Open Access Article
Open Access ArticleThe future of electronic materials is…degradable!
Rajat
Rai
 a and
Daniele
Mantione
a and
Daniele
Mantione
 *ab
*ab
aPOLYMAT University of the Basque Country UPV/EHU, 20018 Donostia-San Sebastián, Spain. E-mail: daniele.mantione@ehu.es
bIKERBASQUE, Basque Foundation for Science, 48009, Bilbao, Spain
First published on 14th August 2023
Abstract
In the last three decades, electronics has passed from a newborn discipline to a consistent part of the material science world. This evolution expands thanks to the rapid development of innovative materials and the quick improvements in their properties. This perspective goes through the last applications, developments, and opportunities that the literature shows about disintegrable or degradable materials for electronic applications. After a brief introduction overviewing the”issue” of plastic pollution and how the literature has taken this subject, the initial discussion covers the disintegrability from a chemistry point of view and presents insights into the bonding structure by ending in a spread vision of the last used materials. This last part is divided into two main areas: supporting materials, intended as the ones which embed the device and bear the whole system and active materials, being in this case, conductive or semiconductive. The vision has the fil rouge of degradability or disintegrability and is strictly related to the last quinquennium, highlighting the most present and cited materials that are opening the way for the future of electronics.
Introduction
We can easily admit that our world now is based on plastic materials, without which much of our civilization and progress would have been different, chemically speaking we could rename this era the “plastic age”. Nowadays, it is almost impossible to think of a society and progress without plastic, the global annual usage of which exceeds 300 million tons.1 Thanks to the excellent physicochemical properties, this family of materials is, on the one hand, substituting the existent non-plastic ones, and on the other hand, creating huge problems derived from these outstanding properties: primarily pollution.2Obviously, we all need to commit to reduce, recycle, and shift to more sustainability, as the rapidity with which we are progressing is not affordable by the planet and will lead naturally to an end. Today, recycled plastic is only 16% higher datum compared to biodegradable plastic, which counts only less than 1%.3 The academic and non-academic research is day by day pushing these materials for more performance and cheaper in production, overlooking, mostly in relation to new-born materials, the degradability, reusability, and recyclability properties. For these new classes of materials, we have to think further than the mere properties: parallelly, we have to explore initiatives and ideas of how these are going to be recycled/reused. The lesson the world is giving us about the most used plastic, and thus, the most pollutant as polyethylene terephthalate (PET), high- and low-density polyethylene (H/LDPE), polyvinyl chloride (PVC), polypropylene (PP) should spur us to do not repeat the same mistakes we have done in the past; in fact, since the 50 s, for half century, the world focused almost only into the properties and the price. We did not think about recycling and recovering those. Keeping plastic and its problems in mind, this principle should be applied to all the “new” materials we are introducing or discovering and should be a central argument of academic and industrial studies. On this line, the more recent studies of flexible electronics and bioelectronics are demanding and discovering new types of materials that will be employed in future devices. Thus, also to these, our attention has to be on both the properties and the challenge of the applications as well as the after-life of the devices.
Components of an electronic device
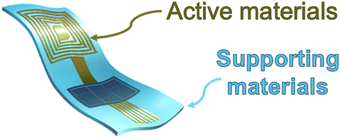
A common (bio)electronic device is formed by two main parts: the active conductive polymer and the supporting material (Fig. 1). This last part consists, normally in the majority of the mass of the device and provides mostly, mechanical properties, for instance, mechanical, adhesive, etc., and is for the most part, a polymeric network.The supporting material, thanks to the wide interest of the scientific community for biodegradable plastic materials and the pushing of the European Union through the initiatives like the EC Plastics Circular Economy, Green Deal or the UN Sustainable Development Goals: # 7 and 13, many initiatives have been explored and appears clear in the literature. Many polymeric matrixes more than the standard polylactic acid (PLA) have been applied, like polylactic-co-glycolic acid (PLGA), polycaprolactone (PCL), and stark blends only to cite a few of them.4 This makes it clear that the support for the active materials has a chance to be biodegradable and, possibly, biosourced. Supporting materials, like jute, banana fibre, coconut warn, and many more, show dielectric properties, work nicely, and are able to provide a bio-sources/degradable alternative.
The active material, instead, appear to be more challenging. Due to their chemical characteristics, conductive or semiconductive materials, instead, are more problematic, as the literature and the industry clearly show this.
A difficult compromise is clearly presented, and until now, the take-home message from all the literature is choral: a good material in terms of electronic behaviour will not be good in terms of both sustainability and degradability. This problem is due to the chemical properties of (semi)conductors. These properties are given by an extended conjugation which parkours an organic backbone. This characteristic is given by the length of this route that normally involves a series of sp2 carbon atoms. This structure, on the other hand, does not contain any breakable linkers resulting in neither disintegrable nor biodegradable.5–8 A widely known example to understand this trend is represented by a material that nowadays the majority of literature refers to: EDOT and, mostly, to the aqueous suspension of PEDOT:PSS used as-it-is. This gold standard, thanks to its great physicochemical properties and mechanical versatility, the suspension can be spin coated, drop cast, spray coated etc., has discouraged the scientific community from performing major variations. This black box has appeared to be difficult to unravel, leading to an enormous disequilibrium between research works using the suspension and research works studying the suspension. The drawback of this is a notable lack of affordable research information: part is patented, and the formulations are covered by industrial secrets. Nonetheless, the PSS, about 2.5 times more in weight in a standard suspension, is totally derived from oil, a non-renewable source forming a fully non-degradable polymer, a derivate of polystyrene.9
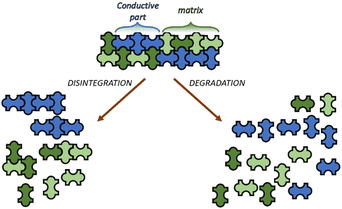
Retaining the electrical and mechanical properties, having, at the same time, degradability or disintegrability (Fig. 2), would lead to great interest and a real scientific breakthrough in (semi)conductive polymers. These materials can replace the existing ones both disposable and non-disposable, paving the way to a closed conductive polymer life cycle and reducing the impact of these in the imminent future by nulling the relative wastes.
To complete the overview, it is mandatory to mention that there still exists a blank in the etymology. If IUPAC clearly includes definitions for the words degradation and biodegradation,10 about the terms, disintegration and biodisintegration are still interpretable and too general, with missing information about the particle sizes, chemically degraded structures, etc.11 Worth to underline that all the derivates of the word upcycling and cradle-to-cradle are not present at all. On this aspect, we can find literature works that elucidate this dictionary, stating a complete degradation as recycle and a partial as disintegration,12 and completing exhaustive tables about degradation and decomposition.13
To complicate this already crowded skyline, the possibility of getting the materials from renewable sources adds one more variable and ravel the dictionary.
In this perspective, we overview the last applications, developments, and opportunities that the literature shows about disintegrable or degradable materials for electronic applications, and, focusing on the last quinquennium, we introduce the chemistry of the degradability and then we take into consideration the different materials used. The general theme is going to be disintegrability and degradability, considering the most cited and promising works.
The chemistry behind degradability
The degradation of both naturally and synthetically derived polymers is dependent on different variables, and the crucial ones are the functional groups and the conditions provided for degradation. It generally happens through the cleavage of the parts of the polymeric chain that leads to the disaggregation of the whole network. The functional group plays an important role; typically, all the amide, sulphonamide, anhydride, carbonate, ether, imide, imine, acetals, ortho esters, phosphonate, ester, thioester, urea, and urethane bonds are the moieties that are more susceptible to hydrolytic degradation. The hydrolysis of these can be proceeded chemically, via acid or base, or even by water. Polymers derived from natural resources incline themselves, mostly, to undergo enzymatic degradation.12,14 Environmental factors like temperature, pH, and time also affect the rate of hydrolysis and eventually, the rate of degradation.15Poly(vinyl alcohol) PVA/chitosan polymer blends were found to be temperature dependent; when the temperature increases, the dissolution time reduces and biodegradation occurs faster.14 Degradability of water-soluble polymer matrixes (polyethylene oxide (PEO), PVA, and gelatine) were studied with or without additives (sodium bicarbonate and citric acid), finding that the concentration plays an important role in dissolution over time. It was observed that when the concentration of the additives increases, the rate of degradation increases.16 Interestingly, nanoparticle composites made of Melanin, a naturally occurring polymer, with PVA were degraded using super worms (z. morios) larvae.17 PVA with poly(glycerol sebacate) (PGS) in corporation with Au nanoparticles (NPs) were found to be degradable in PBS at 37 °C over a period of 28 days.18 The degradation of PVAs leads to acids and aldehydes (Fig. 3d).
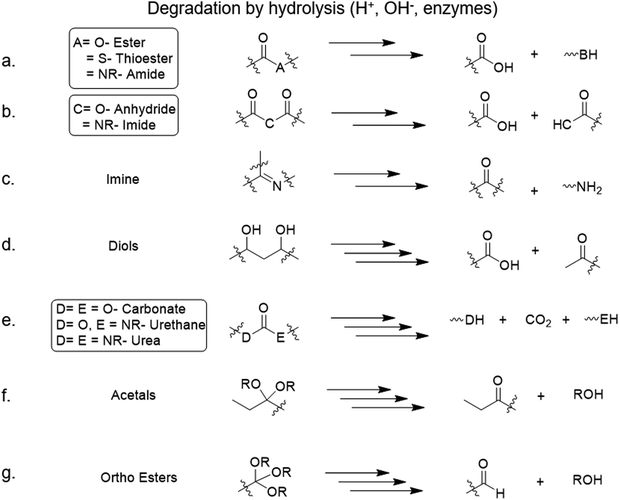 | ||
| Fig. 3 Degradation mechanisms of various moieties by hydrolysis (H+, OH−, enzymes).12,23,24 | ||
PLA is much known for its low toxicity and biodegradebility,19–21 for instance, coordination of PLA with Pt metal can result in the formation of Pt-PLA nanocomposite network structures, which are stable over a broad temperature range for degradability.22 Indium tin oxide (ITO) free organic light emitting diodes (OLEDs) made of PLA were found to be fully biocompatible and degradable at approximately room conditions, i.e., 37 °C, 5% CO2, and 95% relative humidity.19 The general degradation of polyesters leads to acids and aldehydes (Fig. 3a).
Poly imine or poly imide with different substrates like fish colloid, siloxane, and anhydrides were found to be thermally stable, and some of them were found to be water disintegrable, so, overall, they show excellent degradability, biocompatibility, flexibility, recyclability, and extensibility.35–38 Naphthalene diimide based polymers were found to be active and degradable at pH lower than 7; the acidic conditions allow an efficient charge transport through its extended π-conjugation.25 The breakdown of imide groups after degradation leads to acids and amides; also, the imines yield either aldehyde or ketone with amine (Fig. 3b).
Polylactic-co-glycolic acid(PLGA) with polycaprolactone (PCL) composite nanofiber membranes were obtained from electrospinning. They were found to be fully degradable at neutral pH (pH = 7) in phosphate-buffered saline (PBS).26 Elastomer of PCL, instead, showed good degradability at pH 0.5 as the cleavage of the bonds required acidic media.27 Barium titanate nanoparticles with poly(L-lactic-co-glycolic) acid polymer (BT-PLGA) appear as bulk erosion when degraded with PBS at pH = 7.4 and at 37 °C.39 PCL and PLGA are ester derivatives and their degradation simply gives acids and alcohols (Fig. 3a).
Degradation of nanofiber (NF)-reinforced on water-borne polyurethane (NFR-WPU) was studied in phosphate-buffered saline (PBS) at 37 °C.28 Hybrid crosslinked furan-polyurethane (FPU) elastomer with dissociative dynamic bonds also showed excellent recyclability at room temperature with self-healing properties as well.40 Full degradation of PU leads to alcohol, amine, and CO2 (Fig. 3e).
Gelatine is widely studied due to its excellent biodegradability; for example, gelatine with polyacrylic acid (PAA) forms an organohydrogel, with complete degradation in water at 80 °C.29 Gelatine-alginate hydrogel, instead, undergoes degradation by the enzyme Gelatine hydrolase.30 Gelatine has amide groups and its degradation leads to acids and amines (Fig. 3a).
Silk presents a recurrent molecule for degradation purpose. Silk/PEDOT:PSS conductive composites own an efficient enzymatic disintegration at 37 °C.31 Silk fibroin with magnesium41 and melanin42,43 showed excellent degradability with low cytotoxicity and high biocompatibility, in line with possible medical applications. The degradation of silk is environment friendly, and it leads to simple amino acids.44,45
Polysaccharide chains are also widely employed. Poly(3,4-ethylenedioxy-thiophene):polystyrene sulfonate (PEDOT:PSS) on a cellulose diacetate was biodegraded under ISO 14855 standard at 25 °C and found to be 80% degradable.32
Cellulose with imidazolium perchlorate-based membranes with acetate buffer46 or with agarose-based hydrogels47 or cellulose nanofibers with Ag,48 all are found to be biodegradable with different conditions according to their respective substrates and properties. Starch hydrogels have shown biodegradability in water at room temperature.49 Also, chitosan and PEDOT could biodisintegrate in lysozyme solution at pH = 4.5 at room temperature without producing any toxic residues.34 Cellulose, agarose, starch, and chitosan come in the category of polysaccharides. The hydrolysis of these for biodegradation leads to the formation of simple sugars.50–52
From the above discussion, we can see that the conditions for biodegradation are very crucial. Different molecules, even if they intrinsically own the possibility to degrade or disintegrate, require specific conditions. All in all, the amount of work and the generally mild conditions that these works have shown are promising for real degradable possibilities and choices that modern electronics have.
In Table 1, we have summarised how the polymeric systems are being degraded and how they can be altered. We can find, generally, that enzymatic or in vivo degradation has a faster rate than chemical ones. This approach is limited to the relatively high cost of the enzymatic systems and their selectivity. Almost all the in vitro degradation studies have been done either in PBS or DI water, although some cases are reported in acidic media. As expected, naturally occurring polymers are more prone and easily degraded in comparison to synthetic polymers, and combining both systems help faster degradations.
| Polymeric system | Conditons of degradation | Degradation extent | Time required | Factors affecting | Application of the devices | |
|---|---|---|---|---|---|---|
| PVA | Chitosan14 | DI Water at 25–65 °C | ≈100% | ≈120 min | Temperature/time | Sustainable and transient bioelectronics. |
| PEO, Gelatin Matrix16 | DI water at 25 °C | ≈100% | ≈5 min | Concentration of additives like NaHCO3 | Transient materials/electronics | |
| Melanin17 | Using super worms (z. morios) larvae | ≈0.141 mg h−1 | ≈12 h | Increase in the concentration of melanin increases the efficacy of worms | Bioorganic electronics, implantable electronics, edible-electronics, and green eco-electronics | |
| Poly(glycerol sebacate) (PGS) with Au nanoparticles18 | In PBS at 37 °C | — | ≈28 days | A high Young's modulus slows down the degradation | Transient and stretchable electronics | |
| PLA | Iridium complexes19 | 37 °C 5% CO2, 95% relative humidity | — | 48 h | Humidity/CO2 | ITO free OLEDs and flexible optoelectronic devices |
| Pt nanoparticles22 | In PBS at 27 °C | ≈70% | 20 h | — | Miniaturized biosensor for detection of glucose in sweat | |
| Poly imine/imide | Bi thiophene and imine based linkers25 | DI water with trifluoracetic acid (TFA), pH ≈ 0.5 | ≈100% | ≈24 h | Lower pH increase degradation | Transient organic electronics |
| PCL | Polylactic-co-glycolic acid26 | in PBS at 27 °C with neutral pH | ≈80% | 18 days | Higher PLGA content increase degradation | Piezo-capacitive pressure sensor |
| Dialdehyde-functionalized (DPP) and p-phenyldiamine (PPD)-[p(DPP-PPD)]27 | Trifluoroacetic acid (TFA, pH ≈ 0.5) in water | ≈80% | 50 weeks | Higher [p(DPP-PPD)] increase degradation. | Transient electronics | |
| PU | NFs of poly(glycerol sebacate): poly(vinyl alcohol) (PGS:PVA)28 | In PBS at 37 °C | ≈99% | 48 h | The addition of PGS and PVA in the system enhances the rate of degradation | Transient Wearable electronics |
| Gelatin | Polyacrylic acid (PAA)29 | In DI water at 80 °C | ≈99% | 6 h | Temperature | Sustainable electronics |
| Alginate30 | With gelatin hydrolase in DI water at 25 °C | — | 7 days | Higher crosslinking rate slower the degradability | Multifunctional soft electronics | |
| Silk | PEDOT:PSS31 | With Protease XIV from Streptomyces griseus in PBS at 37 °C | ≈99% | 10 days | Enzyme concentration | Temperature sensors |
| Cellulose | PEDOT:PSS32 | ISO 14855 at 25 °C | ≈80% | 11 weeks | — | Electronic display for sustainable electronics |
| Imidazolium perchlorate (ImClO4)33 | With cellulase enzyme in acetate buffer In pH = 4.8 at 50 °C | ≈100% | 4 h | Enzyme concentration | Piezoelectric sensor | |
| Starch | Chitosan34 | Lysozyme in sodium acetate (pH = 4.5) buffer at 25 °C | ≈100% | 8 min | Enzyme concentration | Wearable green electronics |
Supporting materials
As cited in the introduction, this section is impacted by the approach that society nowadays has toward degradable materials. The efforts that some of the biggest economies are making and the orientation that the main research agencies are taking are visible both in the number of studies in the literature and in the large palette of the pret-a-porter materials that anyone can buy. Thanks to this, a wide variety of materials, way more than the semi/conductive ones, is present, together with deeper studies of their physicochemical properties. For ease, we can divide this part into naturally occurring and synthetic substances. Due to the required physical properties, the materials presented are almost for the totality polymeric. (Fig. 4) Readers who want details about (bio)degradability and (bio)derived materials can refer to the works of Chiong et al.53 and Uva et al.54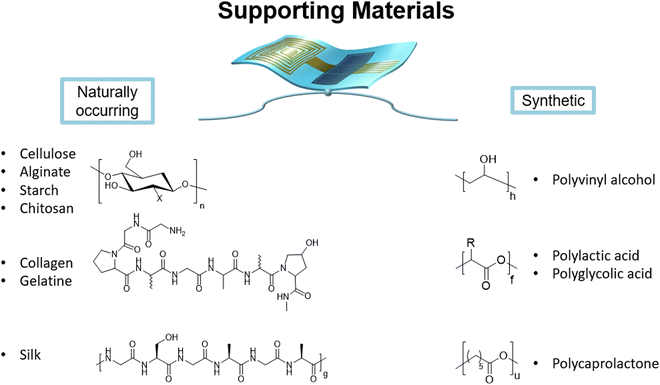 | ||
| Fig. 4 Bioderived supporting materials for electronics-polysaccharides (cellulose,48,56–58,65 alginate,66–70 starch,34,49 chitosan,14,34,59–64), collagen,71 silk31,41–43,72 and synthetically derived supporting materials for electronics-polyvinyl alcohol (PVA),14,16,17,73–75 polyglycolic acid (PGA), polylactic acid (PLA),19–22,76 polycaprolactone (PCL).26,27,38,77–79 | ||
Naturally occurring substrates are derived from living organisms and their resources, owing to the unique characteristic of being, per se, totally degradable and often nontoxic. Depending on the method of processing, we can tune their mechanical, dielectric, semiconductive, and surface morphological properties.
We can easily affirm that the podium of this category goes to cellulose. The polysaccharide chain discovered in 183855 and omnipresent in our life is broadly used, in the form of nanofibers, for instance, for touch sensors56 or moisture sensors.48 Cellulose derived from bacteria is employed for piezoelectric sensors46 or in a acetylated version for OECT57 or electrochromic display.32 Cellulose nanocrystals coupled with agarose have been useful for hydrogel formation for ionic diodes,47 with nanocellulose and nano silicate, instead, as heat sensors.58 Following the (poly)saccharides family, a few molecules are strongly present in the literature: chitosan, alginate, chondroitin, and chitin; among them, chitosan is the most used up to now.
Closely related to cellulose, starch is a carbohydrate and a natural component widely present in plants, wearable electronics, and cutaneous electrodes has been reported.34,49
Chitosan is a linear polysaccharide similar to cellulose but with an amine functionality or an acetamide one. This polysaccharide has been coupled with PEDOT:PSS for making long-term bioelectronic devices59 or via electrospinning technique.60 Coupled with lignin for flexible humidity sensors61 or with polyaniline (PANI) for bioelectronic patch.62 An edible starch-chitosan-based device has been developed for wearable electronics34 or a self-healing and injectable hydrogel based on cellulose-chitosan.63 Chitosan has been found useful even with synthetic polymers like polyvinyl pyrrolidone (PVP) as biodegradable support for flexible devices64 and polyvinyl alcohol (PVA) for transient electronic devices.14 Together with graphene, chitosan, and a poly(glycidyl) formed an innovative skin-inspired tissue.80Alginate is derived from alginic acid and presents a biocompatible and biosourced polysaccharide. It has been used, together with its chemical modifications, in a wide number of applications and setups as bioelectronic implantable hydrogels66 or a silver polyacrylamidehydrogel,68 spacing from the 3D printing67 to self-heal materials.69 The reader interested in these chemical structures can find the complete information in the work of Teng et al.70
Chitin, despite the second most abundant polysaccharide in nature after cellulose, is scarce with respect to other biopolymers. Flexible micro structured as chitin methacrylate has been applied for electronics81 or coupled with silk.82 A complete book chapter including chitin and chitosan has been recently published by Pottathara et al.67
Silk represents one of the most used materials as support. Derived from a natural source, as extracted directly from the cocoon of Bombyx mori silkworm, it is a natural protein presenting great potential thanks to its easy biodegradability.83 Temperature sensors have been developed coupled with PEDOT:PSS31 and with clay for developing a green display.84 It has also been used as dielectric materials for implantable bioelectronics for the detection of epileptic seizure,41 a methacrylate version, in an all-biodegradable device with eumelanine,43 and, with melanin, using the electrospinning technique.42 Silk nanoribbons have also been obtained and used as support in conductive wires.85
Completing the biopolymer derived materials, it is worth citing gelatine and collagen as both are biodegradable and fully biosourced cocktail of molecules: peptides and proteins. In fact, depending on the provenience, lot, and post-treatments, its composition may vary. Usually, more than half of it is formed by glycine, proline, and hydroxyproline, but we can also find alanine, arginine, aspartic and/or glutamic acid. Recent advances in electronics have been reported using them as supporting materials for epidermic mechanical and thermal sensors,30,65,86 as well as in biomemristive devices.71 Cutaneously, a wound healing and motion sensing device has also been performed using gelatine coupled with PEDOT:PSS and carbon nanotubes. Together with PAA, an innovative organohydrogel has been presented, owning a super-fast degradation ability.29
On the other part, synthetic substrates provide excellent control for the efficiency and physiochemical properties of the material, as we can change or modulate the preparation according to our needs.
In this, the major synthetic biodegradable and biocompatible polymers are polyesters, thanks to their easy synthetic process and confirmed (bio)degradability. Different examples are present in the literature, many of which use biobased starting materials.
The first to cite is PLA, poly(lactic acid), is a thermoplastic aliphatic polyester derived from renewable resources, such as sugarcane, corn, or tapioca; even though it was discovered in 1920, it did not attract attention until the end of the past century. PLA has been coupled with carbon nanoallotropes such as graphene in an innovative coated fabric,20 or with carbon nanotubes in a biocompatible films.76 Organic photoelectronics, also exploited this material in OLEDs,19 and organic photovoltaics OPVs.21 Glucose biosensing devices based on PLA–platinum core were also studied and developed using quartz as substrate.22 Copolymers like poly(lactic acid-co-glycolic acid)39 and mixed with poly(caprolactone)26 have been also recently presented.
Poly(caprolactone) (PCL) is, as seen, an important building block for polyester, poly(caprolactone-co-1,8-octanediol-co-citric acid) has been presented by Chu et al. as an innovative polyester for wearable electronics.78 PCL has also been used together with polyurethanes in a fully degradable electronic device,27 and poly(caprolactone-co-trimethylene carbonate) has been employed for light-emitting electrochemical cells.38
Similar in chemical structure, poly(glycerol sebacate) has been suggested as elastic and stretchable support98 and poly(citrate-co-siloxane) as a biodegradable antibacterial elastic device for biomedical applications.99 Poly(hydroxybutyrate-co-lactic acidco-caprolactone) represents another innovative example of a bioderived malleable polyester presented by Yeo et al.79
Finally, polyimides/polyimines, even if not central, consist of an innovative part of the degradable materials for electronics. Diphthalic anhydride-based materials have been presented as biocompatible materials for soft electronics.37 A fluorinated version of the same material represents a fully degradable substrate in ethanol.35 A simpler polyamide network instead has been obtained from aromatic dialdehydes and diamines and applied as a recyclable and flexible membrane.36
An unusual example, to complete the view, is also represented by a polyanhydride:polybutanedithiol 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione pentenoic anhydride (PBTPA) is a degradable supporting material for transient electronics.100
As can be seen by the many examples and the variety that this part has, the supporting materials for electronics are in a healthy period; worth to add that almost the totality of them are really degradable and not disintegrable, and even if we have seen only a few examples of possible recycling,46,101 the molecules in which the materials end up are single block, thus, possibly, with a recycling/upcycling future.
Active materials
As explained in the previous section of this perspective, the conductive or semiconductive materials, the situation is further different from the supporting one, both in the number of examples and in the variety of them. The issue of a continuous conjugated perimeter, as a sp2 conjugation, implies a more difficult degradation, even with some exceptions.102 For ease, we can also divide these materials into naturally occurring and synthetic (Fig. 5).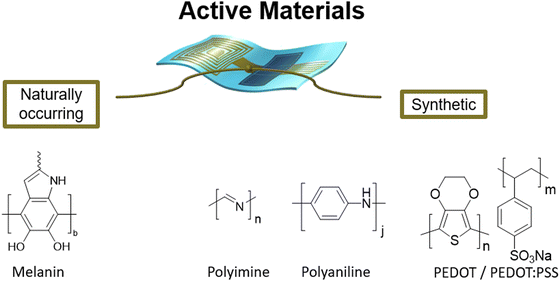 | ||
| Fig. 5 Bioderived active materials for electronics-Melanine,43,87 and synthetically derived active materials for electronics-PEDOT:PSS,31,32,34,48,56,57,59,60,72,75,88–94 polyaniline(PANI),62,95 polyimine,25,96 (the structure of melanin has been adapted97). | ||
As cited in the introduction, the golden standard of synthetic active materials is PEDOT:PSS, which is still one of the most used materials, although it is not presenting degradability but only disintegrability.
The blue suspension of poly(ethylene-3,4-dioxothiophene):poly(styrene sulfonate) has been utilized in combination with silver nanowires in the preparation of a self-heling wearable electronic93 or with chitosan for a piezoelectric device.60 Touch sensor,56 moisture sensor,48 metabolitesensors72,103 and temperature sensor31 have been created combining PEDOT:PSS with cellulose, cellulose nanofibers, or silk. Together with single wall carbon nanotubes (SCNTs) and graphene, Miao et al. presented a biodisintegrable wearable electronics material34 as well as with chitosan for a long term biosensing device.59 Ho et al. presented and demonstrated a disintegrable and recyclable device using PVA/PMMA and PEDOT:PSS.75 Fast degradability of a thermoelectric device has been demonstrated by combining it with cellulose acetate.92 Variation of PEDOT, without PSS but self-doped via a sulfonate group directly connected to the heterocyclic backbone, has been optimized for optoelectronic devices94 and for regenerative engineering.91
Electroactive disintegrable polymers are a rarity, although the literature is overloaded with terms, as biodegradable or recyclable, and is visible in the imbalance towards other types of easier chemical approaches as cited.
Imines disintegrable linkers are often used to glue active materials, such as naphthalene dianhydride/thiophenes25 or diketopyrrolopyrrole,27,104 and in both these works, the disintegration behavior is successfully presented in a day timescale. Thiophene rings have also been linked through this bond and polymeric chains have been obtained via Stille coupling.105 An interesting approach has been studied by coupling carotenoid derivatives and forming conjugated high-degradable polymeric materials.106
A complete discussion of degradable/disintegrable conjugated polymers can be found in the work of Tropp et al.96
Polyanilines derivates are also present in this family of disintegrable materials. Often are oligomeric structures embedded or copolymerized with a degradable polymeric macrostructure. Tetra-aniline, for example, has been used to cap or copolymerize polycaprolactone, obtaining a printable disintegrable device107 or an electrical responsive drug delivery system.108 The same strategy has been applied to hyaluronic acid109 and dextrane,110 leading to innovative hydrogel materials.
A rising star material and worthy representative in the class of the active naturally occurring is represented by melanin. This material is a naturally occurring pigment derived from dopamine and is present in many living organisms. This family of polymers presents a conjugated perimeter and is fully biodegradable. Extracted directly from the black pigment of squids and has been employed successfully as an active material of bioelectronics devices43,87 or as proton conductors.111 The reader who wants to explore this system can refer to the work of Paulin et al.112
Summary and outlook
From this perspective, we have covered the latest news about degradable electronics. The development of new materials, as well as complementary techniques, gave a fruitful environment for the advance and the progressive change of the non-degradable materials to fewer impacting ones. It is really visible in the way that today's society takes. In fact, the supporting materials have a wider variety with respect to the active ones. This, in our opinion, is due thanks to the ease that these materials have. The handling and the synthesis of these molecules, with respect to the active ones, are normally more interdisciplinary friendly. Biodegradable polymers could be bought and formulated without requiring sectorial chemistry skills. The impact on the final devices of this part is more visible and more sellable due to the higher weight that this part owns in the final device. The studies that can be done upon this moiety are, generally, easier in terms of recurses and machinery. This last concept derives a perceptible trend in literature in which thermomechanical analyses are scarce. In this respect, the community should orient to develop degradable conductive and semiconductive materials, fighting with organic chemistry and the instrumentation and skills that this includes. This is really visible browsing the literature, which shows that degradable and biodegradable plastic as polyesters seem to be fully integrated within the system and competitive. Biomolecules, such as cellulose or silk, are excellently employed, answering optimally to the need of the devices nowadays. Instead, for the active materials, the imine-derived disintegrable semiconductive polymers are the only ones redundant in the different works as synthetic polymers. Melanin, on the other hand, has been presented as a nice alternative but still not easily adapted to the need of different devices. Unfortunately, from this part, we are in a status quo and the commercial PEDOT:PSS still prevails. The community should take this into consideration, pushing an interdisciplinary dialog between the engineering, chemical, and biotechnology parts. Deeper studies of degradable conjugated organic molecules are needed and should be given as feedback by the device making and the final properties. The different branches of science should understand the issues of the other parts and adapt, bargain, and possibly haggle, instead of preferring to choose the easy and faster bottled polymers or known supporting formulations. We believe that the words disintegrable and degradable in electronics will be more and more employed and that the literature will turn positively in substituting today's materials into less impacting ones. In these cases, as in all our lives, thinking about the impact of our being is thinking about our future, in which the only things not at zero impact, should be our ideas.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. R. thanks SUINK project funded by the European Union's Horizon Europe research and innovation program under Grant Agreement No. 101070112. D. M. thanks Ayuda RYC2021-031668-I financiada por MCIN/AEI/10.13039/501100011033 y por la Unión Europea NextGenerationEU/PRTR.Notes and references
- E. Macarthur, Foundation; World Economic Forum; McKinsey&Co, Ellen Macarthur Found., 2016, 1–120 Search PubMed.
- M. Hong and E. Y. X. Chen, Green Chem., 2017, 19, 3692–3706 RSC.
- T. P. Haider, C. Völker, J. Kramm, K. Landfester and F. R. Wurm, Angew. Chem., Int. Ed., 2019, 58, 50–62 CrossRef CAS PubMed.
- I. del Agua, S. Marina, C. Pitsalidis, D. Mantione, M. Ferro, D. Iandolo, A. Sanchez-Sanchez, G. G. Malliaras, R. M. Owens and D. Mecerreyes, ACS Omega, 2018, 3, 7424–7431 CrossRef CAS PubMed.
- R. P. Magisetty, A. Surendren, N. S. Cheekuramelli and R. Aepuru, Biodegrad. Polym. Blends Compos., 2022, 551–571 Search PubMed.
- M. Bolognesi, M. Prosa and M. Seri, Sustain. Strateg. Org. Electron, 2022, 297–338 Search PubMed.
- S. Jadoun, U. Riaz and V. Budhiraja, Med. Devices Sensors, 2021, 4, e10141 CAS.
- L. Vallan, E. Istif, I. J. Gómez, N. Alegret and D. Mantione, Polymers, 2021, 13, 1977 CrossRef CAS PubMed.
- D. Mantione, I. del Agua, A. Sanchez-Sanchez and D. Mecerreyes, Polymers., 2017, 9, 354 CrossRef PubMed.
- in The IUPAC Compendium of Chemical Terminology, ed. V. Gold, International Union of Pure and Applied Chemistry (IUPAC), Research Triangle Park, NC, 2019 Search PubMed.
- M. Vert, Y. Doi, K.-H. Hellwich, M. Hess, P. Hodge, P. Kubisa, M. Rinaudo and F. Schué, Pure Appl. Chem., 2012, 84, 377–410 CrossRef CAS.
- V. R. Feig, H. Tran and Z. Bao, ACS Cent. Sci., 2018, 4, 337–348 CrossRef CAS PubMed.
- C. Vasile, Degradation and decomposition, Marcel Dekker Inc, 2000 Search PubMed.
- L. Liu, H. Liang, J. Zhang, P. Zhang, Q. Xu, Q. Lu and C. Zhang, J. Cleaner. Prod., 2018, 195, 786–795 CrossRef CAS.
- E. S. Hosseini, S. Dervin, P. Ganguly and R. Dahiya, ACS Appl. Bio Mater., 2021, 4, 163–194 CrossRef CAS PubMed.
- R. Jamshidi, Y. Chen and R. Montazami, Materials, 2020, 13, 23–28 Search PubMed.
- T. Eom, J. Jeon, S. Lee, K. Woo, J. E. Heo, D. C. Martin, J. J. Wie and B. S. Shim, Part. Part. Syst. Charact., 2019, 36(10), 1900166 CrossRef CAS.
- A. Hanif, G. Ghosh, M. Meeseepong, H. Haq Chouhdry, A. Bag, M. V. Chinnamani, S. Kumar, M. J. Sultan, A. Yadav and N. E. Lee, Micromachines, 2021, 12(9), 1036 CrossRef PubMed.
- C. T. Prontera, F. Villani, I. E. Palamà, M. G. Maglione, P. Manini, V. Maiorano and L. Tammaro, Polym. Adv. Technol., 2022, 33, 1523–1532 CrossRef CAS.
- M. Najafi, M. Zahid, L. Ceseracciu, M. Safarpour, A. Athanassiou and I. S. Bayer, J. Mater. Res. Technol., 2022, 18, 5197–5211 CrossRef CAS.
- M. K. Välimäki, L. I. Sokka, H. B. Peltola, S. S. Ihme, T. M. J. Rokkonen, T. J. Kurkela, J. T. Ollila, A. T. Korhonen and J. T. Hast, Int. J. Adv. Manuf. Technol., 2020, 111, 325–339 CrossRef.
- J. Y. Han, M. Li, H. Li, C. Li, J. Ye and B. Yang, Biosens. Bioelectron., 2020, 170, 112675 CrossRef CAS PubMed.
- Q. Li, S. Ma, S. Wang, Y. Liu, M. A. Taher, B. Wang, K. Huang, X. Xu, Y. Han and J. Zhu, Macromolecules, 2020, 53, 1474–1485 CrossRef CAS.
- G. Herwig, M. M. Perez-Madrigal and A. P. Dove, Biomacromolecules, 2021, 22, 1472–1483 CrossRef CAS PubMed.
- H. Park, Y. Kim, D. Kim, S. Lee, F. S. Kim and B. J. Kim, Adv. Funct. Mater., 2022, 32(2), 2106977 CrossRef CAS.
- M. A. U. Khalid, M. Ali, A. M. Soomro, S. W. Kim, H. B. Kim, B. G. Lee and K. H. Choi, Sens. Actuators, A, 2019, 294, 140–147 CrossRef.
- H. Tran, V. R. Feig, K. Liu, H. C. Wu, R. Chen, J. Xu, K. Deisseroth and Z. Bao, ACS Cent. Sci., 2019, 5, 1884–1891 CrossRef CAS PubMed.
- G. Ghosh, A. Bag, A. Hanif, M. Meeseepong, Y. R. Lee and N. E. Lee, Adv. Funct. Mater., 2022, 2209277, 1–13 Search PubMed.
- L. Fang, J. Zhang, W. Wang, Y. Zhang, F. Chen, J. Zhou, F. Chen, R. Li, X. Zhou and Z. Xie, ACS Appl. Mater. Interfaces, 2020, 12, 56393–56402 CrossRef CAS PubMed.
- X. P. Hao, C. W. Zhang, X. N. Zhang, L. X. Hou, J. Hu, M. D. Dickey, Q. Zheng and Z. L. Wu, Small, 2022, 18(23), 2201643 CrossRef CAS PubMed.
- S. Pradhan and V. K. Yadavalli, ACS Appl. Electron. Mater., 2021, 3, 21–29 CrossRef CAS.
- M. Pietsch, S. Schlisske, M. Held, N. Strobel, A. Wieczorek and G. Hernandez-Sosa, J. Mater. Chem. C, 2020, 8, 16716–16724 RSC.
- J. Lu, S. Hu, W. Li, X. Wang, X. Mo, X. Gong, H. Liu, W. Luo, W. Dong, C. Sima, Y. Wang, G. Yang, J. T. Luo, S. Jiang, Z. Shi and G. Zhang, ACS Nano, 2022, 16, 3744–3755 CrossRef CAS PubMed.
- J. Miao, H. Liu, Y. Li and X. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 23037–23047 CrossRef CAS PubMed.
- L. Chen, H. Yu, M. Dirican, D. Fang, Y. Tian, C. Yan, J. Xie, D. Jia, H. Liu, J. Wang, F. Tang, X. Zhang and J. Tao, Macromol. Rapid Commun., 2020, 41, 1–8 CrossRef.
- H. Xiong, S. Ling, Y. Li, F. Duan, H. Zhu, S. Lu and M. Du, J. Colloid Interface Sci., 2022, 608, 1126–1134 CrossRef CAS PubMed.
- G. Ghosh, M. Meeseepong, A. Bag, A. Hanif, M. V. Chinnamani, M. Beigtan, Y. Kim and N. E. Lee, Mater. Today, 2022, 57, 43–56 CrossRef CAS.
- J. Zimmermann, L. Porcarelli, T. Rödlmeier, A. Sanchez-Sanchez, D. Mecerreyes and G. Hernandez-Sosa, Adv. Funct. Mater., 2018, 28, 1–8 CrossRef.
- S. Selvarajan, A. Kim and S. H. Song, IEEE Access, 2020, 8, 68219–68225 Search PubMed.
- Y. Guo, L. Yang, L. Zhang, S. Chen, L. Sun, S. Gu and Z. You, Adv. Funct. Mater., 2021, 31, 1–7 Search PubMed.
- Y. Zhang, Z. Zhou, Z. Fan, S. Zhang, F. Zheng, K. Liu, Y. Zhang, Z. Shi, L. Chen, X. Li, Y. Mao, F. Wang, Y. L. Sun and T. H. Tao, Small, 2018, 14(35), 1802050 CrossRef PubMed.
- M. Nune, S. Manchineella, T. Govindaraju and K. S. Narayan, Mater. Sci. Eng., C, 2019, 94, 17–25 CrossRef CAS PubMed.
- Y. H. Youn, S. Pradhan, L. P. Da Silva, I. K. Kwon, S. C. Kundu, R. L. Reis, V. K. Yadavalli and V. M. Correlo, ACS Biomater. Sci. Eng., 2021, 7, 2466–2474 CrossRef CAS PubMed.
- C. Guo, C. Li and D. L. Kaplan, Biomacromolecules, 2020, 21, 1678–1686 CrossRef CAS PubMed.
- B. Liu, Y. Wei Song, L. Jin, Z. Jian Wang, D. Yong Pu, S. Qiang Lin, C. Zhou, H. Jian You, Y. Ma, J. Min Li, L. Yang, K. L. P. Sung and Y. Guang Zhang, Colloids Surf., B, 2015, 131, 122–128 CrossRef CAS PubMed.
- J. Lu, S. Hu, W. Li, X. Wang, X. Mo, X. Gong, H. Liu, W. Luo, W. Dong, C. Sima, Y. Wang, G. Yang, J. T. Luo, S. Jiang, Z. Shi and G. Zhang, ACS Nano, 2022, 16, 3744–3755 CrossRef CAS PubMed.
- K. Nyamayaro, P. Keyvani, F. D’Acierno, J. Poisson, Z. M. Hudson, C. A. Michal, J. D. W. Madden, S. G. Hatzikiriakos and P. Mehrkhodavandi, ACS Appl. Mater. Interfaces, 2020, 12, 52182–52191 CrossRef CAS PubMed.
- A. Rivadeneyra, A. Marín-Sánchez, B. Wicklein, J. F. Salmerón, E. Castillo, M. Bobinger and A. Salinas-Castillo, Compos. Sci. Technol., 2021, 208, 108738 CrossRef CAS.
- S. Wan, N. Wu, Y. Ye, S. Li, H. Huang, L. Chen, H. Bi and L. Sun, Small Struct., 2021, 2, 2100105 CrossRef CAS.
- D. W. Cockburn and N. M. Koropatkin, Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease, Elsevier B.V., 2016, vol. 428 Search PubMed.
- G. Ali, M. Sharma, E. S. Salama, Z. Ling and X. Li, Biomass Convers. Biorefinery, 2022 DOI:10.1007/s13399-022-02684-x.
- C. Jiang, Z. Liu, D. Cheng and X. Mao, Biotechnol. Adv., 2020, 45, 107641 CrossRef CAS PubMed.
- J. A. Chiong, H. Tran, Y. Lin, Y. Zheng and Z. Bao, Adv. Sci., 2021, 8, 2101233 CrossRef CAS PubMed.
- A. Uva, A. Lin, J. Babi and H. Tran, J. Chem. Technol. Biotechnol., 2022, 97, 801–809 CrossRef CAS.
- A. Payen, Comptes Rendus, 1838, 7, 1052–1056 Search PubMed.
- H. Ling, R. Chen, Q. Huang, F. Shen, Y. Wang and X. Wang, Green Chem., 2020, 22, 3208–3215 RSC.
- R. Granelli, I. Alessandri, P. Gkoupidenis, I. Vassalini, Z. M. Kovács-Vajna, P. W. M. Blom and F. Torricelli, Small, 2022, 18, 2108077 CrossRef CAS PubMed.
- F. B. Kadumudi, J. Trifol, M. Jahanshahi, T. G. Zsurzsan, M. Mehrali, E. Zeqiraj, H. Shaki, M. Alehosseini, C. Gundlach, Q. Li, M. Dong, M. Akbari, A. Knott, K. Almdal and A. Dolatshahi-Pirouz, ACS Appl. Mater. Interfaces, 2020, 12, 48027–48039 CrossRef CAS PubMed.
- Q. Pan, Q. Wu, Q. Sun, X. Zhou, L. Cheng, S. Zhang, Y. Yuan, Z. Zhang, J. Ma, Y. Zhang and B. Zhu, Sens. Actuators, B, 2022, 373, 132703 CrossRef CAS.
- L. Du, T. Li, F. Jin, Y. Wang, R. Li, J. Zheng, T. Wang and Z. Q. Feng, J. Colloid Interface Sci., 2020, 559, 65–75 CrossRef CAS PubMed.
- L. Wang, Z. Lou, K. Wang, S. Zhao, P. Yu, W. Wei, D. Wang, W. Han, K. Jiang and G. Shen, Research, 2020, 8716847 CAS.
- Y. Yan, L. Travaglini, K. Lau, J. Rnjak-Kovacina, D. Ta, M. Eslami, S. Yang, A. Lauto, D. L. Officer and D. Mawad, ACS Appl. Polym. Mater., 2021, 3, 2541–2552 CrossRef CAS.
- V. Panwar, A. Babu, A. Sharma, J. Thomas, V. Chopra, P. Malik, S. Rajput, M. Mittal, R. Guha, N. Chattopadhyay, D. Mandal and D. Ghosh, J. Mater. Chem. B, 2021, 9, 6260–6270 RSC.
- R. Kumar, S. Ranwa and G. Kumar, J. Phys. Chem. B, 2020, 124, 149–155 CrossRef CAS PubMed.
- M. Baumgartner, F. Hartmann, M. Drack, D. Preninger, D. Wirthl, R. Gerstmayr, L. Lehner, G. Mao, R. Pruckner, S. Demchyshyn, L. Reiter, M. Strobel, T. Stockinger, D. Schiller, S. Kimeswenger, F. Greibich, G. Buchberger, E. Bradt, S. Hild, S. Bauer and M. Kaltenbrunner, Nat. Mater., 2020, 19, 1102–1109 CrossRef CAS PubMed.
- Y. Choi, K. Park, H. Choi, D. Son and M. Shin, Polymers, 2021, 13(7), 1133 CrossRef CAS PubMed.
- Y. B. Pottathara, H. R. Tiyyagura, Z. Ahmad and S. Thomas, Handbook of Chitin and Chitosan, Elsevier, 2020, pp. 71–88 Search PubMed.
- Y. Ohm, C. Pan, M. J. Ford, X. Huang, J. Liao and C. Majidi, Nat. Electron., 2021, 4, 185–192 CrossRef CAS.
- S. Mallakpour, E. Azadi and C. M. Hussain, Adv. Colloid Interface Sci., 2021, 293, 102436 CrossRef CAS PubMed.
- K. Teng, Q. An, Y. Chen, Y. Zhang and Y. Zhao, ACS Biomater. Sci. Eng., 2021, 7, 1302–1337 CrossRef CAS PubMed.
- Y. Zeng, B. Sun, H.-Y. Yu, X. Wang, H. Peng, Y. Chen, S. Zhu, S. Mao and W. Hou, Mater. Today Chem., 2019, 13, 18–24 CrossRef CAS.
- Z. Jia, J. Gong, Y. Zeng, J. Ran, J. Liu, K. Wang, C. Xie, X. Lu and J. Wang, Adv. Funct. Mater., 2021, 31(19), 2010461 CrossRef CAS.
- Y. Liu, H. Wang and Y. Zhu, Adv. Electron. Mater., 2021, 7(9), 2100588 CrossRef CAS.
- X.-J. Zha, S.-T. Zhang, J.-H. Pu, X. Zhao, K. Ke, R.-Y. Bao, L. Bai, Z.-Y. Liu, M.-B. Yang and W. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 23514–23522 CrossRef CAS PubMed.
- J. C. Ho, Y. C. Lin, C. K. Chen, L. C. Hsu and W. C. Chen, Org. Electron., 2022, 100, 106358 CrossRef CAS.
- N. Vicentini, T. Gatti, M. Salerno, Y. S. Hernandez Gomez, M. Bellon, S. Gallio, C. Marega, F. Filippini and E. Menna, Mater. Chem. Phys., 2018, 214, 265–276 CrossRef CAS.
- M. Abdulrhman, A. Zhakeyev, C. M. Fernández-Posada, F. P. W. Melchels and J. Marques-Hueso, Flex. Print. Electron., 2022, 7, 025006 CrossRef.
- X. Chu, R. Wang, H. Zhao, M. Kuang, J. Yan, B. Wang, H. Ma, M. Cui and X. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 16631–16640 CrossRef CAS PubMed.
- J. C. C. Yeo, J. K. Muiruri, J. J. Koh, W. Thitsartarn, X. Zhang, J. Kong, T. T. Lin, Z. Li and C. He, Adv. Funct. Mater., 2020, 30(30), 2001565 CrossRef CAS.
- X. Luo, Y. Liu, R. Qin, F. Ao, X. Wang, H. Zhang, M. Yang and X. Liu, Appl. Mater. Today, 2022, 29, 101576 CrossRef.
- S. Pradhan, K. M. Moore, K. M. Ainslie and V. K. Yadavalli, J. Mater. Chem. B, 2019, 7, 5328–5335 RSC.
- M. Hong, G. Choi, J. Kim, J. Jang, B. Choi, J. Kim, S. Jeong, S. Leem, H. Kwon, H. Hwang, H. Im, J. Park, B. Bae and J. Jin, Adv. Funct. Mater., 2018, 28, 1705480 CrossRef.
- M. Zheng, X. Wang, O. Yue, M. Hou, H. Zhang, S. Beyer, A. M. Blocki, Q. Wang, G. Gong, X. Liu and J. Guo, Biomaterials, 2021, 276, 121026 CrossRef CAS PubMed.
- F. B. Kadumudi, M. Jahanshahi, M. Mehrali, T.-G. Zsurzsan, N. Taebnia, M. Hasany, S. Mohanty, A. Knott, B. Godau, M. Akbari and A. Dolatshahi-Pirouz, Adv. Sci., 2019, 6, 1801241 CrossRef PubMed.
- Q. Niu, X. Huang, S. Lv, X. Yao, S. Fan and Y. Zhang, J. Mater. Chem. A, 2020, 8, 25323–25335 RSC.
- X. Yang, L. Cao, J. Wang and L. Chen, ACS Sustainable Chem. Eng., 2020, 8, 10726–10739 CAS.
- E. Di Mauro, D. Rho and C. Santato, Nat. Commun., 2021, 12, 3167 CrossRef CAS PubMed.
- S. M. Kim, N. Kim, Y. Kim, M. S. Baik, M. Yoo, D. Kim, W. J. Lee, D. H. Kang, S. Kim, K. Lee and M. H. Yoon, NPG Asia Mater., 2018, 10, 255–265 CrossRef CAS.
- A. Fallahi, S. Mandla, T. Kerr-Phillip, J. Seo, R. O. Rodrigues, Y. A. Jodat, R. Samanipour, M. A. Hussain, C. K. Lee, H. Bae, A. Khademhosseini, J. Travas-Sejdic and S. R. Shin, ChemNanoMat, 2019, 5, 729–737 CrossRef CAS PubMed.
- L. Ferlauto, P. Vagni, A. Fanelli, E. G. Zollinger, K. Monsorno, R. C. Paolicelli and D. Ghezzi, Biomaterials, 2021, 274, 120889 CrossRef CAS PubMed.
- J. Tropp, A. S. Mehta, X. Ji, A. Surendran, R. Wu, E. A. Schafer, M. M. Reddy, S. P. Patel, A. J. Petty and J. Rivnay, Chem. Mater., 2023, 35, 41–50 CrossRef CAS.
- J. H. Song, J. Park, S. H. Kim and J. Kwak, ACS Appl. Mater. Interfaces, 2023, 15(2), 2852–2860 CrossRef CAS PubMed.
- R. Yeasmin, S. I. Han, L. T. Duy, B. Ahn and H. Seo, Chem. Eng. J., 2022, 455, 140543 CrossRef.
- J. Y. Kim, S. Nagamani, L. Liu, A. H. Elghazaly, N. Solin and O. Inganäs, Biomacromolecules, 2020, 21, 1214–1221 CrossRef CAS PubMed.
- Y. Kaykha and M. Rafizadeh, Polymer, 2019, 166, 138–147 CrossRef CAS.
- J. Tropp and J. Rivnay, J. Mater. Chem. C, 2021, 9, 13543–13556 RSC.
- M. d’Ischia, A. Napolitano, V. Ball, C.-T. Chen and M. J. Buehler, Acc. Chem. Res., 2014, 47, 3541–3550 CrossRef PubMed.
- M. Held, A. Pichler, J. Chabeda, N. Lam, P. Hindenberg, C. Romero-Nieto and G. Hernandez-Sosa, Adv. Sustain. Syst., 2022, 6(2), 2100035 CrossRef CAS.
- Y. Li, N. Li, J. Ge, Y. Xue, W. Niu, M. Chen, Y. Du, P. X. Ma and B. Lei, Biomaterials, 2019, 201, 68–76 CrossRef CAS PubMed.
- Y. S. Choi, J. Koo, Y. J. Lee, G. Lee, R. Avila, H. Ying, J. Reeder, L. Hambitzer, K. Im, J. Kim, K. Lee, J. Cheng, Y. Huang, S. Kang and J. A. Rogers, Adv. Funct. Mater., 2020, 30, 2000941 CrossRef CAS.
- M. Tavakoli, P. Alhais Lopes, A. Hajalilou, A. F. Silva, M. Reis Carneiro, J. Carvalheiro, J. Marques Pereira and A. T. de Almeida, Adv. Mater., 2022, 34(31), 2203266 CrossRef CAS PubMed.
- S. Tian, Q. Yue, C. Liu, M. Li, M. Yin, Y. Gao, F. Meng, B. Z. Tang and L. Luo, J. Am. Chem. Soc., 2021, 143, 10054–10058 CrossRef CAS PubMed.
- M. Xu, Y. Jiang, S. Pradhan and V. K. Yadavalli, Front. Mater., 2019, 6, 00331 CrossRef.
- J. A. Chiong, Y. Zheng, S. Zhang, G. Ma, Y. Wu, G. Ngaruka, Y. Lin, X. Gu and Z. Bao, J. Am. Chem. Soc., 2022, 144, 3717–3726 CrossRef CAS PubMed.
- H. Tran, S. Nikzad, J. A. Chiong, N. J. Schuster, A. E. Peña-Alcántara, V. R. Feig, Y.-Q. Zheng and Z. Bao, Chem. Mater., 2021, 33, 7465–7474 CrossRef CAS.
- A. Uva, A. Lin and H. Tran, J. Am. Chem. Soc., 2023, 145, 3606–3614 CrossRef CAS PubMed.
- A. Prasopthum, Z. Deng, I. M. Khan, Z. Yin, B. Guo and J. Yang, Biomater. Sci., 2020, 8, 4287–4298 RSC.
- J. G. Hardy, D. J. Mouser, N. Arroyo-Currás, S. Geissler, J. K. Chow, L. Nguy, J. M. Kim and C. E. Schmidt, J. Mater. Chem. B, 2014, 2, 6809–6822 RSC.
- J. Qu, X. Zhao, Y. Liang, Y. Xu, P. X. Ma and B. Guo, Chem. Eng. J., 2019, 362, 548–560 CrossRef CAS.
- B. Guo, J. Qu, X. Zhao and M. Zhang, Acta Biomater., 2019, 84, 180–193 CrossRef CAS PubMed.
- M. Jia, J. Kim, T. Nguyen, T. Duong and M. Rolandi, Biopolymers, 2021, 112(7), e23433 CrossRef CAS PubMed.
- J. V. Paulin and C. F. O. Graeff, J. Mater. Chem. C, 2021, 9, 14514–14531 RSC.
| This journal is © The Royal Society of Chemistry 2023 |