms 1 and 2. Surprisingly, the concentrations in the test Room 2 actually scattered slightly less than in the reference Room 1 (see ESI†). In total, approx. 1.5 g DiBA and 1.5 g DnBA were introduced into each room via the house dust. The quantities of DPP, BBzP and DEHA applied by means of the latex paint were significantly higher at approx. 200 g per substance and room. In comparison to a further experiment in which di-n-butyl phthalate (DnBP, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA = 9.83, log
KOA = 9.83, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps = −3.46 (Pa), calculated using SPARC) was applied as a constituent of latex paint in small and large chambers,34 the concentrations measured here were considerably lower, despite lower air exchange rates. For DPP, which has similar properties to DnBP, concentrations of significantly less than 1 μg m−3 were attained within seven days following the start of the experiment.
Ps = −3.46 (Pa), calculated using SPARC) was applied as a constituent of latex paint in small and large chambers,34 the concentrations measured here were considerably lower, despite lower air exchange rates. For DPP, which has similar properties to DnBP, concentrations of significantly less than 1 μg m−3 were attained within seven days following the start of the experiment.
A similar experimental design had already been realized at an earlier time by Sukiene et al.32,33 Amongst others, DiBA, DnBA and DEHA were applied as deuterium-marked substances via spiked products in test rooms and the concentrations in air and dust were monitored over a period of 12 weeks. The ratio of the air concentrations was analogous to the results described here; the quantities of introduced SVOCs were, however, significantly lower. The absolute concentrations of DiBA, DnBA and DEHA were therefore also smaller by approx. one order of magnitude.
The measured TVOCPAS concentrations were of little significance for the experimental design chosen here. Without cleaning measures, the continuously measured concentrations lay within a range of 300 ppb to 500 ppb (relative to propane). Through the operation of the air purifier, the TVOCPAS concentration was reduced by approx. 25–30% (see ESI†). It must, however, be borne in mind that the photo-acoustic method registers in particular small molecules, such as methane, which the device removes from the air only to a small extent. In this respect, the TVOCPAS value is not a criterion for the efficiency of the air-purifying device.
3.3 The effect of cleaning measures
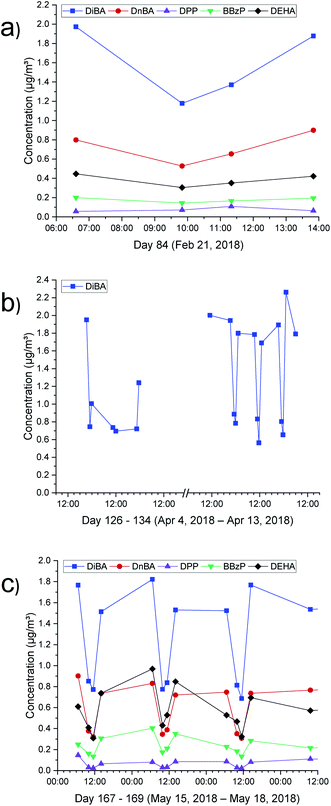
One of the main aspects of this project was the investigation of the influence of cleaning measures on the indoor air concentration of the target substances. The results were comparable for all test conditions and are discussed here using three examples. The air purifier was operated for the first time on day 84 for 5 hours (mode: automatic). The device is conceived for a room area of up to 95 m2. The Clean Air Delivery Rate (CADR) specifies the quantity of air per time unit which is purified of particles of a certain size under defined test conditions.40 The stated value of CADR = 393 m3 h−1 for the operated device pertains to cigarette smoke and therefore provides no information concerning the effectiveness as regards the target substances relevant here.Fig. 4a shows the respective air concentration before, during and following operation of the air purifier. A purifying effect can be clearly observed for DiBA, DnBA and DEHA, but is not evident for DPP and BBzP due to the small concentrations. The effect is, however, only visible during the purifying phase. After switching off the air purifier, the concentration rises back to the original value within 2–3 hours. Fig. 4b shows the results for DiBA from the test protocol between day 126 and day 134. Between day 126 and day 128, the air purifier was operated continuously for 50 hours. On days 132, 133 and 134, the device was switched on for 5 hours per day. Irrespective of the operating time, the concentration of DiBA is reduced from approx. 2 μg m−3 to approx. 0.6 μg m−3, which corresponds to a reduction of 70%. However, the effect that the original concentration is swiftly reattained after switching off the device can also be observed here. Fig. 4c once again shows the effect for all target compounds when the air purifier is operated for 5 hours per day on three consecutive days. Here, in contrast to Fig. 4a, the reduction effect can be clearly seen in all cases.
On days 134, 169 and 176, the floor of Room 2 was partially cleaned for a period of 10 minutes using the vacuum cleaner. For this, the room was divided into three equal segments. During each floor cleaning process, a different segment was vacuumed. None of these vacuuming processes has either a short-term or long-term influence on the indoor air concentration of the five target substances. It is, however, known that vacuuming influences the particle concentration in a room.41
After the end of the experimental phase (day 1–184) both rooms were left unchanged for a certain time (humidifier and radiator on, air cleaner off) and the particle concentrations were continuously measured in Room 1 over five days. During this period the room was vacuumed three times (10 min per event). Without vacuuming activities, the number concentration of ultrafine particles (UFP) (5.6–560 nm) was between 5000 # per cm3 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 # per cm3. Vacuuming significantly increased the UFP level to 15
000 # per cm3. Vacuuming significantly increased the UFP level to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20
000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 # per cm3. In case of larger particles (0.3–10 μm) vacuuming had no significant effect on the particle concentration and size distribution. The results are presented in the ESI.† The concentrations of the five target compounds in the room air were not significantly different from the concentrations during the experimental phase (day 80–184).
000 # per cm3. In case of larger particles (0.3–10 μm) vacuuming had no significant effect on the particle concentration and size distribution. The results are presented in the ESI.† The concentrations of the five target compounds in the room air were not significantly different from the concentrations during the experimental phase (day 80–184).
3.4 SVOCs in materials and house dust
The distribution of an organic compound between gas phase and sedimented dust under equilibrium conditions can be estimated in accordance with Weschler and Nazaroff12 pursuant to eqn (1).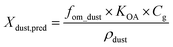 | (1) |
Although the gas/particle distribution was not measured it can be assumed, on the basis of the published gas/particle distribution coefficients Kip (see Table 2), that more than 90% of DiBA, DnBA, BBzP and DPP are present in the gas phase.28 For DEHA, with a Kip value of 2.8 × 10−2 m3 μg−1, the fraction in the particle phase Φ may vary within approximately 20% and 60%, depending on the particle concentration.28 Taking into consideration fOC = 0.43 for the unspiked dust, it can be assumed that the organic proportion fom_dust of the spiked dust lies in the range of 0.8–1. With the conservative assumption of a gas phase concentration of Cg = 0.1 μg m−3 for BBzP, eqn (1) results in Xdust,pred(BBzP) = 4 × 104 μg g−1. Although the calculation of Xdust,pred is subject to errors within an order of magnitude,26 it becomes clear that the value is considerably higher than the concentrations measured in the collected house dust (see Table 3). This shows that even after several months, the system is still far removed from equilibrium conditions.
| Material | Day | DiBA, μg g−1 | DnBA, μg g−1 | DEHA, μg g−1 | BBzP, μg g−1 | DPP, μg g−1 | Pr, μg m−2 |
|---|---|---|---|---|---|---|---|
| Room 2 (test room) (air purifier and vacuum cleaner operated) | |||||||
| Filter air purifier | 096 | 3 | 4 | <LOQ | <LOQ | 2 | |
| Filter air purifier | 119 | 5 | 5 | 8 | 3 | 2 | |
| Filter air purifier | 132 | 21 | 11 | 13 | 2 | 3 | |
| Filter air purifier | 134 | ||||||
| Filter air purifier | 169 | 7 | 3 | 12 | 6 | ||
| Sampling bag (dust) | 134 | 288 | 255 | 23 | 24 | 6533 | |
| Sampling bag (dust) | 169 | ||||||
| Sampling bag (dust) | 176 | 646 | 798 | 49 | 47 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 368 368 |
|
| Carpet sample (uncleaned) | 133 | ||||||
| Carpet sample (uncleaned) | 134 | 3 | 2 | 11 | 5 | 7 | |
| Carpet sample (cleaned) | 134 | 3 | 2 | 11 | 6 | 6 | 2.3 |
| Carpet sample (uncleaned) | 168 | 29 | 46 | 8 | 7 | 86 | 27.6 |
| Carpet sample (uncleaned) | 169 | 4 | 2 | 8 | 7 | 6 | 6.0 |
| Carpet sample (cleaned) | 169 | 4 | 2 | 8 | 7 | 6 | 6.1 |
| Carpet sample (uncleaned) | 176 | 4 | 4 | 16 | 6 | 13 | |
| Carpet sample (cleaned) | 176 | 3 | <LOQ | 7 | 6 | 5 | |
| Carpet sample (freshly cleaned) | 176 | 3 | <LOQ | 9 | 6 | 5 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Room 1 (reference room) | |||||||
| Carpet sample (uncleaned) | 133 | 5 | 6 | 3 | 2 | 6 | |
| Carpet sample (uncleaned) | 134 | 3 | 2 | 7 | 5 | 4 | |
| Carpet sample (uncleaned) | 168 | 4 | 3 | 22 | 7 | 7 | 3.0 |
| Carpet sample (uncleaned) | 169 | 4 | 15 | 12 | 6 | 44 | 8.4 |
| Carpet sample (uncleaned) | 176 | 6 | 5 | 7 | 6 | 7 | |
Generally, only weak correlations can be observed for the ratio of air to dust concentration.9,24 The reasons for this lie in the complexity of the indoor conditions and the uncertainty of the analytical procedures. In the experiments performed here, the dust was finely dispersed in the respective room. This, however, in no way guarantees that the distribution across the entire surface of the respective room is homogeneous. Furthermore, as a result of the spiking, the dust exhibits a very high organic content which limits its dispersibility. Areas with higher and lower dust content are therefore to be expected. This assumption is supported by the greatly varying praseodymium values. Moreover, the dust particles might interact with the fibers of the textile floor covering. It can therefore be assumed that the SVOCs migrate from the dust into the floor covering. Carpet fibers are furthermore a strong sink for SVOCs.42
A further point concerns the sampling of the floor covering. The extracted pieces had an area of 7 cm × 7 cm. In relation to the total area of the respective room of 33.4 m2, this is an area proportion of 1.5 × 10−4. It is therefore unlikely that the sampled area is representative of the carpet. This is also shown in Table 3. On the whole, the SVOC concentrations of the flooring samples in Rooms 1 and 2 are extremely inconsistent, as are the concentrations of the marker praseodymium (see Table 3). The very highest values for the SVOCs and praseodymium were measured in the sample taken on day 168. Melymuk et al.43 also monitored SVOCs in different matrices in a residential environment and found that individual dust samples were not necessarily representative of the average room conditions.
Considerably more significant values are obtained through analysis of the contents of the vacuum cleaner bags. In the test Room 2, 5–6 g of dust were collected when 11 m2 were cleaned. The concentrations lay between 250 μg g−1 and 800 μg g−1 (DiBA and DnBA), 20 μg g−1 and 50 μg g−1 (DEHA and BBzP) as well as 6000 μg g−1 and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg g−1 (DPP). These results are plausible. DiBA and DnBA were constituents of the introduced dust; they are, however, compounds with comparatively high vapor pressures and simultaneously low KOA values. DPP was also added to the dust; the vapor pressure for this substance is, however, three orders of magnitude lower than that of DiBA and DnBA, whilst the KOA value is more than three orders of magnitude higher. DEHA and BBzP were added to the latex paint. Significantly lower concentrations are therefore to be expected for these two compounds, as the enrichment takes place through diffusion via the air pathway.
000 μg g−1 (DPP). These results are plausible. DiBA and DnBA were constituents of the introduced dust; they are, however, compounds with comparatively high vapor pressures and simultaneously low KOA values. DPP was also added to the dust; the vapor pressure for this substance is, however, three orders of magnitude lower than that of DiBA and DnBA, whilst the KOA value is more than three orders of magnitude higher. DEHA and BBzP were added to the latex paint. Significantly lower concentrations are therefore to be expected for these two compounds, as the enrichment takes place through diffusion via the air pathway.
The experimental activities ended after 184 days but both rooms were left unchanged for another 300 days. Carpet samples (50 cm × 50 cm) were taken on day 453 in both rooms and stripes of 2 cm × 7 cm were analyzed for the five target compounds and praseodymium. The results are presented in the ESI.† Again, high SVOC concentrations roughly correlated with high concentrations of praseodymium. As discussed before, the SVOCs had distributed in the entire room but in case of DPP peak concentrations (43.7 μg g−1, 206.5 mg m−2) were only found in Room 1, where no cleaning had occurred. This means that vacuuming is an efficient measure to remove compounds of very low volatility, for which dust provides a reservoir.
3.5 Temperature dependence of the room air concentrations
The mechanism for the release of a substance from a product varies according to the substance and product properties and is correspondingly complex in the case of SVOCs.44 In general, the temperature dependence of release and distribution processes follows a Van't Hoff approach,45 which means that the logarithm of the considered value (here: concentration) is, in accordance with eqn (2), proportional to the reciprocal of the temperature.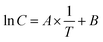 | (2) |
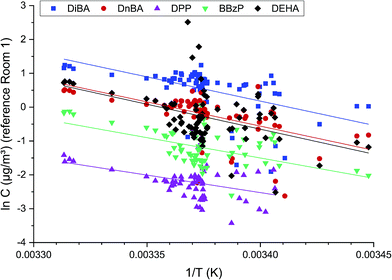
For the five target substances and the reference Room 1, the logarithms of the measured air concentrations against 1/T are shown in Fig. 5. In all cases, a distinct temperature dependence can be observed, i.e. the concentrations increase with rising temperature. The data can be adjusted in a good approximation through a linear function (see eqn (2)), but in some cases the deviations of individual data from the fit curve were in the range of an order of magnitude. The reasons for this are due to the small temperature window of ΔT = 11.8 K and the analytical error, which increases with lower concentrations.46 A calculation of the enthalpy, which theoretically results from the linear gradient, was therefore omitted. The linear gradients show that with a temperature increase of 10 K, the air concentration increases by a factor of 3–4. This value lies within the range of the temperature dependence of the thermodynamic variables for the substances examined here.28 For a substance with the evaporation enthalpy ΔHvap = 100 kJ mol−1, the vapor pressure increases by a factor of 4 when the temperature increases from 291 K to 301 K. This result leads to the conclusion that in this experiment, the distribution processes are controlled by evaporation.
4 Conclusions
The results of the investigations performed here demonstrate the complex dynamic processes within an indoor area, which can only be partially explained by models and predictions.47 The quantities of SVOCs introduced into the respective rooms of the test house via latex paint and house dust were high but not unrealistic, as the proportion of plasticizers in a plastic can certainly amount to 30%.48 The indoor air concentrations were lower than or comparable to air concentrations in chambers and cells,25,36,49 higher than in similar experiments carried out by Sukiene et al.,32,33 and within the range of concentrations in real indoor environments.2,50 Therefore, the measured concentrations can be considered as realistic.Fundamental conclusions could be drawn from the cleaning activities. The operation of an air-purifying device was efficient regarding the five target compounds and reduced their concentrations by approx. 50%. After switching off the device, however, the initial value was reattained within a short time. This shows that the processes leading to the establishment of equilibrium are controlled by evaporation. Vacuum-cleaning of the floor increased the UFP concentration for a short period of time (see ESI†) but did not lead to a reduction of the indoor air concentrations of the target compounds. It must therefore be concluded that the SVOCs had distributed themselves amongst dust, carpet fibers and other surfaces. This also becomes clear from the results of the vacuum cleaner-bag analyses. The DPP added to the dust was still detectable in high concentrations after 176 days, whilst the considerably more volatile compounds DiBA and DnBA were depleted. In this respect, the totality of the room formed the reservoir for the SVOCs after a period of time. However, analysis of the uncleaned (reference Room 1) and cleaned (test Room 2) carpet after 453 days showed that areas of high dust amounts are removed by surface cleaning activities (see Tables SI-1–SI-4†). The losses through air exchange were slight, compared to the quantities introduced. Taking DiBA as an example, the mass removal of this compound through air exchange can be calculated from integration of the data in Fig. 3. When assuming AER = 0.25 h−1 (the mean value in the measured range between 0.2 h−1 and 0.3 h−1) for the entire experiment over 184 days, the total loss of DiBA is less than 0.5 g.
In summary, the results of the study design provide relevant information on the dynamics of SVOCs in indoor areas with and without disturbance of the equilibrium conditions. Depending on the furnishings and features of the respective room and the activities carried out therein, the substances are subject to diverse distribution processes. As a result, as already demonstrated by Weschler et al.,9 the attainment of reliable exposure estimates on the basis of models is, for the individual case, difficult to impossible. Reductions of air pollutant concentrations are only possible through increased ventilation or air-purifying measures.
Conflicts of interest
There are no conflicts of interests to declare.Acknowledgements
This work was financially supported by Philips Consumer Lifestyle, Drachten. The authors are grateful to Ina Kirsch, Nicole Schulz and Alexander Omelan for their experimental support. We also wish to thank Karen McDonald (Kaledonia Kommunikation) for her editorial work. Graphical abstract design: Christian Fauck.References
- R. A. Rudel, D. E. Camann, J. D. Spengler, L. R. Korn and J. G. Brody, Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust, Environ. Sci. Technol., 2003, 37, 4543–4553 CrossRef CAS PubMed.
- H. Fromme, T. Lahrz, M. Piloty, H. Gebhart, A. Oddoy and H. Ruden, Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany), Indoor Air, 2004, 14, 188–195 CrossRef CAS PubMed.
- M. Shoeib, T. Harner, B. H. Wilford, K. C. Jones and J. Zhu, Perfluorinated Sulfonamides in Indoor and Outdoor Air and Indoor Dust: Occurrence, Partitioning, and Human Exposure, Environ. Sci. Technol., 2005, 39, 6599–6606 CrossRef CAS PubMed.
- M. Wensing, E. Uhde and T. Salthammer, Plastics additives in the indoor environment – flame retardants and plasticizers, Sci. Total Environ., 2005, 339, 19–40 CrossRef CAS PubMed.
- G. Leng, E. Berger-Preiß, K. Levsen, U. Ranft, D. Sugiri, W. Hadnagy and H. Idel, Pyrethroids used indoor – ambient monitoring of pyrethroids following a pest control operation, Int. J. Hyg. Environ. Health, 2005, 208, 193–199 CrossRef CAS PubMed.
- B. H. Wilford, M. Shoeib, T. Harner, J. Zhu and K. C. Jones, Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: implications for sources and exposure, Environ. Sci. Technol., 2005, 39, 7027–7035 CrossRef CAS.
- W. Butte and B. Heinzow, Pollutants in house dust as indicators of indoor contamination, Rev. Environ. Contam. Toxicol., 2002, 175, 1–46 CAS.
- C. J. Weschler and W. W. Nazaroff, Semivolatile organic compounds in indoor environments, Atmos. Environ., 2008, 42, 9018–9040 CrossRef CAS.
- C. J. Weschler, T. Salthammer and H. Fromme, Partitioning of phthalates among the gas phase, airborne particles and settled dust in indoor environments, Atmos. Environ., 2008, 42, 1449–1460 CrossRef CAS.
- J. F. Pankow, An absorption model of gas/particle partitioning of organic compounds in the atmosphere, Atmos. Environ., 1994, 28, 185–188 CrossRef CAS.
- A. Finizio, D. Mackay, T. Bidleman and T. Harner, Octanol-air partition coefficient as a predictor of partitioning of semi-volatile organic chemicals to aerosols, Atmos. Environ., 1997, 31, 2289–2296 CrossRef CAS.
- C. J. Weschler and W. W. Nazaroff, SVOC partitioning between the gas phase and settled dust indoors, Atmos. Environ., 2010, 44, 3609–3620 CrossRef CAS.
- C. Rauert, B. Lazarov, S. Harrad, A. Covaci and M. Stranger, A review of chamber experiments for determining specific emission rates and investigating migration pathways of flame retardants, Atmos. Environ., 2014, 82, 44–55 CrossRef CAS.
- I. Liagkouridis, I. T. Cousins and A. P. Cousins, Emissions and fate of brominated flame retardants in the indoor environment: a critical review of modelling approaches, Sci. Total Environ., 2014, 491–492, 87–99 CrossRef CAS PubMed.
- C. Liu, S. Shi, C. Weschler, B. Zhao and Y. Zhang, Analysis of the dynamic interaction between SVOCs and airborne particles, Aerosol Sci. Technol., 2013, 47, 125–136 CrossRef CAS.
- C. J. Weschler and W. W. Nazaroff, Growth of organic films on indoor surfaces, Indoor Air, 2017, 27, 1101–1112 CrossRef CAS PubMed.
- R. Sühring, H. Wolschke, M. L. Diamond, L. M. Jantunen and M. Scheringer, Distribution of organophosphate esters between the gas and particle phase-model predictions vs. measured data, Environ. Sci. Technol., 2016, 50, 6644–6651 CrossRef.
- C. M. A. Eichler, J. Cao, G. Isaacman-VanWertz and J. C. Little, Modeling the formation and growth of organic films on indoor surfaces, Indoor Air, 2019, 29, 17–29 CrossRef CAS.
- Y. Wu, C. M. A. Eichler, W. Leng, S. S. Cox, L. C. Marr and J. C. Little, Adsorption of phthalates on impervious indoor surfaces, Environ. Sci. Technol., 2017, 51, 2907–2913 CrossRef CAS PubMed.
- J. L. Benning, Z. Liu, A. Tiwari, J. C. Little and L. C. Marr, Characterizing gas-particle interactions of phthalate plasticizer emitted from vinyl flooring, Environ. Sci. Technol., 2013, 47, 2696–2703 CrossRef CAS PubMed.
- C. Rauert, S. Harrad, M. Stranger and B. Lazarov, Test chamber investigation of the volatilization from source materials of brominated flame retardants and their subsequent deposition to indoor dust, Indoor Air, 2015, 25, 393–404 CrossRef CAS PubMed.
- Y. Wu, C. M. A. Eichler, J. Cao, J. Benning, A. Olson, S. Chen, C. Liu, E. P. Vejerano, L. C. Marr and J. C. Little, Particle/gas partitioning of phthalates to organic and inorganic airborne particles in the indoor environment, Environ. Sci. Technol., 2018, 52, 3583–3590 CrossRef CAS PubMed.
- P. A. Clausen, V. Hansen, L. Gunnarsen, A. Afshari and P. Wolkoff, Emission of di-2-ethylhexyl phthalate from PVC flooring into air and uptake in dust: emission and sorption experiments in FLEC and CLIMPAQ, Environ. Sci. Technol., 2004, 38, 2531–2537 CrossRef CAS PubMed.
- T. Schripp, C. Fauck and T. Salthammer, Chamber studies on mass-transfer of di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DnBP) from emission sources into house dust, Atmos. Environ., 2010, 44, 2840–2845 CrossRef CAS.
- P. Schossler, T. Schripp, T. Salthammer and M. Bahadir, Beyond phthalates: gas phase concentrations and modeled gas/particle distribution of modern plasticizers, Sci. Total Environ., 2011, 409, 4031–4038 CAS.
- T. Salthammer and T. Schripp, Application of the Junge- and Pankow-equation for estimating indoor gas/particle distribution and exposure to SVOCs, Atmos. Environ., 2015, 106, 467–476 CrossRef CAS.
- K.-U. Goss and R. P. Schwarzenbach, Linear free energy relationships used to evaluate equilibrium partitioning of organic compounds, Environ. Sci. Technol., 2001, 35, 1–9 CrossRef CAS.
- T. Salthammer and K.-U. Goss, Predicting the gas/particle distribution of SVOCs in the indoor environment using poly parameter linear free energy relationships, Environ. Sci. Technol., 2019, 53, 2491–2499 CrossRef CAS PubMed.
- W. Wei, C. Mandin, O. Blanchard, F. Mercier, M. Pelletier, B. Le Bot, P. Glorennec and O. Ramalho, Distributions of the particle/gas and dust/gas partition coefficients for seventy-two semi-volatile organic compounds in indoor environment, Chemosphere, 2016, 153, 212–219 CrossRef CAS.
- B. Kolarik, C.-G. Bornehag, K. Naydenov, J. Sundell, P. Stavova and O. F. Nielsen, The concentrations of phthalates in settled dust in Bulgarian homes in relation to building characteristic and cleaning habits in the family, Atmos. Environ., 2008, 42, 8553–8559 CrossRef CAS.
- M. Venier, O. Audy, S. Vojta, J. Becanova, K. Romanak, L. Melymuk, M. Kratka, P. Kukucka, J. Okeme, A. Saini, M. L. Diamond and J. Klanova, Brominated flame retardants in the indoor environment – Comparative study of indoor contamination from three countries, Environ. Int., 2016, 94, 150–160 CrossRef CAS PubMed.
- V. Sukiene, A. C. Gerecke, Y.-M. Park, M. Zennegg, M. I. Bakker, C. J. E. Delmaar, K. Hungerbühler and N. von Goetz, Tracking SVOCs' transfer from products to indoor air and settled dust with deuterium-labeled substances, Environ. Sci. Technol., 2016, 50, 4296–4303 CrossRef CAS PubMed.
- V. Sukiene, N. Von Goetz, A. C. Gerecke, M. I. Bakker, C. J. E. Delmaar and K. Hungerbühler, Direct and air-mediated transfer of labeled SVOCs from indoor sources to dust, Environ. Sci. Technol., 2017, 51, 3269–3277 CrossRef CAS PubMed.
- C. Bi, Y. Liang and Y. Xu, Fate and transport of phthalates in indoor environments and the influence of temperature: a case study in a test house, Environ. Sci. Technol., 2015, 49, 9674–9681 CrossRef CAS PubMed.
- W. Wei, C. Mandin, O. Blanchard, F. Mercier, M. Pelletier, B. Le Bot, P. Glorennec and O. Ramalho, Temperature dependence of the particle/gas partition coefficient: an application to predict indoor gas-phase concentrations of semi-volatile organic compounds, Sci. Total Environ., 2016, 563, 506–512 CrossRef PubMed.
- T. Schripp, T. Salthammer, C. Fauck, G. Bekö and C. J. Weschler, Latex paint as a delivery vehicle for diethylphthalate and di-n-butylphthalate: predictable boundary layer concentrations and emission rates, Sci. Total Environ., 2014, 494–495, 299–305 CrossRef CAS PubMed.
- VDI 4300-7, Indoor air pollution measurement – measurement of indoor air change rate, Beuth Verlag, Berlin, 2001 Search PubMed.
- ISO 16000-6, Indoor air – Part 6: Determination of volatile organic compounds in indoor air and test chamber air by active sampling on Tenax TA sorbent, thermal desorption and gas chromatography using MS or MS/FID, Beuth Verlag, Berlin, 2011 Search PubMed.
- P. A. Baron and K. Willeke, Aerosol Measurement. Principles, Techniques, and Applications, John Wiley & Sons, New York, 2005 Search PubMed.
- J. A. Siegel, Primary and secondary consequences of indoor air cleaners, Indoor Air, 2016, 26, 88–96 CrossRef CAS PubMed.
- T. Glytsos, J. Ondrácek, L. Dzumbová, I. Kopanakis and M. Lazaridis, Characterization of particulate matter concentrations during controlled indoor activities, Atmos. Environ., 2010, 44, 1539–1549 CrossRef CAS.
- X. Liu, M. R. Allen and N. F. Roache, Characterization of organophosphorus flame retardants' sorption on building materials and consumer products, Atmos. Environ., 2016, 140, 333–341 CrossRef CAS.
- L. Melymuk, P. Bohlin-Nizzetto, S. Vojta, M. Kratka, P. Kukucka, O. Audy, P. Pribylova and J. Klanova, Distribution of legacy and emerging semivolatile organic compounds in five indoor matrices in a residential environment, Chemosphere, 2016, 153, 179–186 CrossRef CAS.
- Z. Liu, W. Ye and J. C. Little, Predicting emissions of volatile and semivolatile organic compounds from building materials: a review, Building and Environment, 2013, 64, 7–25 CrossRef.
- R. P. Schwarzenbach, P. M. Gschwend and D. M. Imboden, Environmental Organic Chemistry, John Wiley & Sons, Hoboken, NJ, 2017 Search PubMed.
- P. R. Bevington and D. K. Robinson, Data reduction and error analysis for the physical sciences, McGraw Hill, New York, 2003 Search PubMed.
- Y. Xu, E. A. Cohen Hubal, P. A. Clausen and J. C. Little, Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: a mechanistic analysis, Environ. Sci. Technol., 2009, 43, 2374–2380 CrossRef CAS.
- Phthalate Esters, ed. C. A. Staples, Springer-Verlag, Berlin, Heidelberg, 2003 Search PubMed.
- E. Uhde, M. Bednarek, F. Fuhrmann and T. Salthammer, Phthalic esters in the indoor environment – test chamber studies on PVC-coated wall coverings, Indoor Air, 2001, 11, 150–155 CrossRef CAS.
- H. Fromme, A. Schütze, T. Lahrz, M. Kraft, L. Fembacher, S. Siewering, R. Burkardt, S. Dietrich, H. M. Koch and W. Völkel, Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3), Int. J. Hyg. Environ. Health, 2016, 219, 33–39 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00121b |
| This journal is © The Royal Society of Chemistry 2019 |