An extended-gate-type organic transistor for monitoring the Menschutkin reaction of tetrazole at a solid–liquid interface†
Yui
Sasaki
 abc,
Kohei
Ohshiro
abc,
Kohei
Ohshiro
 b,
Xiaojun
Lyu
b,
Xiaojun
Lyu
 b,
Takayuki
Kawashima
b,
Takayuki
Kawashima
 b,
Masao
Kamiko
b,
Hikaru
Tanaka
d,
Akari
Yamagami
d,
Yoshinori
Ueno
d and
Tsuyoshi
Minami
b,
Masao
Kamiko
b,
Hikaru
Tanaka
d,
Akari
Yamagami
d,
Yoshinori
Ueno
d and
Tsuyoshi
Minami
 *b
*b
aResearch Center for Advanced Science and Technology, The University of Tokyo, 4-6-1, Komaba, Meguro-ku, Tokyo 153-8904, Japan
bInstitute of Industrial Science, The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505, Japan. E-mail: tminami@g.ecc.u-tokyo.ac.jp
cJST, PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
dCorporate Research Center, Toyobo Co., Ltd., 2-1-1 Katata, Otsu, Shiga, Japan
First published on 13th August 2024
Abstract
We herein propose an approach to visualize the Menschutkin reaction at an interface between a self-assembled monolayer with nucleophilic properties and water containing alkyl halides. An organic field-effect transistor functionalized with a nucleophilic monolayer has detected in situ alkylation depending on differences in the leaving group ability and the bulkiness of electrophilic alkyls.
Self-assembled monolayers (SAMs) have unique properties derived from intermolecular interactions among building blocks.1 SAMs are formed on solid-state substrates by various methods such as vapor deposition and wetting processes.2 The fabrication methods involve the chemistry of SAMs, which affect growth, nucleation, and coalescence.3 In this regard, in situ chemical modification on SAM-attached substrates effectively endows functions with SAMs on demand.4 Surace analysis to identify SAM modification is performed using X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy.4 However, facile methods for monitoring chemical reactions on SAMs have not been fully established. Therefore, we propose a sensor device to detect chemical reactions on SAMs.
An organic field-effect transistor (OFET) is a solution-processable electronic device.5,6 The switching characteristics of OFETs are observed upon applying gate voltages. In this regard, the surface potentials of gate electrodes are manipulated by SAM modification, which affects the performance of OFETs.7 Upon chemical reactions on SAM-attached electrodes, changes in the structure of SAMs cause the difference in surface potentials. Thus, chemical reactions on SAMs modified on gate electrodes can be detected through changes in transistor characteristics (i.e., drain currents (IDSs) and threshold voltages (VTHs)).7 In this study, we applied an extended-gate structure8 for an OFET to detect structure changes of SAMs upon in situ chemical reactions at a solid–liquid interface.
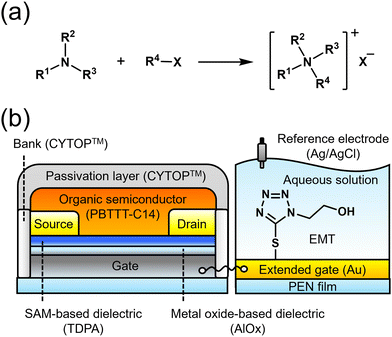
Herein, we employed the Menschutkin reaction to discuss the reactivity based on differences in (1) the leaving group ability and (2) the bulkiness of electrophilic alkyls (Fig. 1(a)).9 The nucleophilic substitution reaction in situ has been monitored using self-assembled materials in solution states,9b,9c whereas the reaction has never been applied to SAMs at solid–liquid interfaces. To detect alkylation using the OFET, 1-hydroxyethyl-5-mercapto-1H-tetrazole (EMT) as a nucleophile was modified on the extended-gate electrode (Fig. 1(b)). Tetrazole is the representative heterocyclic structure comprising four nitrogen atoms, the nucleophilicity of which is a potent property in drug discovery.10 The π electrons of the tetrazole ring are delocalized, whereas the substitution influences π systems.11 Thus, the changes in charges around the tetrazole ring on the extended-gate electrode can be referred to as signals of alkylation. A tetrazole ring thiolated at the 5 position was selected as a building block, considering the following aspects. A hydroxyethyl group introduced into the tetrazole ring at the 1 position can provide intermolecular interactions among building blocks immobilized as a SAM, which contributes a uniform SAM.12 Meanwhile, a substitution reaction at the 4 position of the tetrazole ring occurs in a 1,5-substituted derivative.13 Therefore, the alkylation near the electrode surface can be detected sensitively using the OFET. In this study, the transistor characteristics of the EMT-attached OFET upon alkylation were analyzed using principal component analysis (PCA) to visualize the reactivity of the EMT-based SAM using alkyl halides.
The OFET was fabricated step-by-step (see the ESI†). A double dielectric layer made of aluminum oxide (AlOx) and tetradecylphosphonic acid (TDPA) was employed to achieve device operation at low voltages (<|3| V).14 A π-conjugate polymer, poly{2,5-bis(3-tetradecylthiophen-2-yl)thieno[3,2-b]thiophene} (PBTTT-C14),15 was drop-cast onto a channel region. A hydrophobic material (i.e., CYTOP™) as a passivation layer was entirely covered on the surface of the OFET device for stable operation under ambient conditions (Fig. S1 and S2, ESI†).
The extended-gate Au electrode was fabricated on a polyethylene naphthalate (PEN) film through thermal deposition using a shadow mask. The Au electrode surface was functionalized with EMT under ambient conditions (see the ESI†). The molecular density of EMT was estimated by linear sweep voltammetry, resulting in (1.76 ± 0.44) × 10−10 mol cm−2 (n = 3) (Fig. S3, ESI†). Photoelectron yield spectroscopy (PYS) in air was performed to estimate the work functions of the Au electrode before (4.89 ± 0.04 eV) and after modification of EMT (4.76 ± 0.02 eV) (Fig. S4, ESI†). The shift in the work function to a shallow direction indicates the accumulation of electron-donating groups.8b In addition, an increase in the surface hydrophilicity of the electrode surface functionalized with EMT was observed in a wettability test (Fig. S5, ESI†). Moreover, S 2p and N 1s core-level peaks originating from EMT were identified by XPS measurements (Fig. S6, ESI†). Overall, the modification of EMT was supported by various surface analyses.
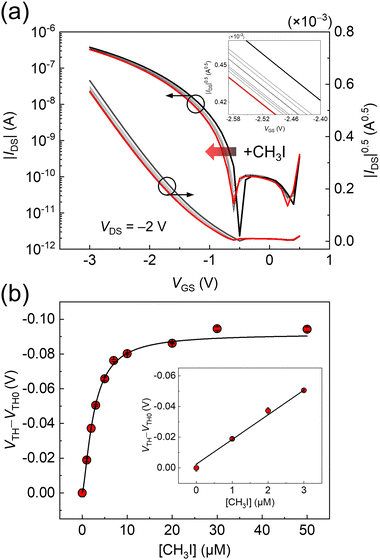
The OFET device and EMT-attached extended-gate electrode were connected using a conductive cable to detect in situ alkylation at the solid–liquid interface. The alkylation of the EMT-based SAM was performed in an aqueous medium containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, HEPES (100 mM) and NaCl (100 mM) at pH 7.4. The extended-gate-type OFET showed time-course negative shifts in transfer curves upon adding an alkyl halide (i.e., iodomethane, CH3I) (100 μM) (Fig. S7, ESI†). A 90% response was observed at 60 min in the time-dependency test. Next, the concentration-dependent test was performed using CH3I. The transistor characteristics at each CH3I concentration were recorded after incubation for 60 min. Shifts in VTHs corresponded to the added CH3I concentration (Fig. 2). The limit of detection value for CH3I was determined to be 57 ppb based on the 3σ method.16 Alkyl halides are widely used reagents for organic synthesis owing to their high reactivity. Meanwhile, the inherent reactivity potentially causes toxicity and mutagenicity in human bodies involving significant diseases.17 Considering the demand for alkyl halide detection in an aqueous environment, the detectability of the EMT-attached OFET for alkyl halides is attractive.
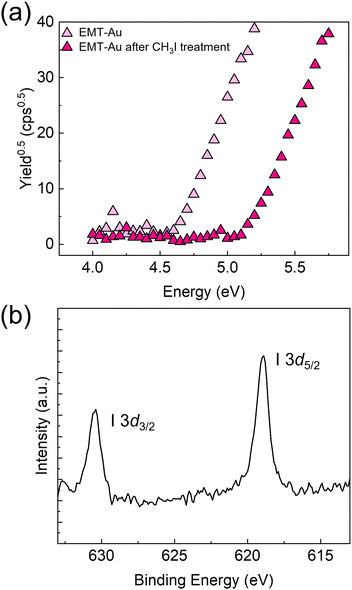
Subsequently, surface analysis was performed to investigate the alkylation of the EMT-based SAM. The Au electrode functionalized with EMT was immersed in an aqueous solution containing CH3I (100 μM) for 60 min under ambient conditions. The treated electrode was gently washed with Milli-Q water, followed by drying with N2 gas. Fig. 3(a) presents PYS measurements of the EMT-attached Au electrode before (light pink triangle) and after the CH3I treatment (deep pink triangle). The deeper shift of the work function implied the accumulation of positively charged species on the Au electrode.8b In addition, XPS measurements of the EMT-attached Au electrode treated with CH3I supported typical peaks originating from I 3d3/2 and I 3d5/2 (Fig. 3(b)). Indeed, the 1H NMR analysis results of a mixture of 1-hydroxyethyl-5-methylthio-1H-tetrazole and CH3I in a solution state shows a new peak at 4.70 ppm corresponding to methyl protons at the 4 position of the tetrazole ring (Fig. S8, ESI†).13 Furthermore, the product after alkylation was also identified using electrospray ionization-mass spectrometry (Fig. S9, ESI†). According to the evaluation in both solution and solid states, the observed changes in the transistor characteristics can be explained by the formation of tetrazolium induced by methylation.
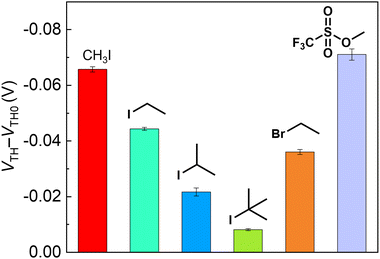
As the next attempt, the reactivity of the EMT-attached OFET was assessed using six electrophiles (i.e., CH3I, iodoethane (CH3CH2I), 2-iodopropane ((CH3)2CH–I), 2-iodo-2-methylpropane ((CH3)3C–I), bromoethane (CH3CH2Br), and methyl trifluoromethanesulfonate (CF3SO3CH3)). Fig. 4 shows changes in the VTH of the OFET upon adding electrophiles (5 μM). The order of VTH changes implied influences of the leaving group ability (CF3SO3− > I− > Br−). In addition, the order depended on the bulkiness of electrophilic alkyls (CH3I > CH3CH2I > (CH3)2CH–I > (CH3)3C–I).
We performed PCA to visualize the reactivity of the EMT-SAM in the Menschutkin reaction. A multidimensional dataset constructed with transistor characteristics was applied to PCA (see the ESI†). PCA can reduce the dimensionality of the dataset as two-dimensional outputs while maintaining the patterns and trends of the initial dataset.18 PCA can determine the correlations between the transistor responses and analyte structures.19Fig. 5 shows the distribution of six clusters of electrophiles at 5 μM. The position of all clusters in the PCA can be classified as dashed lines according to differences in the bulkiness of electrophilic alkyls. We further demonstrated a semi-quantitative assay of six electrophiles at different concentrations using the EMT-OFET. Fig. S15 (ESI†) presents the concentration-dependent cluster distribution by PCA.
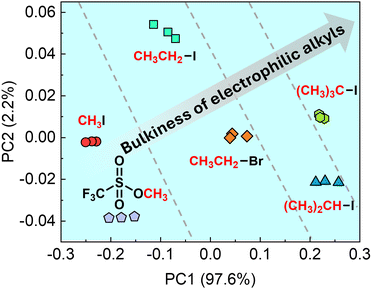 | ||
| Fig. 5 PCA plot for the response patterns of six electrophiles at 5 μM. Three repetitions were carried out for each measurement. | ||
In summary, we designed an OFET-based detection system in situ for the Menschutkin reaction at a solid–liquid interface. Although in situ chemical modification on SAM-attached substrates effectively endows SAMs with desired functions, the reactivity has not been fully discussed. Motivated by this, we aimed to discuss the reactivity of the SAM and alkyl halides based on (1) the leaving group ability and (2) the bulkiness of electrophilic alkyls. In this study, a hydroxyethyl group-attached tetrazole derivative, EMT, was used to monitor alkylation using transistor characteristics based on conversion into a tetrazolium salt. The EMT-attached OFET showed negative shifts in the transfer curves upon adding alkyl halides, which were derived from alkylated products with positive charges. Indeed, the alkylation of the EMT-based SAM on the extended-gate electrode was supported by surface analysis using well-established instrumental methods. In this regard, the transistor characteristics showed unique response orders corresponding to leaving group abilities and the bulkiness of electrophilic alkyls. The responses were applied to pattern recognition for visualizing the reactivity of the EMT-attached SAM and six electrophiles. All clusters in the PCA plot were distributed depending on the bulkiness of electrophilic alkyls. Overall, the EMT-attached OFET achieved not only detection of in situ alkylation but also visualization of its reactivity using a data processing method. We believe the proposed method can be used for facile monitoring of chemical reactions on SAMs at solid–liquid interfaces.
Y. Sasaki gratefully acknowledges the financial support from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No. JP24K17667) and JST PRESTO (Grant No. JPMJPR23H2). T. Minami thanks JSPS KAKENHI (Grant No. JP23H03864 and JP24K01315) and JST CREST (Grant No. JPMJCR2011). K. Ohshiro and X. Lyu thank the JSPS Research Fellow for Young Scientists (DC1) (Grant No. JP23KJ0785 and JP22J23435).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.References
- (a) K. L. Prime and G. M. Whitesides, Science, 1991, 252, 1164–1167 CrossRef CAS PubMed; (b) E. Delamarche, B. Michel, H. A. Biebuyck and C. Gerber, Adv. Mater., 1996, 8, 719–729 CrossRef CAS; (c) K. S. Mali, N. Pearce, S. De Feyter and N. R. Champness, Chem. Soc. Rev., 2017, 46, 2520–2542 RSC.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- J. T. Woodward, I. Doudevski, H. D. Sikes and D. K. Schwartz, J. Phys. Chem. B, 1997, 101, 7535–7541 CrossRef CAS.
- L. Wang, U. S. Schubert and S. Hoeppener, Chem. Soc. Rev., 2021, 50, 6507–6540 RSC.
- G. Horowitz, Adv. Mater., 1998, 10, 365–377 CrossRef CAS.
- T. Minamiki, T. Minami, Y.-P. Chen, T. Mano, Y. Takeda, K. Fukuda and S. Tokito, Commun. Mater., 2021, 2, 8 CrossRef CAS.
- (a) K. Ohshiro, Y. Sasaki, Q. Zhou, P. Didier, T. Nezaki, T. Yasuike, M. Kamiko and T. Minami, Chem. Commun., 2022, 58, 5721–5724 RSC; (b) Y. Sasaki, Y. Zhang, K. Ohshiro, K. Tsuchiya, X. Lyu, M. Kamiko, Y. Ueno, H. Tanaka and T. Minami, Faraday Discuss., 2024, 250, 60–73 RSC.
- (a) R. Kubota, Y. Sasaki, T. Minamiki and T. Minami, ACS Sens., 2019, 4, 2571–2587 CrossRef CAS PubMed; (b) R. Mitobe, Y. Sasaki, W. Tang, Q. Zhou, X. Lyu, K. Ohshiro, M. Kamiko and T. Minami, ACS Appl. Mater. Interfaces, 2022, 14, 22903–22911 CrossRef CAS PubMed.
- (a) S.-D. Yoh, D.-Y. Cheong and O.-S. Lee, J. Phys. Org. Chem., 2003, 16, 63–68 CrossRef CAS; (b) B. W. Purse, A. Gissot and J. Rebek, J. Am. Chem. Soc., 2005, 127, 11222–11223 CrossRef CAS PubMed; (c) K. J. Stanger, J.-J. Lee and B. D. Smith, J. Org. Chem., 2007, 72, 9663–9668 CrossRef CAS PubMed.
- N. Dhiman, K. Kaur and V. Jaitak, Bioorg. Med. Chem., 2020, 28, 115599 CrossRef CAS PubMed.
- N. Sadlej-Sosnowska, J. Org. Chem., 2001, 66, 8737–8743 CrossRef CAS PubMed.
- (a) R. S. Clegg and J. E. Hutchison, Langmuir, 1996, 12, 5239–5243 CrossRef CAS; (b) P. A. Lewis, R. K. Smith, K. F. Kelly, L. A. Bumm, S. M. Reed, R. S. Clegg, J. D. Gunderson, J. E. Hutchison and P. S. Weiss, J. Phys. Chem. B, 2001, 105, 10630–10636 CrossRef CAS.
- I. Yu. Shirobokov, M. V. Chekushina, V. A. Ostrovskii, G. I. Koldobskii and G. B. Erusalimskii, Tetrazoles. 24. Preparation of 1,4-dimethyl- and 2,4-dimethyl-5-aryl-tetrazolium salts in Chemistry of Heterocyclic Compounds, Springer, Berlin, 1988, 24, 413–417 Search PubMed.
- H. Klauk, U. Zschieschang, J. Pflaum and M. Halik, Nature, 2007, 445, 745–748 CrossRef CAS PubMed.
- I. McCulloch, M. Heeney, C. Bailey, K. Genevicius, I. MacDonald, M. Shkunov, D. Sparrowe, S. Tierney, R. Wagner, W. Zhang, M. L. Chabinyc, R. J. Kline, M. D. McGehee and M. F. Toney, Nat. Mater., 2006, 5, 328–333 CrossRef CAS PubMed.
- J. N. Miller and J. C. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson/Prentice Hall, Upper Saddle River, NJ, 2005 Search PubMed.
- (a) Y. Gannot, C. Hertzog-Ronen, N. Tessler and Y. Eichen, Adv. Funct. Mater., 2010, 20, 105–110 CrossRef CAS; (b) W. Chen, S. A. Elfeky, Y. Nonne, L. Male, K. Ahmed, C. Amiable, P. Axe, S. Yamada, T. D. James, S. D. Bull and J. S. Fossey, Chem. Commun., 2011, 47, 253–255 RSC; (c) M.-G. Kang, H. Lee, B. H. Kim, Y. Dunbayev, J. K. Seo, C. Lee and H.-W. Rhee, Chem. Commun., 2017, 53, 9226–9229 RSC.
- P. Anzenbacher, Jr., P. Lubal, P. Buček, M. A. Palacios and M. E. Kozelkova, Chem. Soc. Rev., 2010, 39, 3954–3979 RSC.
- Y. Liu, T. Minami, R. Nishiyabu, Z. Wang and P. Anzenbacher, Jr., J. Am. Chem. Soc., 2013, 135, 7705–7712 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Reagents and materials, apparatuses, fabrication and operation of the OFET, fabrication and characterization of the extended-gate Au electrode, time-dependency test, identification of an alkylated EMT derivative, selectivity test, and semi-quantitative assay. See DOI: https://doi.org/10.1039/d4cc03266g |
| This journal is © The Royal Society of Chemistry 2024 |