Open Access Article
Open Access ArticleGold(I) complexes as powerful photosensitizers – a visionary frontier perspective
Andrea
Pinto
 *ab and
Laura
Rodríguez
*ab and
Laura
Rodríguez
 *ab
*ab
aDepartament de Química Inorgànica i Orgànica, Secció de Química Inorgànica, Universitat de Barcelona, Martí i Franquès 1-11, 08028 Barcelona, Spain
bInstitut de Nanociència i Nanotecnologia (IN2UB), Universitat de Barcelona, 08028 Barcelona, Spain. E-mail: andrea.pintoi@ub.edu; laurarodriguezr@ub.edu
First published on 29th July 2024
Abstract
Singlet oxygen production and its reactivity have significant implications in fields ranging from polymer science to photodynamic therapy. Extensive research has focused on the development of organic-based materials and heavy metal complexes, including Ru(II), Rh(III), Ir(III) and Pt(II). However, metal complexes containing Au(I) have been scarcely explored and warrant further investigation. This review provides a comprehensive analysis of reported compounds, classified based on the ligands coordinated to the gold(I) centre. Additionally, future directions in photosensitizer development and singlet oxygen applications are discussed.
1. Introduction
Long-lived photoexcited triplet states can be efficiently quenched by molecular oxygen through energy transfer.1,2 In this context, for the characterization of emissive species, oxygen must be removed before any measurements on phosphorescence emission spectra, lifetimes, and quantum yields in solutions at ambient temperatures.3 Otherwise, a supramolecular matrix should be added to the solution to isolate the phosphors from oxygen quenching.4–6This process results in the formation of singlet oxygen, 1O2, which can occur, for example, through sensitization. Here, a photosensitizer (PS) in the excited triplet state relaxes to its ground state by transferring energy to ambient oxygen.7 This research area has garnered increasing attention in recent years due to its pivotal role in various processes such as wastewater treatment, blood sterilization, photocatalytic processes, photodynamic therapy, organic synthesis of fine chemicals, and environmental contaminant destruction.7–10
Photosensitizers possessing a populated triplet excited state higher in energy but close to the lower excited state of oxygen, 1O2, are of great interest in this field. The efficiency of singlet oxygen production, represented as singlet oxygen quantum yield, ΦΔ, depends on numerous factors, including not only the rate of intersystem crossing kISC, but also the rates of radiative and non-radiative decay of excited states relative to kISC, the thermal accessibility of excited d-states enabling non-radiative deactivation pathways, and the efficient transfer of the T1 energy to 3O2.11–15
For effective molecular design, an efficient photosensitizer should exhibit high intersystem crossing (ISC) rates and a prolonged triplet lifetime to maximize energy transfer probability. Some examples of good photosensitizers are rose bengal, fluorescein or perinaphthenone. Enhancing ISC can be achieved by increasing spin–orbit coupling (SOC), which can be done by introducing heavy atoms or heteroatoms, or by incorporating donor/acceptor units that facilitate electron transfer steps.7,16–19 The triplet excited-state energy must be greater than the energy required to convert O2 from its fundamental state (3Σg−) to an excited state (1Δg) (0.98 eV).
Transition metal complexes are of great interest in photophysics because their heavy metal centers can promote efficient intersystem crossing (ISC) and populate the triplet excited state, resulting in phosphorescent emitters or enabling energy transfer processes.7,9,13,20–22
In energy transfer processes, these complexes can act as photosensitizers, transferring energy from the photosensitizer (PS) to molecular oxygen to produce reactive singlet oxygen (1O2). The proximity of a heavy atom to the fluorophore is expected to enhance the likelihood of intersystem crossing: the closer the contact, the higher the photosensitizer's efficiency. Therefore, chromophores attached to heavy metals are of particular interest, as they exhibit high spin–orbit coupling (SOC) values and allow for easy tuning of their photophysical properties through ligand modification. Among the metals studied for singlet oxygen production, gold(I) is relatively underexplored, with only a few examples reported in the literature. However, the high phosphorescence emission observed for gold(I) complexes at low temperatures, along with the recorded decrease in fluorescence quantum yields and shorter decay times of their singlet excited states, clearly indicate the promotion of the triplet state by the Au(I) heavy atom effect. These characteristics make gold(I) complexes promising candidates for exploration as singlet oxygen generators. However, understanding the relationship between structure and photophysical properties in gold(I) complexes remains limited. Efficient 1O2 sensitization and its evaluation through singlet oxygen quantum yields, ΦΔ, are other promising areas of research that have been poorly explored for these compounds. To the best of our knowledge, there are very few reports in the literature on gold(I) complexes used as photosensitizers, with yields ranging from low to high. The well-established synthetic methodologies for obtaining a wide variety of gold(I) derivatives, combined with their well-known heavy atom effect promoting ISC, make these compounds worthy of careful analysis and a promising area for further research and development.
2. Methods of 1O2 detection
Two different methods of detection have been described to identify and/or quantify the formation of 1O2 and qualify a compound as a good PS. We will refer to these methods as Method 1 (the indirect method) and Method 2 (the direct method).Method 1 is based on monitoring changes in the absorption or emission spectra of a singlet oxygen (1O2) quencher, typically a chemical probe with aromatic rings such as 9,10-dimethylanthracene or 1,3-diphenylisobenzofuran among others, in the presence of a photosensitizer (PS) over time.10 This is done by irradiating the solution at the absorption maximum wavelength of the PS. The quenching reaction generally results in the formation of an epoxide or a [4 + 2] cycloaddition product, leading to the loss of the large aromaticity and a corresponding decrease in absorption spectra over time (see Fig. 1). The concentration of 1O2 can be quantitatively monitored by measuring the decay in absorption of the quencher at its absorption maximum wavelength. By plotting ln(A0/A) against irradiation time and comparing it with a reference PS, the efficiency of the PS can be quantified. This is the most common method, probably because UV-visible absorption spectrophotometers are easily available in different laboratories.
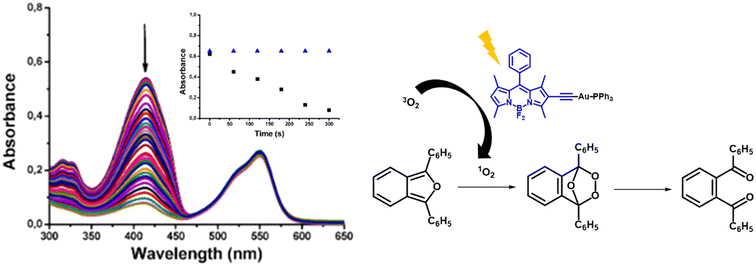 | ||
| Fig. 1 Singlet oxygen mediated bleaching of 1,3-diphenylisobenzofuran (DPBF) in the presence of gold(I) complex 25 and the rates of decay of DPBF monitored at 415 nm, using gold(I) complex and the organic ligand as a reference.10 | ||
Method 2, referred to as “the direct method”, involves directly measuring the characteristic emission of singlet oxygen (1O2) at approximately 1270 nm as it decays to the ground state, 3O2 (see Fig. 2).10,16 This emission is observed after irradiating the photosensitizer (PS) under study at its absorption maximum. The efficiency of the new PS can be quantified by integrating the resulting 1O2 emission band and comparing it to a reference tabulated pattern (such as perinaphthenone or rose bengal), followed by applying the following equation to determine the PS efficiency: 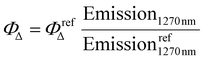 .
.
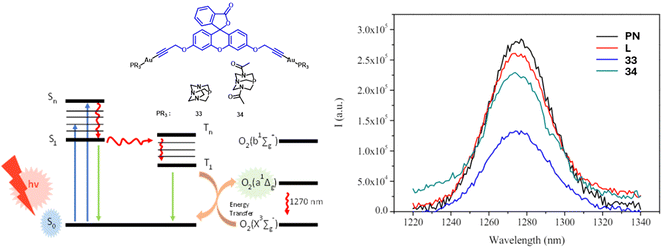 | ||
| Fig. 2 Methods of 1O2 measurement using direct detection of the phosphorescence of the singlet oxygen at 1270 nm of gold(I) complexes 33 and 34, the organic counterpart (L) and perinaphthenone (PN). | ||
This method is substantially less common and is less frequently reported in the literature, likely due to its technical requirements. It necessitates a highly sensitive DSS-IGA020L detector (Indium Gallium Arsenide Solid state detector) coupled to a spectrofluorometer, as well as the optimization of experimental parameters to accurately record the 1O2 emission. Nevertheless, once these conditions are met, we strongly recommend this method because it is based on the direct measurement of an emission spectrum. Additionally, changes in the 1O2 emission lifetimes can be measured when an oxygen quencher molecule undergoes cycloaddition, providing direct evidence of the successful photosensitizing process.
Quantum yields of singlet oxygen production not only depend on the photophysical properties of the PS but may also be considerably influenced by the experimental conditions, such as oxygen concentration nature of the solvent and temperature.7b Some of the PS can be strongly influenced by the polarity of the solvent or the protonation. For example, the quantum yield values of singlet oxygen of 9H-fluoren-9-one goes from 0.83 in benzene to lower than 0.1 in methanol. Therefore, in this review, the solvent used will be also mentioned next to the quantum yields reported.
3. Gold(I) complexes as singlet oxygen photosensitizers
The photophysics of gold(I) compounds remains not fully understood, as many principles and parameters influence the various pathways between ground and excited states, ultimately leading to their deactivation to the ground state. Intersystem crossing (ISC) is typically favoured in these compounds, largely due to the heavy atom effect, which enhances spin–orbit coupling (SOC) and increases the population of triplet excited states.11 As a result, an increase in triplet state population usually correlates with a decrease in fluorescence emission, since these are two competing deactivation mechanisms. However, this trend is not universally applicable, as the chromophore can significantly influence these processes and sometimes favour fluorescence emission over triplet state population.There are very few examples in the literature of gold(I) complexes acting as photosensitizers (PS). This prompted our research efforts a couple of years ago to delve into this intriguing research topic, wherein we adapted our laboratory equipment to successfully measure the corresponding 1O2 emission at 1270 nm (Method 2, direct method). At that time, only two examples of gold(I) PS were reported, both based on well-known organic PS (BODIPY and porphyrin) linked to the gold(I) atom.23,24 The synthetic procedures for obtaining such chromophores typically involve several reaction steps and rigorous purifications, yielding commonly only a few milligrams of the final product. Therefore, our interest was piqued in attempting to synthesize new gold(I) PS containing simpler organic ligands, facilitating easier synthetic methods and potential scalability of the final products in larger quantities, if required.
The resulting data on the reports of gold(I) PS reported in the literature has been analysed herein, leading to the extraction of several conclusions. Discussions have been organized based on the chromophores for clarity and organization purposes (Fig. 3).
Global singlet oxygen production quantum yield values, ΦΔ, are displayed in Table 1 and explained later in more detail.
| Compound | Φ Δ (solvent) | Compound | Φ Δ (solvent) |
|---|---|---|---|
| 1 | 0.96 (toluene) | 18 | 0.09 (dichloromethane) |
| 2 | 0.04 (acetonitrile) | 19 | 0.09 (dichloromethane) |
| 3 | 0.04 (acetonitrile) | 20 | 0.46 (acetonitrile) |
| 4 | 0.06 (acetonitrile) | 21 | 0.43 (acetonitrile) |
| 5 | 0.07 (acetonitrile) | 22 | 0.05 (acetonitrile) |
| 6 | 0.12 (acetonitrile) | 23 | 0.02 (acetonitrile) |
| 7 | 0.18 (acetonitrile) | 24 | 0.07 (acetonitrile) |
| 8 | 0.02 (dichloromethane) | 25 | 0.84 (dichloromethane) |
| 9 | 0.01 (dichloromethane) | 26 | 0.65 (dichloromethane) |
| 10 | 0.10 (dichloromethane) | 27 | 0.09 (C6D6) |
| 11 | 0.02 (acetonitrile) | 28 | 0.17 (C6D6) |
| 12 | 0.02 (acetonitrile) | 29 | 0.16 (ethanol) |
| 13 | 0.03 (acetonitrile) | 30 | 0.39 (ethanol) |
| 14 | 0.21 (dichloromethane) | 31 | 0.33 (ethanol) |
| 15 | 0.26 (dichloromethane) | 32 | 0.93 (acetonitrile) |
| 16 | 0.17 (dichloromethane) | 33 | 0.45 (dichloromethane) |
| 17 | 0.11 (dichloromethane) | 34 | 0.81 (dichloromethane) |
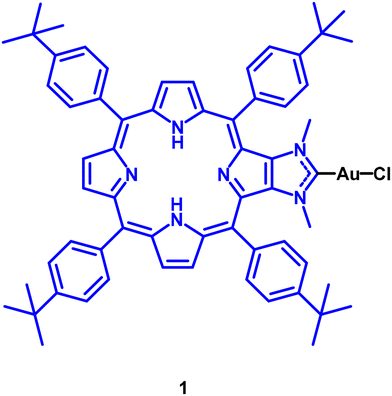
To the best of our knowledge, the earliest gold(I) PS described in the literature dates to 2017 and contains porphyrin, a well-known organic PS (compound 1 in Fig. 4), in its chemical structure.24 It was observed that the efficiency of 1 (ΦΔ = 0.96) is higher than that of the porphyrin organic precursor (ΦΔ = 0.58). This enhancement was attributed to the heavy atom effect, which favours the population of the T1 state and facilitates energy transfer to 3O2.
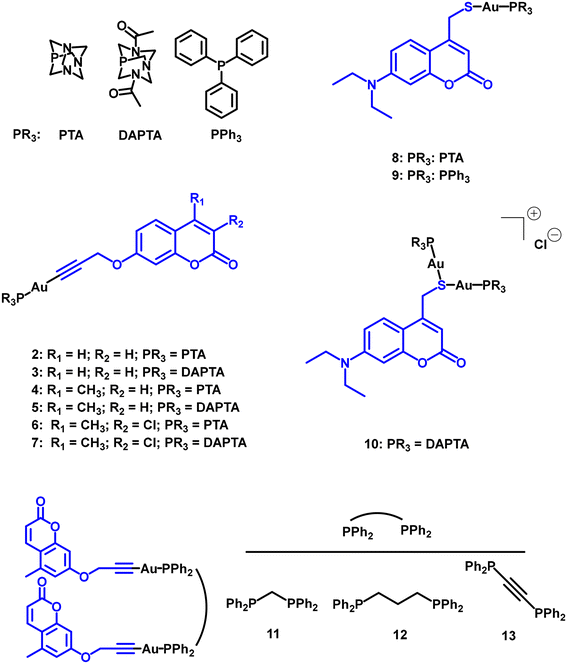
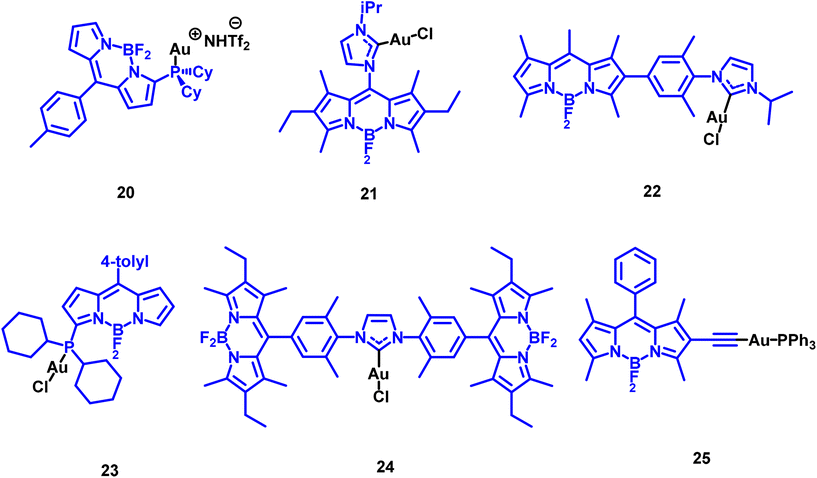
Gold(I) derivatives of coumarin (Fig. 5) were observed to be poor photosensitizers (PS), despite the anticipated enhancement of their triplet excited state population due to the presence of the gold(I) heavy atom. Two groups of coumarin derivatives were explored, with coordination to the metal centre occurring through either an alkynyl unit or a sulphur atom.12,14,25 No significant differences were observed based on this connecting atom. Although the electron-donating or electron-withdrawing characteristics of the substituents on the coumarin rings exert some influence on the resulting ΦΔ, the overall values range from 1% to a maximum of approximately 20%.
The comparison of the first two series of coumarin derivatives, connected through a propargyl group to the gold(I) metal centre (compounds 2–7 and 11–13), leads us to assert that the nuclearity of the compounds does not appear to play a significant role in the PS efficiency. This is likely because the gold-propargylcoumarin moieties act independently, as there is no clear evidence of interconnection or proximity between them. This hypothesis is supported by the coumarin complexes connected through a sulphur atom (compounds 8–10), where the resulting ΦΔ of 10 is approximately one order of magnitude higher than the recorded for 8 and 9. The main difference relies on the fact that compound 10 displays two gold-phosphane units in close proximity. This raises an important point for further exploration: the potential role of Au⋯Au (aurophilic) interactions in the resulting ΦΔ.
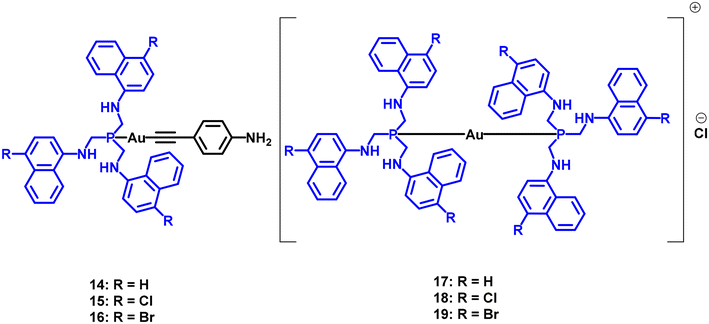
Two series of neutral or ionic gold(I) compounds containing both aniline and naphthalene-substituted chromophores were reported in 2022 (Fig. 6).16
Two important conclusions can be drawn from these data. The first one is that the global charge of the compounds affects their PS efficiency, as the ΦΔ calculated for 14–16 is approximately 2–3 times higher than the values of 17–19.
The presence of two different chromophores might explain the observed differences, particularly since the ionic compounds contain only naphthalene-substituted chromophores. Additionally, the halogen substituents on the naphthalene rings exhibit a heavy atom effect that counteracts the effect of the gold(I) metal centre. This leads to reduced PS efficiency, with chloride- and bromide-substituted naphthalene compounds performing worse than their unsubstituted counterparts in both neutral and ionic forms. BODIPY-derived compounds are another well-known class of organic PS. The corresponding Au-BODIPY PS complexes are displayed in Fig. 7.23,26
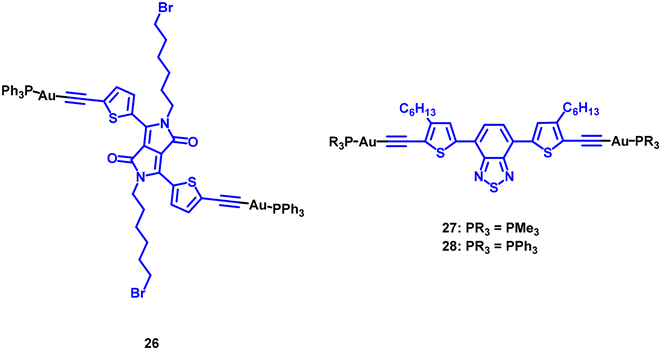
The position of the gold(I) metal centre on the functionalized BODIPY was observed to influence the photosensitizer (PS) efficiency. Generally, the proximity of the heavy metal centre to the BODIPY affects the efficiency, with higher values observed when the BODIPY is closer to the gold atom. Interestingly, complex 23, in which the gold is bonded very close to the BODIPY core, exhibits poor singlet oxygen production. The authors attributed this to the appearance of a deactivation channel through PET-quenching, where an electron transfer from the BODIPY excited state to the metal centre is followed by a non-radiative recombination of the charges. The positive effect of the heavy atom on gold(I)-BODIPY photosensitizers was clearly evidenced when the chromophore structure was functionalized with an alkynyl moiety as a coordination point for the metal centre (Au–PPh3 group) in compound 25, Fig. 6. While the BODIPY organic counterpart does not behave as a PS, the corresponding gold compound 25 presents one of the highest values recorded (84%). The analysis of a di-iodoBODIPY equivalent compound also demonstrates the positive effect of the heavy atom on the 1O2 photogeneration, although the resulting value is slightly smaller than that measured for 25 (79%). A similar effect was observed for 26 in Fig. 8, where the obtained ΦΔ value is enhanced compared to that recorded for the organic free ligand (39% vs. 65%).27 Theoretical calculations show that the organic free ligand has higher reverse intersystem crossing rates than the gold(I) complex. As a result, the ligand more easily returns to the singlet state, reducing the likelihood of its triplet state transferring energy to ground-state 3O2. An important feature not yet explored is the effect of the ancillary phosphane ligand on the resulting PS behaviour. This was analysed for a couple of terthiophene (TBT) derivatives where the 1O2 efficiency of the compound containing PMe3 ligand (compound 27) is the half (9%) than the value calculated for the PPh3 (28) analogous derivative (17%).28 This underscores the importance of taking into account the stereoelectronic properties of the phosphane in future studies within this field.
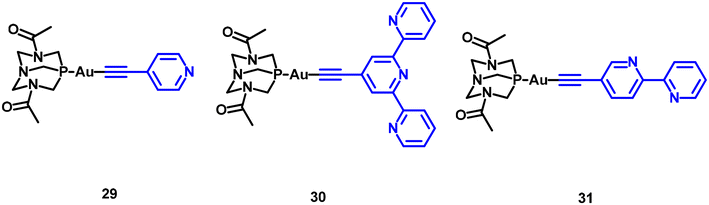
Other chromophores coordinated to the Au centre through an alkynyl moiety have been explored as 1O2 PS. This includes (poly)pyridyl derivatives, as shown in Fig. 9.11,29
The efficiency calculated for these compounds is moderate, but notably higher (approximately double) in the case of bipyridyl and terpyridyl derivatives (31 and 30) compared to the pyridyl compound 29.
The analysis of the photophysical data retrieved by the authors showed that gold(I) complexes demonstrate rapid intersystem crossing rates, significantly influenced by the chromophore structure and the singlet oxygen quantum yields. The inclusion of gold(I) effectively adjusts the excited-state dynamics of the organic counterpart. However, this adjustment does not lead to complete unity in quantum yields of triplet formation, as internal conversion from the singlet state continues to be a competitive pathway.
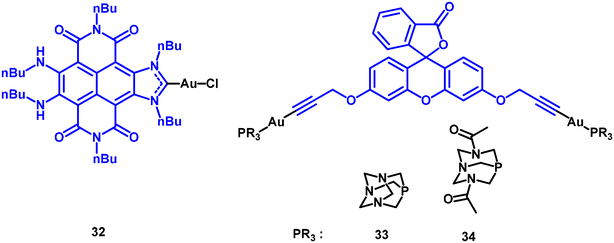
Very recent studies in this field have revealed some of the highest ΦΔ values, around 90%, with naphthalene-diimide-decorated N-heterocyclic carbene (NDI-NHCs) and fluorescein gold(I) derivatives (see Fig. 10).
In both series of compounds, the photophysical (PS) efficiency is not significantly influenced by the heavy atom but does affect the resulting coloration. The NDI-NHC derivative 32 shows efficient catalysis in the endoperoxidation and peroxidation of cyclic and acyclic alkenes, as well as in the selective oxidation of 2-chloroethyl ethyl sulphide to the non-toxic product chloroethyl ethyl sulfoxide.30
Different approaches have been undertaken in the study of the fluorescein derivatives 33 and 34 as PS.5
Singlet oxygen production was also evaluated in doped polymer matrices, with polystyrene exhibiting the strongest signal. Theoretical calculations indicated that gold(I) complexes interact less with the matrix, resulting in increased contact with oxygen. To enhance the active surface area and thus singlet oxygen production, polystyrene microspheres were tested, leading to an observed increase in the signal.
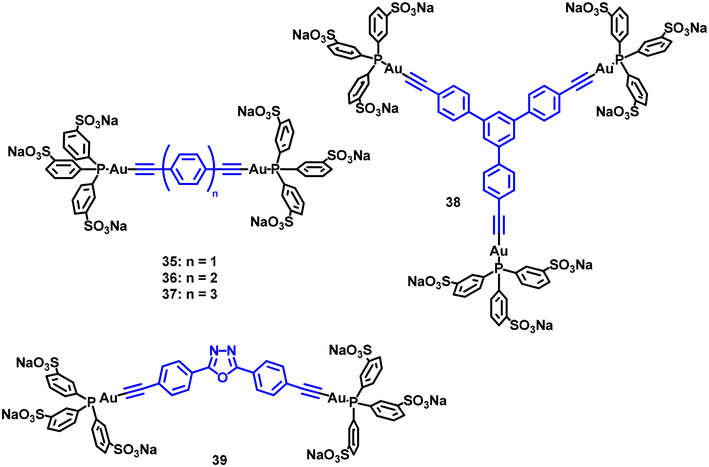
The use of triphenylphosphine-3,3′,3′′-trisulfonate (TPPTS) phosphane was also explored in 2017 with compounds 35–39 (Fig. 11) that also display the chromophore-alkynyl-AuPR3 chemical structure.31 Although the results demonstrate that they are efficient PS, as far as we know, no quantification was reported for them. Interestingly, the authors use the singlet oxygen production as a locally deoxygenation method and a way to activate the phosphorescence pathway both in DMSO and in a gel matrix. The results provide a novel and non-contact approach for promoting phosphorescence at room temperature of metal–organic complexes.
4. Conclusions and outlook
The production of singlet oxygen by Au(I)-based compounds is strongly influenced by the ligand coordinated to gold, where significant electronic transitions occur. Acting as a heavy atom, gold(I) enhances intersystem crossing, accelerating this process's rate constants and populating the triplet state, thereby promoting singlet oxygen production.Literature findings underscore the promising nature of this research area. Critical factors influencing photosensitizing behaviour include the heavy atom effect, metallophilic interactions, chromophore characteristics, and the distance between the chromophore and the gold(I) centre. However, further studies are necessary to establish correlations between chemical structure and singlet oxygen production. Exploration into heterometallic complexes (which facilitate metallophilic interactions) remains in its nascent stages, as does analysis of these processes in solid samples beyond powders (e.g., glass, organic matrices).
The ability to modify the chemical structure of gold(I) compounds easily, owing to their linear coordination motif, coupled with the expanding applications of singlet oxygen in biological and environmental research, positions this research field as highly promising.
Author contributions
AP and LR conceptualized the review, and were involved on formal analysis, writing, review and editing. LR obtained funding and supervised.Data availability
The data supporting this article have been included within the text.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Ministerio de Ciencia e Innovación of Spain (Project number PID2022-139296NB-I00). This article is also based upon work from COST Action CA22131, LUCES Supramolecular LUminescent Chemosensors for Environmental Security, supported by COST (European Cooperation in Science and Technology).References
- V. Balzani, P. Ceroni and A. Juris, Photochemistry and Photophysics-Concepts, Research, Applications, Wiley-VCH, Weinheim, 2014, pp. 82–83 Search PubMed.
- N. J. Turro, V. Ramamurthy and J. C. Scaiano, Principles of Molecular Photochemistry-An Introduction, University Science Books, Sausalito, CA, USA, 2009, pp. 244–245 Search PubMed.
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence-Principles and Applications, Wiley-VCH, Weinheim, 2nd edn, 2012, pp. 275–276 Search PubMed.
- G. Romo-Islas, J. S. Ward, K. Rissanen and L. Rodríguez, Inorg. Chem., 2023, 62, 8101–8111 CrossRef CAS PubMed.
- A. Pinto, A. Llanos, R. M. Gomila, A. Frontera and L. Rodríguez, Inorg. Chem., 2023, 62, 7131–7140 CrossRef CAS PubMed.
- P. Duan, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2013, 135, 19056–19059 CrossRef CAS PubMed.
- (a) P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181–3209 RSC; (b) A. P. Darmanyan and C. S. Foote, J. Phys. Chem., 1993, 97, 4573–4576 CrossRef CAS.
- Y. Wang, Y. Lin, S. He, S. Wu and C. Yang, J. Hazard. Mater., 2024, 461, 132538 CrossRef CAS PubMed.
- G. Romo-Islas, R. M. Gomila, A. Frontera and L. Rodríguez, Inorg. Chem. Front., 2023, 10, 6204–6220 RSC.
- N. Singh, R. Sen Gupta and S. Bose, Nanoscale, 2024, 16, 3243–3268 RSC.
- E. Aguiló, A. J. Moro, M. Outis, J. Pina, D. Sarmento, J. S. Seixas De Melo, L. Rodríguez and J. C. Lima, Inorg. Chem., 2018, 57, 13423–13430 CrossRef PubMed.
- C. Cunha, A. Pinto, A. Galvão, L. Rodríguez and J. S. Seixas De Melo, Inorg. Chem., 2022, 61, 6964–6976 CrossRef CAS PubMed.
- A. De Aquino, J. S. Ward, K. Rissanen, G. Aullón, J. C. Lima and L. Rodríguez, Inorg. Chem., 2022, 61, 20931–20941 CrossRef CAS PubMed.
- A. Pinto, C. Cunha, G. Aullón, J. C. Lima, L. Rodríguez and J. S. Seixas de Melo, J. Phys. Chem. B, 2021, 125, 11751–11760 CrossRef CAS PubMed.
- S. Wan and W. Lu, Angew. Chem., 2017, 129, 1810–1814 CrossRef.
- A. Pinto, J. S. Ward, K. Rissanen, M. Smith and L. Rodríguez, Dalton Trans., 2022, 51, 8795–8803 RSC.
- M. L. Agazzi, J. E. Durantini, N. S. Gsponer, A. M. Durantini, S. G. Bertolotti and E. N. Durantini, ChemPhysChem, 2019, 20, 1110–1125 CrossRef CAS PubMed.
- A. Lázaro, R. Bosque, J. S. Ward, K. Rissanen, M. Crespo and L. Rodríguez, Inorg. Chem., 2023, 62, 2000–2012 CrossRef PubMed.
- G. H. Kim, R. Lampande, J. B. Im, J. M. Lee, J. Y. Lee and J. H. Kwon, Mater. Horiz., 2017, 4, 619–624 RSC.
- A. Rodriguez-Serrano, V. Rai-Constapel, M. C. Daza, M. Doerr and C. M. Marian, Phys. Chem. Chem. Phys., 2015, 17, 11350–11358 RSC.
- A. Lázaro, C. Cunha, R. Bosque, J. Pina, J. S. Ward, K. N. Truong, K. Rissanen, J. C. Lima, M. Crespo, J. S. Seixas De Melo and L. Rodríguez, Inorg. Chem., 2020, 59, 8220–8230 CrossRef PubMed.
- M. Parasram and V. Gevorgyan, Chem. Soc. Rev., 2017, 46, 6227–6240 RSC.
- M. Üçüncü, E. Karakuş, E. Kurulgan Demirci, M. Sayar, S. Dartar and M. Emrullahoglu, Org. Lett., 2017, 19, 2522–2525 CrossRef PubMed.
- J. F. Longevial, K. El Cheikh, D. Aggad, A. Lebrun, A. van der Lee, F. Tielens, S. Clément, A. Morère, M. Garcia, M. Gary-Bobo and S. Richeter, Chem. – Eur. J., 2017, 23, 14017–14026 CrossRef CAS PubMed.
- C. Sobrerroca, I. Angurell, A. de Aquino, G. Romo, C. Jubert and L. Rodríguez, ChemPlusChem, 2023, 88, e202300020 CrossRef CAS PubMed.
- S. Popov and H. Plenio, Eur. J. Inorg. Chem., 2022, e202200335 CrossRef CAS.
- X. Huang, R. Gu, J. Li, N. Yang, Z. Cheng, W. Si, P. Chen, W. Huang and X. Dong, Sci. China: Chem., 2020, 63, 55–64 CrossRef CAS.
- S. Goswami, R. W. Winkel and K. S. Schanze, Inorg. Chem., 2015, 54, 10007–10014 CrossRef CAS PubMed.
- E. Aguiló, A. J. Moro, R. Gavara, I. Alfonso, Y. Pérez, F. Zaccaria, C. F. Guerra, M. Malfois, C. Baucells, M. Ferrer, J. C. Lima and L. Rodríguez, Inorg. Chem., 2018, 57, 1017–1028 CrossRef PubMed.
- C. Ruiz-Zambrana, M. Poyatos and E. Peris, ACS Catal., 2024, 14, 4066–4073 CrossRef CAS.
- S. Wan and W. Lu, Angew. Chem., 2017, 129, 1810–1814 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |