Metal–organic framework-based platforms for implantation applications: recent advances and challenges
Yifan
Liu
ab,
Shuteng
Wang
ab,
Chunhua
Quan
*c,
Shifang
Luan
 ab,
Hengchong
Shi
ab,
Hengchong
Shi
 ab and
Lei
Wang
ab and
Lei
Wang
 *a
*a
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: leiwang@ciac.ac.cn
bSchool of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei 230026, P. R. China
cCentral Laboratory, Affiliated Hospital of Yanbian University, Yanji, Jilin 133002, P. R. China. E-mail: 9000002277@ybu.edu.cn
First published on 13th December 2023
Abstract
The development of minimally invasive technology has promoted the widespread use of implant interventional materials, which play an important role in alleviating patients’ pain during and after surgery. Metal–organic frameworks (MOFs) and their related hybrids formed by bridging ligands and metal nodes via covalent bonds represent one of the smart platforms in implant interventional fields due to their large surface area, adjustable compositions and structures, biodegradability, etc. Significant progresses in the implantation application of MOF-based materials have been achieved recently, but these studies are still in the initial stage. This review highlights the recent advances of MOFs and their related hybrids in orthopedic implantation, cardio-vascular implantation, neural tissue engineering, and biochemical sensing. Each correction between the structural features of MOFs and their corresponding implanted works is highlighted. Finally, the confronting challenges and future perspectives in the implant interventional field are discussed.
Introduction
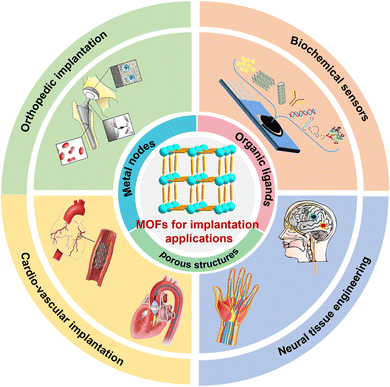
The advancement of minimally invasive technology has led to the rapid development of interventional therapy, which has been known as the third clinical method alongside internal medicine and surgical treatment. The emergence of medical implant devices such as joint prostheses, vascular stents, and catheters has provided a much effective technical means to solve clinical problems. However, commonly used implant intervention materials, including ceramics, polymers, and metals, are all biologically inert materials with much weaker responses to surrounding tissues and cells. In addition, the implantation of these non-biological materials can activate the immune microenvironment to induce local acute or chronic inflammatory reactions.1 Importantly, invasive prosthesis implantation faces the life-threatening risk of bacterial infections, encrustations, thrombosis, bloodstream infections, etc., as device-associated complications.2Porous materials have excellent characteristics, such as high specific surface area, low specific gravity, high permeability, and high noise reduction, which have been widely used in dentistry, ophthalmology, and orthopedics.3,4 For example, porous titanium, tantalum,5 hydroxyapatite, and bioglass6 can provide good scaffolds for tissue growth via enhancing cell adhesion and vascular growth. Their strong shock absorption and buffering performance also make it a good choice for filling damaged tissues. In addition, some porous materials could be suitable carriers for loading biological factors and drugs. Metal–organic frameworks (MOFs) are periodic porous materials bridged by metal nodes and organic ligands via coordination bonds. In addition to the above-mentioned merits of the traditional porous materials, MOFs and their nanoscale particles have their own features of adjustable porosity and compositions, larger specific surface area, biodegradability, and easy surface functionalization.7,8 Currently, there are a number of comprehensive reviews on the biological applications of MOFs materials in cancer treatment, biosensing, biological imaging, antibacterial activities, and others.9 However, few works are focused on the implant intervention usage of MOF-based materials.
Combined with the structural features of MOFs and their individual requirements in different implant intervention scenarios, we first summarize and divide these previous MOF-related results into four sections, namely, orthopedic implantation, cardio-vascular implantation, neural tissue engineering, and implantable biochemical sensors. Then, we highlight the latest advances of implanted MOFs in each scenario. Finally, the potential challenges and future perspectives in the implant intervention applications of MOFs and their composites are given.
MOFs materials in orthopedic implantation
Bone tissue is made up of a mixture of living cells and bioactive minerals.10 Bone grafting is currently the most common method for treating bone defects coming from osteoporosis and osteogenesis imperfection as well as surgery-led injury.11 To solve these, a suitable bone material for clinical implantation should be addressed for several major issues including less wound infection, high mechanical stability, modulus matching comfort, good biocompatibility, and biodegradation.12 Therefore, the design and synthesis of bone tissue materials remain a challenge (Scheme 1).MOFs have been proven to be ideal candidates for bone materials. Similar to those natural tissues, MOFs provide the physical stability, toughness and mechanical strength needed to restore the load-bearing capacity of the injured limb. The contained metal ions in the structure of MOFs, for example ZIF-8,13 can be released slowly under specific simulations, which can not only perform an anti-bacterial function to reduce the wound infection, but also regulate the alkaline phosphatase (ALP) activity during the osteogenesis.14–16 In addition, the porous network and easy post-modification of MOFs could be used as appropriate substances to target in the damaged part and induce the adhesion and proliferation of bond cells, beneficial for the bone regeneration.
Titanium implants have been widely used for bone repair due to their excellent biocompatibility and mechanical properties, but are limited by insufficient bone integration in clinical settings. Chen et al. synthesized a bone microenvironment-responsive biofunctional Ce/Sr MOFs coating on a titanium surface via hydrothermal treatment (Fig. 1a).17 The anchored Ce and Sr ions of MOFs coated with good biocompatibility exhibit an on-demand enzymatic-like activity to decompose ROS in mesenchymal stem cells (MSCs) and restore mitochondrial functions, so that osteogenicity can be enhanced. In addition, p-xylylenebisphosphonate (PXBP) ligands within Ce/Sr MOFs as primary agents for treating osteoporosis can also induce osteoclast apoptosis to inhibit osteoclast-mediated bone resorption. These merits will jointly promote the bone integration of titanium implants in the state of osteoporosis in vivo, thereby improving the clinical osteogenic effect. Wu et al. constructed a Bio-MOF-1 coating on a titanium surface via the alkali-heat treatment.18 The obtained Bio-MOF-1 coating exhibited high thermostability, good biocompatibility, and stable Zn2+ release, which can up-regulate the expression of osteogenesis-related genes for potential application in bone tissue engineering.
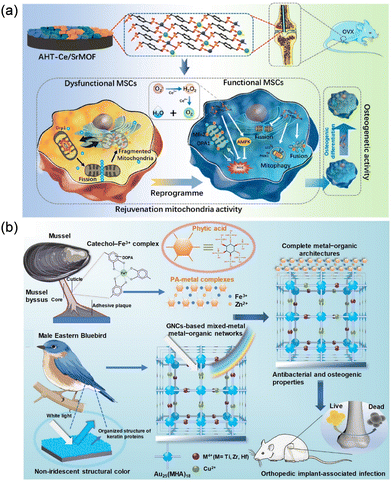 | ||
| Fig. 1 Application of MOFs in orthopedic implants to promote bone integration and new bone formation. (a) Biofunctional Ce/Sr MOFs coating on titanium implants to enhance osteointegration in osteoporosis by amending the mitochondrial function in MSCs. Reprinted from ref. 17 with permission. Copyright 2022 ACS. (b) Bioinspired metal−organic coating strategy involving nanocluster self-assembled films and PA−metal complexes for synergetic antibacterial and osteogenic activities of implants. Reprinted from ref. 26 with permission. Copyright 2022 ACS. | ||
Owing to the controllable structure parameters for enhancing bone tissue regeneration, three-dimensional (3D) printed porous scaffolds combined with bone growth-inducing components are considered ideal bone grafts.19 Zhong et al. incorporated nanoZIF-8 into a composite scaffold composed of polycaprolactone (PCL) and dicalcium phosphate dihydrate (DCPD) using an extrusion-based 3D printing technology. Both in vivo and in vitro experiments demonstrate that nano ZIF-8 has minimal cytotoxicity, good antibacterial activity, and strong bone formation. A flexible fiber membrane containing ZIF-11 and HKUST-1 MOFs was obtained by continuing electrospinning technique.20 After the release of Cu2+ and Zn2+, the as-synthesized MOFs membranes could stimulate the angiogenesis and promote the process of tenogenesis. The abundant pore structures of MOFs can serve as carriers to load biomolecules for synergistic effects. As two commonly used methods for preparing tissue engineering scaffolds, 3D printing technology and electrospinning each has its own characteristics. Owing to the merits of high precision, versatility and structural designability, 3D printing technology can use a variety of biocompatible materials such as polycaprolactone (PCL), polylactic acid (PLA)21 and polyether ketone (PEK),22 to prepare complex scaffolds with well-matched shapes of damaged bones and good mechanical properties. In addition, the soft and deformable characteristics of electrospun scaffolds, combined with the selection of piezoelectric materials, can be used to prepare piezoelectric scaffolds in bone tissue regeneration.23 Besides, the formed fiber scaffolds with flexible and three-dimensional spatial structures can smoothly attach local tissues. Moreover, those two methods can be combined to leverage their respective advantages and prepare scaffolds that are more suitable for orthopedic implantation needs.24 Dong et al. synthesized a multifunctional responsive MOF-based hydrogel coating on a sulfonated long carbon fiber-reinforced polyetheretherketone (LCFRPEEK) implant.25 Under the low pH of immune environment, this MOFs hydrogel can slowly release biological molecules (GA, Mg2+ and Ca2+) to repair the damaged tissue.
Surface modification of implants by MOFs can also achieve additional functions. For example, the Lewis acid–base coordination principle has been utilized to connect inorganic and organic groups in situ through a metal–organic coordination-driven self-assembly process (Fig. 1b).26 They initially constructed a Au25(MHA)18 nanocluster coating with self-assembled amorphous photonic structure on an implant surface, which provides a visual indication of the structural integrity of the implant. Then, a phytic acid (PA) metal coordination complex was further constructed at the coating-bone interface to promote the conversion of surface wettability and interface hydroxyapatite biomineralization. The construction of biomimetic self-assembly coating effectively improves the synergistic antibacterial ability and bone integration effect of metal implants.
Postoperative infections remain another major challenge in orthopedic implants, leading to serious complications and economic burden for patients. Teng et al. immobilized ZIF-8 on the surface of micro-arc oxidative titanium by in situ hydrothermal treatment and then processed to load iodine onto ZIF-8 by vapour deposition (Fig. 2).27 Under NIR light irradiation, the loaded iodine in ZIF-8 was rapidly released in a “triggered burst” manner to achieve anti-infection performance through ROS generation. In addition, this antibacterial system also significantly promoted the bone integration of the coated implants, indicating that the improvement of antibacterial ability did not sacrifice any functional properties of the implants. Besides, there are some works on the preparation of bone-implanted MOFs composite coatings by loading with osteogenic growth peptides and naringin with the aim to enhance the integration of bone and antibacterial functions.28,29 However, there is still a lack of enough clinical results for MOF-based bone materials, and more in vivo experiments are needed to fully understand the degradation and pathway process of MOFs.
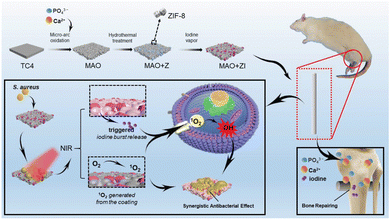 | ||
| Fig. 2 Iodine-immobilized ZIF-8 undergoes an antibacterial process guided by NIR triggering, resulting in a burst release of iodine combined with the generation of 1O2 on the surface. This process is further enhanced by Ca2+, PO43−, and iodine, leading to effective osseointegration both in vitro and in vivo. ref. 27 with permission. Copyright 2021 Wiley. | ||
MOFs materials in cardio-vascular implantation
Blood-contacting devices including catheters and stents have been widely applied to treat various cardiovascular diseases by vascular drug delivery and heart repair.30 However, the bacterial adhesion on the surfaces of those devices as well as coagulation systems and the damage reactions of vascular endothelial cells could cause some cardiovascular complications including catheter-related bloodstream infections (CRBIs) and catheter-related thrombosis (CRT). In addition, the in-stent restenosis (ISR) of the cardiovascular stent reduces its long-term safety in clinic applications.31 Hence, the requirements for blood-contacting devices are to improve the blood compatibility and biological functions of permanent stents and vascular grafts, and to reduce the demand for systemic antithrombotic therapy.Nitric oxide (NO) is a signalling molecule produced by endothelial cells, and it can be used as an anti-platelet adhesion inhibitor to prevent the formation of thrombosis and promote endothelialization.32 As a crucial free-radical gas, high diffusibility and brief life-time are theirs two primary distinguishing characteristics.33 Although NO can have rapid interactions with various cellular targets, less than 2 seconds of biological lifespan limits its applications.34 Meanwhile, the impact of NO is also contingent on its concentration and the spatial distributions within specific microenvironments.35 Since the early 1990s, the understanding of NO fluxes in preserving the thromboresistance of endothelial cell walls has been confirmed. The inherent NO flux from the endothelium typically ranges from 0.5 × 10−10 to 4 × 10−10 mol cm−2 min−1, while a NO surface flux of 3 × 10−8 mol cm−2 min−1 has been documented to inhibit platelet adhesion by up to 87%.36 The key for this NO system is to design biological triggered materials to release NO upon contact with tissues, limited by the amount of NO contained in the substance.37 In response to the urgent need for alternative methods to prolong the release of NO, much effort has recently been devoted to implantation coating that mimics endothelial functions through the catalytic decomposition of the endogenous blood component S-nitrosothiol (RSNO) to continuously generate NO.38 A number of works have shown that fixed copper compounds (such as Cu(II) complexes and copper-polydopamine complexes), copper-doped TiO2 films and polymer coatings containing nano-copper can effectively catalyse the formation of NO by physiological concentrations of RSNOs.39–42 For example, a CuO/SiO2 nanocomposite catalyst was prepared by chemisorption and calcination. The activity of the catalyst to produce NO depends on the specific surface area of the material and the exposure of CuO on the surface, which can be effectively applied via structural and proportional regulation.36
The abundant pore channels and large specific surface area of MOFs provide the innate features for copper ion loadings. Zhou et al. doped copper ions into ZIF-8 particles to prepare MOF-based catalysts for controlling the formation of NO. After 5 times of NO generation cycles, the 75% of catalytic efficiency still maintains, indicating that this Cu2+/ZIF-8 can be potential for long-term biomedical applications. In addition, copper could effectively prevent the formation of biofilms and kill bacteria. Co-administration of the Cu2+/ZIF-8 sample and GSNO resulted in a 45% reduction in biofilm biomass of P. aeruginosa.43
In addition to the pore loading, copper ions can be used as metal nodes to coordinate with organic ligands to prepare copper-based MOFs for NO catalysis. Recently, copper-based MOFs (Cu-MOFs) have been synthesized to catalyse the generation of NO by decomposing NO donors. CuBTC (also known as HKUST-1 or MOF199) and CuBTTri, whether grown in situ on the polymer surface or mixed into the polymer, were considered heterogeneous NO catalysts for the decomposition of endogenous S-nitrosothiols. A polyurethane/HKUST-1 composite can be prepared by a two-step composite and extrusion method.38 CuBTTri MOFs can also be deposited directly onto the surface of carboxyl-functionalized cotton.44 Three kinds of substrates, namely, titanium oxide film, 316L stainless steel, and poly(vinyl chloride) have been used to construct the functional HKUST-1 coating. The polydopamine coating was prepared on the substrates as the hydroxyl terminating surface first. The catechol groups in the polydopamine coating provided chelating sites for fixing copper ions. Subsequently, the HKUST-1 coating was then prepared on the surface of the coating by a liquid-phase epitaxy or layered assembly (LBL) method. This robust HKUST-1 coating imparts antithrombotic and antibacterial activity to the implant. It provides a simple and effective method for blood contact devices without substrate dependence (Fig. 3).45
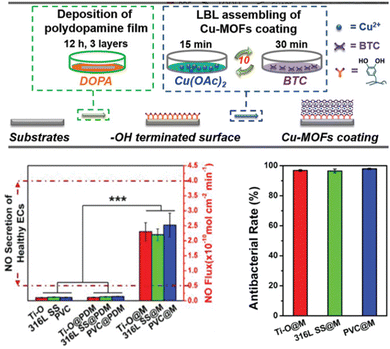 | ||
| Fig. 3 In situ assembly of the substrate-independent HKUST-1 coating by an LBL method on the polydopamine-coated biomaterials. Reprinted from ref. 45 with permission. Copyright 2020 Wiley. | ||
As another important subclass of cardio-vascular implants, cardiovascular stents are currently one of the most effective therapeutic methods for the clinical treatments of atherosclerosis. However, intravascular injury promotes platelet adhesion and activation, and the subsequent acute inflammatory response worsens endothelial dysfunction, leading to excessive proliferation of smooth muscle cells (SMCs), which further leads to late stent thrombosis (LST) and intrastent restenosis (ISR), reducing the long-term safety of cardiovascular stents. To solve these, HKUST-1 nanoparticles were fixed on the surface of titanium with a polydopamine coating. Cu-MOFs catalysed the formation of NO and the delivery of copper ions in situ. The NO and copper ions synergistically inhibit platelet activation induced by gelatin, promote endothelial cell growth, and reduce endometrial hyperplasia. Both In vitro and in vivo experiments show that the Cu-MOF-immobilized coatings have obvious anticoagulation, re-endothelialization, and anti-proliferation properties. This strategy can effectively solve the problems of stent LST and ISR through surface modification of cardiovascular stents (Fig. 4).46
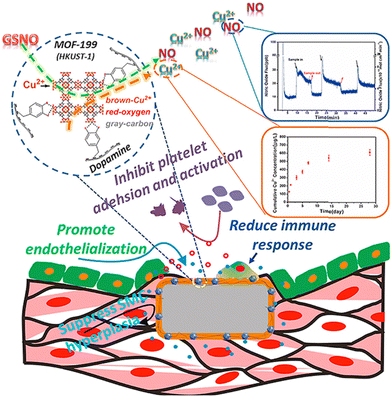 | ||
| Fig. 4 Cu-MOFs with polydopamine coating on cardiovascular stents to prevent the late stent thrombosis and in-stent restenosis. Reprinted from ref. 46 with permission. Copyright 2019 Elsevier. | ||
MOFs materials in neural tissue engineering
The regeneration and repair of neural tissues still pose challenges due to the complexity of anatomy and physiology of the nervous system.47,48 Traumatic injuries to the central nervous system (CNS) of the brain or spinal cord (such as falls, car accidents, and attacks) as well as non-traumatic injuries (such as tumors and neurodegenerative diseases) may lead to lifelong disability in neural tissues.49–52 The development of neural tissue engineering and drug delivery strategies as potential therapeutic methods for neural tissue regeneration has begun to receive widespread attention. Meanwhile, the inhibition of inflammatory and immune response-induced neuronal oxidative damage and apoptosis has also played a key role in the neuroprotective therapy.In recent years, MOFs have been applied in the treatment of neuro-related diseases.53–55 Compared with traditional nanomaterials, MOFs have obvious advantages. First, their relatively weak coordination bonds make MOFs degradable under complex physiological conditions, which can play the function of stimulating reactive drug delivery systems while avoiding long-term accumulation and potential toxicity in vivo.56 Second, the high porosity of MOFs promote the high loading capacity in the development of drug delivery systems (DDSs) to transport several biomedical drugs into damaged neural tissues.57 At last, the controllable compositions, easy post-modification, and tuneable sizes of MOFs particles enable them to cross the blood–brain barrier and to be applied in neuroimaging and biosensing.54 In short, these desirable properties render the MOFs a potential platform for diagnosing/monitoring and effectively treating neurological disorders.
The central nervous system is like a huge information processor, which plays a key role in the dominance of human movement, controlling perception, processing and storing information. As a major part of the central nervous system, the health of the brain is closely related to various life activities of the human body. For traumatic brain injury (TBI), there is no effective strategy to treat it in clinical settings. Achieving angiogenesis and neurogenesis in damaged brain tissues, inhibiting brain shrinkage and promoting its long-term rehabilitation are the key to modern medicine. Appropriate concentrations of NO and electrons, as endogenous signals, can induce various physiological functions in the nervous system and play a positive role in nerve repair. Therefore, a 3D-printed implantable microneedle composed of a magnetoelectric MoCx–Cu MOFs and a NO donor, S-nitrosoglutathione (GSNO), was developed by Chan et al. for the magnetoelectric responsive repair of traumatic brain injury.58 Under the action of alternating magnetic fields (AMFs), GSNO can release NO on demand and promote the differentiation and growth of neural stem cells by synergistic stimulation with eddy currents induced by conductive MOFs (Fig. 5a).
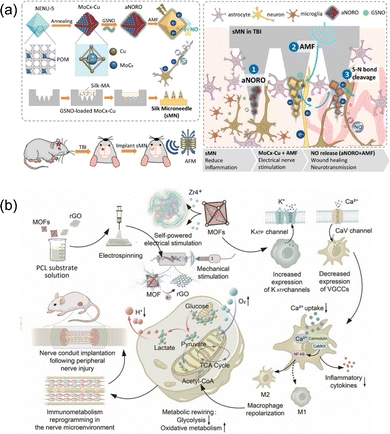 | ||
| Fig. 5 (a) An alternating magnetic field-responsive NO-release system and an electric stimulus for traumatic brain injury treatment. Reprinted from ref. 58 with permission. Copyright 2023 Elsevier. (b) A self-powered nerve bridging scaffold by integrating piezoelectric UiO-66 and rGO into PCL substrates. Reprinted from ref. 64 with permission. Copyright 2023 Elsevier. | ||
Stroke caused by cerebrovascular, focal or overall brain tissue damage, with high morbidity, disability, recurrence and mortality rates, has become the world's second leading cause of death. Ischemic stroke accounts for 75% to 90% of all strokes. The explosive production of reactive oxygen species can cause secondary brain injury after the initial ischemic injury, namely, cerebral ischemic reperfusion injury (CIRI). In order to achieve effective removal of reactive oxygen species, a selenium-containing MOFs (Se-MOF) was used as a carrier to construct ferro-atomic nanocases (Fe2NC) with a multi-enzyme cascade activity to effectively clear intracellular ROS and regulate the related signalling pathways, so as to avoid secondary injury caused by cerebral ischemia reperfusion.59 Besides, CeO2@ZIF-8 and PDA–Mn-MOFs have been used as antioxidants for the treatment of ischemic stroke.60,61
Although central nervous injuries (CNIs) are often more harmful to the human body, the peripheral nervous injuries (PNIs) are more common due to the widespread presence of nerves throughout the body and the lack of the hard bone layer or blood–brain barrier (BBB) that protect the nervous system. Macrophages are key regulators of homeostasis near nerve injury sites. The expression of macrophage function is influenced by both external stimuli and intracellular metabolism. The increase in glycolysis and oxidative metabolism promotes the expression of pro-inflammatory M1 macrophages and pro-healing M2 macrophages, respectively. Therefore, inhibiting glycolytic enzymes in macrophages and re-participating in mitochondrial oxidative metabolism may play a role in preventing excessive inflammation and promoting tissue regeneration. In addition, extracellular electrical signals are closely coupled with intracellular functions through ion channels expressed on the cellular membrane, and the regulation of intracellular signals also plays a crucial role in the phenotype transformation of macrophages. Living cells and bioelectric materials have restricted electrical compatibility. To enhance the neural implant's biocompatibility, and it is crucial to regulate the bioelectric communications between indigenous cells and interfaced substances.62 Compared with other conductive materials, MOFs materials with high specific surface area and high porosity make them more conducive to cell adhesion, thus generating electrical signal regulation on cells and also promoting nerve repair.63 Based on the above-mentioned theories, Yap et al. prepared a rGO@UiO-66/polycaprolactone (PCL) electrospun fiber membrane by an electrospinning process, which was implanted in vivo as a scaffold for 10 mm sciatic nerve defect (Fig. 5b).64 UiO-66 formed with Zr6O4(OH)4 nodes and benzoic acid ligands was first synthesized by Karl et al. from the University of Oslo in 2008.65 Its decomposition temperature is more than 400 °C and exhibits strong corrosion resistance to various chemicals and under high external pressures.66 Using 2-amino-benzenedicarboxylic acid (H2N–H2BDC) as an organic ligand to obtain Zr–UiO-66-NH2 can introduce functional sites without changing the original topology. The as-synthesized Zr–UiO-66-NH2, as a non-centrosymmetric crystalline material, has a piezoelectric response, and generates the accumulation of charges on the surface induced by the polarized internal electric field under the mechanical stimulation of external periodic ultrasonic waves.67 The improved electrical conductivity of MOFs compounded with graphene oxides promotes the effective conduction of electric charge. Compared with the pure PCL scaffold in the control group, the experimental group had a significant regulatory effect on the selective expression of cell membrane ion channels after implantation for 8 weeks, and the metabolism of nerve tissues changed from glycolytic-dominant phenotype to OXPHOS-dominant phenotype. In addition, macrophages changed from M1 phenotype to M2 phenotype, thus restoring the immune metabolism of the neural microenvironment and leading to nerve regeneration. There are also some studies on MOFs as a drug or gene carrier implanted into the human body for spinal cord injury treatment. For example, a β-cyclodextrin metal–organic framework loaded with methylprednisolone sodium succinate is used for transdermal treatment of the injured site via microneedles.68 Zhou et al. used ZIF-8 to support dental pulp stem cells and then loaded them into gelatin methylacrylyl hydrogels and injected them into the injured spinal cord of rats to achieve effective nerve repair.69
MOFs materials in implantable biochemical sensors
Unlike the traditional rigid devices with the incompatible soft tissue mechanics, flexible electronic products can minimize tissue damage and provide comfortable tissue contact,70,71 which have been widely used in health monitoring and disease diagnosis. However, the development of flexible devices for implantable biochemical sensing is still in its early stage. The requirement for long-term use, multi-parameter measurement with smart sensitivity and good stability remains a major challenge. To achieve sensitive and stable chemical sensing, it is necessary to conduct in-depth investigations on new biological functional materials with strong sensing capabilities, high adjustability, and good biocompatibility.Sensitive and stable biochemical sensing can be achieved by incorporating various functional materials such as metal nanoparticles (NPs),72 metal oxides,73 bioactive materials,74 and 2-dimensional (2D) materials.75 However, the activity decreased rapidly over time, and the lack of functional groups or simple spatial configurations resulted in poor sensing performance.76 On the contrary, the highly synthetic design of MOFs and its related materials makes it possible to precisely adjust their optical, electrical, and magnetic properties. For example, MOFs are generally thought of as near-insulators with little or no electrical conductivity (<10−10 S cm−1) and poor electron mobility (<1 cm2 V−1 s−1) for the lack of conjugated structure.77 However, designed MOFs materials with much better proton-conductive,78 semiconductive,79 ferroelectric80 and ionconductive81 performances can be achieved by selecting specific metal ions and organic ligands, adjusting the framework structures,82 or loading other functional materials into the pores;83 typical substrate materials include UiO-66, ZIF-8, HKUST-1, and MOF-5 (Table 1).84 In addition, controlling the morphologies of MOFs has been proven to be an effective strategy for enhancing their electrical conductivities. For instance, two-dimensional π-conjugated MOFs exhibit much higher electrical conductivity and carrier mobility due to their layered structure.85 Besides the conductivity, the mechanical robustness and isotropy should usually be considered in the preparation of hybrid MOFs with heterostructures.86 Large specific surface area, adjustable porosity, high tolerance to heat and solution, and abundant active metal sites involved in REDOX make these materials have broad application prospects in the direction of biochemical sensing.
| MOFs | Components | Method and design | Features | Ref. |
|---|---|---|---|---|
| PXBP: p-xylylenebisphosphonate. 2-MIM: 2-methylimidazole. DCPD: dicalcium phosphate dehydrate. H3BTC: 1,3,5-benzenetricarboxylic acid. MHA: 6-mercaptohexanoic acid. β-CD: β-cyclodextrin. HHTP: 2,3,6,7,10,11-hexahydroxytriphenylene. H4BTEC: pyromellitic acid. TCPP: tetrakis(4-carboxyphenyl)porphyrin. LCFRPEEK: sulfonated long carbon fiber reinforced polyetheretherketone. Ti–O: titanium oxide film. 316L SS: 316L stainless steel. MPSS: methylprednisolone sodium succinate. DPSCs: dental pulp stem cells. CYFRA21-1: cytokeratin 19 fragment antigen21-1. | ||||
| Orthopedic implantation | ||||
| Ce/Sr MOF | Ce3+ ion, Sr2+ ion; tetraethyl-PXBP | Alkali-heat treated Ti; surface coating; facile hydrothermal process | Ce and Sr ions: decompose ROS; PXBP: induce osteoclast apoptosis; MOF: amend mitochondrial function in MSCs, promote new bone formation | 16 |
| ZIF-8 | Zn2+ ion; 2-MIM | Mixed with PCL and DCPD to produce the paste; composite scaffolds; extrusion based 3D printing | Increased mechanical strength and homogeneous structure; MOFs: minimal cytotoxicity, inhibited bacterial activities, promoted osteogenesis | 19 |
| ZIF-11 & HKUST-1 | Zn2+ ion; benzimidazole &Cu2+ ion; H3BTC | Loaded via PLA nanofibers coencapsulation; continuous electrospinning | Zn2+ ion: stimulate collagen synthesis, promote wound healing; Cu2+ ion: activate the VEGF and HIF-1 pathway, improve bone regeneration; MOF: promote the osteogenesis and tenogenesis; | 20 |
| HAP@Mg–GA | nano-hydroxyapatite; Mg2+ ion; gallic acid | Heat-press-saturation-cool molding LCFRPEEK plate; methacryloyl chitosan hydrogels loaded with HAP@Mg–GA grafted onto the surface of the sulfonated LCFRPEEK by UV irradiation grafting | pH-Sensitive biomolecule release; scavenge excess ROS and reduce NO production under the immune environment; GA, Mg2+ and Ca2+: promote angiogenesis and osteogenic differentiation | 25 |
| Ti/Zr/Hf-Cu-MON(MM-MONs) & Fe/Zn–PA | Ti4+, Zr4+, Hf4+, Cu2+ ion; Au25(MHA)18 & Fe3+ ion; Zn2+ ion; phytic acid | Self-assembled coating and PA-metal complexes sequentially constructed on the surface of titanium | MM-MONs: artificial photonic structures, combine mechanical stability and bacteriostatic activity; Fe/Zn–PA: colorless and transparent, hydrophilic, interfacial bio-mineralization of hydroxyapatite | 26 |
| ZIF-8 | Zn2+ ion; 2-MIM | MAO treatment on titanium; ZIF-8 loaded via in situ hydrothermal process; iodine captured via vapor deposition | Responsive iodine release under near-infrared exposure; intracellular ROS oxidation; synergistic antibacterial effect; improve osseointegration of coated implants | 27 |
| Zr-MOF | Zr4+ ion; TCPP | Mineralized collagen coating with MOF deposited on the cathode (Ti) by electrochemically assisted deposition; naringin dropped onto the coating last | Naringin-loaded MOFs coating: control the release of naringin; promote osseointegration and prevent bacterial infection | 29 |
| Cardio-vascular implantation | ||||
| Cu2+/ZIF-8 | Zn2+ ion; Cu2+ ion; 2-MIM | React at room temperature; amount of copper doped was tuned by varying the molar ratio | Achieve controlled NO generation by tuning the copper doping percentages; produced a 10-fold increased amount of NO compared with previous reports | 43 |
| HKUST-1 (CuBTC) | Cu2+ ion; BTC | MOF–PU composite tubing by compounding and extrusion | Facilitate the generation of NO from bioavailable S-nitrosothiols at therapeutic levels; dramatically reduce thrombi formation; elicit 95% reduction in the bacterial attachment of both S. aureus and E. coli; Cu2+ ion realease: prevent the late stent thrombosis and in-stent restenosis associated with stent implantation | 38 |
| Directly deposition onto the surface of carboxyl-functionalized cotton | 39 | |||
| Prepared on three kinds of inert biomaterials (Ti–O, PVC, 316L SS) with PDA as the nucleation center | 45 | |||
| Immobilized onto the titanium surface with PDA | 46 | |||
| Neural tissue engineering | ||||
| CeO2@ZIF-8 | Zn2+ ion; 2-MIM | ZIF-8-capped CeO2 NPs | Catalytic and antioxidative activities; improve stroke therapeutic efficacy | 60 |
| Fe2NC@Se-MOF | Zn2+ ion; 2-MIM | Fe2NC synthesized by wet-chemistry carbonization; synthesize an imidazole derivative containing Se to grow a new layer outside Fe2NC | Counteract oxidative damage and inhibit neural apoptosis after cerebral ischemia-reperfusion injury | 59 |
| UiO-66 | Zr4+ ion; BDC-NH2 | Integrating rGO and MOF into PCL substrate solution for electrospinning | Piezoelectric scaffolds have matched bioadaptability with nerve immunometabolism, facilitate nerve repair | 64 |
| CD-MOF | K+ ion; β-CD | CD-MOF@MPSS mixed in MN needle-like array substrates | A new method of transdural delivering drugs to the spinal cord | 68 |
| ZIF-8 | Zn2+ ion; 2-MIM | ZIF-8-introduced DPSCs loaded into GelMA hydrogel | Promote neural differentiation and angiogenesis of DPSCs for nerve repair | 69 |
| Neural tissue engineering | ||||
| Cu-MOF, Co-MOF | Cu2+ ion; Co(OAc)2·4H2O; H2L | MOFs dispersion solution drop-casted onto the working electrode of the flexible sensor | Continuous monitor important nutriments by redox of metal ions for 20 days | 87 |
| Ni-MOF | Ni2+ ion; HHTP | Integrate cMOF-based layered film electrodes onto the nanocellulose substrate | Selective and accurate detection of vitamin C and uric acid | 88 |
| Ln-MOF | Eu3+ ion; H4BTEC | DA solutions, Eu-BTEC dispersion, and buffer solution mixed for DA detection. | Selectively recognize DA even in complex biological fluids without the interference of other structurally similar neurotransmitters | 89 |
| PCN-224 | Zr4+ ion; TCPP | Inserted functional molecule into MOFs through the carboxyl group | Realize the simultaneous detection and imaging of pH and phosphorylation through the pH-sensitive group piperazine and the ZrIV node | 90 |
| CD-MOF | K+ ion; β-CD | Encapsulate Ru(bpy)32+ in MOF and then adhered to the electrode surface | High sensitivity, low limit of detection and a great linear range for CYFRA21-1 in A549 lung cancer cells | 91 |
Although flexible sensors can be bent and deformed, which can reduce tissue damage and improve comfort when implanted in the body, their specificity, reliability and sensitivity in chemical sensing are slightly insufficient compared with rigid sensors. Combining the functionality of MOFs with the merits of flexible materials may be a promising approach to fabricate implantable chemical sensors in vivo. Ling et al. mixed the grinded nMOFs (Cu-MOF and Co-MOF) and 10 wt% of Nafion uniformly through ultrasound to obtain a MOFs dispersion solution (Fig. 6a).87 Then, they were dropwise injected into the working electrode obtained through screen printing to achieve surface functionalization modification. Through the oxidation of metal nodes in the selected MOFs materials, specific recognition and detection of nutrients such as ascorbic acid, L-tryptophan, glycine, and glucose are achieved, thereby monitoring human metabolism and circulation processes. Compared to other surface coating materials, the peak current of the working electrode after MOFs modification increases and the bias voltage greatly decreases, resulting in a decrease in overall energy consumption and avoiding hydrolysis damage and tissue damage caused by overvoltage. The continuous working time of the sensor is also increased within 20 days. By distinguishing the bias voltage required for different nutrients to participate in oxidation reactions, simultaneous detection and effective differentiation of multiple substances in different parts can be achieved, enabling the preparation of multi-channel implantable sensors. In addition, compared with the performance of Cu-MOFs and Co-MOFs, it was found that there were significant differences in their conductivity when the surface area and pore size were close, leading to differences in response and catalytic activity during actual detection. This result indicates that the resolution and specificity of the sensor can be adjusted by the structure and composition of MOFs, and the pore channels of MOFs also provide the possibility of introducing other conductive fillers such as graphene oxides. Similarly, a wearable sweat metabolite detection sensor was prepared by combining conductive Ni-MOFs with flexible nanocellulose (Fig. 6b).88 The nanocellulose substrate endows the sensor with flexibility and breathability, enabling the fit of the human skin and wet adhesion. The selected MOFs with their large specific surface area and multiple reaction sites can achieve in situ detection by selective electrocatalytic oxidation of trace amounts of vitamin C in sweat.
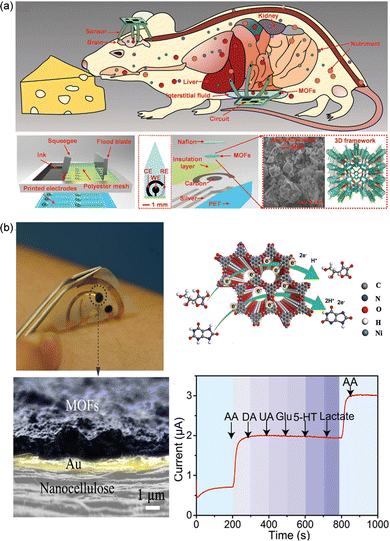 | ||
| Fig. 6 (a) Conductive Cu-MOFs and Co-MOFs drop-casted onto the working electrode of the flexible sensor to monitor nutriments continuously. Reprinted from ref. 87 with permission. Copyright 2018 Wiley. (b) Ni-MOF-based wearable sweat sensors for selective and accurate detection of vitamin C and uric acid. Reprinted from ref. 88 with permission. Copyright 2022 Wiley. | ||
As one of the important neurotransmitters, the abnormal concentration of dopamine (DA) will lead to a series of diseases related to the central nervous system and cardiovascular system. Therefore, the detection of DA through biosensing technology is a useful tool for the disease diagnosis. Lanthanide MOFs with tuneable and strong multiple luminescent centres have been widely utilized as fluorescent sensors. The designability of the Ln-MOF structure provides the possibility for material modular design and structural programming. In addition, the Ln-MOF-based artificial receptor DA biosensor has been designed successfully to achieve highly selective and dual-response detection of dopamine.89 In addition to specific detection of nutrients, neurotransmitters, and so on, MOF-based biochemical sensors are used for disease assessment and cancer cell monitoring, mainly through the identification of changes in environmental pH and protein phosphorylation under inflammatory conditions, as well as specific biomarkers.90,91
Conclusions and perspectives
The development of minimally invasive technology has promoted the widespread application of implant interventional devices, which can be roughly divided into bone implantation, cardiovascular implantation, nerve repair and biochemical sensing according to different application sites and performance requirements. Compared with the traditional porous materials, MOFs materials have a greater potential application value in the modification of implant interventional materials due to their unique features of adjustable pore size, diverse and biodegradable compositions, easier chemical post-modification, and so on. MOFs are inorganic–organic hybrid materials with periodic porous structure formed by metal ions or metal oxygen clusters and organic bridging ligands through coordination bonds. Combined with the characteristics of the MOFs themselves, the biological function of the implant interventional material can be endowed by the structural design to better serve in the human body.First, the metal ions contained in the MOFs, which can be slowly released under specific conditions, not only have antibacterial functions and reduce wound infection, but also regulate the activity of ALP during osteogenesis, so as to achieve the bone-promoting and antibacterial properties of bone implant materials. Specific metal ions such as copper ions have been shown to catalyze the breakdown of the endogenous blood component S-nitroso mercaptan (RSNO), enabling the continuous production of nitric oxide (NO). On the one hand, NO, as a signalling molecule produced by endothelial cells, can inhibit platelet adhesion, prevent thrombosis and promote endothelialization. On the other hand, the appropriate concentration of NO, as an endogenous signal, can induce various physiological functions of the nervous system, play an active role in nerve repair, and play a key role in coping with traumatic brain injury. Some valence-variable metal ions, such as cerium ions, have antioxidant effects and can avoid further oxidative damage caused by the explosive production of ROS during cerebral ischemia reperfusion. The surface of the electrode can be modified by MOFs, and the metal nodes of MOFs can recognize the nutrients in the human body through selective electrocatalytic oxidation, so as to realize human biochemical sensing. Besides, the selection of organic ligands plays an important role in the realization of MOF functionalization. For example, bio-MOF-1 materials are generated by the coordination of zinc ions and adenine ligands, which have good biocompatibility and osteogenic potential and work synergically with zinc ions to enhance early bone integration at the bone-implant interface. Similarly, gallic acid (GA) in the pH-sensitive Mg–GA MOF materials promotes angiogenesis and osteogenic differentiation. The selection of some photosensitive ligands can give the material photo-responsive bactericidal properties. In addition, by modifying the functional groups of the organic ligands, the non-central symmetry of the crystalline MOFs material is increased, so that its piezoelectric properties are improved. The electromechanical coupling effect of MOF materials realizes the surface charge generation, which plays an important role in nerve repair. Last but not least, the abundant pore structure of MOFs increases the contact area and improves the accessibility of reaction sites. On the other hand, it can be used as a carrier to support bioactive substances and achieve synergistic effects. For example, the loading of hydroxyapatite and osteogenic growth peptides enables the material to promote bone properties. The loading of Chinese herbs, antibiotics, and metal iodine gives the material better preventive or bactericidal properties. The loading of conductive nano-fillers such as graphene oxides improves the effective carrier migration within the MOFs material to achieve better electrical properties.
Compared with the antibacterial and anticancer applications both in vitro and in vivo, the application of MOFs materials in the implant interventional field is still in its initial stage. Although recent works with great progresses have been reported on the implant interventional applications of MOFs and their related structures, there are still some problems that limit their further development.
Design and property of implanted MOFs
There are still many questions about the functional design, so extensive research on MOF materials with new functions is needed. At present, most studies on MOF materials as coatings of bone implants focus on Zn-based MOFs, while less attention is paid to Mg-based, Ca-based, and Sr-based MOFs. In addition to the role of metal nodes in promoting bone healing, the deep mechanism affecting cell behaviour and the role of ligands need to be further studied. In terms of cardiovascular implantation and nerve repair, the influence of the shape, size regulation and surface chemical composition of nMOF materials on the final clinical effect also needs further experimental verification. As for biochemical sensing, there is no clear conclusion on the specific mechanism of metal nodes participating in redox reactions and whether they have catalytic activity conducive to the electron transfer of sensing. The effect of introducing other conductive materials to form heterogeneous structures on the catalytic capacity also needs to be further studied. Long-term studies in animal models and humans are needed to evaluate the performance, stability and bioactivity of MOFs as surface coatings.Stability and modulus match
Most studies focus only on the mechanism of action of MOFs and the evaluation of related properties, often ignoring the change in physical and chemical properties of MOF-modified composite materials. MOFs are usually introduced onto the surface of metal implants as coatings or into the polymers by means of blending extrusion, solution blending, electrospinning, and other processes to prepare composite implanting interventional materials. As an inorganic–organic composite, the interface compatibility between MOFs and matrix materials will affect the overall mechanical properties and other biological properties of the composite. How to improve the interaction force between MOFs and the matrix materials to avoid accidental shedding, and how to match the modulus to enhance the mechanical properties are still the key problems that need to be solved when MOFs materials are really used in clinical implantation interventional devices.Biodegradation
Different implantation scenarios put forward different demands on the degradability of MOFs materials. In terms of biochemical sensing, MOFs coatings modified on the surface of metal electrodes need to have good stability to ensure that metal nodes can be used as active centers to achieve long-term monitoring of substances in the body. In the field of bone implantation, MOFs degradation is usually required to release metal ions to achieve the dual functions of bone healing and antibacterial activity. In terms of cardiovascular implantation and nerve repair, metal ion release accelerates the endothelialization process while catalysing the synergistic effect of NO production. In addition, additional functions are usually achieved by loading bioactive molecules in MOFs pore channels. However, biodegradable MOFs have a high degradation rate and poor stability, resulting in the sudden release of loaded drugs or ions at the initial stage, which affects their biocompatibility, long-term release and antibacterial performance. Therefore, it is necessary to further improve the drug loading efficiency and slow-release ability. At present, most of the mechanism studies of sustained release systems are speculative, and the intelligent release mechanism of drugs needs to be further studied and explained. In addition, since the in vitro degradation conditions are difficult to simulate the real in vivo scene, the mechanism and pathway of MOFs degradation in vivo need to be systematically studied. Although antibiotics are the most commonly used and effective antimicrobial means in clinical settings, considering the long-term resistance problem, the development of new functional MOFs materials to achieve dynamic sterilization, especially based on physical triggers, is a more sensible choice.Toxicity issues
The primary problem to be solved in introducing MOFs into implant intervention materials is their toxicity. At present, there are more or less toxicity problems of heavy metals and ligands in known MOFs. It could not be ignored that the released metal ions from unstable MOFs can gradually accumulate on certain tissues under physiological conditions. To address this issue, more in vivo experiments should be carried out to fully evaluate the potential impact of these ions on tissue health and function. Besides, it is necessary to develop new MOFs materials that use endogenous or bioactive molecules as ligands and highly biocompatible metal ions (such as Zn, Ca, and Fe) as metal nodes. In addition, agglomeration caused by uneven dispersion of MOFs in composite implanting interventional materials will produce additional toxic effects, and hence, it is necessary to develop MOFs with surface functionalization and size control strategies to adjust the surface potential and improve the binding force of MOFs with polymer or metal materials.In general, MOFs have unique advantages and great prospects as implant intervention materials, but the research is still in its infancy. Academically, there are still many challenging fundamental questions to be addressed. On the one hand, by comprehensively exploring the matching and selection of metal ions and ligands, the physicochemical and biological characteristics of MOFs can be studied, focusing on the biological behaviour of each component of the composite materials and the synergistic effect between each component. On the other hand, systematic in-depth studies are needed to reveal the structure–property relationship. True practical applications of MOFs need to be evaluated from the perspective of long-term stability of implant materials, reproducibility for large-scale manufacturing, and commercial costs.
Abbreviations
| ALP | Alkaline phosphatase |
| MSCs | Mesenchymal stem cells |
| GA | Gallic acid |
| PA | Phytic acid |
| NIR | Near infrared |
| CRBIs | Catheter-related bloodstream infections |
| CRT | Catheter-related thrombosis |
| ISR | In-stent restenosis |
| NO | Nitric oxide |
| RSNO | S-Nitrosothiols |
| GSNO | S-Nitrosoglutathione |
| LBL | Layer-by-layer self-assembly |
| SMCs | Smooth muscle cells |
| LST | Late stent thrombosis |
| CNS | Central nervous system |
| DDSs | Drug delivery systems |
| TBI | Traumatic brain injury |
| AMF | Alternating magnetic field |
| CIRI | Cerebral ischemic reperfusion injury |
| CNIs | Central nervous injuries |
| PNIs | Peripheral nervous injuries |
| BBB | Blood–brain barrier |
| DA | Dopamine. |
Author contributions
Yifan Liu: investigation, methodology, formal analysis, writing – original draft. Shuteng Wang: investigation, formal analysis. Chunhua Quan: supervision, writing – review & editing. Shifang Luan: writing – review & editing, conceptualization. Hengchong Shi: supervision, funding acquisition, writing – review & editing. Lei Wang: conceptualization, project administration, supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Jilin Provincial Key Research and Development Plan Project (Project No. 20230204105YY), the National Natural Science Foundation of China (Project No. 52373049), Youth Innovation Promotion Association of CAS (Grant No. Y2021066), and Chinese Academy of Sciences-Wego Group Hightech Research & Development Program.Notes and references
- M. Eger, S. Hiram-Bab, T. Liron, N. Sterer, Y. Carmi, D. Kohavi and Y. Gabet, Front. Immunol., 2018, 9, 2963 CrossRef CAS PubMed.
- C. R. Arciola, D. Campoccia and L. Montanaro, Nat. Rev. Microbiol., 2018, 16, 397–409 CrossRef CAS.
- A. Aarvold, J. O. Smith, E. R. Tayton, S. A. Lanham, J. B. Chaudhuri, I. G. Turner and R. O. C. Oreffo, J. Biomed. Mater. Res., Part A, 2013, 101, 3431–3437 CrossRef PubMed.
- I. Bružauskaitė, D. Bironaitė, E. Bagdonas and E. Bernotienė, Cytotechnology, 2016, 68, 355–369 CrossRef PubMed.
- A. Rodríguez-Contreras, J. Guillem-Marti, O. Lopez, J. M. Manero and E. Ruperez, Colloids Surf., B, 2019, 182, 110317 CrossRef PubMed.
- B. Kundu, S. K. Nandi, S. Dasgupta, S. Datta, P. Mukherjee, S. Roy, A. K. Singh, T. K. Mandal, P. Das, R. Bhattacharya and D. Basu, J. Mater. Sci.: Mater. Med., 2011, 22, 705–720 CrossRef CAS PubMed.
- Y. Xue, G. Zhao, R. Yang, F. Chu, J. Chen, L. Wang and X. Huang, Nanoscale, 2021, 13, 3911–3936 RSC.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed.
- L. Wang, M. Zheng and Z. Xie, J. Mater. Chem. B, 2018, 6, 707–717 RSC.
- M. Li, S. Yin, M. Lin, X. Chen, Y. Pan, Y. Peng, J. Sun, A. Kumar and J. Liu, J. Mater. Chem. B, 2022, 10, 5105–5128 RSC.
- N. Aslankoohi, D. Mondal, A. S. Rizkalla and K. Mequanint, Polymers, 2019, 11, 1437–1468 CrossRef CAS PubMed.
- W. Wang and K. W. K. Yeung, Bioact. Mater., 2017, 2, 224–247 Search PubMed.
- M. d J. Velásquez-Hernández, R. Ricco, F. Carraro, F. T. Limpoco, M. Linares-Moreau, E. Leitner, H. Wiltsche, J. Rattenberger, H. Schröttner, P. Frühwirt, E. M. Stadler, G. Gescheidt, H. Amenitsch, C. J. Doonan and P. Falcaro, CrystEngComm, 2019, 21, 4538–4544 RSC.
- M. Shyngys, J. Ren, X. Liang, J. Miao, A. Blocki and S. Beyer, Front. Bioeng. Biotechnol., 2021, 9, 603608 CrossRef PubMed.
- L. Q. Fu, X. Y. Chen, M. H. Cai, X. H. Tao, Y. B. Fan and X. Z. Mou, Front. Bioeng. Biotechnol., 2020, 8, 576348 CrossRef PubMed.
- C. Liu, X. Xu, W. Cui and H. Zhang, Eng. Regener., 2021, 2, 105–108 Search PubMed.
- M. Chen, D. Wang, M. Li, Y. He, T. He, M. Chen, Y. Hu, Z. Luo and K. Cai, ACS Nano, 2022, 16, 15397–15412 CrossRef CAS.
- J. Wu, S. Jiang, W. Xie, Y. Xue, M. Qiao, X. Yang, X. Zhang, Q. Wan, J. Wang, J. Chen and X. Pei, J. Mater. Chem. B, 2022, 10, 8535–8548 RSC.
- L. Zhong, J. Chen, Z. Ma, H. Feng, S. Chen, H. Cai, Y. Xue, X. Pei, J. Wang and Q. Wan, Nanoscale, 2020, 12, 24437–24449 RSC.
- R. Yang, Y. Zheng, Y. Zhang, G. Li, Y. Xu, Y. Zhang, Y. Xu, C. Zhuang, P. Yu, L. Deng, W. Cui, Y. Chen and L. Wang, Adv. Healthcare Mater., 2022, 11, 2200072 CrossRef CAS PubMed.
- B. Zhang, L. Wang, P. Song, X. Pei, H. Sun, L. Wu, C. Zhou, K. Wang, Y. Fan and X. Zhang, Mater. Des., 2021, 201, 109490 CrossRef CAS.
- K. Banerjee, M. Debroy, V. K. Balla and S. Bodhak, J. Mater. Res., 2021, 36, 3877–3893 CrossRef CAS.
- R. A. Surmenev, A. N. Ivanov, A. Cecilia, T. Baumbach, R. V. Chernozem, S. Mathur and M. A. Surmeneva, Open Ceram., 2022, 9, 100237 CrossRef CAS.
- X. Liu, M. Chen, J. Luo, H. Zhao, X. Zhou, Q. Gu, H. Yang, X. Zhu, W. Cui and Q. Shi, Biomaterials, 2021, 276, 121037 CrossRef CAS PubMed.
- W. Dong, S. Zhao, Y. Wang, X. Zhou, J. Jiang, J. Dang, D. Sun, X. Dai, M. Zhang and Z. Jiang, Mater. Des., 2023, 225, 111485 CrossRef CAS.
- C. Zhang, G. Chu, Z. Ruan, N. Tang, C. Song, Q. Li, W. Zhou, J. Jin, H. Haick, Y. Chen and D. Cui, ACS Nano, 2022, 16, 16584–16597 CrossRef CAS PubMed.
- W. Teng, Z. Zhang, Y. Wang, Y. Ye, E. Yinwang, A. Liu, X. Zhou, J. Xu, C. Zhou, H. Sun, F. Wang, L. Zhang, C. Cheng, P. Lin, Y. Wu, Z. Gou, X. Yu and Z. Ye, Small, 2021, 17, 2102315 CrossRef CAS PubMed.
- B. Tao, W. Yi, X. Qin, J. Wu, K. Li, A. Guo, J. Hao and L. Chen, J. Mater. Sci. Technol., 2023, 146, 131–144 CrossRef CAS.
- M. Yu, D. You, J. Zhuang, S. Lin, L. Dong, S. Weng, B. Zhang, K. Cheng, W. Weng and H. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 19698–19705 CrossRef CAS PubMed.
- M. Badv, F. Bayat, J. I. Weitz and T. F. Didar, Biomaterials, 2020, 258, 120291 CrossRef CAS PubMed.
- I. H. Jaffer, J. C. Fredenburgh, J. Hirsh and J. I. Weitz, J. Thromb. Haemostasis, 2015, 13, S72–S81 CrossRef PubMed.
- A. de Mel, F. Murad and A. M. Seifalian, Chem. Rev., 2011, 111, 5742–5767 CrossRef CAS PubMed.
- D. D. Thomas, X. Liu, S. P. Kantrow and J. R. Lancaster Jr., Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 355–360 CrossRef CAS PubMed.
- D. D. Thomas, Redox Biol., 2015, 5, 225–233 CrossRef CAS PubMed.
- C.-G. Dai, J.-L. Wang, Y.-L. Fu, H.-P. Zhou and Q.-H. Song, Anal. Chem., 2017, 89, 10511–10519 CrossRef CAS PubMed.
- K. Kulyk, L. Azizova, J. M. Cunningham, L. Mikhalovska, M. Borysenko and S. Mikhalovsky, J. Mater. Chem. B, 2020, 8, 4267–4277 RSC.
- N. Naghavi, A. de Mel, O. S. Alavijeh, B. G. Cousins and A. M. Seifalian, Small, 2013, 9, 22–35 CrossRef CAS PubMed.
- J. L. Harding and M. M. Reynolds, J. Mater. Chem. B, 2014, 2, 2530–2536 RSC.
- M. J. Neufeld, J. L. Harding and M. M. Reynolds, ACS Appl. Mater. Interfaces, 2015, 7, 26742–26750 CrossRef CAS PubMed.
- F. Zhang, Q. Zhang, X. Li, N. Huang, X. Zhao and Z. Yang, Biomaterials, 2019, 194, 117–129 CrossRef CAS PubMed.
- J. Pant, M. J. Goudie, S. P. Hopkins, E. J. Brisbois and H. Handa, ACS Appl. Mater. Interfaces, 2017, 9, 15254–15264 CrossRef CAS PubMed.
- T. C. Major, D. O. Brant, C. P. Burney, K. A. Amoako, G. M. Annich, M. E. Meyerhoff, H. Handa and R. H. Bartlett, Biomaterials, 2011, 32, 5957–5969 CrossRef CAS PubMed.
- Y. Zhou, T. Yang, R. Namivandi-Zangeneh, C. Boyer, K. Liang and R. Chandrawati, J. Mater. Chem. B, 2021, 9, 1059–1068 RSC.
- M. J. Neufeld, J. L. Harding and M. M. Reynolds, ACS Appl. Mater. Interfaces, 2015, 7, 26742–26750 CrossRef CAS PubMed.
- Y. Zhang, Q. Zhao, W. Li, J. Liu, J. Chen, Y. Fan and Y. Weng, Adv. Mater. Interfaces, 2020, 7, 1902011 CrossRef CAS.
- Y. Fan, Y. Zhang, Q. Zhao, Y. Xie, R. Luo, P. Yang and Y. Weng, Biomaterials, 2019, 204, 36–45 CrossRef CAS PubMed.
- G. J. Hankey, Stroke, 2005, 36, 218–221 CrossRef PubMed.
- G. J. Hankey, Lancet, 2017, 389, 641–654 CrossRef PubMed.
- A. Flemming, Nat. Rev. Immunol., 2019, 19, 473 CrossRef CAS PubMed.
- S. Matsumoto, M. Murozono, M. Kanazawa, T. Nara, T. Ozawa and Y. Watanabe, Acute Med. Surg., 2018, 5, 213–221 CrossRef PubMed.
- X. R. Lee and G. L. Xiang, Clin. Neurol. Neurosurg., 2018, 167, 157–161 CrossRef PubMed.
- S. Bhattacharjee, B. Jun, L. Belayev, J. Heap, M. A. Kautzmann, A. Obenaus, H. Menghani, S. J. Marcell, L. Khoutorova, R. Yang, N. A. Petasis and N. G. Bazan, Sci. Adv., 2017, 3, e1700735 CrossRef PubMed.
- H. Niu, H. Bu, J. Zhao and Y. Zhu, Small, 2023, 19, 2206575 CrossRef CAS PubMed.
- C. Qiao, R. Zhang, Y. Wang, Q. Jia, X. Wang, Z. Yang, T. Xue, R. Ji, X. Cui and Z. Wang, Angew. Chem., Int. Ed., 2020, 59, 16982–16988 CrossRef CAS PubMed.
- J. Han, M. Zhang, G. Chen, Y. Zhang, Q. Wei, Y. Zhuo, G. Xie, R. Yuan and S. Chen, J. Mater. Chem. B, 2017, 5, 8330–8336 RSC.
- X. Ma, X. Ren, X. Guo, C. Fu, Q. Wu, L. Tan, H. Li, W. Zhang, X. Chen, H. Zhong and X. Meng, Biomaterials, 2019, 214, 119223 CrossRef CAS PubMed.
- L. Meng, N. Ren, M. Dong, S. Zhang, A. Wang, Z. Zhuang, J. Wang, C. Sun and H. Liu, Adv. Funct. Mater., 2023, 2309974 CrossRef.
- Y.-C. Chan, Y.-H. Lin, H.-C. Liu, R.-S. Hsu, M.-R. Chiang, L.-W. Wang, T.-C. Chou, T.-T. Lu, I. C. Lee, L.-A. Chu and S.-H. Hu, Nano Today, 2023, 51, 101935 CrossRef CAS.
- L. He, G. Huang, H. Liu, C. Sang, X. Liu and T. Chen, Sci. Adv., 2020, 6, eaay9751 CrossRef CAS PubMed.
- R. Tian, H. Ma, W. Ye, Y. Li, S. Wang, Z. Zhang, S. Liu, M. Zang, J. Hou, J. Xu, Q. Luo, H. Sun, F. Bai, Y. Yang and J. Liu, Adv. Funct. Mater., 2022, 32, 2204025 CrossRef CAS.
- J. Wang, Y. Wang, X. Xiaohalati, Q. Su, J. Liu, B. Cai, W. Yang, Z. Wang and L. Wang, Adv. Sci., 2023, 10, 2206854 CrossRef CAS PubMed.
- S. Zhao, A. S. Mehta and M. Zhao, Cell. Mol. Life Sci., 2020, 77, 2681–2699 CrossRef CAS PubMed.
- M.-X. Wu and Y.-W. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef PubMed.
- X. Yao, Z. Yan, A. Liu, L. Zhan, Y. Liu, C. Huang, Y. Ouyang, H. Ruan, Y. Qian and C. Fan, Nano Today, 2023, 49, 101814 CrossRef CAS.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- C. Zhang, D. Lei, C. Xie, X. Hang, C. He and H. L. Jiang, Adv. Mater., 2021, 33, e2106308 CrossRef PubMed.
- X. Zhai, K. Chen, X. Wei, H. Zhang, H. Yang, K. Jiao, C. Liu, Z. Fan, J. Wu, T. Zhou, H. Wang, J. Li, M. Li, Y. Bai and B. Li, J. Controlled Release, 2023, 360, 236–248 CrossRef CAS PubMed.
- H. Zhou, S. Jing, W. Xiong, Y. Zhu, X. Duan, R. Li, Y. Peng, T. Kumeria, Y. He and Q. Ye, J. Nanobiotechnol., 2023, 21, 316 CrossRef CAS PubMed.
- W. Ling, J. Yu, N. Ma, Y. Li, Z. Wu, R. Liang, Y. Hao, H. Pan, W. Liu, B. Fu, K. Wang, H. Wang, L. Li, X. Sheng, H. Peng, B. Ning, J. Yang and X. Huang, Adv. Funct. Mater., 2020, 30, 2002644 CrossRef CAS.
- Y. Yu, H. Y. Y. Nyein, W. Gao and A. Javey, Adv. Mater., 2020, 32, 1902083 CrossRef CAS PubMed.
- R. M. Pallares, N. T. K. Thanh and X. Su, Nanoscale, 2019, 11, 22152–22171 RSC.
- D. Nunes, A. Pimentel, A. Gonçalves, S. Pereira, R. Branquinho, P. Barquinha, E. Fortunato and R. Martins, Semicond. Sci. Technol., 2019, 34, 043001 CrossRef CAS.
- K. Kim, C. H. Lee and C. B. Park, Chem. Soc. Rev., 2020, 49, 5446–5472 RSC.
- K. Khan, A. K. Tareen, M. Aslam, R. Wang, Y. Zhang, A. Mahmood, Z. Ouyang, H. Zhang and Z. Guo, J. Mater. Chem. C, 2020, 8, 387–440 RSC.
- Y. Li, W. Ling, X. Liu, X. Shang, P. Zhou, Z. Chen, H. Xu and X. Huang, Nano Res., 2021, 14, 2981–3009 CrossRef CAS.
- S. K. Bhardwaj, N. Bhardwaj, R. Kaur, J. Mehta, A. L. Sharma, K.-H. Kim and A. Deep, J. Mater. Chem. A, 2018, 6, 14992–15009 RSC.
- X.-X. Xie, Y.-C. Yang, B.-H. Dou, Z.-F. Li and G. Li, Coord. Chem. Rev., 2020, 403, 213100 CrossRef CAS.
- Q.-Q. Huang, Y.-J. Lin, R. Zheng, W.-H. Deng, C. Kashi, P. N. Kumar, G.-E. Wang and G. Xu, Inorg. Chem. Commun., 2019, 105, 119–124 CrossRef CAS.
- M. Guo, H.-L. Cai and R.-G. Xiong, Inorg. Chem. Commun., 2010, 13, 1590–1598 CrossRef CAS.
- M. Sadakiyo, H. Kasai, K. Kato, M. Takata and M. Yamauchi, J. Am. Chem. Soc., 2014, 136, 1702–1705 CrossRef CAS PubMed.
- J. Yang, Z. Ma, W. Gao and M. Wei, Chem. – Eur. J., 2017, 23, 631–636 CrossRef CAS PubMed.
- A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. El Gabaly, H. P. Yoon, F. Léonard and M. D. Allendorf, Science, 2014, 343, 66–69 CrossRef CAS PubMed.
- S. Amirjalayer, M. Tafipolsky and R. Schmid, Angew. Chem., Int. Ed., 2007, 46, 463–466 CrossRef CAS PubMed.
- V. Rubio-Giménez, S. Tatay and C. Martí-Gastaldo, Chem. Soc. Rev., 2020, 49, 5601–5638 RSC.
- C. Liu, J. Wang, J. Wan and C. Yu, Coord. Chem. Rev., 2021, 432, 213743 CrossRef CAS.
- W. Ling, G. Liew, Y. Li, Y. Hao, H. Pan, H. Wang, B. Ning, H. Xu and X. Huang, Adv. Mater., 2018, 30, 1800917 CrossRef PubMed.
- X. Yang, J. Yi, T. Wang, Y. Feng, J. Wang, J. Yu, F. Zhang, Z. Jiang, Z. Lv, H. Li, T. Huang, D. Si, X. Wang, R. Cao and X. Chen, Adv. Mater., 2022, 34, 2201768 CrossRef CAS PubMed.
- L. Yu, L. Feng, Z. Wei, S. Wang, Y. Feng, Y. Shen, J. Cai, J. Wu and Y. Xiao, Adv. Funct. Mater., 2023, 33, 2300309 CrossRef CAS.
- J. Li, N. Zhao, W. Zhang, P. Li, X. Yin, W. Zhang, H. Wang and B. Tang, Angew. Chem., Int. Ed., 2023, 62, e202215178 CrossRef CAS PubMed.
- Y. Wang, Y. Li, X. Zhuang, C. Tian, X. Fu and F. Luan, Biosens. Bioelectron., 2021, 190, 113371 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |