Open Access Article
Open Access ArticleA cradle-to-gate life cycle assessment of green methanol production using direct air capture†
Nicholas Badger *,
Rahim Boylu
*,
Rahim Boylu ,
Valentine Ilojianya,
Mustafa Erguvan
,
Valentine Ilojianya,
Mustafa Erguvan and
Shahriar Amini
and
Shahriar Amini *
*
Department of Mechanical Engineering, College of Engineering, University of Alabama, Tuscaloosa, Alabama, USA. E-mail: nsbadger@crimson.ua.edu; sean.amini@ua.edu
First published on 20th August 2024
Abstract
This study presents a comprehensive cradle-to-gate life cycle assessment (LCA) of synthetic methanol production, integrating low-temperature solid sorbent direct air capture (DAC) systems with renewable energy sources and green hydrogen to evaluate the environmental impacts of various renewable energy configurations for powering the DAC-to-methanol synthesis processes. Renewable energy-powered configurations result in significantly lower greenhouse gas (GHG) emissions than traditional methanol production methods and DAC systems powered by conventional grid energy. Energy configurations analyzed are current US grid mix, solar photovoltaic (PV) in Alabama and Arizona, USA, onshore wind, run-of-river hydroelectric, and geothermal. Notably, hydroelectric and wind power in the western United States emerge as the most sustainable options, showing the lowest global warming potential (GWP) impacts at −2.53 and −2.39 kg CO2 eq. per kg methanol produced, respectively, in contrast to the +0.944 kg CO2 eq. from traditional steam methane reforming. Furthermore, this research investigates the use of various heat sources for regenerating low-temperature solid sorbent DAC, emphasizing the potential integration of new experimental results of novel microwave-based regeneration compared to industrial waste heat. Through the analysis of renewable energy scenarios and DAC regeneration heat sources, the research emphasizes the pivotal role of sustainable energy sources in climate change mitigation. This study introduces a new approach by comparing both various renewable energy sources and DAC heat sources to identify the most optimal configurations. This work is also distinguished by its integration of new experimental data on microwave DAC regeneration, offering a unique contribution to the existing body of knowledge. This LCA scrutinizes the environmental impacts of renewably powered DAC-to-methanol systems and compares them with traditional methanol production methods, revealing the significant potential for carbon neutrality. The findings highlight the importance of strategic technology and energy source optimization to minimize environmental impacts, thus guiding the scaling up of DAC and renewable energy technologies for effective climate mitigation. By recognizing the environmental advantages of integrating renewable energy sources with DAC-to-methanol technologies, this research marks a significant step forward in advancing DAC technology and pushes the boundaries of green methanol production toward true sustainability.
Introduction
The urgency to address climate change has escalated with rising global carbon dioxide (CO2) levels, pushing Earth's climate system towards a critical state. The Intergovernmental Panel on Climate Change (IPCC) highlights the impacts of human-induced climate change on global weather patterns, including droughts, heatwaves, and extreme precipitation, attributing these changes to the significant increase in measured global temperatures driven by human activity.1,2 Despite international efforts to curb emissions, traditional mitigation strategies are falling short of the ambitious targets set by the Paris agreement, while fossil fuels continue to demonstrate an importance to global economies. To bridge this gap, synthetic fuel production is a promising area of research, particularly when integrated with net-negative CO2 technologies such as direct air capture.3 DAC, when considered with its full life cycle emissions, holds the potential to offset current and past emissions, emerging as a crucial technology in the quest for net-zero emissions.4 This technology is poised to become as significant as solar photovoltaics, wind energy, batteries, and electrolyzers in the arsenal of climate change mitigation tools.5 Additionally, CO2 which is removed from ambient air through DAC can be used as feedstock to create synthetic fuel, which alleviates the burden of introducing new CO2 to the carbon cycle through traditional fossil fuels.Exploring methanol synthesis through renewable energy and advanced carbon capture technologies highlights a strategic approach in reducing GHG emissions and fostering sustainable chemical production. Nemet and Brandt6 along with Taghdisian et al.7 study the economic and environmental impacts of DAC and its eco-friendly design within methanol synthesis frameworks. These considerations are furthered by Daggash et al.,8 who emphasize the climate mitigation effectiveness of DAC through a comparative analysis of power-to-fuel and power-to-DAC strategies. In parallel, the environmental advantages of integrating clean energy into such processes are explored by the LCAs conducted by Chen et al.9 and Al-Qahtani et al.,10 while Meunier et al.11 and Khojasteh-Salkuyeh et al.12 emphasize the significance of CO2 valorization and renewable energy advancements. Sollai et al.13 and Cormos14 further assess the techno-economic viability, illustrating how policy and technological progress are making green methanol increasingly competitive.
For specific production methodologies, Lin et al.15 assess methanol production through various CO2 reduction approaches, pinpointing the substantial environmental impact of electrochemical routes due to their high energy demands. Yet, they advocate for the mitigation of these impacts through renewable energy integration. This perspective is echoed in the analyses conducted by Rigamonti and Brivio,16 Luu et al.,17 Schreiber et al.,18 Zang et al.,19 Trudewind et al.,20 and Ling et al.,21 who explore the environmental impacts of methanol production using carbon capture and utilization (CCU) technologies and highlight the potential of such processes to significantly reduce GHG emissions, especially when leveraging renewable energy sources and innovative production methods. Nguyen et al.22 explore methanol production combining dry methane reforming and partial oxidation to minimize CO2 emissions. By integrating process design, simulation, and cradle-to-gate LCA, they identify optimal conditions that notably achieve low process emissions at 0.81 kg CO2 per kg of methanol produced. Khojasteh-Salkuyeh et al.23 demonstrate in their LCA that methanol synthesis from CO2 through CCU and tri-reforming of methane significantly reduces life cycle GHG emissions to 1.75 and 0.41 kg CO2 per kg MeOH, respectively, compared to conventional natural gas-based methods. Ryoo et al.24 study the CO2 to methanol conversion processes, evaluating four different methods: coal gasification, coal coking, hydrogenation, and photocatalytic conversion, across various technological readiness levels and find photocatalytic conversion to be the least burdensome for global warming potential (GWP). Photocatalytic conversion is also explored in a LCA by Robbins et al.25 Barati et al.26 highlight the electrified combined reforming of methane for CO2 to methanol conversion, showing enhanced efficiency and significant reductions in GHG emissions across Canadian provinces.
The integration of renewable energy within chemical production is further showcased by Biernacki et al.,27 who analyze the environmental impacts of converting renewable electricity into methanol. Artz et al.28 provide a comprehensive review of CCU technology development, emphasizing the essential role of LCA in optimizing these technologies for economic and environmental sustainability. This narrative is extended by studies like those of Cordero-Lanzac et al.29,30 and Galusnyak et al.,31 and Sternberg et al.32 who examine the production of green methanol from CO2 and renewable hydrogen, underscoring the importance of process design and the utilization of renewable energy sources.
Including broader environmental sustainability assessments, Garcia-Garcia et al.33 and Rosental et al.34 review the impacts of CCU technologies, including methanol production, advocating for the reduction of global warming impacts through harmonized LCA methodologies. This is complemented by the work of Matzen and Demirel,35 Maimaiti et al.,36 and Nizami et al.,37 who highlight the environmental benefits of renewable energy-powered methanol production, from wind-derived hydrogen and photovoltaics to solar energy, emphasizing the sector's transition towards sustainability.
Within the context of DAC technology, Van der Giesen et al.,38 Liu et al.,39 Deutz and Bardow,40 Terlouw et al.,41 and Micheli et al.42 recognize the pivotal role of renewable energy in enhancing the environmental and economic outcomes of DAC technologies. They explore various applications, from liquid hydrocarbon production to aviation sector decarbonization, showcasing the significant benefits of renewable-powered DAC pathways. This collective body of research underlines the necessity of transitioning towards sustainable practices in methanol production, with renewable energy and carbon capture positioned as key components in global climate mitigation efforts, despite the challenges posed by data availability for comprehensive LCA of DAC-based systems. Recent studies highlight the varying efficiencies and social costs associated with different carbon capture technologies. For instance, Jacobson43 emphasizes that synthetic direct air carbon capture and storage and CCU technologies, when powered by natural gas, capture a net of only 10.5–10.8% of CO2 emissions over 20 years and 20–31% over 100 years due to uncaptured emissions from natural gas combustion and upstream processes.
Building upon the insights of these prior studies, this research marks a distinctive contribution to the field by conducting a comparative analysis of different electrical energy sources and DAC heat sources within the context of synthetic methanol production, including the incorporation of new experimental data on microwave DAC regeneration. This side-by-side examination of these energy sources is novel, enhancing the granularity of LCA methodologies applied to DAC systems and providing a comprehensive assessment of their environmental performance. By integrating new data and contrasting multiple energy pathways, the study sets a new precedent for evaluating the potential of DAC-to-methanol systems, offering unique insights that could shape future research directions, influence policy decisions, and catalyze innovation in the pursuit of sustainable CO2 mitigation strategies.
LCA goal and scope
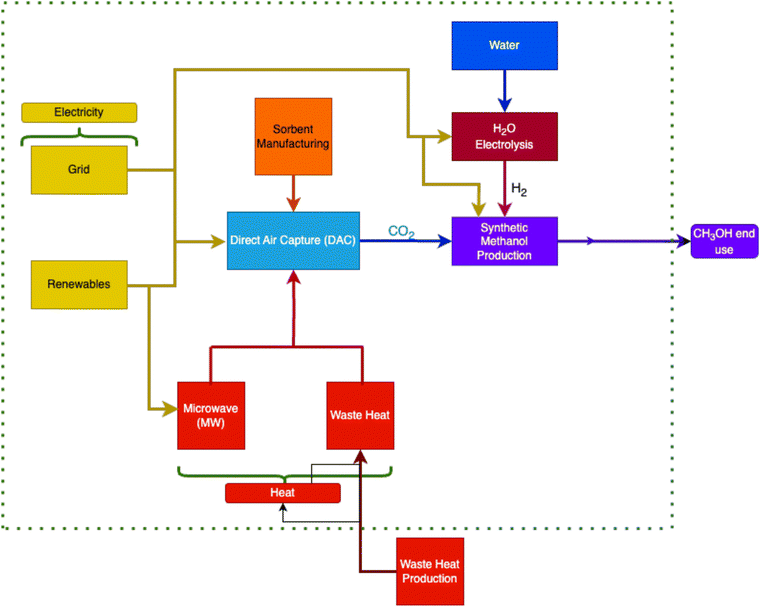
The goal of this work is to analyze multiple sustainable energy scenarios where renewably powered green methanol production and DAC to identify the most sustainable operational methods for GHG reduction. While some sources such as geothermal or hydroelectric would require co-location at a pre-existing or planned plant, others such as PV or wind-powered production could be planned as an off-the-grid, sustainable solution to mitigate climate change in many different areas, from urban to remote. This flexibility is a key advantage to certain renewables which could benefit these processes greatly.Fig. 1 illustrates the proposed system which contains three main components: the CO2 hydrolysis methanol production plant, the low temperature solid sorbent DAC plant, and the electrolysis processes for H2 production. Through this study, a comprehensive investigation on the environmental impact of integrating renewably powered DAC with synthetic methanol production is conducted to lower demand for naturally drilled methanol and thereby close the carbon loop. Using captured CO2 from the DAC plant as an input to synthetic methanol has the added benefit of potentially offsetting the production cost through the sale of green fuel, which could lower the net cost of DAC. This study extends the scope of previous life cycle assessments of DAC systems by focusing on the environmental implications and potential for integrating renewably powered DAC with hydrogen and green methanol production systems.
This study's scope is cradle-to-gate, examining all processes from the extraction of raw materials to production of synthetic methanol. While the end use of methanol is not considered in the scope, all other end of life (EOL) processes associated with DAC and the construction are included. The results of this study will be compared to traditional methanol production methods, also cradle-to-gate, to understand the improvements to the environmental impacts which could be made through investments in green methanol production. All results reported are relative to the functional unit (FU), defined as “1 kg of synthetic methanol produced from carbon dioxide”, one of the FU's recommended by DOE guidance for LCA of carbon dioxide utilization44 with similar variations used in many other studies.15,22,27,29,34–36,45 This FU allows for the comparison of different energy configurations, with a particular focus on the integration of renewables. The research will benchmark the environmental performance of the DAC and methanol system with different energy supply scenarios. Five different electrical power sources are considered for comparison: US grid mix, solar photovoltaic power, onshore wind power, geothermal power, and run-of-river hydroelectric power.
The largest contributor to energy usage for DAC is well established to be during the regeneration phase.46 Hence, this study will analyze two different DAC regeneration heat sources: microwave (MW)-based heat and industrial waste heat (WH). MW heat is explored based on the results of novel experimentation by the research team in the Decarbonization Laboratory of the University of Alabama. Industrial WH is an enticing option for DAC heat due to its potential to be used with low burden as a waste product.40 However, because waste steam is a useful commodity, an exergy-based allocation is used to capture that the WH could have been used for other projects.
Life cycle inventory analysis
The second phase of the LCA methodology is to gather all inputs and outputs associated with the unit processes of the system boundary before the impact results can be calculated.47 In this study, some of the most important unit processes included are methanol production, DAC operations, energy consumption, construction, process chemical production, waste management, and decommissioning. The assumptions for the construction and EOL treatment of the infrastructure is available in the electronic supplementary information (ESI).†Methanol production
Aspen Plus software was used to simulate the process flow for synthetic methanol production via CO2 hydrogenation using an adiabatic fixed-bed catalytic reactor based on the processes studied by Sollai et al.13 and Meunier et al.,11 via eqn (1).11,48Eqn (1) Methanol synthesis reaction.
| CO2 + 3H2 ↔ CH3OH + H2O | (1) |
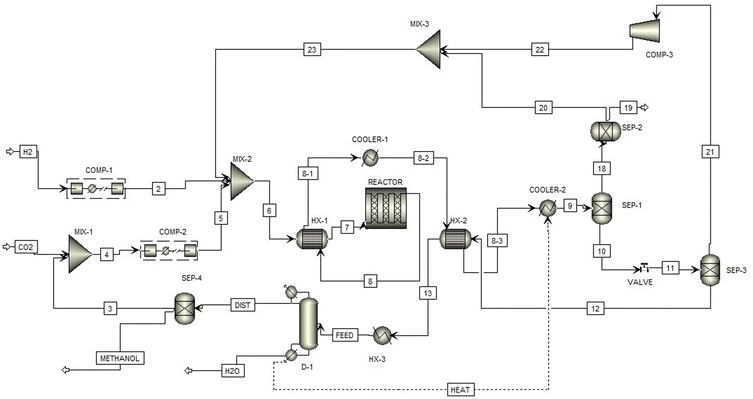
Fig. 2 illustrates the flowsheet of the methanol production process. The CO2 input to the process is concentrated by the DAC system while the H2 is formed through water electrolysis on-site as described in Section 3.3. Prior to entering the reactor, these inputs are mixed and compressed to 6.5 MPa, and they pass through the first heat exchanger to increase to the operating temperature of 210 °C.13 Following the reaction, the majority of unreacted CO2 and H2, and the produced H2O is separated from the outlet stream.11 Most of the remaining H2O is removed in the distillation column, and the remaining CO2 is returned to the inlet.11 Overall, 281 kg h−1 of H2O is calculated to be removed during purification, assumed in the LCA to be sent to water treatment.
The resultant methanol stream is found to have a purity of 95.3%. Most of the separated CO2 and H2 is recycled back to the inlet of the reactor, but the methanol stream still contains trace amounts of CO2 and H2O11 which are purged and flared to remove them from the circuit.13 This purge flow does increase CO2 emissions slightly, but this contribution is almost negligible since it is only about 3% of the total.13 It was also found that there is a CO2 and H2 loses to the atmosphere during the separation processes with the flow rates of 36.32 kg h−1 and 9.64 kg h−1, respectively. These atmospheric release purges slightly lower the net amount of CO2 removed from the air by about 5%, which is accounted for as an air emission.
The catalyst for this reaction is Cu/Zn/Al, which is well documented to be an efficient and economically viable catalyst material.11,13,49,50 The selection of this catalyst is based on its proven capability to operate effectively in fixed-bed catalytic reactors at the required temperatures (250–300 °C) and pressures (50–100 bar), conditions that are ideal for methanol synthesis from CO2 and H2.13 This catalyst has been verified to have a lifetime of at least four years, and eight years has been observed.13,51 Four years is assumed for this study.
Table 1 lists the assumptions for the methanol production plant. The inlet mass-flow rates, temperature, operating pressure requirements, and general flows are based on the research by Sollai et al.,13 while the proportion of unreacted inputs which are recycled derives from Meunier et al.11 The resultant CO2, H2, and H2O outlet flows, and electrical load are calculated in Aspen Plus and align well with the previous values as determined by Sollai.13 The annual design capacity of the methanol plant is based on the conversion of 4 kt CO2 per year.
| Parameter | Value |
|---|---|
| Design capacity | 2.63 kt yr−1 |
| CO2 inlet mass flow | 723 kg h−1 (ref. 13) |
| H2 inlet mass flow | 104 kg h−1 (ref. 13) |
| CO2 outlet mass flow to atmosphere | 36.32 kg h−1 |
| H2 outlet mass flow to atmosphere | 9.64 kg h−1 |
| H2O outlet mass flow to treatment | 281 kg h−1 |
| Methanol outlet mass flow | 500 kg h−1 (ref. 13) |
| Electricity load | 108.64 kW |
| Catalyst amount | 292.4 kg Cu/Zn/Al13 |
| Catalyst lifetime | 4 yr13 |
Direct air capture
The type of DAC analyzed is a low-temperature solid sorbent system of an annual capacity of capacity 4000 tons CO2, similar to the nominal plant capacity analyzed by Deutz and Bardow.52 Auxiliary electrical load for fans and other DAC electronics is assumed to be 0.555 kW h per kg CO2.53 Fan electricity is well documented to play only a small role in overall energy consumption when compared to regeneration heat.40,41,46,53The decision to employ DAC with adsorption rather than absorption is primarily driven by the significant advancements and cost efficiencies associated with low-temperature solid sorbents.54 Compared to absorption, adsorption processes typically require lower energy inputs for regeneration, particularly when utilizing WH from industrial processes. Recent studies,55–57 highlight that the use of solid sorbents can reduce overall heat supply costs, making the technology more economically viable. The continuous improvements in sorbent materials have led to higher CO2 capture capacities and enhanced stability, further lowering the operational costs and enhancing the commercial feasibility of DAC technologies. The main parameters of the DAC system are included in Table 2.
| Parameter | Value |
|---|---|
| DAC design capacity | 4 kt CO2 per year40 |
| Capacity factor | 0.95 |
| DAC equipment lifetime | 20 yr40,41,46 |
| DAC location (PV-powered) | Tuscaloosa, AL, USA and Phoenix, AZ, USA |
| DAC location (grid-powered) | United States of America |
| DAC location (wind, geothermal, hydro-powered) | Western United States of America |
| Base electricity load | 0.555 kW h per kg CO253 |
| Heat load (MW) | 4.8 MJ per kg CO2 captured58 |
| Heat load (WH) | 7.72 MJ per kg CO2 captured59 |
| Electrical source | U.S. grid mix, PV, wind, geothermal, hydroelectric |
| Heat source | MW, WH |
| Sorbent type | Zeolite 13X on a cordierite substrate |
| Sorbent consumption rate | 0.788 g per kg CO2 captured60 |
To compare the life cycle impacts of regeneration heat sources, heat is assumed to be supplied to the DAC system from each of two sources: MW and industrial WH. MW-based heat is based on two the principles of microwave swing desorption (MSD) as studied by Erguvan et al.,61 who examined the potential of using MW technology for the desorption of CO2 from zeolite 13X. MW as a source for regeneration heat for carbon capture and DAC had previously been proposed by others.62–65 Erguvan et al. focused on simulated flue gas capture, and subsequent work was performed to determine the regeneration heat requirements of a similar setup but for ambient air through DAC.58 During this LCA, MW-based regeneration heat will be a scaled-up DAC regeneration system based on the results of experimental MW DAC work performed by Boylu, Erguvan, and Amini58 as shown in Fig. 3.
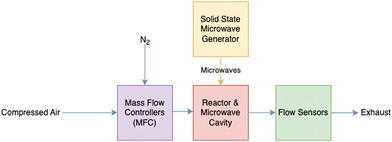 | ||
| Fig. 3 General flow diagram of the laboratory experimental setup for the DAC with MW regeneration and zeolite 13X sorbent. | ||
The efficiency of two key processes in MW-based DAC–adsorption and desorption processes were investigated using a mono-mode microwave unit. Microwave power and regeneration temperature were varied from 4 to 30 W. Three grams of zeolite 13X were placed in the reactor, and the microwave unit was set to reach a target temperature of 33 °C, 50 °C, and 100 °C.
The sorbent, zeolite 13X, was preconditioned in a conventional oven at 350 °C overnight to eliminate volatiles, following the method by Ellison et al.66 Subsequently, the reactor was purged with nitrogen gas at a flow rate of 200 mL min−1 for 20 minutes to create a CO2-free environment. During the adsorption phase, compressed air was introduced with a flow rate of 750 mL min−1 to simulate fluidized bed behaviors. The adsorption process was deemed complete when the sorbent reached CO2 saturation within two hours.
Following adsorption, the feed gas to the reactor was switched to N2 with the same flow rates to purge any desorbed CO2. Concurrently, the microwave heating was initiated at each power level to achieve and maintain the desired temperature for regeneration. The power output of the MW generator was adjusted dynamically to maintain the temperature as close as possible to the target. The regeneration phase was concluded once the CO2 concentration in the effluent gas dropped to 30 ppm. This meticulous control of microwave power ensured that the regeneration temperature remained stable, illustrating the precise management of the desorption process under the influence of microwave heating.
It was found that the most efficient microwave parameters for DAC regeneration are using a MW power setting of 4 W and with a regeneration temperature of 33 °C which achieved a regeneration energy demand of 4.8 MJ per kg CO2.58 This regeneration heat value is assumed for all MW DAC configurations.
Zeolite 13X was selected as the sorbent for the WH case as well to facilitate closer comparison to MW. The details of the inventory for zeolite manufacturing and lifetime are included in the ESI.† When determining the heat requirements for desorption of CO2 with WH, there is some variance in the literature regarding regeneration heat requirements for zeolite 13X. Generally, sources have demonstrated a heat load of 3–6 MJ per kg CO2 in temperature ranges of 125–200 °C;59,67–70 in this study, we will use the value of 7.72 MJ kg−1 at 100 °C for the case of WH,59 as it falls near the middle of the values reported in the literature, and is a temperature of heat readily available in industry.
The availability of WH for DAC desorption is substantial, particularly in industrial settings such as power plants, chemical plants, and refineries. In industrial facilities, including power plants, about 60–70% of the energy produced is discarded as waste heat.71 This makes WH a practical and cost-effective option for DAC systems. Typically, WH for the proposed DAC system with a capacity of 4 kt per year would require 0.978 MW heat on average. For a 1000 MW fossil fuel plant at the average efficiency of 36%,71 this corresponds to up to 640 MW waste heat available. Some previous studies have not considered the burden due to use of WH, considering it free energy.40 In this study, an exergy calculation is applied as the allocation to demonstrate the usefulness potential of the steam, as shown in eqn (2). Q is given as the heat requirement of the steam, T0 is the assumed ambient temperature of 25 °C, and T is the saturated required steam temperature of 100 °C.59
WH and MW heating each offer unique benefits for DAC desorption. WH, commonly found in industrial settings, can provide a cost-effective source of thermal energy by repurposing heat that would otherwise be lost. This makes WH a practical option where industrial processes produce ample excess heat. However, its availability and efficiency depend on the specific industrial context and the quality of the heat source, and DAC is limited to a location near an ample WH source. Conversely, MW heating has the potential to offer superior energy efficiency and greater operational flexibility. Even at lab scale, MW heating can achieve lower regeneration energy demands and precise, rapid heating directly to the sorbent material, making it especially useful in locations where WH is unavailable. This technology's ability to deliver targeted heating with lower energy consumption is expected to improve with further research.
Eqn (2) exergy calculation used in the allocation of waste steam, where Q is the heat requirement, T0 is the ambient temperature, and T is the system temperature, with both temperatures are units of absolute temperature.72
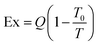 | (2) |
Hydrogen production
Hydrogen production as an input to the catalytic hydrogenation of CO2 for synthetic methanol production is assumed to take place on location with methanol production using H2O electrolysis through a polymer electrolyte membrane (PEM) electrolyzer and using the mass and energy flows as researched by Sollai et al.13 The selection of this technology was influenced by its quick activation from a cold state, enhanced adaptability, and thus, a more effective integration with variable and intermittent power sources, including renewable energies like solar and wind, which makes this production method easily adaptable to the renewable sources analyzed.13 Despite these advantages, PEM electrolysis technology faces challenges, including higher costs and limited operational lifespan. Current systems typically last around 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours before requiring stack replacement due to degradation, though advancements are expected to extend this to 85
000 hours before requiring stack replacement due to degradation, though advancements are expected to extend this to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours. The specific energy consumption ranges from 50 to 80 kWh per kilogram of hydrogen produced. For optimal environmental benefits, it is essential to power PEM electrolyzers with renewable energy, as demonstrated in the e-methanol production study by Sollai et al., which utilized overgenerated renewable electricity to minimize carbon footprint and enhance sustainability.13
000 hours. The specific energy consumption ranges from 50 to 80 kWh per kilogram of hydrogen produced. For optimal environmental benefits, it is essential to power PEM electrolyzers with renewable energy, as demonstrated in the e-methanol production study by Sollai et al., which utilized overgenerated renewable electricity to minimize carbon footprint and enhance sustainability.13
In our study, the produced H2 is used as an input to the methanol production process. The outlet flow of O2 is assumed to be released to the atmosphere as an unwanted product. The construction of the electrolyzer is assumed to be included in the infrastructure construction of the methanol plant. Sensitivity analysis has been conducted to confirm the impacts to the study results based on construction inventories. The mass flow rate of the hydrogen production unit has been listed in Table 3.
Energy scenarios
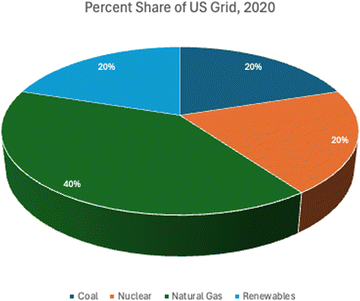
As discussed earlier, five different electrical energy scenarios, US grid mix, solar photovoltaic, onshore wind power, geothermal power, and run-of-river hydroelectric power, are analyzed in this study. All processes are assumed to take medium voltage electricity, which is standard for most industrial applications. US grid mix is the average energy mix for the United States based on 2020 data.73 By 2020 the US grid has converted power generation by fuel type to about 20% coal, 20% nuclear, 40% natural gas, and 20% renewables as shown in Fig. 4, down from 49% coal, 19% nuclear, 22% natural gas, 2% oil, and just 8% renewables in 2007.74 As the Unites States continues to drive development of renewables, nuclear, and clean natural gas with carbon capture, the grid will continue to improve its carbon footprint. However, the purpose of this research is to identify the most favorable scenarios for implementation of sustainably powered methanol production. Therefore, the US grid is intended to serve mainly as a comparison tool; the results will show that powering these processes through dedicated renewables will have a more favorable impact to the environment.For wind, geothermal, and hydroelectric power, ecoinvent 3.8 processes were used.75 The assumption is that the methanol and DAC facilities are developed in proximity to pre-existing power plants of the respective type, since the power consumption is relatively small compared to the full capacity of many of these facilities; therefore, construction is only accounted for as a component of the use inventories developed by ecoinvent. Inventories from the Western Electricity Coordinating Council (WECC) for wind, hydroelectric, and geothermal power were used because the WECC reports separately by renewable energy type in ecoinvent while other US sources do not. Wind is assumed to be 1–3 MW onshore turbines which would be more than capable of supplying the electrical load for the facility. According to ecoinvent, as of the year in which data was collected for wind power inventories in 2014, 93.2% of all wind farms in the western United States use 1–3 MW turbines.73 For this study, geothermal power is assumed to be deep well, and hydroelectric is run-of-river type.
PV-based electricity scenarios are set in two locations: Tuscaloosa, Alabama (AL) and Phoenix, Arizona (AZ). Tuscaloosa was chosen since it is a location with medium-high sunlight, medium humidity, and sufficient land area for development. Since the University of Alabama's Decarbonization Laboratory is based in Tuscaloosa, this analysis will help to demonstrate the viability for future renewably-powered DAC projects. Phoenix, Arizona was chosen through an optimization process which analyzed the annual solar irradiation, cloud cover, and available land near utilities infrastructure and support systems to find the most beneficial location for PV in the U.S.76,77
The sizing of PV was completed in both locations for each of the two regeneration heat sources since the total annual electrical load varies depending on heat source. The solar arrays are sized to provide on average the full annual power requirement of all processes: methanol production, hydrogen electrolysis, and DAC. Input parameters and calculated nominal capacities required are shown in Table 4. The solar arrays are assumed to be connected to the grid to supply additional electricity needed for night and times of low PV production while supplying renewable energy to the grid when producing excess for an average annual net grid electrical consumption of zero. All solar irradiation and PV data is provided by the Global Solar Atlas developed by World Bank Group, which uses complex PV modeling algorithms, paired with historical solar irradiation data, to estimate monthly and annual electrical energy production based on user inputs.77 Construction and EOL processes are fully considered: the construction of the plant includes the ecoinvent infrastructure process of a 570 kWp PV plant scaled based on the nominal PV size required to supply the annual electrical load for the respective energy configuration and reference location in order to capture all the PV electrical infrastructure and construction rather than simply the solar panels. EOL recycling and waste treatment processes are based on research for user-scale smart grid treatment by Rossi et al.78,79 and Latunussa et al.80
| Solar Parameter | Tuscaloosa, AL | Phoenix, AZ |
|---|---|---|
| Global tilted irradiation | 1885 kW h m−2 yr−1 | 2437 kW h m−2 yr−1 |
| Optimal angle | 30° | 32° |
| Azimuth | 180° | 180° |
| Power requirement (MW) | 38.79 GW h yr−1 | 38.79 GW h yr−1 |
| Power requirement (WH) | 33.72 GW h yr−1 | 33.72 GW h yr−1 |
| System capacity (MW) | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 436 kWp 436 kWp |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491 kWp 491 kWp |
| System capacity (WH) | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 kWp 111 kWp |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 813 kWp 813 kWp |
| System area (MW) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 m2 476 m2 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 495 m2 495 m2 |
| System area (WH) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799 m2 799 m2 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 m2 340 m2 |
For all power scenarios, two possibilities for DAC regeneration heat were considered: MW and industrial WH. While each case is considered for purposes of comparison, some are less likely to be implemented in practicality; for instance, if a DAC plant is located nearby a geothermal power plant, it is likely that WH could be used as a heat source as in a current implementation by Climeworks.40 Therefore, needing MW-generated heat would likely be less likely than in a remote location powered by PV. These cases are all included in the study for full comparison, but the practicality of implementation depends on the specific application.
Impacts of weather
When exploring the viability of any system powered by renewable energy such as wind or solar, it is imperative to consider the inherent variability and dependency on weather conditions of the primary renewable energy inputs. These sources can fluctuate substantially in output since their availability and efficiency are directly influenced by weather patterns, which can vary significantly over time and location. As a result, the assumptions regarding energy supply for the DAC-to-methanol system are based on average values derived from historical weather and usage data and projected trends. However, this introduces a degree of variability and uncertainty into the energy supply estimates, impacting the overall efficiency and environmental performance of the DAC system. The solar PV cases analyzed have arrays sized to overproduce to the grid during times of high solar irradiation while using grid energy during low production with no net grid usage annually, although in practicality this can vary due to weather. Therefore, when assessing the practicality and reliability of such renewable-powered systems, it is crucial to implement strategies that mitigate the risks associated with their weather-dependent nature to ensure stable and efficient operations.The performance of DAC systems using moisture-swing sorbents is notably sensitive to ambient weather conditions, especially temperature and humidity.81,82 These systems are more effective in hot, dry climates where the absence of humidity facilitates the release of CO2 during the regeneration phase. In contrast, operation in cold and moist climates can be less efficient and may require adaptations, such as the addition of a mild thermal swing to improve performance.82 Zeolite 13X performance may be significantly degraded in high humidity such as 60% because the interactions between the zeolite and water molecules are so strong that they cannot be removed without significant heating.83 Due to the variable nature of the weather and humidity, actual performance could vary, and careful consideration should be taken when selecting the location for a DAC facility.
Life cycle impact assessment
In this study, a comprehensive LCA has been conducted to evaluate the environmental impacts and efficiency of DAC-to-methanol systems powered by low-carbon energy sources. The inventories for each case were calculated in SimaPro 9.5, and unit processes used were primarily from the ecoinvent 3.8 database.75 Based on guidelines from the US DOE regarding conducting a LCA on DAC systems, a modified version of EPA's TRACI version 2.1 method (US, 2008) was used to characterize the impact categories of ozone depletion, global warming, smog formation, acidification, eutrophication, carcinogenics, non-carginogenics, respiratory effects, ecotoxicity, and fossil fuel depletion.47 Since TRACI 2.1 was last updated in 2012, it does not reflect recent GWP characterization factors from the IPCC.47 Hence, the TRACI 2.1 method was modified for global warming to add or change the characterizations to reflect the recent IPCC 2021 report.84Table 5 demonstrates the comparative results of methanol production with a MW heat source for each energy scenario, compared to traditional production via steam methane reforming (SMR), while Table 6 similarly shows results for the WH cases. Renewably powered MW emerges as the better heat source for environmental impacts, which opens the possibility for more sustainable DAC deployment in remote locations away from WH sources, although co-location with an industrial WH source is shows great improvement in many impact categories over traditional production.
| Impact category | Unit | MW, Grid | MW, PV (AL) | MW, PV (AZ) | MW, Wind | MW, Hydro | MW, Geothermal | SMR |
|---|---|---|---|---|---|---|---|---|
| Ozone depletion | kg CFC-11 eq. | 4.78 × 10−7 | 1.27 × 10−7 | 1.01 × 10−7 | 7.75 × 10−9 | −2.91 × 10−9 | 3.74 × 10−8 | 2.33 × 10−7 |
| Global warming | kg CO2 eq. | 5.57 × 100 | −1.36 × 100 | −1.60 × 100 | −2.39 × 100 | −2.53 × 100 | −1.47 × 100 | 9.44 × 10−1 |
| Smog | kg O3 eq. | 1.76 × 10−1 | 4.26 × 10−2 | 2.96 × 10−2 | −1.17 × 10−2 | −1.87 × 10−2 | 3.43 × 10−2 | 2.51 × 10−2 |
| Acidification | kg SO2 eq. | 2.04 × 10−2 | 6.50 × 10−3 | 5.32 × 10−3 | 1.41 × 10−3 | 6.98 × 10−4 | 4.83 × 10−3 | 1.99 × 10−3 |
| Eutrophication | kg N eq. | 4.18 × 10−2 | 4.14 × 10−3 | 3.21 × 10−3 | 1.91 × 10−4 | −5.22 × 10−4 | 2.98 × 10−3 | 5.51 × 10−4 |
| Carcinogenics | CTUh | 1.48 × 10−6 | 1.22 × 10−6 | 1.17 × 10−6 | 1.16 × 10−6 | 9.90 × 10−7 | 1.24 × 10−6 | 2.48 × 10−8 |
| Non-carcinogenics | CTUh | 3.02 × 10−6 | 2.00 × 10−6 | 1.86 × 10−6 | 1.41 × 10−6 | 1.29 × 10−6 | 1.54 × 10−6 | 5.82 × 10−8 |
| Respiratory effects | kg PM2.5 eq. | 1.43 × 10−2 | 2.69 × 10−4 | 5.77 × 10−6 | −8.08 × 10−4 | −9.50 × 10−4 | 1.63 × 10−4 | 2.07 × 10−4 |
| Ecotoxicity | CTUe | 1.89 × 102 | 1.99 × 102 | 1.83 × 102 | 1.48 × 102 | 1.19 × 102 | 1.33 × 102 | 3.65 × 100 |
| Fossil fuel depletion | MJ surplus | 7.12 × 100 | 4.40 × 10−1 | 2.41 × 10−1 | −3.73 × 10−1 | −5.29 × 10−1 | 1.15 × 10−1 | 5.06 × 100 |
| Impact category | Unit | MW, Grid | MW, PV (AL) | MW, PV (AZ) | MW, Wind | MW, Hydro | MW, Geothermal | SMR |
|---|---|---|---|---|---|---|---|---|
| Ozone depletion | kg CFC-11 eq. | 4.40 × 10−7 | 1.35 × 10−7 | 1.20 × 10−7 | 3.18 × 10−8 | 2.25 × 10−8 | 5.75 × 10−8 | 2.33 × 10−7 |
| Global warming | kg CO2 eq. | 4.80 × 100 | −1.22 × 100 | −1.31 × 100 | −2.12 × 100 | −2.24 × 100 | −1.32 × 100 | 9.44 × 10−1 |
| Smog | kg O3 eq. | 1.58 × 10−1 | 4.22 × 10−2 | 3.84 × 10−2 | −4.97 × 10−3 | −1.11 × 10−2 | 3.50 × 10−2 | 2.51 × 10−2 |
| Acidification | kg SO2 eq. | 1.86 × 10−2 | 6.53 × 10−3 | 6.39 × 10−3 | 2.11 × 10−3 | 1.49 × 10−3 | 5.08 × 10−3 | 1.99 × 10−3 |
| Eutrophication | kg N eq. | 3.65 × 10−2 | 3.78 × 10−3 | 3.37 × 10−3 | 3.44 × 10−4 | −2.76 × 10−4 | 2.76 × 10−3 | 5.51 × 10−4 |
| Carcinogenics | CTUh | 1.41 × 10−6 | 1.19 × 10−6 | 1.20 × 10−6 | 1.13 × 10−6 | 9.87 × 10−7 | 1.21 × 10−6 | 2.48 × 10−8 |
| Non-carcinogenics | CTUh | 2.82 × 10−6 | 1.93 × 10−6 | 1.86 × 10−6 | 1.42 × 10−6 | 1.32 × 10−6 | 1.53 × 10−6 | 5.82 × 10−8 |
| Respiratory effects | kg PM2.5 eq. | 1.23 × 10−2 | 1.88 × 10−4 | 9.33 × 10−5 | −7.48 × 10−4 | −8.72 × 10−4 | 9.57 × 10−5 | 2.07 × 10−4 |
| Ecotoxicity | CTUe | 1.78 × 102 | 1.87 × 102 | 1.75 × 102 | 1.42 × 102 | 1.17 × 102 | 1.29 × 102 | 3.65 × 100 |
| Fossil fuel depletion | MJ surplus | 6.56 × 100 | 7.47 × 10−1 | 6.56 × 10−1 | 4.08 × 10−2 | −9.51 × 10−2 | 4.65 × 10−1 | 5.06 × 100 |
Results in Fig. 5 demonstrate that all renewably powered DAC-to-methanol systems which utilize MW or WH for regeneration are net negative and much more favorable for GWP than traditional methanol production routes. This study finds that the most favorable renewable energy source to power the DAC-to-methanol processes is run-of-river hydroelectric, followed by wind power. These two sources generally were the most favorable among the renewables simply based on the low emissions cataloged in the WECC inventory based on historical plant data.75 The PV cases and geothermal power also demonstrate the potential for improvements in GWP impact compared to traditional methanol production via SMR.
 | ||
| Fig. 5 Relative impacts for methanol production with various energy sources from MW-based DAC compared to traditional production via SMR, per kg of methanol produced. | ||
The difference in impacts between AL and AZ is due to addition to the higher solar irradiation and greater capacity factor associated with Phoenix, which allows smaller sized PV infrastructure to be needed for the required annual capacity. The most favorable DAC regeneration heat scenario is the use of WH from outside industrial processes, as this source provides less burden than the other heat sources. In all cases, the methanol hydrogenation catalyst demonstrated only negligible effects to the overall system life cycle compared to energy usage and construction. Further results for each energy configuration are available in the ESI.†
Comparison to traditional methanol production
The results of the study are also compared to traditional methanol production in Tables 5 and 6 through SMR using the ecoinvent process for methanol production, which derives its values from literature on plant design and efficiency.75 Notably, while all renewably powered cases demonstrate favorable GWP benefits compared to traditional SMR production, SMR shows lower impacts than many of the energy scenarios for smog, acidification, acidification, eutrophication, carcinogenics, non-carcinogenics, and ecotoxicity, as demonstrated in Fig. 5.The largest contributor to each of these impacts in the DAC-to-methanol system is hydrogen production via hydrolysis, due to the high amount of energy required. The electrolysis process for green hydrogen production via hydroelectric energy was compared to hydrogen production via SMR in Table 7 to identify whether this method could be a favorable replacement process. Results demonstrate that despite the high electrical energy requirement, electrolysis remains less impactful than SMR except for ecotoxicity and carcinogenics. As with the other processes in this DAC-to-methanol system, ensuring electrolysis is integrated with renewable sources is key to minimizing most impacts.
| Impact category | Unit | Hydrogen via SMR | Electrolysis |
|---|---|---|---|
| Ozone depletion | kg CFC-11 eq. | 2.45 × 10−6 | 2.28 × 10−8 |
| Global warming | kg CO2 eq. | 1.79 × 100 | 2.73 × 10−1 |
| Smog | kg O3 eq. | 1.30 × 10−1 | 2.27 × 10−2 |
| Acidification | kg SO2 eq. | 1.57 × 10−2 | 1.11 × 10−3 |
| Eutrophication | kg N eq. | 3.93 × 10−3 | 5.56 × 10−4 |
| Carcinogenics | CTUh | 5.73 × 10−8 | 1.54 × 10−7 |
| Non-carcinogenics | CTUh | 1.39 × 10−7 | 6.14 × 10−8 |
| Respiratory effects | kg PM2.5 eq. | 1.41 × 10−3 | 5.19 × 10−4 |
| Ecotoxicity | CTUe | 4.77 × 100 | 6.02 × 100 |
| Fossil fuel depletion | MJ surplus | 2.12 × 101 | 2.14 × 10−1 |
This analysis assumes that the O2 produced during hydrolysis is compressed and saved as a co-product, which claims a credit based on the process inventory for production via cryogenic air separation, the most efficient and cost-effective form of O2 production.75 The total GWP credit for O2 is substantial at −2.03 kg CO2 eq. per kg methanol produced. As such, retaining the produced O2 for industrial use or resale should be considered as some processes may fail to reach net negative emissions, even when powered by renewable energy.
Sensitivity analysis
Due to unknowns in the construction inventories, sensitivity analysis was performed per DOE guidance44,47 by varying the DAC construction inventories by ±50%. Table 8 shows the impacts for each DAC regeneration heat source with hydroelectric power, since hydroelectric demonstrates the least environmental burden of all cases examined, and therefore is most sensitive to changes in construction. Results demonstrate that GWP varied by less than 1%, which substantiates previous studies that construction of DAC generally has a low life cycle impact when compared to the lifetime of operation energy use.85 Ozone depletion varied the most by up to 27% in the MW case due to the high ozone burden of the MW inventory. Other impact categories were generally between 1–12% of the baseline results in Table 5.| Impact category | Unit | MW – low construction | MW – high construction | WH – low construction | WH – high construction |
|---|---|---|---|---|---|
| Ozone depletion | kg CFC-11 eq. | −3.61 × 10−9 | −2.21 × 10−9 | 2.19 × 10−8 | 2.31 × 10−8 |
| Global warming | kg CO2 eq. | −2.54 × 100 | −2.52 × 100 | −2.25 × 100 | −2.23 × 100 |
| Smog | kg O3 eq. | −1.94 × 10−2 | −1.80 × 10−2 | −1.17 × 10−2 | −1.05 × 10−2 |
| Acidification | kg SO2 eq. | 6.21 × 10−4 | 7.75 × 10−4 | 1.43 × 10−3 | 1.55 × 10−3 |
| Eutrophication | kg N eq. | −5.68 × 10−4 | −4.75 × 10−4 | −3.10 × 10−4 | −2.42 × 10−4 |
| Carcinogenics | CTUh | 9.83 × 10−7 | 9.96 × 10−7 | 9.82 × 10−7 | 9.92 × 10−7 |
| Non-carcinogenics | CTUh | 1.29 × 10−6 | 1.30 × 10−6 | 1.31 × 10−6 | 1.32 × 10−6 |
| Respiratory effects | kg PM2.5 eq. | −9.65 × 10−4 | −9.35 × 10−4 | −8.83 × 10−4 | −8.60 × 10−4 |
| Ecotoxicity | CTUe | 1.17 × 102 | 1.20 × 102 | 1.16 × 102 | 1.17 × 102 |
| Fossil fuel depletion | MJ surplus | −5.37 × 10−1 | −5.21 × 10−1 | −1.01 × 10−1 | −8.91 × 10−2 |
Interpretation
Assessment of GWP impact
The results of this study indicate a significant potential for carbon removal, aligning with the objectives of global carbon neutrality goals. Notably, energy source and energy efficiency with capture technology were found to be the most critical in determining the life cycle carbon emissions and energy efficiency of these systems. Analysis reveals that DAC-to-methanol systems utilizing renewable energy sources exhibit markedly lower life cycle GHG emissions compared to those powered by conventional fossil fuels and energy mixes, as observed for DAC systems by previous studies.17,18,21,22 This stresses the importance of integrating DAC with sustainable energy systems to maximize environmental benefits. However, this study advances the conversation by providing a nuanced understanding of how specific low-carbon energy sources can optimize the performance and sustainability of DAC systems. This contributes valuable insights into the ongoing efforts to develop scalable and economically viable solutions for atmospheric carbon reduction. Future DAC research should focus on optimizing these systems for broader implementation, including exploring innovative regeneration energy solutions and sorbent technologies.The main findings indicate that the most detrimental energy configurations for climate change are grid or fossil-fuel based sources, as did McQueen et al.86 who found that the integration of renewable energy sources with a DAC-based system provides profound environmental implications over natural gas. The GWP impacts of each energy scenario, shown in Fig. 6, highlight the substantial benefits of integrating renewable energy sources with synthetic fuel production and DAC technologies. Scenarios utilizing solar PV energy in AL and AZ demonstrate an improvement in GWP contributions compared to traditional sources but are impacted greatly due to the heavy construction and EOL environmental burden of PV infrastructure. The integration of hydroelectric, wind, and geothermal energy sources with green methanol and DAC technologies significantly lowers the GWP when compared to the US grid, further reinforcing that renewable energy sources are pivotal to the environmental sustainability. This research agrees with the study by Khojasteh-Salkuyeh et al.,12 which found that the CO2 hydrogenation process used for methanol production can be environmentally friendly but should be assessed on a regional level, and only where low-carbon energy sources are available.
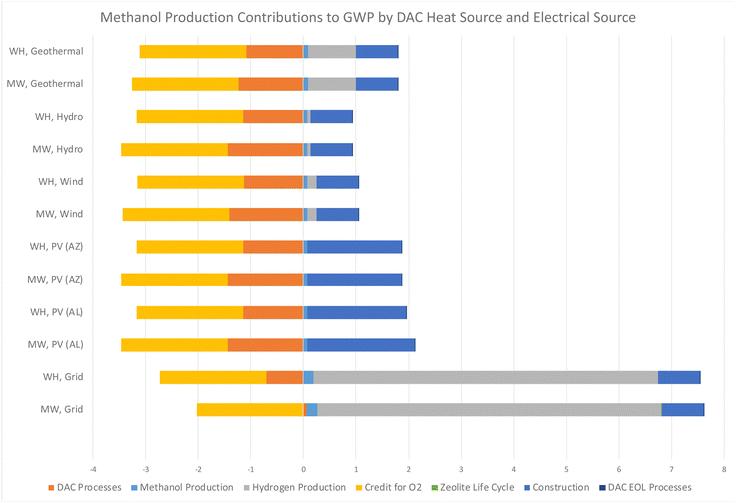 | ||
| Fig. 6 Contributions to GWP for each configuration of a DAC regeneration heat source and electrical energy supply, per kg of synthetic methanol produced, in units of kg CO2 eq. | ||
The variability in environmental impacts across different DAC heat configurations highlights the importance of technology selection. The results indicate that scenarios employing WH recovery exhibit low GWP contributions, highlighting the efficiency and environmental benefits of leveraging WH in DAC processes in agreement with the study conducted by Deutz and Bardow.40 This suggests that optimizing DAC technology selection, based on environmental impact assessments, is crucial for minimizing the carbon footprint of methanol production systems. Research to further maximize the efficiency of MW technology for DAC regeneration demonstrates the life cycle improvements this technology can have on overall impacts, and continued research into this area is warranted.
The study's outcomes have profound implications for the implementation of DAC and carbon utilization technologies. By demonstrating the environmental advantages of renewable energy integration and the critical role of technology selection, the findings of this study provide a solid foundation for policies that encourage the adoption of sustainable green energy systems. The variability in GWP contributions across different scenarios brings to light the necessity for region-specific strategies that leverage local renewable energy resources, optimizing the sustainability of DAC-integrated methanol production. This study points to the need for further investigation focusing on optimizing DAC processes and technologies, particularly in the context of exploring new methods to improve energy efficiency and implementing new MW regeneration technology or WH recovery with renewable energy sources.
Assessment of other impacts
Traditional methanol production via SMR shows substantially higher impacts compared to any of the renewable energy sources. This is primarily due to the chemical and energy-intensive processes involved in SMR, which contribute significantly to ozone-depleting substances. In contrast, hydroelectric, wind, and PV technologies exhibit much lower ozone depletion potential, which demonstrates the environmental advantages of using renewable energy for DAC processes.For smog formation, hydroelectric and wind energy emerge as net negative over the life cycle. This is attributed to their minimal emissions of nitrogen oxides (NOx) and volatile organic compounds (VOCs), which are primary contributors to smog. Interestingly, SMR slightly outperforms the other renewables, except for hydro and wind, indicating its relatively lower contribution to smog formation compared to PV and the grid mix.
When considering acidification and eutrophication, SMR performs comparably to hydroelectric and wind energy. These impacts, resulting from emissions that lead to soil and water acidification and nutrient enrichment, show that SMR can be competitive with renewables when it comes to managing acidification and eutrophication. Although PV and the grid mix have higher impacts, SMR's controlled emissions offer a balanced performance similar to wind and hydroelectric.
Carcinogenic and non-carcinogenic impacts are significantly lower for SMR compared to all other energy sources. The controlled environment of SMR helps reduce the release of carcinogenic and toxic substances, making it a cleaner option. In contrast, renewables and grid-based electricity, which involve broader and less controlled emission sources during manufacturing and operation phases, exhibit higher impacts in these categories.
For respiratory effects, SMR's impact is comparable to most renewable sources, except for hydroelectric and wind, which are net negative, which indicates that these two sources could potentially reduce the potential for respiratory-related health issues. SMR, while not as beneficial as hydro and wind, performs similarly to other renewable options, making it an acceptable alternative in this regard.
Ecotoxicity, which measures the potential harm to ecosystems from pollutants, shows that SMR performs far better than all other cases. The PV systems, particularly in Alabama, exhibit the highest ecotoxicity, followed by the U.S. grid mix and PV in Arizona. The production and disposal processes of PV panels contribute significantly to ecotoxicity, whereas SMR's more contained processes result in lower impacts.
Lastly, fossil fuel depletion is a measure of the consumption of non-renewable energy resources. SMR has a very high fossil fuel depletion rate, second only to the U.S. grid mix. Renewable energy sources, including hydroelectric, wind, and PV, show much-improved performance in this category. Notably, hydroelectric power is the only option with a net negative fossil fuel depletion, indicating it offsets fossil fuel use.
Scalability and infrastructure requirements
The scalability and infrastructure requirements for deploying DAC-to-fuel systems such as synthetic methanol are significant yet surmountable challenges that hinge on strategic integration with renewable energy sources and efficient heat management for DAC regeneration. This study has demonstrated the pivotal role of leveraging renewable energy configurations such as PV, wind, hydroelectric, and geothermal energy sources in driving down GHG emissions associated with synthetic methanol production. However, the transition from conventional to green methanol production via DAC systems necessitates a comprehensive approach to scalability and infrastructure development. This includes the co-location of DAC facilities with renewable energy sources or existing industrial operations to utilize WH effectively, thereby addressing the substantial energy demands of DAC regeneration processes while potentially lessening the infrastructure burden through shared infrastructure.Renewable energy sources, such as solar and wind, are integral to decarbonizing the energy sector and providing sustainable power for DAC processes. However, it is important to clarify that while these renewable energy sources promote decarbonization, the mineral extraction process for manufacturing PV panels and wind turbines is highly costly. Additionally, the CO2 emissions associated with the entire production process, water consumption in maintenance, land use, and subsequent recycling are significant. These factors must be considered to fully understand the environmental impacts of deploying renewable energy technologies on a large scale.
Recent studies and industry developments demonstrate the feasibility and potential of scaling up DAC in conjunction with renewable energy sources and electrolysis. Climeworks' “Orca” plant in Iceland, which uses geothermal energy, has successfully demonstrated the feasibility of DAC at an industrial scale, removing almost 4000 tons of CO2 annually.87 Integrating DAC with renewable energy sources, such as photovoltaic panels and wind turbines, is essential for sustainable scaling.88 The energy-intensive nature of DAC requires the use of low-carbon energy to minimize additional emissions. For example, solvent DAC plants use high-temperature heat, typically from natural gas, while sorbent plants can utilize low-temperature heat from renewable sources or WH. Furthermore, the scalability and environmental impact of DAC technologies are enhanced through continuous improvements in sorbent materials and modular plant designs, reducing energy requirements and costs.89 Scaling DAC responsibly involves understanding its environmental and social impacts. Proper siting and community engagement are crucial to minimizing negative impacts and maximizing benefits.87 These advancements make DAC a more viable option for large-scale carbon removal. The successful implementation of large-scale DAC will depend on strategic planning, responsible scaling practices, and continuous innovation.
The exploration of novel MW-based regeneration techniques, as compared to industrial WH, presents an innovative avenue for reducing energy consumption and improving system efficiency. Moreover, the integration of these systems with green hydrogen production, derived from water electrolysis powered by renewable energy, further enhances the environmental sustainability of the DAC-to-fuel process. This new regeneration heat method should be targeted for future research to improve on the scalability to meet the requirements of modern, full scale DAC systems when industrial WH may not be an option.
Policy and market dynamics
In 2021, the global methanol market was valued at over $37.4 billion and is projected to reach nearly $61.7 billion by 2030, with a continuous annual increase in production worldwide.90 In 2022, global methanol production was estimated to have exceeded 111 million metric tons, growing by nearly four percent from the previous year. The United States plays a significant role in the methanol market, both as a top exporter and a major importer. While methanol plays an important role in the US and world economies, it is important to shift toward more sustainable production and less dependence on fossil fuels to mitigate climate change.Incorporating DAC systems and synthetic fuel production into the US market and with the current policy landscape is a crucial step toward mitigating climate change through carbon neutrality. This shift is reinforced by substantial federal support such funding allocations in legislation such as the Infrastructure Investment and Jobs Act,91 which has earmarked $3.5 billion for carbon management technologies, and the Inflation Reduction Act of 2022,92 which has devoted an estimated $370 billion total to energy security and climate change initiatives. Tax incentives such as the expanded 45Q tax credit92 further demonstrate the financial viability of DAC projects. These policy frameworks are complemented by dynamic market forces, where both start-ups and established corporations are venturing into DAC and synthetic fuels, spurred by potential carbon offset markets and sustainability objectives. Collaborations across private, governmental, and academic spheres are crucial for surmounting technical hurdles and scaling solutions. Yet, the commercial deployment of DAC technologies and synthetic fuel production is fraught with challenges, chiefly cost and energy efficiency. Innovations aimed at reducing these barriers are vital for competitive parity with traditional fuels and carbon capture methods, marking a pivotal era for DAC systems and synthetic fuel production in aligning economic incentives with climate goals.
Comparison to other studies
While the literature on methanol production via point source liquid amine based CCU is extensive, DAC integrated with methanol synthesis is less commonly studied. This discrepancy limits the possibility of direct comparisons between the two methods. However, understanding the differences in GHG impacts between traditional CC and innovative DAC methods is crucial for evaluating the potential and efficiency of newer technologies like DAC in achieving lower emissions. Therefore, the results for GWP from this study were compared to the assessment by Cordero-Lanzac et al.,29 who compared the impacts of green methanol production from amine-based point source carbon capture with two different energy configurations: renewable and non-renewable sources in the EU. Their results, converted to this study's functional unit, found that renewables demonstrated a GWP impact of about −1.45 kg CO2 eq. while non-renewables impacted by about 1.36 kg CO2 eq. These results demonstrate, similarly to this study, the potential for CO2 reduction via using renewably powered processes.This study aligns well with the findings of Galusnyak et al.,31 who performed an LCA of methanol production and conversion into various chemical intermediates and products. Both studies highlight the significant influence of energy sources on environmental impacts. Galusnyak et al. assessed three energy scenarios: EU grid mix, wind power, and hydro power. They found that using renewable energy sources substantially reduces environmental impacts. Similarly, our study demonstrates that hydroelectric power has the lowest impacts across various categories, while the US grid mix shows the highest impacts. For GWP, Galusnyak et al. reported substantial reductions when using wind and hydro power compared to the grid mix, aligning with our findings where hydro and wind exhibit the lowest GWP. Both studies emphasize the importance of renewable energy in achieving better environmental performance for methanol production and highlight the trade-offs involved in different energy sources.
Our findings are also compared to Kibria Nabil et al.,45 which performed a comparative LCA of electrochemical upgrading of CO2 to fuels and feedstocks. The authors highlighted that the carbon intensity of electrochemical routes is highly sensitive to the electricity emission intensity, achieving climate benefits only when coupled with low-emission electricity (<0.25 kg CO2 eq. per kW h). Similarly, our study found that the US grid mix results in the highest impacts, whereas renewables show the lowest. For other impact categories, both studies report that electrochemical processes can have significant energy requirements, particularly in conversion and separation phases, which can offset climate benefits unless powered by renewable energy sources. Our findings of high impacts associated with PV systems in Alabama, for example, mirror the authors’ emphasis on the need for renewable optimization to ensure overall environmental benefits. Overall, both studies highlight the potential of renewable energy-based systems to substantially reduce GWP and other environmental impacts, while also emphasizing the challenges and trade-offs involved in optimizing these technologies.
Similarly, this study's results are compared to Maimaiti et al.,36 who examined renewable energy-based methanol production in China. Both studies highlight the critical influence of energy sources on environmental impacts, with hydroelectric and wind energy showing the lowest impacts. Maimaiti et al. reported a GWP of 0.105 kg CO2 eq. per kg methanol for a photovoltaic-based system with battery storage, similar to our findings where hydroelectric and wind power exhibit the lowest impacts. Both studies also show significant reductions in fossil fuel depletion and GWP for renewable systems compared to conventional methods. However, both studies highlight higher impacts for photovoltaic systems in human toxicity and ozone depletion due to polycrystalline silicon production. This study corroborates Maimaiti et al.'s findings, demonstrating the substantial environmental benefits of renewable energy-based methanol production while noting the specific challenges associated with photovoltaic systems.
The comparison of our results with existing studies reveals the critical role of renewable energy in reducing GWP and other environmental impacts of synthetic methanol production. While renewables consistently show lower impacts, specific technologies like PV may present challenges in certain impact categories. These comparisons highlight the potential and importance of optimizing renewable energy use in DAC-integrated methanol synthesis to achieve sustainable and environmentally friendly outcomes.
Conclusion
This comprehensive cradle-to-gate LCA of green methanol production through the integration of DAC systems powered by renewable energy sources elucidates a clear pathway towards achieving substantial reductions in GHG emissions compared to conventional methanol production methods. Notably, the employment of run-of-river hydroelectric and onshore wind power as energy sources for DAC systems highlights the profound potential of renewable energies in enhancing the sustainability of methanol production processes, although all renewable energy methods demonstrate more favorable impacts than traditional methanol production. Furthermore, the exploration of various DAC regeneration heat sources, with a special focus on the utilization of industrial WH, showcases a significant advancement in enhancing energy efficiency and waste management practices within the DAC process. Moreover, the novel exploration of microwave-based regeneration as a DAC heat source, based on cutting-edge experimental data, signifies a leap forward in DAC technology.This study's findings underscore the critical importance of strategic technology selection and the optimization of energy sources in minimizing environmental impacts and promoting sustainable energy solutions. By showcasing the environmental benefits of integrating renewable energy with DAC and methanol production technologies, this research contributes valuable insights to the ongoing efforts in scaling up these technologies for global climate mitigation.
As the world seeks viable solutions to combat climate change, the integration of DAC with green methanol production emerges as a promising pathway towards carbon neutrality, offering a significant step forward in the quest for sustainable and environmentally friendly energy solutions. This study lays a foundation for future research and policy development, aiming to accelerate the deployment of DAC and renewable energy technologies and thereby contributing to the overarching goal of mitigating global warming and achieving a sustainable future. By presenting a comprehensive analysis of renewable energy scenarios for DAC-to-methanol synthesis, the study contributes a significant chapter to the body of knowledge necessary for the advancement of these technologies towards global climate mitigation efforts. The findings advocate for a nuanced approach to technology integration, emphasizing the promise of DAC with green methanol production as a sustainable path towards achieving carbon neutrality and fostering a greener future.
Nomenclature
| Al | Aluminum |
| AL | Alabama, USA |
| AZ | Arizona, USA |
| C | Celsius |
| CCU | Carbon capture and utilization |
| CFC-11 | Trichlorofluoromethane |
| CO2 | Carbon dioxide |
| CTUe | Comparative toxic unit for ecosystems |
| CTUh | Comparative toxic unit for health |
| Cu | Copper |
| DAC | Direct air capture |
| DOE | United States Department of Energy |
| EOL | End of life |
| eq | Equivalent |
| EPA | United States Environmental Protection Agency |
| EU | European Union |
| FU | Functional unit (of LCA) |
| GHG | GHG |
| GW h | Gigawatt-hour |
| GWP | Global warming potential |
| h | Hour |
| IPCC | Intergovernmental Panel for Climate Change |
| kg | Kilogram |
| kt | Metric kiloton |
| kW | Kilowatt |
| kW h | Kilowatt-hour |
| LCA | Life cycle assessment |
| LCI | Life cycle inventory analysis |
| LCIA | Life cycle impact assessment |
| m | Meter |
| min | Minute |
| mL | Milliliter |
| MJ | Megajoule |
| MSD | Microwave swing desorption |
| MW | Microwave |
| N | Nitrogen |
| NET | Negative emissions technology |
| NOx | Nitrogen oxides |
| O2 | Oxygen |
| O3 | Ozone |
| PEM | Polymer electrolyte membrane |
| PM2.5 | Particulate matter <2.5 microns |
| PV | Solar photovoltaic |
| SO2 | Sulfur dioxide |
| SMR | Steam methane reforming |
| Ton | Metric ton |
| TRACI | Tool for reduction and assessment of chemicals and other environmental impacts |
| VOC | Volatile organic compound |
| W | Watt |
| WECC | Western Electricity Coordinating Council |
| yr | Year |
| Zn | Zinc |
Data availability
This paper was written based on publicly available information, and all values used in calculations are explained or cited. An extensive life cycle inventory and detailed results are available in the ESI.†Conflicts of interest
There are no conflicts of interest to declare.References
- Core Writing Team, H. Lee and J. Romero, IPCC, 2023: Sections, In: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Geneva, Switzerland, 2023.
- M. Erans, E. S. Sanz-Pérez, D. P. Hanak, Z. Clulow, D. M. Reiner and G. A. Mutch, Energy Environ. Sci., 2022, 15, 1360–1405 RSC.
- N. von Der Assen, J. Jung and A. Bardow, Energy Environ. Sci., 2013, 6, 2721–2734 RSC.
- D. Sandalow, J. Friedmann, C. McCormick and S. McCoy, Fifth Innovation for Cool Earth Forum.
- C. Breyer, M. Fasihi, C. Bajamundi and F. Creutzig, Joule, 2019, 3, 2053–2057 CrossRef.
- G. F. Nemet and A. R. Brandt, Energy J., 2012, 33(1), 53–82 CrossRef.
- H. Taghdisian, M. R. Pishvaie and F. Farhadi, J. Cleaner Prod., 2015, 103, 640–650 CrossRef CAS.
- H. A. Daggash, C. F. Patzschke, C. F. Heuberger, L. Zhu, K. Hellgardt, P. S. Fennell, A. N. Bhave, A. Bardow and N. MacDowell, Sustainable Energy Fuels, 2018, 2, 1153–1169 RSC.
- Z. Chen, Q. Shen, N. Sun and W. Wei, J. Cleaner Prod., 2019, 220, 408–416 CrossRef CAS.
- A. Al-Qahtani, A. González-Garay, A. Bernardi, Á. Galán-Martín, C. Pozo, N. Mac Dowell, B. Chachuat and G. Guillén-Gosálbez, Appl. Energy, 2020, 265, 114718 CrossRef CAS.
- N. Meunier, R. Chauvy, S. Mouhoubi, D. Thomas and G. De Weireld, Renew. Energy, 2020, 146, 1192–1203 CrossRef CAS.
- Y. Khojasteh-Salkuyeh, O. Ashrafi, E. Mostafavi and P. Navarri, J. CO2 Util., 2021, 50, 101608 CrossRef CAS.
- S. Sollai, A. Porcu, V. Tola, F. Ferrara and A. Pettinau, J. CO2 Util., 2023, 68, 102345 CrossRef CAS.
- C. C. Cormos, Appl. Therm. Eng., 2023, 231, 120943 CrossRef CAS.
- X. Lin, J. J. Foo and W. J. Ong, Sustainable Mater. Technol., 2023, 37, e00663 CrossRef CAS.
- L. Rigamonti and E. Brivio, Int. J. Greenhouse Gas Control, 2022, 115, 103616 CrossRef CAS.
- M. T. Luu, D. Milani, A. Bahadori and A. Abbas, J. CO2 Util., 2015, 12, 62–76 CrossRef CAS.
- A. Schreiber, A. Peschel, B. Hentschel and P. Zapp, Front. Energy Res., 2020, 8, 533850 CrossRef.
- G. Zang, P. Sun, A. Elgowainy and M. Wang, Environ. Sci. Technol., 2021, 55, 5248–5257 CrossRef CAS PubMed.
- C. A. Trudewind, A. Schreiber and D. Haumann, J. Cleaner Prod., 2014, 70, 27–37 CrossRef CAS.
- G. Z. S. Ling, J. J. Foo, X. Q. Tan and W. J. Ong, ACS Sustainable Chem. Eng., 2023, 11, 5547–5558 CrossRef CAS.
- T. T. H. Nguyen, T. Yamaki, S. Taniguchi, A. Endo and S. Kataoka, J. Cleaner Prod., 2021, 292, 125970 CrossRef CAS.
- Y. Khojasteh-Salkuyeh, O. Ashrafi, E. Mostafavi and P. Navarri, Can. J. Chem. Eng., 2023, 101, 5446–5459 CrossRef CAS.
- S. G. Ryoo, H. S. Jung, M. J. Kim and Y. T. Kang, Energy, 2021, 229, 120626 CrossRef CAS.
- B. Robbins, A. Gaona, A. Tavasoli, J. A. Bergerson, B. A. Saville and H. L. MacLean, J. CO2 Util., 2024, 79, 102638 CrossRef CAS.
- K. Barati, Y. Khojasteh-Salkuyeh, O. Ashrafi and P. Navarri, Energy Convers. Manage., 2023, 287, 117096 CrossRef CAS.
- P. Biernacki, T. Röther, W. Paul, P. Werner and S. Steinigeweg, J. Cleaner Prod., 2018, 191, 87–98 CrossRef CAS.
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 434–504 CrossRef CAS PubMed.
- T. Cordero-Lanzac, A. Ramirez, A. Navajas, L. Gevers, S. Brunialti, L. M. Gandía, A. T. Aguayo, S. Mani Sarathy and J. Gascon, J. Energy Chem., 2022, 68, 255–266 CrossRef CAS.
- T. Cordero-Lanzac, A. Ramirez, M. Cruz-Fernandez, H. J. Zander, F. Joensen, S. Woolass, A. Meiswinkel, P. Styring, J. Gascon and U. Olsbye, J. CO2 Util., 2023, 67, 102337 CrossRef CAS.
- S. C. Galusnyak, L. Petrescu, D. A. Chisalita and C. C. Cormos, Energy, 2022, 259, 124784 CrossRef CAS.
- A. Sternberg, C. M. Jens and A. Bardow, Green Chem., 2017, 19, 2244–2259 RSC.
- G. Garcia-Garcia, M. C. Fernandez, K. Armstrong, S. Woolass and P. Styring, ChemSusChem, 2021, 14, 995–1015 CrossRef CAS PubMed.
- M. Rosental, T. Fröhlich and A. Liebich, Front. Climate, 2020, 2, 586199 CrossRef.
- M. Matzen and Y. Demirel, J. Cleaner Prod., 2016, 139, 1068–1077 CrossRef CAS.
- S. Maimaiti, Y. Gu, Q. Chen and Z. Tang, J. Cleaner Prod., 2023, 425, 139002 CrossRef CAS.
- M. Nizami, Slamet and W. W. Purwanto, J. CO2 Util., 2022, 65, 102253 CrossRef CAS.
- C. Van Der Giesen, C. J. Meinrenken, R. Kleijn, B. Sprecher, K. S. Lackner and G. J. Kramer, Environ. Sci. Technol., 2017, 51, 1024–1034 CrossRef CAS PubMed.
- C. M. Liu, N. K. Sandhu, S. T. McCoy and J. A. Bergerson, Sustainable Energy Fuels, 2020, 4, 3129–3142 RSC.
- S. Deutz and A. Bardow, Nat. Energy, 2021, 6, 203–213 CrossRef CAS.
- T. Terlouw, K. Treyer, C. Bauer and M. Mazzotti, Environ. Sci. Technol., 2021, 55, 11397–11411 CrossRef CAS PubMed.
- M. Micheli, D. Moore, V. Bach and M. Finkbeiner, Sustainability, 2022, 14, 10658 CrossRef CAS.
- M. Z. Jacobson, Energy Environ. Sci., 2019, 12, 3567–3574 RSC.
- T. J. Skone, M. Mutchek, M. Krynock, S. Moni, S. Rai, J. Chou, D. R. Carlson, M. Jamieson, E. Dale, G. Cooney and A. Kumar DOI:10.2172/1845020.
- S. Kibria Nabil, S. McCoy and M. G. Kibria, Green Chem., 2021, 23, 867–880 RSC.
- K. Madhu, S. Pauliuk, S. Dhathri and F. Creutzig, Nat. Energy, 2021, 6, 1035–1044 CrossRef CAS.
- U.S. DOE, Best Practices for Life Cycle Assessment (LCA) of Direct Air Capture with Storage (DACS), Washington, DC, 2022.
- E. Van-Dal and C. Bouallou, J. Cleaner Prod., 2013, 57, 38–45 CrossRef CAS.
- M. Mureddu, F. Ferrara and A. Pettinau, Appl. Catal., B, 2019, 258, 117941 CrossRef CAS.
- S. Szima and C. C. Cormos, J. CO2 Util., 2018, 24, 555–563 CrossRef CAS.
- F. Asinger, Methanol: The Basic Chemical and Energy Feedstock of the Future, Springer, Berlin, 2014 Search PubMed.
- P. Trendafilova, Global Thermostat Unveils Its Demonstration Direct Air Capture Plant, https://carbonherald.com/global-thermostat-unveils-its-demonstration-direct-air-capture-plant/, (accessed 22 April 2023).
- E. Ping, M. Sakwa-Novak and P. Eisenberger, in International Conference on Negative CO2 Emissions, Göteborg, 2018.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1076 RSC.
- M. Fasihi, O. Efimova and C. Breyer, J. Cleaner Prod., 2019, 224, 957–980 CrossRef CAS.
- L. Jiang, W. Liu, R. Q. Wang, A. Gonzalez-Diaz, M. F. Rojas-Michaga, S. Michailos, M. Pourkashanian, X. J. Zhang and C. Font-Palma, Prog. Energy Combust. Sci., 2023, 95, 101069 CrossRef.
- G. Leonzio, P. S. Fennell and N. Shah, Appl. Sci., 2022, 12, 8321 CrossRef CAS.
- R. Boylu, M. Erguvan and S. Amini, Experimental Investigation of Microwave-Based Direct Air Capture Technology Using Zeolite 13X in a Fluidized Bed Reactor, AIChE Annual Meeting, 2024 Search PubMed.
- M. Clausse, J. Merel and F. Meunier, Int. J. Greenhouse Gas Control, 2011, 5, 1206–1213 CrossRef CAS.
- R. Gonzalez-Olmos, A. Gutierrez-Ortega, J. Sempere and R. Nomen, J. CO2 Util., 2022, 55, 101791 CrossRef CAS.
- M. Erguvan, A. Doroshenko and S. Amini, Energy Technol., 2024, 12(5), 2301492 CrossRef CAS.
- T. Chronopoulos, Y. Fernandez-Diez, M. M. Maroto-Valer, R. Ocone and D. A. Reay, Microporous Mesoporous Mater., 2014, 197, 288–290 CrossRef CAS.
- T. Chronopoulos, Y. Fernandez-Diez, M. M. Maroto-Valer, R. Ocone and D. A. Reay, Energy Procedia, 2014, 63, 2109–2115 CrossRef CAS.
- T. N. van Schagen, P. J. van der Waals and D. W. F. Brilman, Chem. Eng. J. Adv., 2022, 9, 100187 CrossRef CAS.
- E. Meloni, M. Martino, P. Pullumbi, F. Brandani and V. Palma, Chem. Eng. Process., 2021, 160, 108291 CrossRef CAS.
- C. Ellison, J. Hoffman and D. Shekhawat, Int. J. Greenhouse Gas Control, 2021, 107, 103311 CrossRef CAS.
- G. N. Nikolaidis, E. S. Kikkinides and M. C. Georgiadis, Chem. Eng. Res. Des., 2018, 131, 362–374 CrossRef CAS.
- K. T. Chue, J. N. Kim, Y. J. Yoo, S. H. Cho and R. T. Yang, Ind. Eng. Chem. Res., 1995, 34, 591–598 CrossRef CAS.
- L. Joss, M. Gazzani, M. Hefti, D. Marx and M. Mazzotti, Ind. Eng. Chem. Res., 2015, 54, 3027–3038 CrossRef CAS.
- R. Morales-Ospino, R. G. Santiago, R. M. Siqueira, D. C. S. de Azevedo and M. Bastos-Neto, Adsorption, 2020, 26, 813–824 CrossRef CAS.
- US EPA, CHP Benefits, https://www.epa.gov/chp/chp-benefits, (accessed 29 July 2024).
- R. Terzi, Application of Exergy Analysis to Energy Systems, in Application of Exergy, ed. T. Taner, Royal Society of Chemistry, Cambridge, 2019, ch. 6 Search PubMed.
- ecoinvent, Data Release: ecoinvent v3.9, https://ecoinvent.org/the-ecoinvent-database/data-releases/ecoinvent-3-9/#1610466712317-fe0cb20b-47401632217981603, (accessed 29 November 2023).
- BloombergNEF, Sustainable Energy in America 2023 Factbook, 2023.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess, 2016, 21, 1218–1230 CrossRef.
- R. J. Schmidli and A. Jamison, NOAA Technical Memorandum NWS WR-177: Climate OF Phoenix, Arizona, Third Revision, 1996.
- World Bank Group, Global Solar Atlas, https://globalsolaratlas.info, accessed June 2024.
- F. Rossi, M. L. Parisi, S. Maranghi, R. Basosi and A. Sinicropi, Sci. Total Environ., 2020, 700, 134814 CrossRef CAS PubMed.
- F. Rossi, M. L. Parisi, S. Maranghi, R. Basosi and A. Sinicropi, Data Brief, 2020, 28, 104895 CrossRef PubMed.
- C. E. L. Latunussa, F. Ardente, G. A. Blengini and L. Mancini, Sol. Energy Mater. Sol. Cells, 2016, 156, 101–111 CrossRef CAS.
- Y. A. Guta, J. Carneiro, S. Li, G. Innocenti, S. H. Pang, M. A. Sakwa-Novak, C. Sievers and C. W. Jones, ACS Appl. Mater. Interfaces, 2023, 15, 46790–46802 CrossRef CAS PubMed.
- X. Shi, H. Xiao, H. Azarabadi, J. Song, X. Wu, X. Chen and K. S. Lackner, Angew. Chem., Int. Ed., 2020, 59, 6984–7006 CrossRef CAS PubMed.
- Micromeritics Instrument Corp., CO2 Adsorption in Zeolite 13X, https://www.micromeritics.com/Repository/Files/Zeolite13X-CO2-Analysis.pdf, accessed June 2024.
- C. Smith, T. Gasser, Z. Nicholls, K. Armour, W. Collins, P. Förster, M. Meinshausen, M. Watanabe, A. Pirani, S. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. Matthews, T. Maycock, T. Waterfield, O. Yelekçi, R. Yu and B. Zhou, The Earth's Energy Budget, Climate Feedbacks and Climate Sensitivity Supplementary Material, Cambridge, UK, and New York, NY, USA, 2021 Search PubMed.
- T. Skone, in Solutions for Today | Options for Tomorrow, National Energy Technology Laboratory, 2021.
- N. McQueen, M. J. Desmond, R. H. Socolow, P. Psarras and J. Wilcox, Front. Climate, 2021, 2, 618644 CrossRef.
- C. Haertel, M. McNutt, M. Ozkan, E. Aradóttir, K. Valsaraj, P. Sanberg, S. Talati and J. Wilcox, Chem, 2021, 7, 2831–2834 CAS.
- K. Lebling, H. Leslie-Bole, P. Psarras, E. Bridgwater, Z. Byrum and H. Pilorgé, Direct Air Capture: Assessing Impacts to Enable Responsible Scaling, World Resources Institute, 2022 DOI:10.46830/WRIWP.21.00058.
- P. Webb, H. Muslemani, M. Fulton and N. Curson, Scaling Direct Air Capture (DAC): A moonshot or the sky's the limit? OIES Paper: CM, No. 07, Oxford: The Oxford Institute for Energy Studies, Oxford, 2023.
- Statista, Methanol - statistics & facts, https://www.statista.com/topics/11339/methanol/#topicOverview, (accessed 29 June, 2024).
- P. A. DeFazio, Infrastructure Investment and Jobs Act, U.S. Congress, Washington, D.C., 2021.
- J. A. Yarmuth, Inflation Reduction Act of 2022, U.S. Congress, Washington, D.C., 2022.
Footnote |
| † Electronic supplementary information (ESI) available: Supp. 1 (Methods), Supp. 2 (Results), Supp. 3 (Inventory). See DOI: https://doi.org/10.1039/d4ya00316k |
| This journal is © The Royal Society of Chemistry 2024 |