Open Access Article
Open Access ArticleA waste-minimized approach for the synthesis of iodinated organic borazines†
Dario
Marchionni
,
Daniele
Gernini
,
Alireza
Nazari Khodadadi
,
Ejdi
Cela
,
Fan
Huang
and
Luigi
Vaccaro
 *
*
Laboratory of Green S.O.C., Dipartimento di Chimica Biologia e Biotecnologie, Università degli Studi di Perugia, Via Elce di Sotto, 8, 06123 Perugia, Italy. E-mail: luigi.vaccaro@unipg.it; Web: https://www.dcbb.unipg.it/greensoc
First published on 9th April 2024
Abstract
We herein report a waste-minimized process for the synthesis of iodinated hexa-aryl borazines in different iodination patterns and amounts. Access to these widely promising B–N-containing materials features several synthetic challenges including low solubility, narrow stability/reactivity, and limited selectivity. Therefore, the development of synthetic procedures also faces evident sustainability challenges. We have tackled these aspects by merging unconventional activation techniques with metrics-oriented synthetic design. Herein, we report a protocol that enables a fast access route to iodinated borazines which are useful substrates to access different post-functionalized more complex materials. The protocol uses simple and benign chemicals, is waste minimized (E-factor 2.79), and produces iodinated hexa-aryl borazines in good to excellent yields. An evaluation of green metrics, including EcoScale, is provided to quantify the advantages associated with the newly defined protocol.
Introduction
Great efforts have recently been dedicated to the exploration of BN-doped carbon structures.1,2 This interest arises from the wide range of new isosteric motifs and intriguing features that can result from the replacement of carbon–carbon (C–C) bonds with boron–nitrogen (B–N) isoelectronic bonds, both in small molecules and in larger structures.The exceptional performance of several BN-doped materials arises from the ability of the boron–nitrogen bonds to alter the electronic structure. For example, these bonds can widen the HOMO–LUMO gap which in many cases allows for the tailoring and fine-tuning of the optical and electronic properties of the original all-carbon-based material.3 Doping can also be introduced by separated boron or nitrogen substitution at different non-adjacent positions,4 creating B–N bonds,5 B–N rings,6 or larger BxNy domains.7 Arguably, some of the most investigated B–N doped materials are the borazine-based systems.8
Simple borazine, being a 6-membered cyclic system consisting only of boron and nitrogen, can be considered the inorganic counterpart of benzene. Although it bears several physical similarities to benzene, it has a significantly different electronic distribution which has led to discussion about its aromatic nature. Recent research tends to suggest that borazine possesses a modest degree of aromaticity.9,10 Substituted organic borazines have emerged as an important class of BNC materials and in particular, hexa-aryl borazines (HABs) featuring bulky groups at the ortho position of the boron-ring display excellent thermal and hydrolytic stability.11
HABs have shown interesting optoelectronic properties in solution and devices such as LEDs or LECs.12–14 They have been used for the preparation of complex molecular architectures with promising applications. For example, borazines have been used for the construction of dendrimeric polyphenylenes,15 or for assembling doped organogels.16
Borazine-doped nanographenes have been prepared for the first time via planarization.6,17 Borazines have been polymerized on metal surfaces to form BN-doped carbon networks,18 used as additives in the preparation of corrosion-resistant coatings,19 or as precursors for low dielectric constant resins.20
Despite the broadly interesting properties of HABs and their potential application in many different fields, synthetic access to this class of molecules still presents significant limitations. Due to the very harsh reaction conditions generally required for their synthesis, there are many restrictions in terms of functional groups and the amount of material that can be prepared.
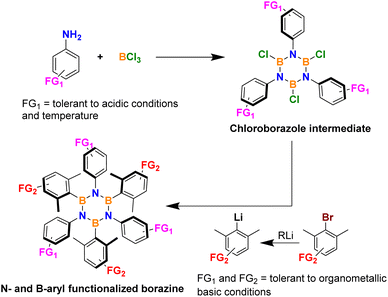
In fact, the borazine ring is usually constructed through a one-pot procedure in which an aniline is reacted with a boron halide, and the resulting adduct is cyclized through the loss of the corresponding acid to form an air and moisture unstable haloborazole intermediate. This intermediate is then purified from HX and arylated by nucleophilic substitution at the boron center with a suitable aryl nucleophile such as aryllithium or aryl-Grignard reagent with the former being by far the more popular option in recent literature together with boron trichloride for the first step (Scheme 1).
As a direct consequence of these synthetic conditions, the preparation of borazines with useful chemical functionalities for their study or even more interesting further elaboration is not straightforward. Only groups that can tolerate strong acidic/electrophilic or basic/nucleophilic conditions can be used on the N-aryl ring, such as alkyls, aryls, fluorides, chlorides, and bromides.
A slightly wider chemical flexibility exists for the substitution on the B-ring as this functionalization occurs at the second organometallic step where groups need to tolerate basic and nucleophilic conditions only.
A small number of decorated borazines have been accessed either through the use of protecting groups, yielding useful chemical handles after deprotection,15 or through acylation or bromination.11,21 However much is still left to do to make the preparation of HABs efficient and their functionalization easier, or anyway comparable to the all-carbon atom hexa-aryl benzene analogue, where most functional groups and substitution patterns have been accessed.22
Aryl iodides are pivotal intermediates in organic chemistry, generally more reactive than aryl bromides and chlorides, they easily undergo metal-catalyzed cross-coupling processes in short times, high yields and mild conditions allowing for extensive functionalization capabilities. These properties are highly desirable in molecules such as hexa-aryl borazines where the three-fold symmetry leads to the necessity of achieving multiple functionalization process at once.
In this contribution, we report our efforts to generate different functionalization patterns on hexa-aryl borazines through iodination. We have also evaluated and considered the sustainability aspects to find the best route for accessing novel chemical spaces for HABs. Specifically, we outline our efforts to establish an efficient iodination protocol for the post-synthetic functionalization of HABs and their representative utilization to prepare derivatives not otherwise accessible.
Results and discussion
Initially, we developed a solution-based iodination approach adapted from literature procedures23 using silver salts as mild and selective oxidants in combination with iodine.24 This reaction was found to work in high yields and selectivity, however, the use of silver salts (featuring a toxic and ecotoxic heavy metal),25,26 and large amounts of non-recoverable halogenated solvents are necessary, clearly posing some issues in terms of green chemistry.In solution vs. mechanochemical conditions
Depending on the substituents HABs dissolve very specifically in a limited number of solvents while they are generally poorly soluble. To develop a greener process, we therefore envisaged the possibility of iodinating these large molecules by using minimal amounts of more benign solvents evaluating the possibility of reducing waste via solvent recovery and reuse.However, upon testing several solvents we concluded that this strategy has some limitations as several borazines display extremely low solubility in non-halogenated solvents at room temperature. We report some solubility data on the representative iodoborazine 11 and the corresponding starting material, borazine 1 in the ESI (Tables S1–3†).
At high temperatures, several non-halogenated less polar solvents dissolve borazine 11. However, heating the iodinated borazine in non-anhydrous conditions under air led, in some cases, to a partial degradation with the formation of some unidentified byproducts. Using dry DMF under argon no degradation was observed.
Green solvents like ethyl acetate or 2MeTHF in this specific case could be used effectively but very large amounts are required compared to DCM or chloroform that in small amounts, are capable of dissolving 11 at room temperature.
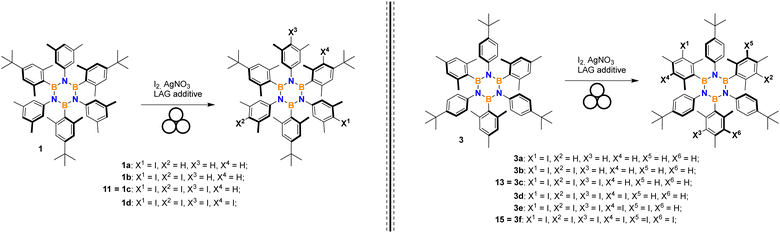
Thus, we directed our attention to mechanochemistry, which is well-known to be highly efficient in the case of heterogeneous reaction conditions. We investigated this route by considering the conditions already developed for in-solution iodination (I2, AgNO3, DCM/MeCN) of borazine 2.24 Results reported in Table 1 have been obtained by partially or entirely removing the solvent and adopting ball-milling conditions.
| Entry | Borazine 0.08 mmol | I2 (equiv.) | AgNO3 (equiv.) | Time (min) | MeCN (μL) | Iodinated productsa (%) | Isolated yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMc | a | b | c | d | e | f | |||||||
| a Area percentage of intermediates including 0–6 iodine atoms from HPLC analysis of the crude mixture. b In solution, using 1.28 mL of DCM. c Starting material (1 or 3). | |||||||||||||
| 1b | 1 | 3.1 | 3 | 60 | 320 | 0 | 0 | 0 | 99 | 1 | 0 | 0 | 92 |
| 2b | 3 | 3.1 | 3 | 60 | 320 | 0 | 0 | 1 | 97 | 2 | 0 | 0 | 90 |
| 3 | 1 | 3.3 | 3.1 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 1 | 3.3 | 3.1 | 60 | 40 | 0 | 0 | 0 | 86 | 14 | 0 | 0 | 85 |
| 5 | 3 | 3.3 | 3.1 | 20 | 40 | 0 | 3 | 12 | 69 | 7 | 7 | 2 | — |
| 6 | 3 | 3.3 | 3.1 | 40 | 40 | 0 | 0 | 1 | 60 | 15 | 16 | 8 | — |
| 7 | 3 | 3.3 | 3.1 | 60 | 40 | 0 | 0 | 0 | 39 | 37 | 18 | 6 | — |
| 8 | 3 | 3.0 | 3.0 | 40 | 40 | 0 | 0 | 2 | 69 | 29 | 0 | 0 | 80 |
| 9 | 3 | 3.0 | 2.9 | 30 | 40 | 1 | 3 | 17 | 79 | 0 | 0 | 0 | — |
| 10 | 3 | 3.0 | 2.9 | 40 | 40 | 0 | 0 | 2 | 90 | 8 | 0 | 0 | 85 |
| 11 | 3 | 2.9 | 2.8 | 60 | 40 | 0 | 1 | 10 | 79 | 7 | 2 | 1 | — |
| 12 | 3 | 2.7 | 2.4 | 60 | 40 | 0 | 1 | 5 | 82 | 9 | 2 | 1 | 87 |
While entries 1 and 2 are the results obtained by performing the reaction in solution, reported for comparison, removing completely the solvent (Table 1, entry 3) led to degradation of the starting material and formation of an inseparable mixture of unknown products.
Therefore, to restore the original reactivity, 40 μL of acetonitrile were added as a LAG (liquid assisted grinding) additive.27 In these conditions, parameter η (defined as the ratio of solvent volume to sample weight) ranges between 0.22 and 0.25 across entries 4–12 reported in Table 1. The η LAG range used is very close to solvent-free grinding conditions, the small amount of solvent used was enough to make the reaction effective again albeit with a lower selectivity (entry 4) than the original procedure in solution.
It should be mentioned that the iodination of HABs can progress to incorporate three, six or more iodine atoms depending on the substrate. Control of selectivity poses a challenge when multiple positions are sterically unhindered.
We moved to borazine 3 to further study and optimize conditions.
By changing the reaction time (entries 5–7) selectivity did not improve much although the products distribution varied with longer milling time. After 1 h (entry 7) considerable overiodination was observed meaning that iodine reacted with 3 even without generating silver iodide. This phenomenon can be attributed to the in situ formation of nitric acid, as a byproduct of the reaction. While in solution the dilution slows down significantly the oxidation of iodine by nitric acid, under mechanochemical stirring the concentration is much higher and part of the iodine can be readily oxidized and react with the borazine.
Consequently, we decreased the amounts of both iodine and silver nitrate (entries 8–12). Under the conditions of entry 12 the distribution of products favoured the desired tri-iodinated product, but unfortunately selectivity was still low.
We therefore searched alternative methods to further explore the use of mechanochemical conditions.
Choice of the iodinating system
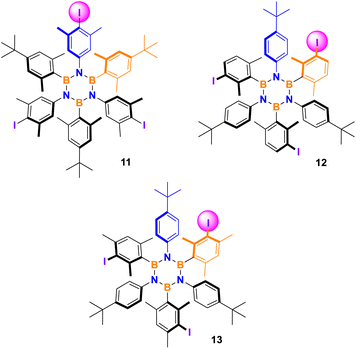
A screening of various combinations of the most common iodinating agents, oxidants, and acids was conducted adapting literature procedures known to be effective on mildly activated aromatics such as alkylbenzenes. Three borazines were selected as representative and tested to evaluate the method's efficiency and selectivity performance. Results are shown in Table 2. Entries 1–2 present the previous results using silver nitrate and iodine in solution and under ball-milling, respectively, repeated here for comparison. Oxidation of iodide using oxone28 was attempted but no reaction was observed (entry 3). A procedure employing iodine, p-toluenesulfonic acid (TsOH) and sodium nitrate29 was tested with success on the three selected borazines (Table 2, entry 4).| No. | Iodinating system | Product yield (selectivity) | ||
|---|---|---|---|---|
| 11 | 12 | 13 | ||
| a General conditions: borazine 0.08 mmol, (1) I2 3.1 eq., AgNO3 3 eq., DCM/MeCN 4/1 0.05 M, RT, 1 h; (2) I2 2.7 eq., AgNO3 2.4 eq., MeCN 40 μL, RT, 30 Hz, 1 h; (3) KI 3 eq., oxone 3 eq., RT, 30 Hz, 1 h; (4) I2 1.5 eq., NaNO3 3 eq., TsOH 3 eq., RT, 30 Hz, 1 h; (5) NaI 3 eq., H2O2 6 eq. 30% aq., TsOH 3 eq., RT, 30 Hz, 1 h; (6) NIS 3 eq., TsOH 3 eq., RT, 30 Hz, 1 h. | ||||
| 1 | I2, AgNO3 | 92 (>95) | 77 (>95) | 90 (>95) |
| 2 | I2, AgNO3 | 89 (>80) | 71 (>80) | 87 (>80) |
| 3 | KI, oxone | 0 | 0 | 0 |
| 4 | I2, NaNO3, TsOH | 86 (>90) | 78 (>90) | 85 (>90) |
| 5 | NaI, H2O2, TsOH | 0 | 0 | 0 |
| 6 | NIS, TsOH | 90 (>95) | 76 (>95) | 84 (>95) |
The strongly acidic and oxidative conditions were able to completely oxidize I2 with almost perfect iodine atom economy, conversely the selectivity was found to be inferior when compared to the solution conditions. However, we noticed the formation of nitrogen oxides29 (NOx) a severe drawback on safety and sustainability.30 In these conditions work-up could be simplified, as all the byproducts are soluble in methanol, thus, after retrieving the powder from the milling jar, the remaining sodium nitrate, acid and iodine were ultimately removed through methanol dissolution.
Additionally, the use of H2O2 was attempted in combination with KI and TsOH31 but we found once again that the reaction did not proceed at all (Table 2, entry 5).
We also investigated different conditions using N-iodosuccinimide as the iodine source and TsOH as the additive leading to a very selective and generally higher yielding procedure (Table 2, entry 6). In conclusion, the screening of the mechanochemical procedures led only in three cases to the formation of the desired products with different selectivity.
Green assessment
In our research, we have always tried to compare different reaction techniques, such as flow chemistry,32–34 microwave irradiation,35 ball-milling36 and green solvents37–39 using green metrics40,41 to assess the actual benefit in terms of sustainability.Accordingly, at this stage of the study, we have quantified the potential advance in terms of sustainability in the iodination of HABs, comparing the different conditions, including the only previously reported literature method.
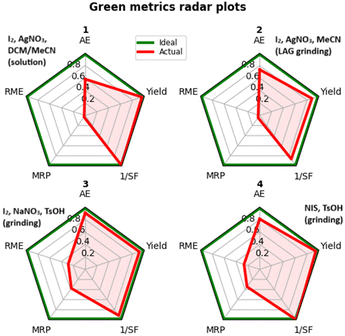
We evaluated some green metrics by calculating AE, 1/SF, RME, and MRP for the iodination of borazine 1. On a gram scale, it was possible to recover methanol by distillation for those methods not employing silver nitrate (Table 3, entries 3 and 4).
| Method | AE | RME | MRP | 1/SF | Yield | VMR | E-Factor |
|---|---|---|---|---|---|---|---|
| Abbreviations: AE: atom economy, RME: reaction mass efficiency, MRP: mass recovery parameter, SF: stoichiometric factor, VMR: vector magnitude ratio. | |||||||
| I2/AgNO3/DCM/MeCN | 0.58 | 0.02 | 0.03 | 0.99 | 0.96 | 0.67 | 57.45 |
| I2/AgNO3/MeCN(LAG) | 0.74 | 0.02 | 0.04 | 0.86 | 0.88 | 0.64 | 40.45 |
| I2/NaNO3/TsOH | 0.91 | 0.29 | 0.38 | 0.91 | 0.91 | 0.74 | 2.49 |
| NIS/TsOH | 0.81 | 0.26 | 0.34 | 1.00 | 0.95 | 0.74 | 2.79 |
| Ideal | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
In other cases, a mixture of low boiling point solvents was obtained making the distillation troublesome.
It is immediately noticeable how the removal of reaction solvent has the expected considerable effect on the reduction of wastes (Table 3, E-factor entries 1 and 2). However, much of the solvent mass comes from the workup; in this case, the production of silver iodide as an extremely insoluble waste represents the major hindrance to further solvent mass reduction as either the scarcely soluble borazine or insoluble AgI must be dissolved selectively to allow the separation.
Thus, a first conclusion is that methods using silver salts, including the previously published iodination method in solution, are inherently less green, so we directed our attention to the other reagent systems.
Adapting the previously reported halogenation procedures, such as bromination, to iodine, proved to be unhelpful. Besides the different reactivity of the two halogens, the protocols are far from ideal in terms of green chemistry. The protocol described by Nagasawa11 employs carbon tetrachloride as a solvent, products are obtained non-selectively as a mixture of tri- and dibromo derivatives, and purification procedures are poorly described. The more recent protocol by Bettinger21 and co-workers was developed for non-ortho-substituted borazines, which are significantly less stable substrates and employ liquid bromine diluted in large amounts of DCM.
Oxidative iodination methods for alkylbenzenes with molecular iodine usually employ very harsh oxidative, acidic or combined conditions in many cases using radical generating agents.42 It is also reported that several oxidant systems degrade hexa-aryl borazines.43
We found that the in situ production of nitric acid was able to oxidize iodine without considerably degrading the starting material (Table 1, entry 12), therefore we tried to replicate the same conditions in Table 2 entry 4 using sodium nitrate and TsOH to mimic the same oxidant system but using solids instead of liquid concentrated nitric acid.
Considering only the mass-based metrics in Table 3, entries 3 and 4 do not differ significantly, the former possessing a higher atom economy and the latter a better stoichiometric factor, leading to a very similar overall vector magnitude ratio (VMR). Despite this similarity we can assert that using sodium nitrate in combination with iodine is worse for the sustainability of the reaction. In fact, oxidation of iodine is accompanied by the formation of nitrites during the course of the process.44
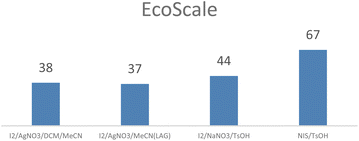
We also compared EcoScale45 values for the four processes (Fig. 1) confirming an advantage for the combination NIS/TsOH.
Furthermore the use of this combination is also recommended by the GSK sustainable reagent guide.46
To summarize, the results in terms of chemical efficiency and sustainability data, including quantification, highlight the value of using metrics assessment, unconventional activation techniques, and sustainable reagent guides. These tools are instrumental in defining convincing synthetic protocols, even for challenging substrates like hexa-aryl borazines. Therefore, we extended the use of the NIS/TsOH procedure to differently substituted borazines, confirming the broader applicability to a wider scope and allowing us to quantify the corresponding yields and selectivities.
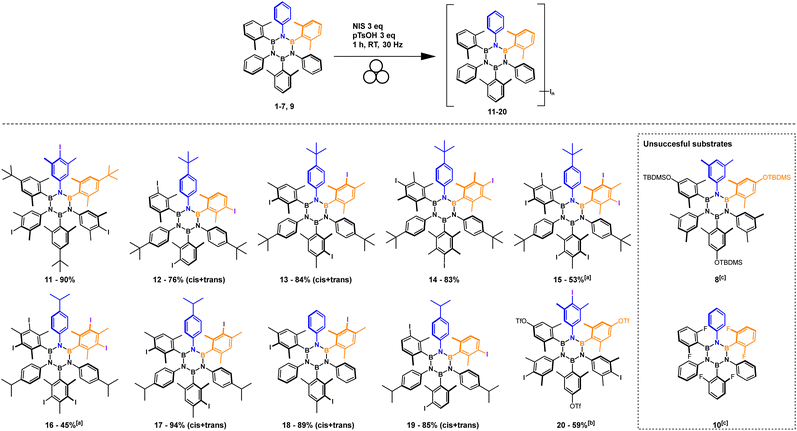
Substrate scope
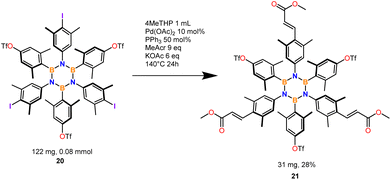
The optimized conditions were extended to 10 substrates (Scheme 2) successfully accessing eight tri-iodinated products in good to excellent yields and two hexa-iodinated products in fair yields considering the 6 processes involved. The reaction was also tested on fluorinated substrate 10 which unfortunately was found to decompose in the reaction conditions and substrate 8 which presumably furnished a mixture of iodination products (NMR analysis).Most importantly the procedure was able to iodinate substrate 9 to product 20, an important and useful substrate for making further HABs functionalization accessible via our iodination protocol. On this substrate we tested the Mizoroki–Heck reaction that preferentially works with iodinated aromatics.41 Only iodine reacted leaving OTf intact (Scheme 3).
Conclusions
In conclusion, we have studied the conditions to define a green protocol for the preparation of iodinated hexa-aryl borazines, synthetic intermediates that cannot be prepared otherwise and that allow access to HABs decorated at different positions. The study progressed by quantifying and comparing the chemical and sustainability features of various iodinating and solvent systems thereby determining the potential sustainability advantages of the desired process. We have found that mechanochemistry can be of great utility for B–N materials that are satisfactory soluble only in chlorinated toxic solvents while poorly soluble in green solvents requiring larger volumes. We have therefore defined a mechanochemistry-based process using NIS/TsOH as iodinating system. This process achieved the three- or six-fold iodination of HABs in 1–2 hours utilizing safe and benign chemicals. In addition, a simple and low-waste purification procedure was defined as proven by a quantified sustainability analysis exemplified by a low value of E-factor of 2.79, typical of much less complicated and bulk procedures. Furthermore, in this contribution we have showed how the combination of unconventional activation techniques such as mechanochemistry can be used in synergy with green chemistry principles and sustainability assessments to tackle challenging transformations accessing valuable B–N materials in accordance with the principles of green chemistry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the European Union's Horizon 2020 Research and Innovation programme under the Marie Sklodowska-Curie entitled STiBNite (N_ 956923). We also acknowledge funding by the European Union – NextGenerationEU under the Italian Ministry of University and Research (MUR) National Innovation Ecosystem grant ECS00000041 – VITALITY. D. M. and L. V. wish also to thank INPS for the Ph.D. grant and training and Graphene XT for useful discussions. D. G. and L. V. thank CIRCC (Progetti Competitivi 2021/CMPT212338 e 2022/CMPT222955, MIUR) for the financial support.References
- A. K. Thakur, K. Kurtyka, M. Majumder, X. Yang, H. Q. Ta, A. Bachmatiuk, L. Liu, B. Trzebicka and M. H. Rummeli, Adv. Mater. Interfaces, 2022, 9, 2101964 CrossRef CAS.
- P. G. Campbell, A. J. V. Marwitz and S.-Y. Liu, Angew. Chem., Int. Ed., 2012, 51, 6074–6092 CrossRef CAS PubMed.
- X. Chen, D. Tan and D.-T. Yang, J. Mater. Chem. C, 2022, 10, 13499–13532 RSC.
- P. Chen, R. A. Lalancette and F. Jäkle, Angew. Chem., Int. Ed., 2012, 51, 7994–7998 CrossRef CAS PubMed.
- T. Hatakeyama, S. Hashimoto, T. Oba and M. Nakamura, J. Am. Chem. Soc., 2012, 134, 19600–19603 CrossRef CAS PubMed.
- J. Dosso, T. Battisti, B. D. Ward, N. Demitri, C. E. Hughes, P. A. Williams, K. D. M. Harris and D. Bonifazi, Chem. – Eur. J., 2020, 26, 6608–6621 CrossRef CAS PubMed.
- Y. Gao, Y. Zhang, P. Chen, Y. Li, M. Liu, T. Gao, D. Ma, Y. Chen, Z. Cheng, X. Qiu, W. Duan and Z. Liu, Nano Lett., 2013, 13, 3439–3443 CrossRef CAS PubMed.
- D. Marchionni, S. Basak, A. N. Khodadadi, A. Marrocchi and L. Vaccaro, Adv. Funct. Mater., 2023, 33, 2303635 CrossRef CAS.
- R. Báez-Grez and R. Pino-Rios, RSC Adv., 2022, 12, 7906–7910 RSC.
- B. Kiran, A. K. Phukan and E. D. Jemmis, Inorg. Chem., 2001, 40, 3615–3618 CrossRef CAS PubMed.
- K. Nagasawa, Inorg. Chem., 1966, 5, 442–445 CrossRef CAS.
- S. Kervyn, O. Fenwick, F. Di Stasio, Y. S. Shin, J. Wouters, G. Accorsi, S. Osella, D. Beljonne, F. Cacialli and D. Bonifazi, Chem. – Eur. J., 2013, 19, 7771–7779 CrossRef CAS PubMed.
- A. Wakamiya, T. Ide and S. Yamaguchi, J. Am. Chem. Soc., 2005, 127, 14859–14866 CrossRef CAS PubMed.
- I. H. T. Sham, C.-C. Kwok, C.-M. Che and N. Zhu, Chem. Commun., 2005, 3547–3549 RSC.
- D. Marinelli, F. Fasano, B. Najjari, N. Demitri and D. Bonifazi, J. Am. Chem. Soc., 2017, 139, 5503–5519 CrossRef CAS PubMed.
- J. Dosso, H. Oubaha, F. Fasano, S. Melinte, J.-F. Gohy, C. E. Hughes, K. D. M. Harris, N. Demitri, M. Abrami, M. Grassi and D. Bonifazi, Chem. Mater., 2022, 34, 10670–10680 CrossRef CAS PubMed.
- J. Dosso, J. Tasseroul, F. Fasano, D. Marinelli, N. Biot, A. Fermi and D. Bonifazi, Angew. Chem., Int. Ed., 2017, 56, 4483–4487 CrossRef CAS PubMed.
- C. Sánchez-Sánchez, S. Brüller, H. Sachdev, K. Müllen, M. Krieg, H. F. Bettinger, A. Nicolaï, V. Meunier, L. Talirz, R. Fasel and P. Ruffieux, ACS Nano, 2015, 9, 9228–9235 CrossRef PubMed.
- A. Renaud, L. Bonnaud, L. Dumas, T. Zhang, Y. Paint, F. Fasano, O. Kulyk, E. Pospisilova, B. Nysten, A. Delcorte, D. Bonifazi, P. Dubois, M.-G. Olivier and M. Poorteman, Eur. Polym. J., 2018, 109, 460–472 CrossRef CAS.
- K. Guo, H. Qi, F. Wang and Y. Zhu, Mater. Sci. Eng., B, 2014, 186, 7–14 CrossRef CAS.
- M. Müller, C. Maichle-Mössmer, P. Sirsch and H. F. Bettinger, ChemPlusChem, 2013, 78, 988–994 CrossRef PubMed.
- S. Suzuki, Y. Segawa, K. Itami and J. Yamaguchi, Nat. Chem., 2015, 7, 227–233 CrossRef CAS PubMed.
- L. Tanwar, J. Börgel, J. Lehmann and T. Ritter, Org. Lett., 2021, 23, 5024–5027 CrossRef CAS PubMed.
- D. Marchionni, A. Nazari Khodadadi, E. Cela, F. Huang and L. Vaccaro, Adv. Synth. Catal., 2024, 3, 494–501 CrossRef.
- N. Hadrup and H. R. Lam, Regul. Toxicol. Pharmacol., 2014, 68, 1–7 CrossRef CAS PubMed.
- P. Courtois, A. Rorat, S. Lemiere, R. Guyoneaud, E. Attard, C. Levard and F. Vandenbulcke, Environ. Pollut., 2019, 253, 578–598 CrossRef CAS PubMed.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418–426 RSC.
- N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, Synth. Commun., 2002, 32, 2319–2324 CrossRef CAS.
- M. S. Yusubov, V. D. Filimonov, H.-W. Jin and K.-W. Chi, Bull. Korean Chem. Soc., 1998, 19, 400–401 CAS.
- W. de Vries, Curr. Opin. Environ. Sci. Health, 2021, 21, 100249 CrossRef.
- J. Iskra, S. Stavber and M. Zupan, Synthesis, 2004, 1869–1873 CrossRef CAS.
- G. Brufani, F. Valentini, G. Rossini, L. Carpisassi, D. Lanari and L. Vaccaro, Green Chem., 2023, 25, 2438–2445 RSC.
- N. Salameh, F. Ferlin, F. Valentini, I. Anastasiou and L. Vaccaro, ACS Sustainable Chem. Eng., 2022, 10, 3766–3776 CrossRef CAS.
- S. Santoro, F. Ferlin, L. Ackermann and L. Vaccaro, Chem. Soc. Rev., 2019, 48, 2767–2782 RSC.
- E. Petricci, C. Risi, F. Ferlin, D. Lanari and L. Vaccaro, Sci. Rep., 2018, 8, 10571 CrossRef PubMed.
- V. Trombettoni, A. Franco, A. G. Sathicq, C. Len, G. P. Romanelli, L. Vaccaro and R. Luque, ChemCatChem, 2019, 11, 2537–2545 CrossRef CAS.
- F. Valentini, H. Mahmoudi, L. A. Bivona, O. Piermatti, M. Bagherzadeh, L. Fusaro, C. Aprile, A. Marrocchi and L. Vaccaro, ACS Sustainable Chem. Eng., 2019, 7, 6939–6946 CrossRef CAS.
- F. Ferlin, L. Luciani, S. Santoro, A. Marrocchi, D. Lanari, A. Bechtoldt, L. Ackermann and L. Vaccaro, Green Chem., 2018, 20, 2888–2893 RSC.
- D. Rasina, A. Lombi, S. Santoro, F. Ferlin and L. Vaccaro, Green Chem., 2016, 18, 6380–6386 RSC.
- V. Hessel, M. Escribà-Gelonch, J. Bricout, N. N. Tran, A. Anastasopoulou, F. Ferlin, F. Valentini, D. Lanari and L. Vaccaro, ACS Sustainable Chem. Eng., 2021, 9, 9508–9540 CrossRef CAS.
- J. L. Osorio-Tejada, F. Ferlin, L. Vaccaro and V. Hessel, Green Chem., 2023, 25, 9760–9778 RSC.
- S. Stavber, M. Jereb and M. Zupan, Synthesis, 2008, 1487–1513 CrossRef CAS.
- D. Bonifazi, F. Fasano, M. M. Lorenzo-Garcia, D. Marinelli, H. Oubaha and J. Tasseroul, Chem. Commun., 2015, 51, 15222–15236 RSC.
- H. G. Brittain and M. J. McLeish, Anal. profiles drug subst. Excip, Academic Press, 1988, vol. 25, p. 106 Search PubMed.
- K. V. Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3 Search PubMed.
- J. P. Adams, C. M. Alder, I. Andrews, A. M. Bullion, M. Campbell-Crawford, M. G. Darcy, J. D. Hayler, R. K. Henderson, C. A. Oare, I. Pendrak, A. M. Redman, L. E. Shuster, H. F. Sneddon and M. D. Walker, Green Chem., 2013, 15, 1542–1549 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc00699b |
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Borazine 0.08 mmol, NIS 6 eq., TsOH 3 eq., RT, 30 Hz, 2 h.
Borazine 0.08 mmol, NIS 6 eq., TsOH 3 eq., RT, 30 Hz, 2 h.