Glycerol and its derivatives as potential C-3 bio-based building blocks for accessing active pharmaceutical ingredients
Romain
Morodo†
a,
Loïc
Bovy†
a,
Diana V.
Silva-Brenes
ab and
Jean-Christophe M.
Monbaliu
 *abc
*abc
aCenter for Integrated Technology and Organic Synthesis, MolSys Research Unit, University of Liège, B-4000 Liège (Sart Tilman), Belgium. E-mail: jc.monbaliu@uliege.be; Web: https://www.citos.uliege.be
bFloW4all Flow Technology Resource center, University of Liège, B-4000 Liège (Sart-Tilman), Belgium
cWEL Research Institute, Avenue Pasteur 6, B-1300 Wavre, Belgium
First published on 11th July 2024
Abstract
This review discusses the underexploited potential of renewable glycerol and its derivatives for the preparation of active pharmaceutical ingredients, some of which are on the World Health Organization list of essential medicines. The regulatory challenges faced by industries regarding the replacement of petro-based building blocks with renewably sourced ones are described before diving into pharmaceutical ingredients that could potentially incorporate these bio-based atoms. The active pharmaceutical ingredients (APIs) are sorted by their therapeutical potential, including entities treating cardiovascular diseases, musculoskeletal drugs and compounds endowed with anti-infective properties. Finally, polymeric drugs and more eclectic substrates such as dietary supplements, radiosensitizers or chemotherapeutical agents are considered in the last two sub-sections. The broad spectrum of presented substrates relying on glycerol or potentially glycerol-derived reagents in their synthetic pathway emphasizes the potential contribution of bio-based substrates in already developed industrial processes. The examples in this review hint toward a future chemical development in which APIs may be constructed with increasing percentages of bio-sourced atoms.
1. Introduction
The concept of bio-based chemicals involves the use of biomass to replace petrol as a source of chemicals. A sector of chemists has turned to starch, cellulose, lignin, and oil, among other biomass, as raw materials to provide new chemical platforms that can fit consumer needs. To be usable, biomass must be degraded to its constitutive compounds, principally sugars, which can then be further modified to obtain chemical building blocks.1 The development of these bio-based platforms is meant to provide an alternative to conventional petroleum-derived products,2 decreasing the environmental impact of current chemical processes and the reliance on ever-decreasing petrol reserves. Furthermore, the adoption of widely available biomass as a source of chemicals, as opposed to geographically limited petrol sources, will have strong economic and even political implications.A great deal of effort has gone into identifying the most promising candidates for bio-based platforms to help focus the attention of both chemists and investors to strategies that have the best chance of success. Among the building blocks that have been highlighted, glycerol stands out particularly due to its exceptionally high availability.
Glycerol, or 1,2,3-propanetriol (1, CAS 56-81-5), is one of the main components of triglycerides found in fats and oils (Fig. 1). Its currently abundant production is due to it being a by-product of the biodiesel, soap, and cooking oil recycling industries. Most of the glycerol on the market is currently bio-sourced, with trends suggesting it will continue to be increasingly the case. A report from 2004 estimated that 25% of the glycerol on the market was being produced synthetically,1 whereas 2016 reports placed the amount of synthetic glycerol on the market at around 12%.3 The production of glycerol has been significantly boosted by the biodiesel industry, which in turn has continued to increase with the support of tax incentives and legislation. Around 1 ton of biodiesel results in the formation of 100 kg of glycerol as by-product.3,4 The cost of glycerol has continued to experience a downward trend for the past decades. In 2019, European transaction prices for glycerol (≥99.5% purity) were around 856 USD per metric ton (USD per mt),5 whereas export prices of crude glycerol averaged 266 USD per mt.6 As a point of comparison, propylene (≥99.5% pure) was valued at 980 USD per mt (2019).7
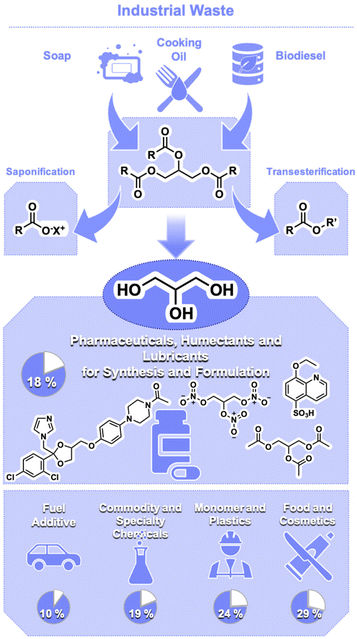 | ||
| Fig. 1 Sources to produce bio-based glycerol (1) and current main applications of glycerol on the market alongside estimations of its end use. | ||
Because glycerol is obtained as a by-product of the biodiesel industries, its current cost of production is virtually negligible. The factor determining the cost of glycerol is the refinement process, with small biorefineries often finding landfilling to be more economical than refining glycerol. A 2004 estimate placed the cost of refining at around $0.23–$0.38 per kg (230–380 USD per mt) depending on the size of the facility.3 However, even with this cost for its refinement, the large production of crude glycerol, and consequent low price, point to an extraordinary potential to develop its use to replace products that were typically obtained from petroleum.8
Traditional uses of glycerol include pharmaceuticals, personal care products, foams, and foods, with these sectors comprising almost 60% of its usage.9 Due to its hygroscopicity, it is frequently used as a humectant, lubricant or to improve the softness of materials. In the food and pharmaceutical sectors, it serves as a humectant and texture-enhancing additive. Glycerol (1) is also used as a sweetener and to prolong the shelf-life of products in the food industry. It has also applications as a moisturizer in cosmetics and personal care products, or in the preparation of plastics to improve their flexibility. In the historical period of World War II, glycerol was in particularly high demand for the preparation of nitro-glycerine explosives (Fig. 1).9
Glycerol's low cost due to current overproduction has led many to propose various derivatizations of the compound, in efforts to open new ways to valorise the derivative.10 Typical C-3 building blocks that can be synthesized from 1 encompass allyl alcohol (2),11–13 which can be further oxidized to yield higher derivatives such as acrolein (3)10 and acrylic acid (4).10 Additionally, 1,3-dihydroxyacetone (5),14,15 1,3-propanediol (6),16 lactic acid (7),17 ethyl acrylate (8) and solketal (9),18 among other derivatives, are also attainable.
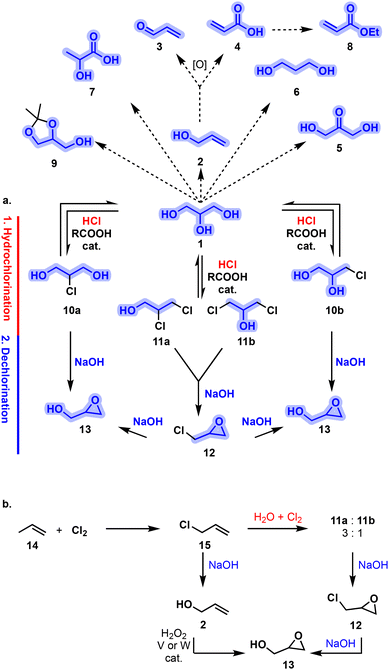
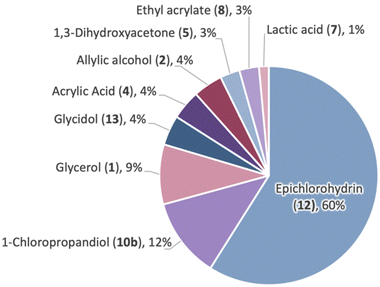
The most notable and extensively utilized C-3 building blocks potentially originating from 1, however, are monochlorohydrins 10a,b, dichlorohydrins 11a,b, and the epoxides derived therefrom: epichlorohydrin (12) and glycidol (13). The Epicerol (Solvay) and the GTE (Dow) processes19,20 are staple transformations connecting 1 with the formation of reactive C-3 oxiranes 12 and 13 (Fig. 2). The overall transformation involves a first hydrochlorination of 1 toward 10, 11 with aqueous HCl and a simple (di)carboxylic acid catalyst. In a second stage, the dechlorination of compounds 10, 11 under alkaline conditions opens a gateway toward oxirane derivatives 12 and 13. Appropriate control of the reaction parameters as well as catalyst selection is particularly relevant to tune the reaction selectivity.21 Such a process has been also intensified under continuous flow conditions.22 These processes stand as a robust alternative to the conventional chlorination of petro-based propylene (14) for the obtaining these versatile building blocks (Fig. 2).
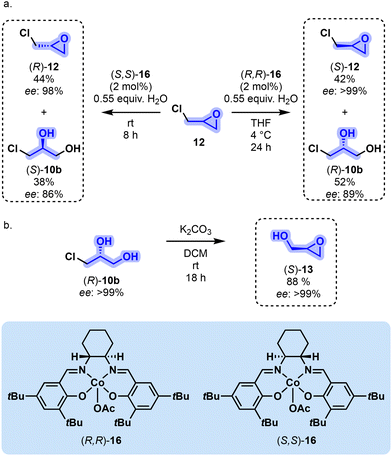
Another potentially appealing feature not shared with parent 1 resides in the presence of a stereogenic center at C2 on both 12 and 13. Enantioenriched oxiranes 12 and 13 can be accessed through a hydrolytic kinetic resolution (HKR) as introduced by Jacobsen in the late 1990s. The procedure relies on the enantioselective hydrolysis of a terminal epoxide in the presence of a Co-salen complex. It yields two distinct valuable products, namely an enantioenriched epoxide and the corresponding enantioenriched diol. To illustrate this point with rac-12, the optimized protocol utilizes (R,R)-16 as a catalyst along with 0.55 equivalents of H2O in THF at 4 °C (24 h). This leads to (S)-12 with a remarkable enantiomeric excess (ee) of >99% in 42% yield. Concurrently, monochlorohydrin (R)-10b was obtained in a 52% yield (ee of 89%) (Fig. 3a). Swapping the catalyst to its enantiomer (S,S)-16, (R)-12 and (S)-10b can be generated. Furthermore, enantiomerically pure (S)-12 can be directly synthesized from (R)-10b under alkaline conditions (88% yield, >99% ee) (Fig. 3b). HKR on rac-13 leads to a lower yield due to undesired side oligomerizations. The access to enantioenriched glycerol-derived reactive oxiranes and monochlorohydrins holds significant potential for the development of chiral derivatives, as are frequently encountered, for example, in the pharmaceutical industry.
This review presents a compilation of active pharmaceutical ingredients (APIs) the synthesis of which contains 1 or some of the compounds that could potentially be derived from it. Fig. 4 gives a distribution of the number of molecules here reported by the nature of the glycerol (1) derivative used in their synthesis. More than half of the APIs that we have managed to identify are due to the incorporation of epichlorohydrin (12). The presence of two reactive electrophilic sites in the C-3 molecule makes it a versatile building block, and previous reports agree on how their distinct reactivity makes them an attractive option to incorporate potentially bio-based C-3 building blocks in the chain of value.23,24
 | ||
| Fig. 4 Distribution of the APIs included in this review according to the potentially glycerol-derived building block incorporated as part of the synthesis. | ||
The following section will discuss more in depth the advantages and the challenges of incorporating more bio-based atoms into currently existing APIs, many of which are listed as WHO essential medicines and/or frequently prescribed medications.
2. The case for the use of glycerol derivatives
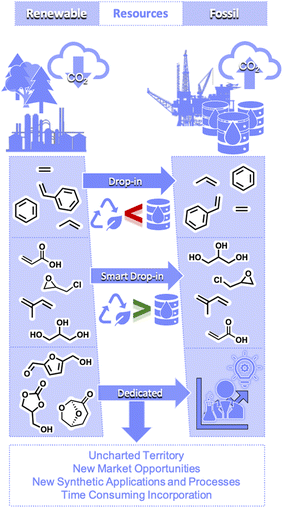
Efforts promoting low-carbon-intensity pathways have been gaining momentum in the chemical industry in general,25–28 including the pharmaceutical industry.29,30 In support of these efforts, we have taken an in-depth look into the feasibility of turning to bio-sourced reagents for the pharmaceutical industry, and particularly, to the use of 1 and derivatives.One of the main reasons that glycerol (1) has been identified as an important bio-based building block is because it can be used in the preparation of highly useful derivatives with established use in the chemical industry. The nova-Institute,31 a leading thinker for the transition to climate neutrality, has outlined three categories to classify bio-based chemicals,32 depending on how they can be integrated into current chemical pathways (Fig. 5). Drop-in platforms correspond to compounds that hold established commercial processes and markets. These platforms were initially developed using petro-based compounds; however, they can be replaced by the drop-in counterpart, obtained from biomass using alternative synthetic routes. A subcategory of these compounds, smart drop-in platforms, refers to compounds whose synthesis starting from bio-based sources is potentially more advantageous (e.g. shorter or with lower energy requirements) than the route used to obtain them from petro-based sources. Finally, the category of dedicated platform is assigned to bio-based molecules which do not match existing petro-based building blocks, thus paving the way to new uncharted territories. Glycerol (1) can be considered a smart drop-in, as it is directly obtained from the hydrolysis of oils. Expanding the uses of 1 is therefore one of the most efficient ways of increasing the use of bio-sourced atoms in current industrial processes, including pharmaceuticals (Fig. 5).
Epichlorohydrin (12) obtained from 1 is listed as another smart drop-in platform. As highlighted in Fig. 2, the process starting from propylene is much more energy intensive, relies on dangerous reagents and requires additional steps when compared with the process relying on 1, therefore confirming the status of smart drop-in of 12. This clear advantage in the production of bio-based epichlorohydrin (12), coupled with the increasing amounts of glycerol by-product from the biodiesel industry, has propelled the steady rise in production of bio-sourced 12 during recent years, specifically for the polymer industry.33–35
Despite these advantages, the incorporation of bio-sourced options for the pharmaceutical industries is slow to come. Bio-sourced compounds are tied to the fear of variability, one such being the change of seasons.36 On occasions, the bio-sourced glycerol obtained does not have the necessary specifications. For example, after the flooding of biodiesel glycerol on the market prompted DOW chemicals to close its synthetic glycerol production facilities in the United States in 2005,37 some pharmaceutical clients were unable to find bio-sourced glycerol substitutes with sufficient quality to sustain their processes.36
On the other hand, many pharmaceutical plants were able to switch to by-product glycerol, with the reduced price of material compensating for the research expenses tied to this change.36 This points to one of the main challenges toward incorporating bio-based atoms into already marketed drugs. Even a seemingly straightforward switch from a petro-based building block to its structurally identical bio-based counterpart entails an administrative burden related to market authorization. Any change made to the manufacturing process of an authorized API necessitates approval from the pertinent authority. For instance, in Europe, centrally authorized medicines are handled through the European Medicines Agency (EMA), while each Member State's competent authorities (i.e. the Federal Agency for Medicines and Health Products, FAMPH, in Belgium)38 manage the process for nationally authorized medicines. In the US, these matters are under the authority of the U.S. Food and Drug Administration (FDA).39
The EMA indicates that the extent of modifications to a given process toward a marketed pharmaceutical dictates the necessary follow-up steps to be undertaken by the Marketing Authorization Holder (MAH). The MAH must demonstrate that the change, e.g. in supplier, does not compromise the quality of the finished API product.40,41 In general, variations can be categorized into minor variations (Type IA and Type IB under the EMA) and major variations (Type II for EMA), each associated with an increasing administrative/economic burden for the MAH.42 Briefly, for Type IA variations, the MAH has 12 months to present the documentation to the competent authority, but can implement the variation while continuing to supply the API on the market, whereas for Type IB variations, the MAH must notify the variations and wait for 30 days before implementing them. A variation is considered minor only if all of the following conditions are fulfilled: (a) it does not change the qualitative and quantitative impurity profile and/or the physico-chemical properties of the final API product, (b) no alterations to the synthetic route are introduced, (c) no new reagents, catalysts or solvents are used in the process, and all intermediates remain the same and (d) the specifications of the API product or its intermediates are unaffected. Type II Major variations require extensive documentation and reviewing, as well as an extended timeline (usually 60 days prior to a decision). This could also lead to the possibility of requiring the filing of a new Drug Master File (DMF) with a new market approval application with the necessity for extensive documentation of (im)purity profiles.42
Strictly speaking, bio-based and petro-based compounds are identical molecular entities, and there are no specific regulations on replacing one for the other in a process toward a marketed API product. However, the change of supplier or of manufacturing site that would likely be required to switch to bio-based does constitute a variation. Whether the impact of such a variation is considered as minor or major will depend on each case. The main aspect to consider will be the proximity of the synthetic step in which the change is introduced to the final API. To better understand this difference, some familiarity with the concept of Good Manufacturing Practices (GMP) and Regulatory Starting Materials (RSMs) is helpful. These concepts are developed in the guidelines of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH).43
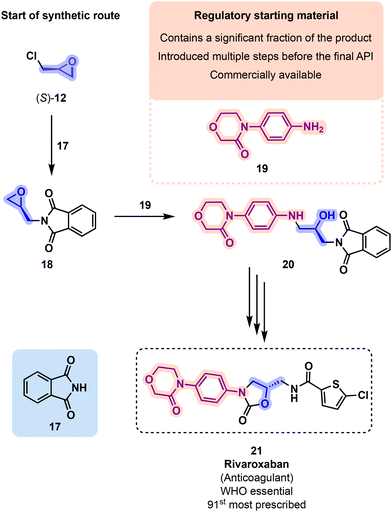
The (Regulatory) Starting Material (RSM) is a raw material, an intermediate, or an API component that bears a significant structural fragment from the final API structure (i.e., it is not a solvent or reagent, Fig. 6).44 The decision on which compound in the synthetic pathway is the RSM is made with the regulators and will impact the rigor with which a step will be monitored. The RSM is selected among the steps that impact the impurity profile of the final drug, and should have multiple steps before arriving at the API.45 The reason is that a greater amount of steps will provide more intermediate purification procedures, which in turn provide greater chances of removing impurities.
Introduction of the RSM into the synthesis marks the step under which Good Manufacturing Practices (GMP) must begin.44 GMP intends to provide a framework to ensure that APIs meet specific quality and purity criteria. Changing structurally identical petro-based to bio-based glycerol derivatives raises concerns regarding the impurity profiles of the corresponding APIs, and therefore, their Critical Quality Attributes (CQAs). CQAs are specific physical, chemical, biological or microbiological properties or inherent features that should be within an appropriate limit, range, or distribution to ensure the desired product quality.46 The manufacturing process should identify key material attributes and process conditions affecting API CQAs. This includes understanding the fate and removal of impurities originating from raw materials, which is critical for ensuring the quality of the final API.45
The proposed RSM must meet appropriate specifications and be justified, with supporting analytical procedures suited to the detection of impurities and their fate added in subsequent steps. Changes to the specifications of RSMs are therefore type II variations, subjected to post-approval requirements. Additional purification steps may be necessary for commercially available commodities to ensure consistent quality, with specifications provided for both incoming and purified materials.45 In general, if the synthetic pathway of a compound has been described as part of the drug filing, any modifications will entail a reglementary impact. While the ICH does not explicitly cover upstream changes, it advocates using fundamental science and risk-based concepts to assess their impact.
Aside from its use as or to produce starting materials, glycerol has been used on occasions as a solvent. Specific guidance on replacing petro-based solvents with bio-based ones is currently unavailable. Both the EMA and FDA are committed to supporting the adoption of environmentally friendly manufacturing technologies, a commitment that aligns with the European Union's “Strategic Approach to Pharmaceuticals in the Environment”.47 However, a change of solvent will be treated as a major variation by regulatory agencies, strongly disincentivizing an expansion of the use of glycerol for currently approved APIs. Furthermore, some commercially available bio-based solvents have not yet been included in FDA solvent classes48 or CHEM21 solvent selection guides.49
The following sections review a variety of marketed APIs according to their primary pharmaceutical and biological activity classes, focusing notably on cardiovascular medications. Other pharmaceutical classes include antidepressants, antihistaminics, UV-B protection, alimentary supplements, contrast agents, cholerectics and antihypertensives, among others. This review fits in the overall effort to reduce the environmental burden associated with waste-extensive processes and their inherent reliance on exhaustible petro-based resources. This paves the avenue toward the incorporation of bio-based atoms within the backbone of common pharmaceuticals.
3. Active pharmaceutical ingredients derived from glycerol
3.1 Cardiovascular pharmaceuticals
Among the various classes of APIs featuring a glycerol-related backbone, numerous examples of pharmaceutical blockbusters concern cardiovascular medications (Fig. 7). Beta blocker agents (section 3.1.1.) are one of the highest selling medications in Western countries. They are aimed at reducing heart rate and contraction force, hence lowering blood pressure. They usually feature a common β-amino alcohol moiety as the central feature. Epichlorohydrin 12 and glycidol 13 became centerpieces for the convenient preparation of such derivatives through an etherification/aminolysis sequence. It is worth mentioning that oxirane 12, with its inherent ambivalent electrophilicity, is commonly preferred over 13. Section 3.1.2 concerns the use of glycerol-based building blocks to access Na+ channel blockers. Na+ channel blockers regulate cardiac action potentials and are prescribed for the treatment of cardiac arrhythmias. Other cardiovascular medications include anticoagulants and vasodilators, which prevent blood clotting and lower the blood pressure, respectively. These medications can also be potentially accessed through glycerol-derived building blocks and are discussed in sections 3.1.3 and 3.1.4, respectively.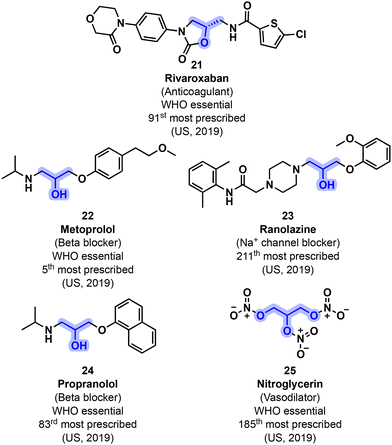 | ||
| Fig. 7 Representative examples of various classes of cardiovascular medications that could potentially incorporate bio-based glycerol and its derived building blocks. | ||
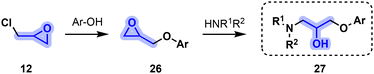 | ||
| Fig. 8 Common synthetic route toward beta blockers 27 featuring a β-amino alcohol moiety starting from epichlorohydrin (12). | ||
Among the various beta blockers derived from 12, six APIs are part of the WHO list of essential medicines51 and are amongst the most prescribed drugs in the US (Table 1).52 On top of these, twelve additional beta blockers are listed in Table 1 alongside their structure, indications and class.
In addition to containing the familiar amino alcohol scaffold, carteolol (41) incorporates another glycerol-derived building block in its cyclic moiety, namely, acrylic acid (4). Phenol-derived intermediate 46 is obtained by reacting acrylic acid (4) with α-β unsaturated ketone 44 before reducing the resulting intermediate with carbon-supported palladium and hydrogen. Williamson etherification between epichlorohydrin (12) and 46 leads to glycidyl ether 47, the latter then undergoing ring-opening aminolysis by tert-butylamine to obtain carteolol53 (41, Fig. 9).
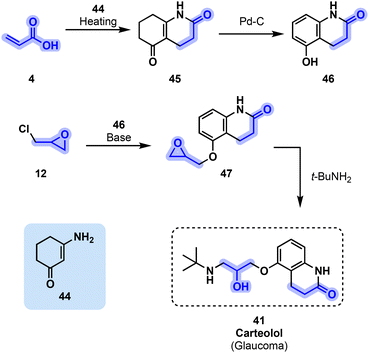 | ||
| Fig. 9 Preparation of beta-blocker 41, highlighting the incorporation of acrylic acid (4) and epichlorohydrin (12). | ||
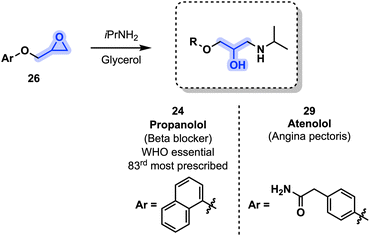
Aside from being a renewable source of valuable oxiranes, glycerol (1) can act as a green polar protic solvent. As such, and taking advantage of the protic nature of 1, Hussain, Kumar and coworkers developed a procedure toward propranolol (24) and atenolol (29) from aryl glycidyl ethers (26) and isopropyl amine in the presence of glycerol as both solvent and catalyst (Fig. 10). Propranolol (24) and atenolol (29) were obtained with 97% and 93% yields respectively in under 3 h. Additionally, glycerol was easily recycled and retained its reactivity even after up to 4 cycles: on the 5th cycle, yields started to drop below the 90% mark.54
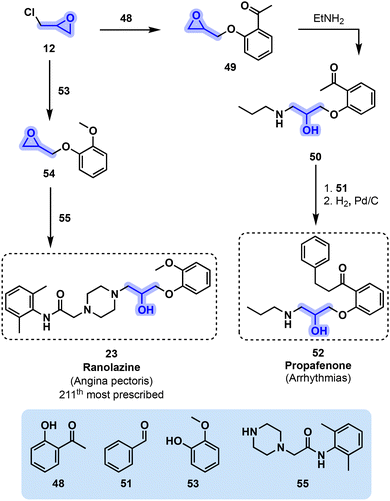 | ||
| Fig. 11 Synthetic pathways toward Na+ channel blockers propafenone (52) and ranolazine (23) starting from epichlorohydrin (12). | ||
Ranolazine (23), another β-amino alcohol derivative with Na+ channel blocking properties, was the 211th most prescribed drug in the U.S. (2.5 million) in 2019.56 It can be obtained by reacting 12 with guaiacol (53), another potentially bio-based building block, leading to glycidyl ether intermediate 54 (Fig. 11). Using piperazine-derived reactant 55 in conjunction with 54 allows ranolazine (23) to be obtained.
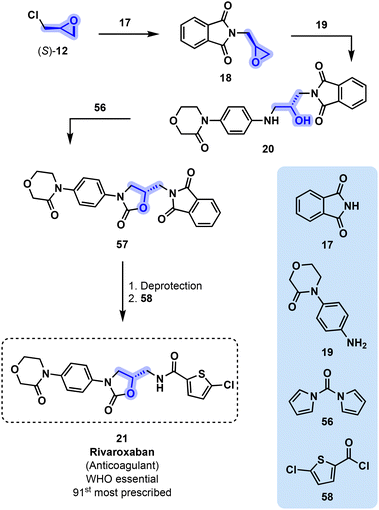
Rivaroxaban (21) was developed by Bayer Healthcare and patented in 2007. Its preparation starts from (S)-12 and phthalimide 17 (Fig. 12). Intermediate 18 is next reacted with aniline derivative 19 to yield diamino alcohol 20, which is next cyclized with 56 toward oxazolidinone 57. Final deprotection followed by a reaction with thiophene 58 ultimately gives 21.57 Rivaroxaban is currently marketed by Bayer and by Janssen (in the U.S.) since 2011. It was prescribed 8.8 million times in the U.S. in 2019 and is present on the WHO's list of essential medicines.51
Among vasodilators, a subclass regroups various organic molecules featuring nitrate functional groups. Metabolism of these derivatives leads to the generation of nitric oxide (NO), a strong natural vasodilator. For instance, nitroglycerin (25) has been used to treat angina pectoris for more than 150 years. Compound 25 can be directly sourced from glycerol (1) by using a nitration mixture (HNO3/H2SO4) (Fig. 13).59 Nitroglycerin was prescribed more than 3.1 million times (US, 2019) and is considered a WHO essential medication.51,52
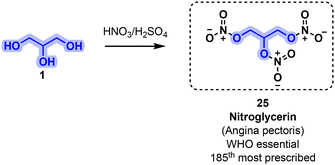 | ||
| Fig. 13 Industrial process used for the manufacture of nitroglycerin (25) starting from glycerol (1). | ||
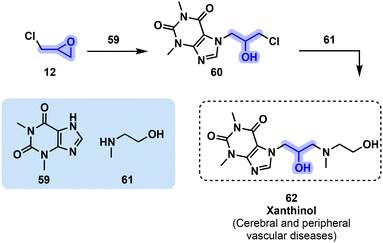
Xanthinol (62) is another vasodilator agent that can be sourced from theophylline (59) and oxirane 12. Oxirane 12 reacts with 59 to yield chlorohydrin intermediate 60, which is then further reacted with amino alcohol derivative 61 to produce xanthinol (62, Fig. 14). The usual formulation includes a nicotinate salt.60
As opposed to beta amino alcohols described above, eprosartan (70) is a molecule exhibiting angiotensin II receptor blocking properties, allowing the reduction of vasoconstriction and decrease of blood pressure through a different biological mechanism. It is currently marketed by Abbot industries in the US after purchasing Kos Pharmaceuticals which was evaluating a scope of antihypertensive drugs.
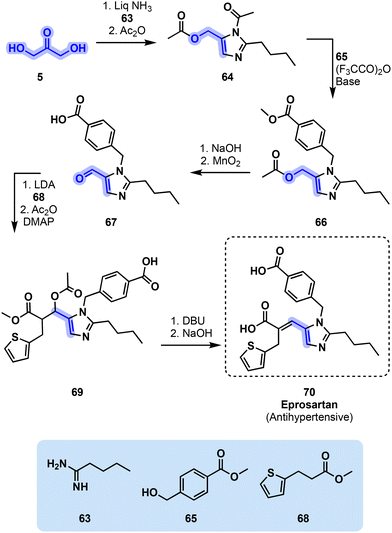
Eprosartan (70) can be obtained following several synthetic pathways. GSK patented a procedure which relies on 1,3-dihydroxyacetone (5) for the synthesis of pyrazole intermediate 64. Dihydroxyacetone (5), a potentially glycerol-derived molecule, is first reacted with amidine 63 in the presence of liquefied ammonia before undergoing acetylation by acetic anhydride. The cyclic intermediate is then reacted with benzyl alcohol derivative 65 in the presence of a base and trifluoroacetic anhydride to yield the corresponding diester 66. The latter is then hydrolyzed by aqueous NaOH, and the alcohol thus obtained is oxidized into its corresponding aldehyde by MnO2. The penultimate synthetic step consists in an aldol condensation of aldehyde intermediate 67 with thiophene derivative 68 in the presence of LDA. The resulting alcohol is then acetylated to yield intermediate 69. Finally, a DBU-triggered elimination reaction results in eprosartan (70, Fig. 15). This route manages to incorporate all three carbon atoms of 5.61
3.2 Musculoskeletal medications
Myorelaxants can be divided into 3 subclasses: (a) drugs used during surgery or assisted ventilation, (b) drugs for the treatment of spasms and (c) short-term pain relievers for spasms arising from musculoskeletal conditions.62 The latter includes common over-the-counter medicines and gathers a variety of drugs that can be produced from glycerol and derivatives.For instance, guaifenesin (72) was marketed in the 1930s and as of today is still used as an expectorant. Now available as a generic medication, 72 was still the 256th most prescribed drug in the U.S. in 2019.52 Guaifenesin can be prepared from bio-based guaiacol (53) and glycerol (1), or one of its activated derivatives such as monochlorohydrin (10b) or oxiranes 12, 13. The most common preparation of 72 involves a straightforward Williamson ether synthesis (Fig. 16, R = OMe).63
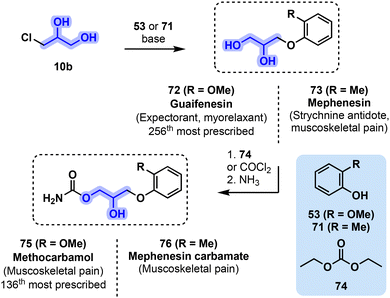 | ||
| Fig. 16 Common synthetic pathways toward guaifenesin (72, R = OMe), mephenesin (73, R = Me) and their carbamate derivatives methocarbamol (75, R = OMe) and mephenesin carbamate (76, R = Me). | ||
Methocarbamol (75), a carbamate derivative of 72, can be subsequently obtained by using diethyl carbonate (74) and ammonia toward methocarbamol (75, Fig. 16, R = OMe).64 Methocarbamol (75) was approved by the US Food and Drug Administration (FDA) in 1957 and is used for the short-term management of musculoskeletal conditions.65 Now available as a generic prescription drug, 75 was the 136th most prescribed drug in the U.S. in 2019.56
Mephenesin (73) is structurally closely related to 72, yet its applications have been limited to some cases of strychnine poisoning in combination with barbiturates as an antidote.66 It was however never prescribed as a myorelaxant, owing to severe deleterious side-effects including respiratory conditions and addiction. Compound 73 is currently discontinued in the U.S. and in France, yet it still finds applications as an ingredient for ointments in various countries.67 The preparation of 73 is similar to 72 and involves the reaction of a monochlorohydrin 10b with phenol derivative 71 (Fig. 16, R = Me).68
A carbamate derivative 76 is currently used for various musculoskeletal pain conditions and can be directly obtained from 73 after a treatment with phosgene and ammonia (Fig. 16, R = Me).69 Mephenesin carbamate (76) was marketed in the U.S. starting from 1954.70
Oxazolidinone myorelaxants can be directly derivatized from vicinal diols such as 72 and analogs. For instance, mephenoxalone (77) is obtained from guaifenesin (72) through the reaction of 72 with urea (Fig. 17a).71 Mephenoxalone (77) is prescribed for the treatment of muscular spasms and as an anxiolytic. It was approved in the US since 1961.72 Metaxalone (79) is another oxazalidinone myorelaxant that can also be obtained by treating 78 with urea (Fig. 17b).73 Metaxalone was approved in 1962 and is mainly used for the management of musculoskeletal pain.74 It was the 332nd most prescribed drug in the U.S. in 2018 and is available as a generic medication.75
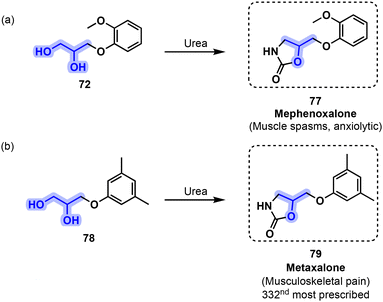 | ||
| Fig. 17 Preparation of the oxazolidinone-based muscle relaxants (a) mephenoxalone (77) and (b) metaxalone (79) from vicinal diols 72 and 78 in the presence of urea. | ||
Another 1,2-propanediol derivative related to pharmaceuticals 72 and 73 is chlorphenesin 81 (Fig. 18), but while it presents myorelaxant properties, safer alternatives are available. It is nowadays mostly used as an antibiotic and antifungal (see section 3.1.1, vide infra).
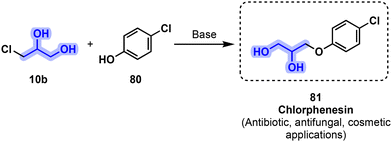 | ||
| Fig. 18 Synthesis of antibiotic and antifungal chlorphenesin (81) through a Williamson etherification with monochlorohydrin 10b. | ||
3.3 Antimicrobial medications
Antimicrobial drugs include all medications that kill or weaken the growth of microorganisms and are generally classified based on the type of pathogen affected, which includes antibiotics, antifungals, antiparasitics and antivirals. APIs that are efficient against multiple classes of microorganisms are defined as broad-spectrum therapeutics.76 Nowadays, antimicrobial resistance (AMR) is considered by the WHO as one of the biggest threats to global health, food security and development.77 In 2019, an estimated 1.27 million deaths were attributed to AMR.78 Drug-resistant diseases could lead to 10 million deaths per year by 2050 if no proper mitigation strategy is established.79 The development of new antimicrobial medications especially with narrow-spectrum applications in addition to other approaches such as phage therapy or vaccines is therefore of critical importance.80–82 Antimicrobial API sourcing is highly dependent on petro-based resources similarly to other APIs, yet several examples including some essential medicines present central moieties that can be constructed from glycerol (1) or its derived building blocks. A (partially) bio-based synthetic pathway of key antimicrobials could secure an alternative sourcing of these important drugs against high volatility price and availability of raw materials.As discussed in section 3.2, 81 was first used for its muscle relaxant properties but is nowadays mostly used as biocide and fungicide in cosmetic applications. It is used against Gram-positive and Gram-negative bacteria as well as various fungi. In 2011 it was used in more than 1300 cosmetic preparations.83 Its preparation is relatively similar to other 1,2-diol muscle relaxants and consists of a Williamson ether synthesis between 10b and an adapted phenol derivative 80 in the presence of a base (Fig. 18).
A major breakthrough in the advancement of antibiotics happened in the early 2000s with the development of linezolid, the first FDA-approved oxazolidinone used for the treatment of bacterial infection.84 Active against a large variety of Gram-positive bacteria, linezolid (86, Fig. 19) is used to treat skin conditions, pneumonia and tuberculosis involving drug-resistant pathogens.85
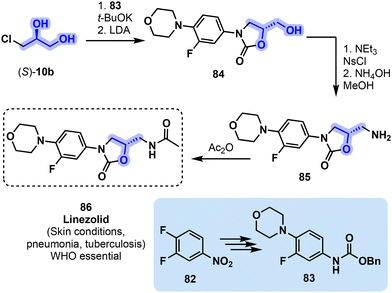 | ||
| Fig. 19 Process-scale synthesis of linezolid (86) involving building block (S)-10b. LDA = lithium diisopropylamide, NsCl = 4-nitrobenzenesulfonyl chloride. | ||
This WHO essential medicine51 is now available as a generic medication. Linezolid was developed by scientists from Pharmacia & Upjohn (now part of Pfizer) in the 1990s and approved by the FDA in 2000.86 The original process-scale synthesis of 86 involves the use of (S)-10b in conjunction with derivative 83 (obtained from 82) to form the central oxazolidinone cycle toward 84 under basic conditions. Next, an amine derivative 85 is obtained from 84 to finally form the amide moiety of linezolid (86) with acetic anhydride (Fig. 19).84 An alternative 7-step synthesis of 86 using (S)-epichlorohydrin ((S)-12) was recently reported.87
Tedizolid (90, Fig. 20) is a second oxazolidinone antibiotic structurally related to 86 that was approved in 2014 by the FDA for the treatment of acute skin infections.88 Tedizolid (90) is effective against Gram-positive pathogens through protein synthesis inhibition and shows a higher potency for the treatment of staphylococci- and enterococci-related infections compared to linezolid.89 The drug was originally discovered by South Korean company Dong-A Pharmaceuticals and developed by Cubist Pharmaceuticals (USA, now part of Merck & Co.).90
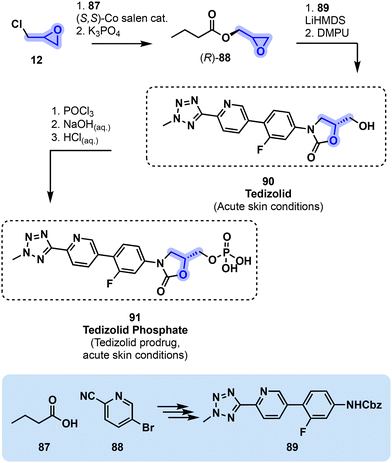 | ||
| Fig. 20 Process-scale synthesis of tedizolid (90) and its phosphate prodrug 91 involving epichlorohydrin (12). LiHMDS = lithiumbis(trimethylsilyl)amide, DMPU = N,N′-dimethylpropyleneurea. | ||
Preparation of 90 involves a critical intermediate (R)-88 that can be produced from epichlorohydrin (12) through an enantioselective reaction involving a Co-salen complex. (R)-88 is then reacted with tetrazole derivative 89, which itself is obtained from commercially available pyridine derivative 88 in a multistep process. Tedizolid (90) is finally obtained from (R)-88 and 89 using LiHMDS and DMPU. The corresponding phosphate prodrug 91 is directly obtained from 90 using POCl3 (Fig. 20).91–93
Another common structure frequently encountered in antibiotic medications is related to nitrofurans. Nifuratel (96) presents a characteristic nitrofuran moiety additionally to an oxazolidinone ring. It is used as a broad-spectrum treatment for vaginitis. Nifuratel (96) is efficient against Chlamydia trachomatis and presents antifungal and antiprotozoal activity. A combination with another drug, nystatin, is frequently used to ensure the elimination of all pathogens that could cause a vaginal inflammation.94 Nifuratel (96) is available and approved in various European and Asian countries. Its preparation involves epichlorohydrin (12) which is first converted to 92 using MeSH in the presence of a base. Next, hydrazine is used to form 93 from 92, which is subsequently reacted with carbonate 74 to form oxazolidone 94. Nitrofuran 95 is finally reacted with 94 toward nifuratel (Fig. 21, 96).95,96
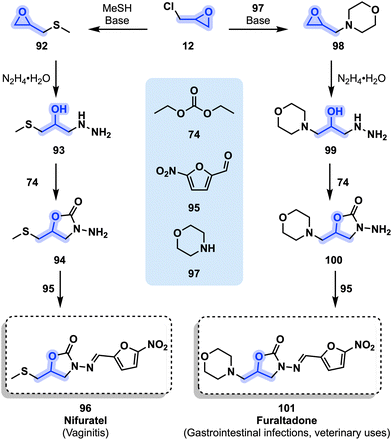 | ||
| Fig. 21 Synthesis of nifuratel (96) and furaltadone (101) from epichlorohydrin (12) and a nitrofuran derivative 95. | ||
Furaltadone (101) is only differentiated from 96 by a morpholine ring in place of a methylthioether. Furaltadone (101) is a veterinary drug mostly used to treat gastrointestinal infections of livestock. Its synthesis is similar to nifuratel (96) and starts with epichlorohydrin (12) and morpholine (97) in the presence of a base toward a first epoxide intermediate (98). Treatment with hydrazine leads to 99 which is further reacted with diethyl carbonate (74) leading to oxazolidinone 100. Finally, 100 and nitrofuran 95 are reacted to form furaltadone (101, Fig. 21).97,98
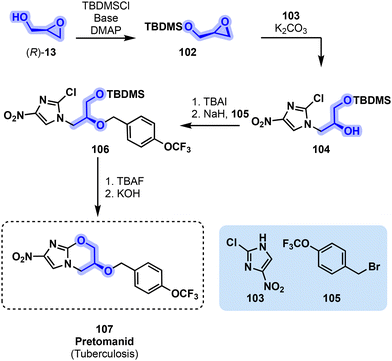
A recently approved nitroimidazole derivative pretomanid (107) is another important antibiotic. It is used in the treatment of multidrug-resistant tuberculosis in combination with other antibiotics including linezolid (86). Pretomanid (107) was developed by the Global Alliance for Tuberculosis Drug Development located in South Africa and was approved by the FDA in 2019 as a first-in-class medication.99,100 A synthetic pathway toward pretomanid (107) involves the use of (R)-glycidol ((R)-13) for the production of protected epoxide 102. A nitroimidazole derivative 103 is next reacted with 102 in the presence of a base, leading to 104. The unprotected alcohol of 104 is next functionalized with bromobenzyl derivative 105, affording compound 106. A final deprotection of the primary alcohol followed by cyclization affords pretomanid (107, Fig. 22).90,101,102
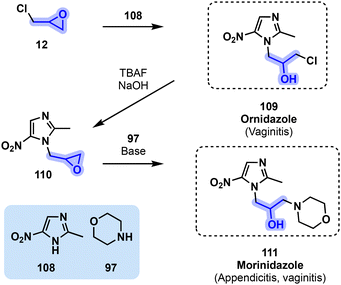
Another nitroimidazole antibiotic, ornidazole (109, Fig. 23), is used in the treatment of infections caused by anaerobic bacteria and is itself an intermediate for the synthesis of morinidazole (111). Both 109 and 111 have been found to display broad-spectrum antiparasitic properties in addition to their antibacterial applications. They are used in the treatment of various types of vaginitis including trichonomiasis.103 Morinidazole can also be used in appendicitis events. Ornidazole (109) is available in various European and Asian countries while morinidazole (111) was approved by the China Food and Drug Administration (CFDA) in 2014.90 Ornidazole (109) can be directly obtained through an acid-catalyzed reaction between epichlorohydrin (12) and nitroimidazole 108. A ring closure of 109 under basic conditions affords the corresponding epoxide 110 that can be directly converted to morinidazole (111) through an aminolysis with morpholine derivative 97 (Fig. 23).90,104
Terbinafine (117) is part of the allylamine family of antifungal agents. It is considered as an essential medicine by the WHO and was the 277th most prescribed drug in the US in 2019.51,52 It is used in the treatment of a variety of nail and skin infections. It is available since the 1990s in Europe and in the US.108,109 A potential synthetic pathway toward 117 starts from epichlorohydrin (12) and secondary amine 112 to generate intermediate 113 under basic conditions. Next, 113 is reacted with organolithium derivative 114 in the presence of a Lewis acid (BF3·OEt2) toward β-amino alcohol 115. Terbinafine (117) is finally obtained through the activation of 115 with MsCl and a base followed by addition of DBU and heating (Fig. 24).109
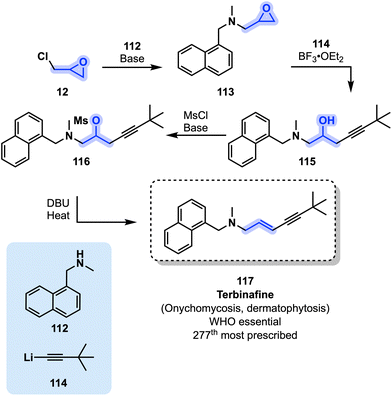 | ||
| Fig. 24 Synthesis of antifungal drug terbinafine (117) from epichlorohydrin (12). MsCl = methanesulfonyl chloride, DBU = 1,8-diazabicyclo(5.4.0)undec-7-ene. | ||
Another main class of antifungals relates to azole compounds. Developed in the 1970s by Janssen Pharmaceutica, azole antifungals rapidly emerged as an important branch of medications treating various types of mycosis. Ketoconazole (125b, Fig. 25), an imidazole derivative, was approved by the FDA in 1981 and is still one of the most prescribed drugs in the US (171st, 2019).52 As of today, the use of oral ketoconazole (125b) is strictly limited because of its hepatotoxicity and only indicated when other alternatives are not available. The topical use of 125b, however, is still considered safe and is employed in the treatment of various conditions.110 Terconazole (125a) is another antifungal azole medication developed by Janssen Pharmaceutica that is structurally related to 125b. It was approved for medical use by the FDA in 1987 and is mainly used in the topical treatment of vaginitis caused by Candida fungi.111,112 Itraconazole (125c) is a critically important azole antifungal developed concomitantly with 125a and 125b in the 1970s. Itraconazole (125c) displays antifungal activity against a broader spectrum of pathogens than ketoconazole (125a) and is used to treat a large variety of mycoses.105,113,114 It was approved by the FDA in 1992, is part of the list of essential medicines of the WHO and was designated as an orphan drug by both the FDA and the EMA for the treatment of basal cell carcinoma nevus syndrome and the prevention of invasive aspergillosis, respectively.51,115,116
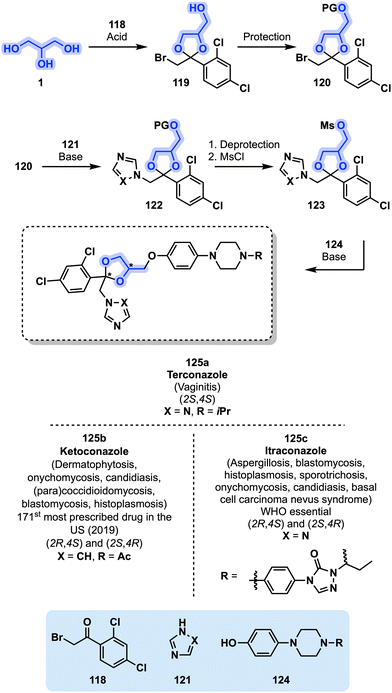 | ||
| Fig. 25 General synthetic pathway toward antifungal azoles 125a–c bearing a central dioxolane ring from glycerol (1). PG = protecting group, MsCl = methanesulfonyl chloride. | ||
A general synthetic procedure toward azole antifungals 125a–c starting from glycerol (1) can be found in the original patents from Janssen.117,118 Acetalization using 1 and arylketone 118 allows the generation of the central dioxolane core 119 in which the primary alcohol is protected leading to 120. Next, depending on the desired product, an imidazole or triazole derivative 121 is employed in a substitution reaction leading to 122. A deprotection of 122 followed by functionalization using MsCl allows the generation of mesylate 123 which can be reacted with piperazine derivative 124 to finally generate antifungal azoles 125a–c (Fig. 25).
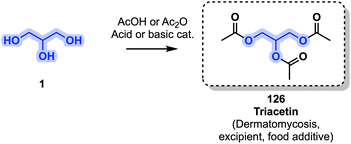
Triacetin (126) is mostly used in cosmetic applications, as a food additive (E1518) and as an excipient, and finds further use as a solvent or humectant.119 The first synthesis of triacetin dates back to the 19th century and it is generally produced through an esterification using acetic acid or acetic anhydride with an optional acid or basic catalyst (Fig. 26).120 Antifungal properties of 126 have been observed, due to decomposition and the release of acetic acid by esterases located in fungi. It is used in the treatment of superficial dermatomycoses.119,121
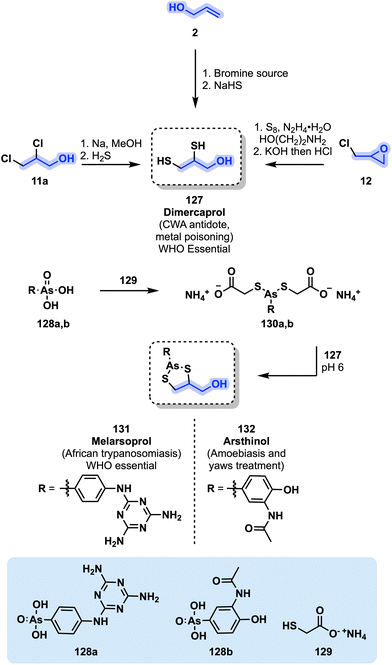
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in 1990)123 and which is caused by some species of Trypanosoma brucei protozoa generally transmitted by infected tsetse fly bites. After an initial stage of fever, headaches, joint pains, and itching, a second stage ensues resulting in confusion, poor coordination and sleeping disorder symptoms leading to death without proper treatment.124 Melarsoprol (131) is a first-line treatment for the second stage of African trypanosomiasis and is considered as an essential medicine by the WHO.51 The synthesis of 131 is shown in Fig. 27.
000 in 1990)123 and which is caused by some species of Trypanosoma brucei protozoa generally transmitted by infected tsetse fly bites. After an initial stage of fever, headaches, joint pains, and itching, a second stage ensues resulting in confusion, poor coordination and sleeping disorder symptoms leading to death without proper treatment.124 Melarsoprol (131) is a first-line treatment for the second stage of African trypanosomiasis and is considered as an essential medicine by the WHO.51 The synthesis of 131 is shown in Fig. 27.
 | ||
| Fig. 27 Preparation of chelating agent dimercaprol (127) and antiparasitic drugs melarsoprol (131) and arsthinol (132) from allylic alcohol (2), dichlorohydrin (11a) or epichlorohydrin (12). | ||
Preparation of 131 usually involves the use of dimercaprol (127), which is itself part of the WHO's essential medicine list. It is employed as a chelating agent for heavy metal poisoning (e.g., Hg, As, Pb). More recently, dithiol 127 was accepted by the FDA as a treatment against Wilson's disease, a condition consisting of the accumulation of copper in the brain and liver with neurological consequences.125 Though varying methods toward dimercaprol were developed, the first one relied on allyl alcohol (2), which is brominated before being treated by NaHS.126 Similarly, the treatment of dichlorohydrin 11a with a solution of sodium and MeOH saturated with H2S can afford 127 (Fig. 27). Finally, dimercaprol can be obtained starting from epichlorohydrin (12) and sulfur with hydrazine to yield thiol oligomers. The latter are then treated by KOH and HCl to obtain the corresponding monomers.127 Dimercaprol was originally used as an antidote for Lewisite, an organoarsenic chemical warfare agent used during World War II.128 Arsenic acid derivative 128a can be treated with ammonium thioglycolate (129) affording compound 130a. Chelation of 130a with dimercaprol finally leads to the formation of melarsoprol (131).129 The same reactions can be applied to acetarsol 128b, a drug initially used to treat syphilis, to prepare arsthinol 132, a drug used to treat yaws and amoebiasis130 (Fig. 27).
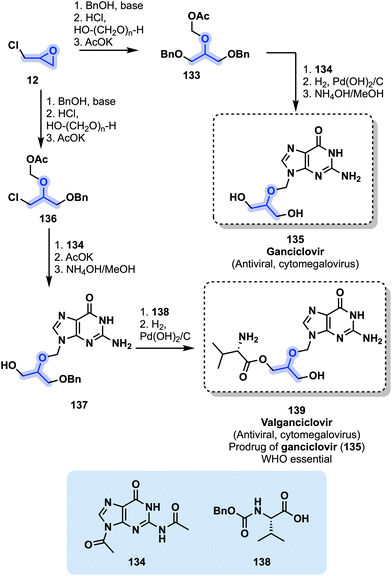 | ||
| Fig. 28 Preparation of ganciclovir (135) and its prodrug valganciclovir (139) from epichlorohydrin (12). | ||
Valganciclovir (139) is considered as an essential medicine by the WHO.51 Synthetic processes toward both 135 and 139 share similar patterns and can start from epichlorohydrin (12) (Fig. 28). Use of benzyl alcohol (BnOH) in conjunction with 12 under basic conditions enables the production of two 1,3-protected alcohols (Fig. 28). Following a chloromethylation with HCl and paraformaldehyde and acetylation with AcOK, derivative 133 is obtained. N,N′-Diacetylguanine (134) is next used in a condensation reaction with 133 to generate ganciclovir (135) after a deprotection and a deacetylation reaction. The preparation of valganciclovir (139) follows a similar pathway and starts with the synthesis of mono-benzylated derivative 136 from 12 (Fig. 28). Condensation between 136 and 134 leads to 137 after an acetalization followed by a deprotection in the presence of NH4OH/MeOH. Installation of the L-valyl scaffold (138) followed by a final deprotection provides valganciclovir (139).135 Valganciclovir was developed by F. Hoffmann-La Roche and approved by the FDA in 2001.136
Another alternative drug to deal with cytomegalovirus infections in immunocompromised hosts is cidofovir (144), which is particularly relevant to treat cytomegalovirus retinitis conditions. Cidofovir was developed by Gilead Sciences and was approved by the FDA in 1996.137 A practical synthesis of 144 starting from (R)-glycidol (R)-13 was developed by scientists from Bristol-Myers Squibb (Fig. 29).
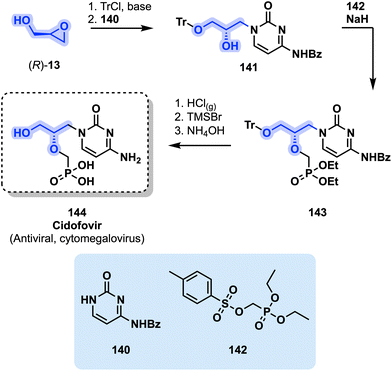 | ||
| Fig. 29 Preparation of cidofovir (144) from (R)-glycidol ((R)-13). TrCl = trityl chloride, TMSBr = trimethylsilyl bromide. | ||
A tritylation of (R)-13 followed by a ring-opening with cytosine derivative 140 afforded intermediate 141. Alkylation of 141 with a tosylphosphonate reactant 142 in the presence of NaH allowed the production of 143 which subsequently afforded cidofovir (144) after a deprotection protocol.138
3.4 Polymeric drugs
The impact of polymeric materials for the pharmaceutical industry spans a variety of applications. In most cases, polymers are used as delivery vehicles, such as in micellar formulations, or used as conjugates with proteins, aptamers or drugs.139 A few cases exist, however, in which the polymer itself is the active component. This use of polymers as drugs is attractive due to their extraordinary multivalency.140 A subcategory of polymeric drugs that can highly benefit from multivalency is polymeric sequesterants.139The use of polymers to sequester unwanted molecules received a significant push from the work of George Whitesides and the foundation of GelTex Pharmaceuticals Inc.141 A few years after its foundation, GelTex Pharmaceuticals obtained FDA approval for sevelamer hydrochloride (RenaGel®), a polymer designed to reduce the level of serum phosphorous in patients with end-stage renal disease.142 Sevelamer (146, Fig. 30) is synthesized by crosslinking a poly(allylamine) (145) with epichlorohydrin (12).143 The polymer is designed to contain a high fraction of amine groups that, when protonated at a pH below 7, provide a polycationic matrix which binds phosphate. This in turn leads to a reduced absorption of dietary phosphorous.144 Currently sevelamer is the recommended alternative treatment in cases when calcium-based phosphate binders cannot be used.145 A second formulation in which the hydrochloride salt is replaced by bicarbonate (sevelamer carbonate) is also available for cases where the acidosis was experienced as a side effect.146
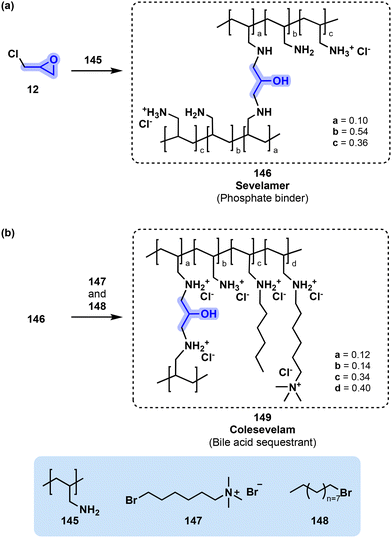 | ||
| Fig. 30 (a) Synthesis of sevelamer (146) through the reaction of epichlorohydrin (12) with poly(allylamine) (145). The fraction of monomeric units is reported in the FDA package insert.112 (b) Post-polymerization modification leading to the preparation of bile sequestrant colesevelam (149). The ratio of monomeric units is taken from the FDA chemistry review.147 | ||
GelTex Pharmaceuticals also developed the bile acid sequestrant colesevelam hydrochloride (Welchol®), following a similar rationale as for sevelamer. Physiologically, the sequestration of bile acids decreases lipid levels by limiting their solubility and therefore their absorption. Furthermore, the depletion of bile acids triggers their replenishment through the synthesis of new bile acids which competes with that of cholesterol, decreasing its levels. Colesevelam is one of only three bile acid sequestrants approved in the United States.148
Like sevelamer (146), colesevelam (149, Fig. 30) is synthesized through the crosslinking of a poly(allylamine) (145) with epichlorohydrin (12). However, the drug has been engineered to increase interaction with bile acids by incorporating hydrophobic sidechains. To this end, the crosslinked poly(allylamine) is alkylated with (6-bromohexyl) trimethylammonium (147) and bromodecane (148) to obtain colesevelam (149, Fig. 30).149 The length of the sidechains has been modulated to provide a complementary fit to bile acids and therefore increased affinity.150 Colesevelam (149) has been demonstrated to have higher potency than the other available bile acid sequesterants.151 Bile acid sequestering also helps regulate glucose levels.120 Colesevelam (149) is the only bile acid sequestrant to receive FDA approval for the treatment of type 2 diabetes.152,153
In both cases (sevelemar and colesevelam), the use of epichlorohydrin (12) as crosslinker has been considered as advantageous due to its low molecular weight and its hydrophilicity. These properties contribute to the increased water-holding capacity of the resulting hydrogel. Its low cost and high availability further contribute to the attractiveness of this choice.154 The interest in these polymeric drugs as sequestrants is evidenced by their commercial success, which led to the purchase of GelTex Pharmaceuticals by Genzyme (2000), and eventually by Sanofi (2011).155
3.5 Miscellaneous
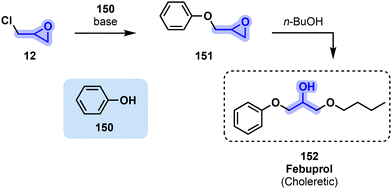
Drugs stimulating hepatic bile production such as febuprol 152 are called choleretics. Despite the preparation of febuprol (152) being reported in 1954 by Minor et al., its use was only patented in 1974 by Klinge-Pharma for its choleretic properties.158,159 Production of febuprol (152) begins by a nucleophilic substitution of epichlorohydrin (12) by a phenol (150) molecule in the presence of a base to yield 151, a glycidyl ether. This molecule then undergoes ring opening by n-butanol to swiftly obtain febuprol (152) as a solid (Fig. 31).
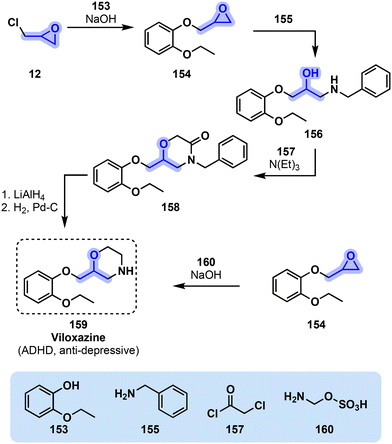
The synthetic scheme for viloxazine as originally reported is shown in Fig. 32. The synthesis begins with the reaction of o-ethoxyphenol (153) with epichlorohydrin (12) under basic conditions. The resulting aryloxypropylene oxide (154) is reacted with benzylamine (155), followed by chloroacetyl chloride (157) and cyclization, to arrive at intermediate 158. Reduction with a metal hydride and deprotection of the amine affords the final compound.160 A modification to this synthesis uses 2-aminoethyl hydrogen sulfate (160) to access the final product directly from intermediate 154.50,163
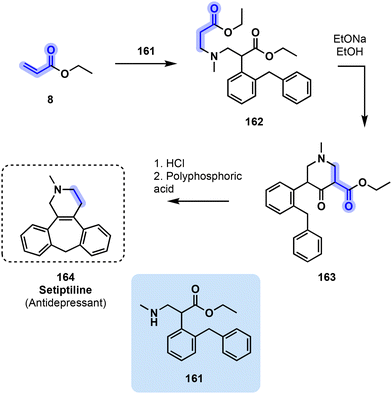 | ||
| Fig. 33 3-Step synthetic process toward antidepressant 164 starting from glycerol-derived ethyl acrylate (8). | ||
The synthesis of tetracyclic 164 incorporates glycerol-derived ethyl acrylate 8 into its preparation. The first step consists in a hydroamination reaction between ethyl acrylate (8) and secondary amine 161, yielding tertiary amine intermediate 162. Deprotonation in alpha of the ester function from 162 by sodium ethoxide leads to cyclization and intermediate 163. Finally, an HCl-catalyzed cyclization followed by decarbonylation with polyphosphoric acid provided antidepressant setiptiline (164, Fig. 33).164
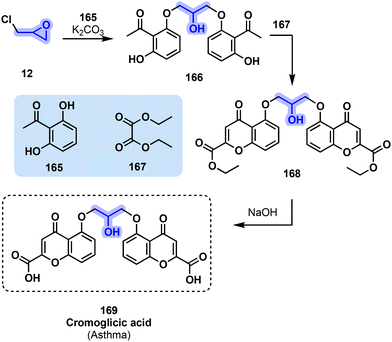
The chemical synthesis of cromolyn (169) uses one molecule of epichlorohydrin (12) to react with two molecules of 2,6-dihydroxyacetophenone (165) during a 48 h reflux to afford bischromone (166). This compound is then reacted with diethyl oxalate (167) to form diester derivative 168, which is hydrolyzed with sodium hydroxide to arrive at the final product (169).166,167 Alternatively, the intermediate 166 can be prepared using 1,3-dibromo-2-hydroxypropane instead of epichlorohydrin (12).166,167
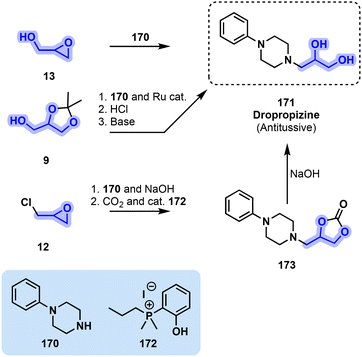
The synthesis of 171 was first reported in 1962 and consisted of the aminolysis of glycidol (13) by secondary amine 170 in the presence of a base.168 Despite this streamlined procedure, 171 can be obtained through other pathways. In 2016, Kann and coworkers169 reported a ruthenium-catalyzed hydrogen borrowing procedure to access beta amino alcohols from solketal (9) and a suitable amine. One such was phenylpiperazine (170) which provided the desired antitussive with an 86% yield over three synthetic steps. Five years later, Werner and coworkers170 found an alternative method for the production of 171 starting from epichlorohydrin (12) and amine 170. The two were reacted in the presence of a base to obtain the epoxide intermediate. The isolated epoxide was further used to trap CO2 in the presence of phosphonium catalyst 172 to obtain the corresponding cyclic carbonate (173), which can be viewed as protected dropropizine. The subsequent deprotection step was then implemented in the presence of NaOH to finally obtain the antitussive API. The last two steps can be decoupled or performed as a one-pot process, the latter however leading to a drop in yield from 95 to 61%.
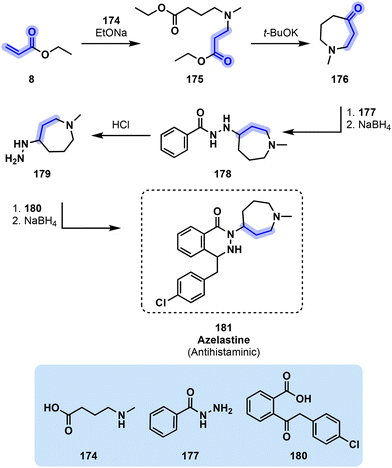
Azelastine (181) synthesis begins with the hydroamination of acrylic ester 8 by 174 toward 175 in the presence of EtONa. 175 is then cyclized in the presence of a base to obtain ketone intermediate 176 which is then subsequently coupled to benzhydrazide 177 and reduced by NaBH4 to obtain 178. Upon acid treatment, newly formed hydrazine 179 is released from 178. Ketone derivative 180 finally reacts with hydrazine 179 before undergoing reduction and cyclization into azelastine (181, Fig. 36).
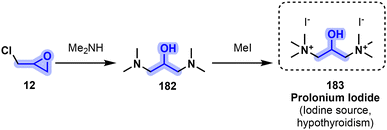
Prolonium iodide (183) can be obtained following a two-step synthesis, the first step consisting of aminolysis and nucleophilic substitution of epichlorohydrin (12) by an excess of dimethylamine to yield 1,3-bis(dimethylamino)-2-propanol 182. This intermediate is then swiftly methylated in the presence of 2 equiv. of methyl iodide to yield the desired iodide salt 183 (Fig. 37).172
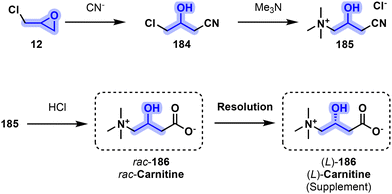
While both D- and L-carnitine exist, only the latter is naturally synthesized, with D-carnitine inhibiting the effect of L-carnitine. Endogenous carnitine is synthesized from lysine, a proteogenic amino acid. In contrast to the biological pathway, the initial industrial synthesis of carnitine starts with the ring opening of epichlorohydrin (12) by a cyanide ion to obtain 4-chloro-3-hydroxybutyronitrile (184). The resulting chlorinated derivative is then reacted with trimethylamine to yield 185 (carnitine nitrile chloride). The nitrile moiety is then reacted with aqueous HCl to convert it into the corresponding carboxylic acid to yield racemic carnitine rac-186, which then requires chiral resolution to obtain biologically relevant L-carnitine (L)-186 (Fig. 38).175
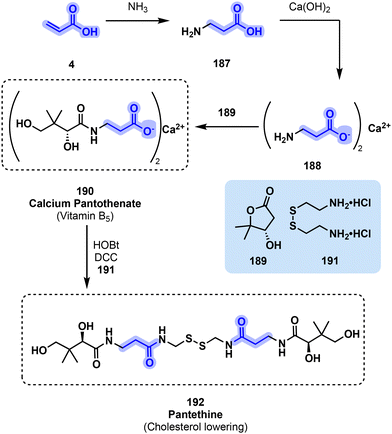
Vitamin B5 (190) production begins with the amination of acrylic acid 4 by ammonia to yield beta alanine176 (187) which is then neutralized by Ca(OH)2 into the corresponding salt177 (188). Finally, the amino acid skeleton is lengthened by the reaction with lactone 189 to obtain calcium pantothenate (190, Fig. 39), the commercial form of vitamin B5 supplements. A single extra step which consists in the coupling of two molecules of calcium pantothenate to one of cystamine (191) in the presence of HOBt and DCC as coupling agents178 is required to go from 190 to 192.
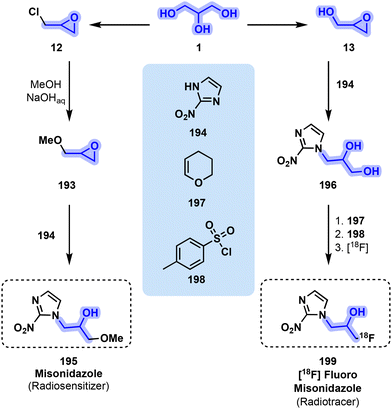
While 199 and other parent molecules allow the detection of the area needing treatment, misonidazole (195) is a drug with radiosensitizing properties, enhancing the therapeutic impact of radiation treatment on cancerous patients. Both molecules follow similar synthetic routes, starting from 12 or 13. In the case of 195, methyl glycidyl ether is the first intermediate before undergoing epoxide opening in the presence of 2-nitroimidazole (194) to obtain 195. When synthesizing [18F] fluoromisonidazole, 13 ring opening by 2-nitroimidazole (194) is the first step, quickly followed by protection by dihydropyran (197) and activation by tosyl chloride (198) of the two alcohol moieties. The final and most important step consists of radiolabelling with 18F and deprotection to retain as much radiochemical activity as possible95,181–183 to finally yield 199 (Fig. 40).
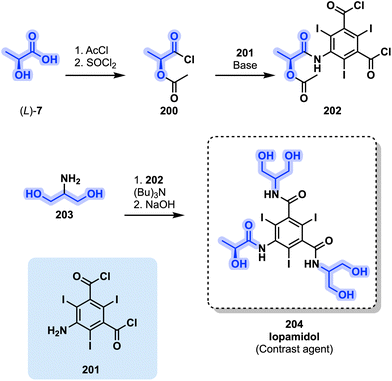 | ||
| Fig. 41 Synthetic pathway leading to contrast agent iopamidol (204) incorporating two different glycerol derivatives: L-lactic acid ((L)-7) and 2-aminoglycerol (203). | ||
The classic and initial method to obtain 204 integrates two different potential glycerol derivatives throughout its synthetic pathway. Firstly, L-lactic acid ((L)-7) is acylated to protect its hydroxyl moiety before being activated into the corresponding acyl chloride, yielding 200. The latter is then coupled to the iodine-bearing aromatic core 201 in the presence of a base to obtain 202. Secondly, the two remaining acyl chloride functions on the central aromatic ring react with a pair of 2-aminoglycerol (203) molecules in the presence of (Bu)3N. Finally, the hydrolysis of the protecting acetyl group of L-lactic acid ((L)-7) completes the synthetic route toward iopamidol (204, Fig. 41). Alternative methods have since been developed and published with the aim of having safer and greener synthetic steps. One of such relies on mechanochemistry for the reaction between 2-aminoglycerol and intermediate 202. Mechanochemistry uses mechanical energy transfered by rapidly moving bearings to activate the reagents in an enclosed milling reactor, instead of solely relying on thermal energy for the chemical reaction to proceed. This allows for a drastic reduction in solvent waste both during the reaction as well as the workup. Lattuada and coworkers reported in 2020 a method relying on a fivefold excess of 2-aminoglycerol (203) over intermediate 202 in the presence of an N-methylmorpholine as proton scavenger. No solvent was added and after 30 min of milling, 204 was obtained in an excellent 98% crude yield.185
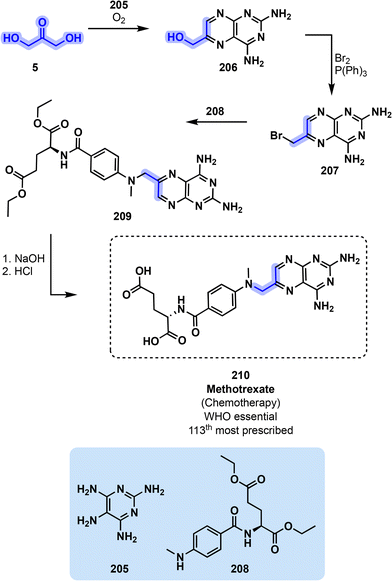
210 can be accessed from glycerol derivative 1,3-dihydroxyacetone (5) by reacting it with symmetrical pyrimidine 205 before oxidizing the resulting bicyclic compound into intermediate 206 (Fig. 42). The remaining alcohol function is then replaced by bromine in the presence of triphenylphosphine to activate 207 for the following reaction with secondary amine 208. The diester can then be hydrolyzed in the presence of aqueous NaOH followed by HCl to obtain the desired API 210 (Fig. 42).189 It is noteworthy that reagent 208 is prepared from glutamic acid ester, a DOE Top 10 recipient of bio-based molecules with promising applications and the corresponding acyl chloride.190
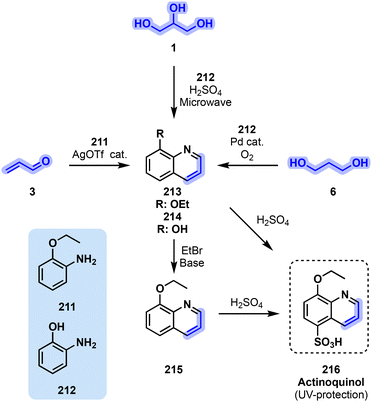
Two methods toward actinoquinol (216, Fig. 43) precursors were reported in 2014. Len and coworkers reported a greener alternative to this reaction by performing it under microwave irradiation while replacing nitrobenzene, which originally acted as solvent and oxidizer, by water.193 This solvent switch was however accompanied by the need for more sulfuric acid for the dehydration of glycerol (1) and that of the final ring to compensate for the loss of the oxidizing agent. The microwave-assisted reaction took place over 10 min at 200 °C, affording 8-hydroxyquinoline (214) in a rather low 34% yield.
The second actinoquinol (216) intermediate synthesis was reported by Zhang and Xu.194 The disclosed methodology is based on the silver-mediated condensation of ene-carbonyl or yne-carbonyl molecules with anilines to yield a broad scope of substituted quinolines. One such ene-carbonyl substrates is acrolein (3), which would logically be yielded from glycerol (1) dehydration in a standard Skraup synthesis. AgOTf and HOTf (5 mol%) in toluene sufficed to convert amine 211 and acrolein (3) into intermediate 213 after 4 h under reflux with a 62% yield. Interestingly, no oxidizer had to be added to the reaction medium for the final step, with dissolved air being enough for that task. Finally, Jiang et al.195 developed a third general method toward quinoline derivatives, among which was 214. This was achieved through palladium catalysis and aerobic oxidation of aniline 212 and 1,3-propanediol (6) as both solvent and reagent. The catalytic system, consisting of 5 mol% of Pd(OAc)2, 10 mol% of 2,4,6-collidine as the ligand and 20 mol% of trifluoro acetic acid under an O2 atmosphere, allowed 214 to be obtained in 55% yield after stirring the reaction medium at 150 °C for 16 h. Intermediate 214 was then reacted toward 215 which would undergo sulfonylation to finally afford UV-B-protecting 216.
4. Conclusions
The accessibility of extensive building block libraries derived from the transformation of oil-derived resources at the beginning of the 20th century gave rise to the ability to synthesize complex molecules on a large scale. This in turn opened the door to the preparation of APIs capable of treating a wide variety of human ailments. With the passage of time, the drawbacks of our reliance on petrochemicals have become evident. Not only is this dependence detrimental to the environment, but it also creates inequalities since petrochemicals are finite and unequally distributed geographically. This realization has motivated the efforts to establish biomass as the source of starting materials for a new chemical industry. These efforts have led to the identification of new bio-based platforms that can be directly derived from biomass and transformed into useful building blocks. Numerous reports on clever and efficient methods to obtain useful bio-based platforms from biomass have been published. The field has developed to the point where concrete applications of these developments now exist, for example, for the preparation of bio-based polymers. Among these platforms, glycerol (1) stands out as highly available compound that is currently akin to waste, but that possesses great potential to be used as a source of bio-based atoms.This review aimed at focusing chemists’ attention on the application of bio-based platforms to the field of pharmaceutical synthesis. Specifically, it sheds light on how the incorporation of bio-sourced atoms is already possible using glycerol (1) and its derivatives. The therapeutic applications and synthesis of all relevant APIs included in this review are recapitulated in Table 2. In total, almost 70 different APIs were reported, highlighting each time how already existing synthetic pathways can provide venues to incorporate bio-based atoms. In most examples, the potential incorporation of bio-based glycerol comes in the form of its derivatization toward current common building blocks such as epichlorohydrin (12).
| Medication name | Medication type | Glycerol derivative | Steps | Alt. route |
|---|---|---|---|---|
| Acebutolol | Beta-blocker | 12 | 2 | |
| Actinoquinol | UV-B ocular protection | 1 | 2 | 3, 6 |
| Alprenolol | Beta-blocker | 12 | 2 | |
| Arotinolol | Beta-blocker | 12 | 2 | |
| Arsthinol | Antiparasitic | 2 | 2 | 11a, 12 |
| Atenolol | Beta-blocker | 12 | 2 | |
| Azelastine | Antihistaminic | 8 | 7 | |
| Betaxolol | Beta-blocker | 12 | 2 | |
| Bisoprolol | Beta-blocker | 12 | 2 | |
| Bupranolol | Beta-blocker | 12 | 2 | |
| Calcium panothenate | Supplement | 4 | 3 | |
| Carnitine | Supplement | 12 | 3 | |
| Carteolol | Beta-blocker | 12 | 2 | 4 |
| Carvedilol | Beta-blocker | 12 | 2 | |
| Celiprolol | Beta-blocker | 12 | 2 | |
| Chlorphenesin | Antibiotic/antifungal | 10b | 1 | |
| Cidofovir | Antiviral | (R)-13 | 6 | |
| Colesevelam | Bile acid sequestrant | 12 | 1 | |
| Cromoglicic acid | Asthma | 12 | 3 | |
| Dimercaprol | Poisoning antidote | 2 | 2 | 11a, 12 |
| Dropropizine | Antitussive | 12 | 1 | 13, 9 |
| Eprosartan | Anti-hypertensive | 5 | 9 | |
| Esmolol | Beta-blocker | 12 | 2 | |
| Febuprol | Choleretic | 12 | 2 | |
| Furaltadone | Antibiotics veterinary | 12 | 4 | |
| Ganciclovir | Antiviral | 12 | 6 | |
| Guaifenesin | Myorelaxant | 10b | 1 | |
| Iopamidol | Contrast agent | 7 | 5 | |
| Itraconazole | Antifungal | 1 | 6 | |
| Ketoconazole | Antifungal | 1 | 6 | |
| Linezolid | Antibiotic | (S)-10b | 4 | |
| Melarsoprol | Antiparasitic | 2 | 2 | 11a, 12 |
| Mephenesin | Strychnine poisoning | 10b | 1 | |
| Mephenesin carbamate | Myorelaxant | 10b | 4 | |
| Mephenoxalone | Myorelaxant | 10b | 4 | |
| Metaxalone | Myorelaxant | 10b | 4 | |
| Methocarbamol | Myorelaxant | 10b | 3 | |
| Methotrexate | Chemotherapy | 5 | 5 | |
| Metoprolol | Beta-blocker | 12 | 2 | |
| Misonidazole | Radiosensitizer | 12 | 2 | |
| 18F-Misonidazole | Radiotracer | 13 | 4 | |
| Morinidazole | Antibiotics | 12 | 3 | |
| Nadolol | Beta-blocker | 12 | 2 | |
| Nifuratel | Antibiotics | 12 | 4 | |
| Nitroglycerin | Vasodilator | 1 | 1 | |
| Ornidazole | Antibiotics | 12 | 1 | |
| Oxprenolol | Beta-blocker | 12 | 2 | |
| Pantethine | Supplement | 4 | 4 | |
| Penbutolol | Beta-blocker | 12 | 2 | |
| Pindolol | Beta-blocker | 12 | 2 | |
| Pretomanid | Antibiotic | (R)-10 | 4 | |
| Prolonium iodide | Hypothyroidism | 12 | 2 | |
| Propafenone | Na+ channel blocker | 12 | 4 | |
| Propranolol | Beta-blocker | 12 | 2 | |
| Ranolazine | Na+ channel blocker | 12 | 2 | |
| Rivaroxaban | Anticoagulant | (S)-12 | 5 | |
| Setiptline | Antidepressant | 8 | 4 | |
| Sevelamer | Phosphate sequestrant | 12 | 1 | |
| Tedizolid | Antibiotics | 12 | 2 | |
| Tedizolid phosphate | Antibiotics | 12 | 3 | |
| Terbinafine | Antifungal | 12 | 4 | |
| Terconazole | Antifungal | 1 | 6 | |
| Timolol | Beta-blocker | 12 | 2 | |
| Triacetin | Antifungal | 1 | 1 | |
| Valganciclovir | Antivirals | 12 | 8 | |
| Viloxazine | ADHD | 12 | 5 | |
| Xanthinol | Vasodilator | 12 | 2 |
There are still significant challenges before bio-sourced APIs become a reality, especially concerning the strict regulation surrounding the preparation of APIs. However, the number of molecules that could be potentially prepared and the variety of purposes that they cover justifies the need for a careful consideration of this possibility. Some of the pharmaceuticals presented here are categorized as essential medicines by the World Health Organization, whereas several others constitute part of the list of the top 300 most prescribed drugs in the US in 2019.
The examples presented in this review are focused on replacing fragments of existing APIs with bio-sourced atoms. However, the use of biomass offers more direct access to building blocks that were less accessible under the petro-based paradigm. A more efficient use of bio-sourced atoms would benefit from taking these molecules into consideration in the early stages of the production of molecular libraries and drug discovery. This approach could allow efforts in green chemistry to go beyond the production of (smart) drop-in molecules that mimic historically relevant building blocks originating in the petrol industry. Instead, future developments should look toward the incorporation of so-called dedicated platforms, and from there, to the production of entirely bio-sourced APIs.
Author contributions
R. M. designed the original plan of the manuscript, selected the target APIs and prepared a preliminary draft. L. B. and D. V. S. B. prepared the manuscript. J. C. M. M. revised the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Interreg V-A Euregio Meuse-Rhine (EMR) program (IN FLOW) for funding. IN FLOW was granted a € 2.1 M total budget from the European Regional Development Fund (ERDF). With the investment of EU funds in Interreg projects, the European Union directly invests in economic development, innovation, territorial development, social inclusion and education in the Euregio Meuse-Rhine. The authors also thank the University of Liège and the F.R.S.-FNRS (Incentive grant for scientific research MIS F453020F, J. C. M. M.). The authors also thank Dr Pierre Francotte (ULiège, Department of Pharmacy), Dr Damien Cornut (Mithra Pharmaceuticals), Dr Laurent Provins (UCB Pharma), Dr Jean Fournier (Oril Industrie), Maryse Phan (Oril Industrie) and the EMA Pharmaceutical Quality Office for their answers related to the regulatory aspects. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.References
- T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass: Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas, Office of Biomass Program Report DOE/GO-102004-1992, U.S. Department of Energy, United States, 2004.
- FACT SHEET: Overview of USDA's BioPreferred Program, https://www.usda.gov/media/press-releases/2016/02/18/fact-sheet-overview-usdas-biopreferred-program, (accessed Nov 2, 2023).
- M. J. Biddy, C. Scarlata and C. Kinchin, Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential, N. R. E. L. (NREL) Report NREL/TP-5100-65509, U.S. Department of Energy, Golden, CO (USA), 2016.
- R. Christoph, B. Schmidt, U. Steinberner, W. Dilla and R. Karinen, Ullmann's Encyclopedia of Industrial Chemistry, 2006 Search PubMed
.
- Glycerol Prices | Historical and Current, https://www.intratec.us/chemical-markets/glycerol-price, (accessed April 1, 2024).
- World - Crude Glycerol, Glycerine Waters And Lyes - Market Analysis, Forecast, Size, Trends And Insights, https://www.indexbox.io/search/crude-glycerol-market/, (accessed April 1, 2024).
- Propylene Prices | Current and Forecast, https://www.intratec.us/chemical-markets/propylene-price, (accessed April 1, 2024).
- https://www.biodieselmagazine.com/articles/7662/the-pure-potential-of-glycerin , (accessed April 1, 2024).
-
M. Pagliaro and M. Rossi, in The Future of Glycerol, The Royal Society of Chemistry, Cambridge, UK, 2nd edn., 2010, ch. 1, pp. 1–28 Search PubMed
.
- B. Katryniok, S. Paul, V. Bellière-Baca, P. Rey and F. Dumeignil, Green Chem., 2010, 12, 2079–2098 RSC
.
- X. Li and Y. Zhang, ACS Catal., 2016, 6, 143–150 CrossRef CAS
.
- N. N. Tshibalonza and J.-C. M. Monbaliu, Green Chem., 2017, 19, 3006–3013 RSC
.
- E. Arceo, P. Marsden, R. G. Bergman and J. A. Ellman, Chem. Commun., 2009, 3357–3359 RSC
.
- P. M. Walgode, R. P. V. Faria and A. E. Rodrigues, Catal. Rev., 2021, 63, 422–511 CrossRef CAS
.
- L. Luo, W. Chen, S.-M. Xu, J. Yang, M. Li, H. Zhou and H. Duan,
et al.
, J. Am. Chem. Soc., 2022, 144, 7720–7730 CrossRef CAS PubMed
.
- M. Główka and T. Krawczyk, ACS Sustainable Chem. Eng., 2023, 11, 7274–7287 CrossRef
.
- N. Razali and A. Z. Abdullah, Appl. Catal., A, 2017, 543, 234–246 CrossRef CAS
.
- M. R. Nanda, Y. Zhang, Z. Yuan, W. Qin, H. S. Ghaziaskar and C. Xu, Renewable Sustainable Energy Rev., 2016, 56, 1022–1031 CrossRef CAS
.
- B. M. Bell, J. R. Briggs, R. M. Campbell, S. M. Chambers, P. D. Gaarenstroom, J. G. Hippler and C. P. Wolfe,
et al.
, Clean: Soil, Air, Water, 2008, 36, 657–661 CAS
.
- G. M. Lari, G. Pastore, C. Mondelli and J. Pérez-Ramírez, Green Chem., 2018, 20, 148–159 RSC
.
- E. Santacesaria, R. Vitiello, R. Tesser, V. Russo, R. Turco and M. Di Serio, Ind. Eng. Chem. Res, 2014, 53, 8939–8962 CrossRef CAS
.
- R. Morodo, R. Gérardy, G. Petit and J.-C. M. Monbaliu, Green Chem., 2019, 21, 4422–4433 RSC
.
- P. Prete, D. Cespi, F. Passarini, C. Capacchione, A. Proto and R. Cucciniello, Curr. Opin. Green Sustainable Chem., 2022, 35, 100624–100624 CrossRef CAS
.
- G. S. Singh, K. Mollet, M. D'Hooghe and N. De Kimpe, Chem. Rev., 2013, 113, 1441–1498 CrossRef CAS PubMed
.
-
Bold Goals for U.S. Biotechnology and Biomanufacturing, White House Office of Science and Technology Policy, Washington D.C., USA, 2023 Search PubMed
.
-
J. Spekreijse, K. Vikla, M. Vis, K. Boysen-Urban, G. Philippidis and R. M'Barek, Bio-based value chains for chemicals, plastics and pharmaceuticals – A comparison of bio-based and fossil-based value chains, European Commission Joint Research Centre Publications Office, 2021 Search PubMed
.
-
T. Ronzon, T. Lammens, J. Spekreijse, M. Vis and C. Parisi, Insights into the European market for bio-based chemicals – Analysis based on 10 key product categories, European Commission Joint Research Centre, Publications Office, 2019 Search PubMed
.
-
L. Nattrass, C. Biggs, A. Bauen, C. Parisi, E. Rodríguez-Cerezo and M. Gómez-Barbero, The EU bio-based industry – Results from a survey, Joint Research Centre- Institute for Prospective Technological Studies Publications Office, 2016 Search PubMed
.
- G. Kaisin, L. Bovy, Y. Joyard, N. Maindron, V. Tadino and J.-C. M. Monbaliu, J. Flow Chem., 2023, 13, 77–90 CrossRef PubMed
.
- L. Wollensack, K. Budzinski and J. Backmann, Curr. Opin. Green Sustainable Chem., 2022, 33, 100586 CrossRef CAS
.
- https://nova-institute.eu , (accessed April 4, 2024).
- M. Carus, L. Dammer, Á. Puente, A. Raschka and D. O. Arendt, Bio-based drop-in, smart drop-in and dedicated chemicals, Report Version 2017-12, nova-Institut GmbH, 2017.
-
R. Chinthapalli, P. Skoczinski, M. Carus, W. Baltus, D. de Guzman, H. Käb and J. Ravenstijn, et al., Bio-based Building Blocks and Polymers – Global Capacities, Production and Trends 2018–2023, nova-Institut GmbH, Hürth, Germany, 2019 Search PubMed
.
-
P. Skoczinski, M. Carus, G. Tweddle, P. Ruiz, D. de Guzman, J. Ravenstijn and A. Raschka, et al., Bio-based Building Blocks and Polymers Global Capacities, Production and Trends 2022–2027, nova-Institut GmbH, Hürth, Germany, 2023 Search PubMed
.
-
P. Skoczinski, M. Carus, G. Tweddle, P. Ruiz, N. Hark, A. Zhang and A. Raschka, et al., Bio-based Building Blocks and Polymers Global Capacities, Production and Trends 2023–2028, nova-Institut GmbH, Hürth, Germany, 2024 Search PubMed
.
- Synthetic glycerine is back (but never really went away)! https://www.outsourcing-pharma.com/Article/2008/10/16/Synthetic-glycerine-is-back-but-never-really-went-away, (accessed April 4, 2024).
- Combating The Glycerin Glut, https://biodieselmagazine.com/articles/combating-the-glycerin-glut-1123, (accessed April 4, 2024).
- FAMHP, https://www.famhp.be/en, (accessed February 29, 2024).
- https://www.fda.gov/ , (accessed February 29, 2024).
- Commission Regulation (EC) No 1234/2008 of 24 November 2008 concerning the examination of variations to the terms of marketing authorisations for medicinal products for human use and veterinary medicinal products, https://data.europa.eu/eli/reg/2008/1234/2021-05-13, (accessed April 8, 2024).
- European Medicines Agency post-authorisation procedural advice for users of the centralised procedure, Human Medicines Evaluation Division Report EMEA-H-19984/03 Rev. 107, European Medicines Agency, Amsterdam, Netherlands, 2024.
- Guidelines on the details of the various categories of variations, on the operation of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 of 24 November 2008 concerning the examination of variations to the terms of marketing authorisations for medicinal products for human use and veterinary medicinal products and on the documentation to be submitted pursuant to those procedures, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.C_.2013.223.01.0001.01.ENG&toc=OJ%3AC%3A2013%3A223%3ATOC, (accessed April 8, 2024, 56).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), https://www.ich.org/, (accessed February 29, 2024).
- International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Q7, ICH Harmonised Tripartite Guideline 2000.
- International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use Development And Manufacture Of Drug Substances (Chemical Entities And Biotechnological/Biological Entities) Q11, ICH Harmonised Tripartite Guideline 2012.
- International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use Pharmaceutical Development Q8(R2), ICH Harmonised Tripartite Guideline 2009.
- Communication From The Commission To The European Parliament, The Council And The European Economic And Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52019DC0128&qid=1605854880622, (accessed February 29, 2024).
- Q3C—Tables and List Guidance for Industry, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q3c-tables-and-list-rev-4, (accessed July 25, 2023).
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC
.
-
A. Kleemann, J. Engel, B. Kutscher and D. Reichert, Pharmaceutical Substances: Syntheses, Patents and Applications of the most relevant APIs, Georg Thieme Verlag, Stuttgart, 4th edn, 2009 Search PubMed
.
- WHO model list of essential medicines, World Health Organization 2021
.
- The Top 300 of 2021, https://clincalc.com/DrugStats/Top300Drugs.aspx, (accessed April 5, 2024).
-
Y. Tamura, K. Nakagawa, S. Yoshizaki and N. Murakami, US Pat, US3910924A, 1975 Search PubMed
.
- M. Saquib, M. F. Khan, J. Singh, B. Khan, Priti, P. Kumar and M. K. Hussain, Sustainable Chem. Pharm., 2022, 30, 100860 CrossRef CAS
.
-
H. Lietz, US Pat, US4474986A, 1984 Search PubMed
.
- The Top 300 of 2019, https://clincalc.com/DrugStats/Top300Drugs.aspx, (accessed Dec 10, 2022).
-
E. Perzborn and T. Krahn, International Pat, WO2007/039134Al, 2009 Search PubMed
.
- Y. C. Loh, C. S. Tan, Y. S. Ch'ng, M. Ahmad, M. Z. Asmawi and M. F. Yam, Molecules, 2016, 21, 495 CrossRef PubMed
.
- P. G. Wang, M. Xian, X. Tang, X. Wu, Z. Wen, T. Cai and A. J. Janczuk, Chem. Rev., 2002, 102, 1091–1134 CrossRef CAS PubMed
.
- L. Schjelderup and A. J. Aasen, Acta Chem. Scand., 1986, 40, 505–507 CrossRef
.
-
J. A. Finkelstein, R. M. Keenan and J. Weinstock, US Pat, US5185351A, 1993 Search PubMed
.
-
O. Melen, Muscle Relaxants, in Essentials of Pain Medicine and Regional Anesthesia, ed. H. T. Benzon, S. N. Raja, R. E. Molloy, S. S. Liu and S. M. Fishman, Churchill Livingstone, Philadelphia, 2nd edn, 2005, ch. 17, pp. 159–165 Search PubMed
.
- A. M. Truscello, C. Gambarotti, M. Lauria, S. Auricchio, G. Leonardi, S. U. Shisodia and A. Citterio, Green Chem., 2013, 15, 625–628 RSC
.
-
C. Brunnengräber, Germany Pat, DE1249852B, 1967 Search PubMed
.
-
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Methocarbamol, https://www.ncbi.nlm.nih.gov/books/NBK548286/, (accessed November 11, 2023) Search PubMed
.
- B. J. Maron, J. R. Krupp and B. Tune, J. Pediatr., 1971, 78, 697–699 CrossRef CAS PubMed
.
- Décontractyl (méphénésine) : retrait des autorisations de mise sur le marché à compter du 28 juin 2019, https://ansm.sante.fr/actualites/decontractyl-mephenesine-retrait-des-autorisations-de-mise-sur-le-marche-a-compter-du-28-juin-2019, (accessed Feb 19, 2023).
- M. B. Hadimani, M. K. Purohit, C. Vanampally, R. Van der Ploeg, V. Arballo, D. Morrow and L. P. Kotra,
et al.
, J. Med. Chem., 2013, 56, 5071–5078 CrossRef CAS PubMed
.
- H. L. Yale, E. J. Pribyl, W. Braker, F. H. Bergeim and W. A. Lott, J. Am. Chem. Soc., 1950, 72, 3710–3716 CrossRef CAS
.
- Mephenesin, https://drugs.ncats.io/substance/7B8PIR2954, (accessed Feb 19, 2023).
- C. D. Lunsford, R. P. Mays, J. A. Richman Jr and R. S. Murphey, J. Am. Chem. Soc., 1960, 82, 1166–1171 CrossRef CAS
.
- Mephenoxalone, https://drugs.ncats.io/drug/CZ87T54W8W).
-
S. Reddy, US Pat, US20110306773A1, 2011 Search PubMed
.
- Drug Approval Package: Skelexin (Metaxalone) Tablets, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/013217s044_SkelexinTOC.cfm, (accessed February 10, 2023).
- Metaxalone, https://drugs.ncats.io/drug/1NMA9J598Y, (accessed Feb 20, 2023).
- A. Firth and P. Prathapan, Curr. Res. Pharmacol. Drug Discov., 2021, 2, 100011 CrossRef PubMed
.
- https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance , (accessed Feb 20, 2023).
- C. J. L. Murray, K. S. Ikuta, F. Sharara, L. Swetschinski, G. Robles Aguilar, A. Gray and M. Naghavi,
et al.
, Lancet, 2022, 399, 629–655 CrossRef CAS PubMed
.
- New report calls for urgent action to avert antimicrobial resistance crisis, https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis, (accessed Feb 20, 2023).
- J. S. Gerber, R. K. Ross, M. Bryan, A. R. Localio, J. E. Szymczak, R. Wasserman and A. G. Fiks,
et al.
, J. Am. Med. Assoc., 2017, 318, 2325–2336 CrossRef PubMed
.
- D. M. Lin, B. Koskella and H. C. Lin, World J. Gastrointest. Pharmacol. Ther., 2017, 8, 162–173 CrossRef PubMed
.
- R. P. N. Mishra, E. Oviedo-Orta, P. Prachi, R. Rappuoli and F. Bagnoli, Curr. Opin. Microbiol., 2012, 15, 596–602 CrossRef CAS PubMed
.
- A. A. Bredikhin, D. V. Zakharychev, A. T. Gubaidullin, A. I. Samigullina and Z. A. Bredikhina, Cryst. Growth Des., 2021, 21, 3211–3224 CrossRef CAS
.
- M. R. Barbachyn and C. W. Ford, Angew. Chem., Int. Ed., 2003, 42, 2010–2023 CrossRef CAS PubMed
.
- The Selection and Use of Essential Medicines (2015), W. T. R. Series Report TRS 994, World Health Organization, 2015.
- Linezolid (oral/injection), https://www.drugs.com/mtm/linezolid-oral-injection.html, (accessed Feb 20, 2023).
- M. G. Russell and T. F. Jamison, Angew. Chem., Int. Ed., 2019, 58, 7678–7681 CrossRef CAS PubMed
.
- Tedizolid (oral/injection), https://www.drugs.com/mtm/tedizolid-oral-injection.html, (accessed Feb 20, 2023).
- G. G. Zhanel, R. Love, H. Adam, A. Golden, S. Zelenitsky, F. Schweizer and J. A. Karlowsky,
et al.
, Drugs, 2015, 75, 253–270 CrossRef CAS PubMed
.
- A. C. Flick, H. X. Ding, C. A. Leverett, R. E. Kyne, K. K. C. Liu, S. J. Fink and C. J. O'Donnell, Bioorg. Med. Chem., 2016, 24, 1937–1980 CrossRef CAS PubMed
.
-
C. A. Costello, J. A. Simson, R. J. Duguid and D. Phillipson, WIPO (PCT) Pat, WO2010042887A2, 2010 Search PubMed
.
-
D. Phillipson, WIPO (PCT) Pat, WO2010091131A1, 2010 Search PubMed
.
- W. Li, S. S. Thakur, S.-W. Chen, C.-K. Shin, R. B. Kawthekar and G.-J. Kim, Tetrahedron Lett., 2006, 47, 3453–3457 CrossRef CAS
.
- W. Mendling and F. Mailland, Arzneimittelforschung, 2002, 52, 8–13 CAS
.
- M. Bastrakov and A. Starosotnikov, Pharmaceuticals, 2022, 15, 705 CrossRef CAS PubMed
.
-
 ,
China Pat, CN102863434A, 2014 Search PubMed
,
China Pat, CN102863434A, 2014 Search PubMed .
- J. Shu, L. He, H. Ding, L. Wang, H. Guo, Y. Gao and Z. Zeng,
et al.
, Anal. Methods, 2014, 6, 2306–2313 RSC
.
- A. Leitner, P. Zöllner and W. Lindner, J. Chromatogr. A, 2001, 939, 49–58 CrossRef CAS PubMed
.
- FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs, https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-resistant-forms-tuberculosis-affects-lungs, (accessed Feb 20, 2023).
-
New drug therapy approvals 2019, Center For Drug Evaluation And Research U.S. Food and Drug Administration, Silver Spring, MD, 2019 Search PubMed
.
- M. Sato, H. Azuma, A. Daigaku, S. Sato, K. Takasu, K. Okano and H. Tokuyama, Angew. Chem., Int. Ed., 2017, 56, 1087–1091 CrossRef CAS PubMed
.
-
B. Parthasaradhi Reddy, K. Rathnakar Reddy, A. Venkat Narsimha Reddy and B. Vamsi Krishna, India Pat, IN201641030408, 2016 Search PubMed
.
-
M. H. Wilcox, in Infectious Diseases, ed. J. Cohen, W. G. Powderly and S. M. Opal, Elsevier, 4th edn, 2017, pp. 1261–1263 Search PubMed
.
- Z. Junsong, Z. Guangming, J. Chunxiao and M. Duobin, Chin. J. Pharm., 2004, 644–660 Search PubMed
.
-
K. Liaras and M. Soković, in Antifungal Compounds Discovery, Elsevier, Amsterdam, 2021, ch. 5, pp. 167–262 Search PubMed
.
-
M. Soković and K. Liaras, in Antifungal Compounds Discovery, ed. M. Soković and K. Liaras, Elsevier, Amsterdam, 2021, ch. 3, pp. 49–66 Search PubMed
.
- W. W. Hope, L. McEntee, J. Livermore, S. Whalley, A. Johnson, N. Farrington and J. H. Rex,
et al.
, mBio, 2017, 8, e01157–e01117 CrossRef PubMed
.
- S. Krishnan-Natesan, Expert Opin. Pharmacother., 2009, 10, 2723–2733 CrossRef CAS PubMed
.
-
K. Karimian, R. C. H. S. Leung-Toung, Y. Li and T. F. Tam, US Pat, US005817875A, 1998 Search PubMed
.
- A. K. Gupta and D. C. A. Lyons, J. Cutaneous Med. Surg., 2015, 19, 352–357 CrossRef CAS PubMed
.
- G. Sood, P. Nyirjesy, M. V. Weitz and A. Chatwani, Infect. Dis. Obstet. Gynecol., 2000, 8, 240–243 CrossRef CAS PubMed
.
- Terconazole, https://www.drugs.com/monograph/terconazole.html, (accessed Feb 20, 2023).
- Itraconazole, https://www.drugs.com/monograph/itraconazole.html, (accessed Feb 20, 2023).
- D. J. Sheehan, C. A. Hitchcock and C. M. Sibley, Clin. Microbiol. Rev., 1999, 12, 40–79 CrossRef CAS PubMed
.
- Search Orphan Drug Designations and Approvals: Itraconazole, https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=473715, (accessed Feb 20, 2023).
- Orphan designation for the prevention of invasive aspergillosis, Report EU/3/18/2024, European Medicines Agency, 2018.
-
J. Heeres, L. J. J. Backx and J. H. Mostmans, US Pat, US4223036A, 1980 Search PubMed
.
-
J. Heeres, L. J. J. Backx and J. H. Mostmans, US Pat, US4144346A, 1979 Search PubMed
.
- M. Z. Fiume and F. A. Andersen, Int. J. Toxicol., 2003, 22, 1–10 CrossRef PubMed
.
- M. Berthelot, Ann. Chim. Phys., 1854, 41, 216–319 Search PubMed
.
- PubChem Annotation Record for TRIACETIN, https://pubchem.ncbi.nlm.nih.gov/source/hsdb/585, (accessed Feb 20, 2023).
- Neglected tropical diseases, https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_2, (accessed Feb 20, 2023).
- H. Wang, M. Naghavi, C. Allen, R. M. Barber, Z. A. Bhutta, A. Carter and C. J. L. Murray,
et al.
, Lancet, 2016, 388, 1459–1544 CrossRef PubMed
.
- Trypanosomiasis, human African (sleeping sickness), https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness), (accessed Feb 20, 2023).
- L. Leggio, G. Addolorato, L. Abenavoli and G. Gasbarrini, Int. J. Immunopathol. Pharmacol., 2005, 18, 7–14 CrossRef CAS PubMed
.
-
P. R. Albert, S. L. Arthur, T. R. H. Stewart, W. F. Neville, M. A. Frank and G. E. James, US Pat, US2432797A, 1947 Search PubMed
.
- E. P. Levanova, V. A. Grabel'nykh, E. N. Sukhomazova, I. A. Zemirova, N. V. Russavskaya, A. I. Albanov and N. A. Korchevin,
et al.
, Russ. J. Org. Chem., 2008, 44, 1428–1433 CrossRef CAS
.
- R. A. Peters, L. A. Stocken and R. H. S. Thompson, Nature, 1945, 156, 616–619 CrossRef CAS PubMed
.
- S. Gibaud, R. Alfonsi, P. Mutzenhardt, I. Fries and A. Astier, J. Organomet. Chem., 2006, 691, 1081–1084 CrossRef CAS
.
- E. A. H. Friedheim, Am. J. Trop. Med. Hyg., 1949, s1–29, 185–188 CrossRef PubMed
.
- G. Thébaud, J. Chadœuf, M. J. Morelli, J. W. McCauley and D. T. Haydon, Proc. R. Soc. B, 2010, 277, 809–817 CrossRef PubMed
.
- S. J. R. da Silva, J. C. F. do Nascimento, R. P. Germano Mendes, K. M. Guarines, C. Targino Alves da Silva, P. G. da Silva and L. Pena,
et al.
, ACS Infect. Dis., 2022, 8, 1758–1814 CrossRef CAS PubMed
.
- Ganciclovir, https://medlineplus.gov/druginfo/meds/a605011.html, (accessed Feb 20, 2023).
- Valganciclovir, https://medlineplus.gov/druginfo/meds/a605021.html, (accessed Feb 20, 2023).
-
R. Vardanyan and V. Hruby, in Synthesis of Best-Seller Drugs, ed. R. Vardanyan and V. Hruby, Academic Press, Boston, 2016, pp. 687–736 Search PubMed
.
- valGANciclovir https://www.drugs.com/monograph/valganciclovir.html, (accessed Feb 20, 2023).
- Cidofovir, https://www.drugs.com/mtm/cidofovir.html, (accessed Feb 20, 2023).
- P. R. Brodfuehrer, H. G. Howell, C. Sapino and P. Vemishetti, Tetrahedron Lett., 1994, 35, 3243–3246 CrossRef CAS
.
- R. Duncan and M. J. Vicent, Adv. Drug Delivery Rev., 2013, 65, 60–70 CrossRef CAS PubMed
.
- J. Li, F. Yu, Y. Chen and D. Oupicky, J. Controlled Release, 2015, 219, 369–382 CrossRef CAS PubMed
.
- Take My Fat Please, https://www.bloomberg.com/news/articles/1998-09-21/take-my-fat-please, (accessed March 1, 2023).
- Drug Approval Package: Renagel (Sevelamer Hydrochloride) Capsules, https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/020926.cfm, (accessed March 1, 2023).
-
S. R. Holmes-Farley, W. H. Mandeville and G. M. Whitesides, US Pat, US5667775A, 1995 Search PubMed
.
- D. P. Rosenbaum, S. R. Holmes-Farley, W. H. Mandeville, M. Pitruzzello and D. I. Goldberg, Nephrol., Dial., Transplant., 1997, 12, 961–964 CrossRef CAS PubMed
.
- C. R. Nolan and W. Y. Qunibi, Kidney Int., 2005, 67, S13–S20 CrossRef PubMed
.
- Drug Approval Package- Renvela (Sevelamer Carbonate) Tablets, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022127s000TOC.cfm, (accessed March 2, 2024).
- Drug Approval Package Welchol (colesevelam HCL) for Oral Suspension, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022362_welchol_toc.cfm, (accessed March 27, 2023).
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bile Acid Resins or Sequestrants, https://www.ncbi.nlm.nih.gov/books/NBK548342/, (accessed March 1, 2023).
-
I. W. Harry Mandeville III and S. R. Holmes-Farley, US Pat., US5693675A, 1995 Search PubMed
.
- A. Corsini, E. Windier and M. Farnier, Eur. J. Cardiovasc. Prev. Rehabil., 2009, 16, 1–9 CrossRef PubMed
.
- M. H. Davidson, M. A. Dillon, B. Gordon, P. Jones, J. Samuels, S. Weiss and S. K. Burke,
et al.
, Arch. Intern. Med., 1999, 159, 1893–1900 CrossRef CAS PubMed
.
- V. A. Fonseca, Y. Handelsman and B. Staels, Diabetes, Obes. Metab., 2010, 12, 384–392 CrossRef CAS PubMed
.
- Prescribing information - Welchol (colesevelam) https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022362s007lbl.pdf, (accessed March 1, 2024).
-
I. W. Harry Mandeville III and S. R. Holmes-Farley, US Pat., 5679717A, 1997 Search PubMed
.
- Sanofi-aventis To Acquire Genzyme For $74.00 In Cash Per Share Plus Contingent Value Right, https://www.pharmaceuticalonline.com/doc/sanofi-aventis-to-acquire-genzyme-for-7400-0001, (accessed March 1, 2023).
- A. Hoffman, Biochem. J., 1963, 89, 57–68 CrossRef PubMed
.
- S. L. Gorbach, Gastroenterology, 1971, 60, 1110–1129 CrossRef CAS
.
- W. F. Minor, R. R. Smith and L. C. Cheney, J. Am. Chem. Soc., 1954, 76, 2993–2996 CrossRef CAS
.
-
H. Hoffmann, H. Grill, J. Wagner and G. Hofrichter, US Pat, US3839587A, 1974 Search PubMed
.
- K. B. Mallion, A. H. Todd, R. W. Turner, J. G. Bainbridge, D. T. Greenwood, J. Madinaveitia and B. A. Whittle,
et al.
, Nature, 1972, 238, 157–158 CrossRef CAS PubMed
.
- R. L. Findling, S. A. Candler, A. F. Nasser, S. Schwabe, C. Yu, J. Garcia-Olivares and J. H. Newcorn,
et al.
, CNS Drugs, 2021, 35, 643–653 CrossRef CAS PubMed
.
- Drugs@FDA: FDA-Approved Drugs, https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=211964, (accessed March 7, 2023).
-
S. Lee, US Pat, US3712890A, 1970 Search PubMed
.
-
W. J. V. D. Burg, US Pat, US4002632, 1977 Search PubMed
.
-
K. S. Gregson and J. D. Bennett, in Pharmacology and Therapeutics for Dentistry, ed. F. J. Dowd, B. S. Johnson and A. J. Mariotti, Mosby, 7th edn, 2017, ch. 27, pp. 392–403 Search PubMed
.
-
C. Fitzmaurice and T. Lee, US Pat, US3777033A, 1973 Search PubMed
.
- H. Cairns, C. Fitzmaurice, D. Hunter, P. B. Johnson, J. King, T. B. Lee and J. S. G. Cox,
et al.
, J. Med. Chem., 1972, 15, 583–589 CrossRef CAS PubMed
.
-
F. B. H. Morren, Germany Pat, DE1178435B, 1964 Search PubMed
.
- A. Said Stålsmeden, J. L. Belmonte Vázquez, K. van Weerdenburg, R. Rae, P.-O. Norrby and N. Kann, ACS Sustainable Chem. Eng., 2016, 4, 5730–5736 CrossRef
.
- Y. Hu, Z. Wei, A. Frey, C. Kubis, C.-Y. Ren, A. Spannenberg and T. Werner,
et al.
, ChemSusChem, 2021, 14, 363–372 CrossRef CAS PubMed
.
-
R. A. Goldsby, Immunology, W.H. Freeman, New York, 5th edn, 2003 Search PubMed
.
-
C. Jurgen, US Pat, US1526627A, 1925 Search PubMed
.
- M. B. Zimmermann, P. L. Jooste and C. S. Pandav, Lancet, 2008, 372, 1251–1262 CrossRef CAS PubMed
.
- M. Ahmed, Lancet, 2008, 372, 88 Search PubMed
.
- K. Strijbis, F. M. Vaz and B. Distel, IUBMB Life, 2010, 62, 357–362 CrossRef CAS PubMed
.
-
J. S. H. Babcock and B. R. Baker, US Pat, US2376334A, 1945 Search PubMed
.
-
F. Hoffmann, Aktiengesellschaft Switerland Pat, CH221847A, 1942 Search PubMed
.
-
E. E. Snell, E. L. Wittle and J. A. Moore, US Pat, US2625565A, 1953 Search PubMed
.
- P. A. Jerabek, T. B. Patrick, M. R. Kilbourn, D. D. Dischino and M. J. Welch, Int. J. Radiat. Appl. Instrum., Part A, 1986, 37, 599–605 CrossRef CAS PubMed
.
- A. G. Sorace, A. A. Elkassem, S. J. Galgano, S. E. Lapi, B. M. Larimer, S. C. Partridge and A. D. Smith,
et al.
, Semin. Nucl. Med., 2020, 50, 488–504 CrossRef PubMed
.
- R. Matthes and H. Frey, Biomacromolecules, 2022, 23, 2219–2235 CrossRef CAS PubMed
.
- F. Wilde, C. Chamseddin, H. Lemmerhirt, P. J. Bednarski, T. Jira and A. Link, Arch. Pharm., 2014, 347, 153–160 CrossRef CAS PubMed
.
- E. Nieto, R. Alajarín, J. Álvarez-Builla, I. Larrañaga, E. Gorospe and M. A. Pozo, Synthesis, 2010, 3700–3704 CAS
.
-
Concise Medical Dictionary, ed., E. Martin, Oxford University Press, Oxford, UK, 2015 Search PubMed
.
- A. Barge, F. Baricco, G. Cravotto, R. Fretta and L. Lattuada, ACS Sustainable Chem. Eng., 2020, 8, 12825–12830 CrossRef CAS
.
-
Methotrexate, ed. B. N. Cronstein and J. R. Bertino, Birkhäuser Verlag, Basel, Switerland, 2000 Search PubMed
.
- P. T. Rajagopalan, Z. Zhang, L. McCourt, M. Dwyer, S. J. Benkovic and G. G. Hammes, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 13481–13486 CrossRef CAS PubMed
.
- The Top 300 of 2020, https://clincalc.com/DrugStats/Top300Drugs.aspx, (accessed Nov 4, 2023).
-
J. A. Ellard, US Pat, US4080325A, 1978 Search PubMed
.
-
J. E. Holladay, J. F. White, J. J. Bozell and D. Johnson, Top Value Added Chemicals From Biomass, Pacific Northwest National Laboratory (PNNL) and National Renewable Energy Laboratory (NREL), Springfield, VA, 2004 Search PubMed
.
- X.-F. Shang, S. L. Morris-Natschke, Y.-Q. Liu, X. Guo, X.-S. Xu, M. Goto and K.-H. Lee,
et al.
, Med. Res. Rev., 2018, 38, 775–828 CrossRef CAS PubMed
.
- B. R. Hammond, B. A. Johnson and E. R. George, Exp. Eye Res., 2014, 129, 135–150 CrossRef CAS PubMed
.
- H. Saggadi, D. Luart, N. Thiebault, I. Polaert, L. Estel and C. Len, RSC Adv., 2014, 4, 21456–21464 RSC
.
- X. Zhang and X. Xu, Chem. – Asian J., 2014, 9, 3089–3093 CrossRef CAS PubMed
.
- J. Li, J. Zhang, H. Yang and G. Jiang, J. Org. Chem., 2017, 82, 3284–3290 CrossRef CAS PubMed
.
Footnote |
| † Equal contributions. |
| This journal is © The Royal Society of Chemistry 2024 |