γ-P4S3I2: a new metal-free infrared second-order nonlinear optical crystal designed by polymorphism strategy†
Xin
Zhao
 ab,
Chensheng
Lin
ab,
Chensheng
Lin
 a,
Shunda
Yang
a,
Haotian
Tian
ab,
Chao
Wang
ab,
Tao
Yan
a,
Shunda
Yang
a,
Haotian
Tian
ab,
Chao
Wang
ab,
Tao
Yan
 *ad,
Jian
Zhang
*ad,
Jian
Zhang
 d,
Bingxuan
Li
a,
Ning
Ye
c and
Min
Luo
d,
Bingxuan
Li
a,
Ning
Ye
c and
Min
Luo
 *a
*a
aKey Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: lm8901@fjirsm.ac.cn; yantao@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cTianjin Key Laboratory of Functional Crystal Materials, Institute of Functional Crystal, Tianjin University of Technology, Tianjin 300384, China
dState Key Laboratory of Crystal Materials, Shandong University, Jinan, 250100, China
First published on 19th December 2022
Abstract
A non-centrosymmetric metal-free thiophosphate, γ-P4S3I2, was successfully synthesized utilizing polymorphism strategy in this study. γ-P4S3I2 crystallized in the space group of P43 and featured paralleled (P4S3I2)n molecular clusters. Importantly, it exhibited promising nonlinear optical (NLO) performances, such as a phase-matchable second harmonic generation (SHG) efficiency (0.5 × AgGaS2), wide band gap (2.38 eV), large birefringence (0.118@2050 nm), and wide infrared transparency, suggesting that γ-P4S3I2 may be a potential IR NLO candidate.
Introduction
Exploring non-centrosymmetric (NCS) inorganic compounds is of great significance in the development of piezoelectric, pyroelectric, ferroelectric, and especially nonlinear optical (NLO) materials.1–6 NLO materials are indispensable for applications in medical treatment, atmospheric detection and optical communication relying on frequency conversion.7,8 To date, many well-known NLO crystals, such as β-BaB2O4, LiB3O5 and CsLiB6O10 as well as KTiOPO4 and LiNbO3, have basically met the needs in the UV and visible (vis) regions.9–12 However, commercially available NLO crystals used in the infrared (IR) region are still inhibited by some intrinsic drawbacks, such as the poor laser damage threshold (LDT) of AgGaS2 and AgGaSe2 and the two-photon absorption (TPA) of ZnGeP2,13 which have restricted their further practical applications. Therefore, exploring new IR NLO crystals is still of current research interest.Generally, inorganic crystals possess a strict structurally non-centrosymmetric space group, which is the prerequisite for NLO materials. However, about 70% of inorganic compounds are crystallize in centrosymmetric space groups,14 which indicates that NCS compounds are not easily accessible. Thus, some strategies, including chemical substitution, mixing anions and salt-inclusion, have been proposed to obtain NCS compounds, which have resulted in plenty of IR NLO crystals being obtained, such as Li2ZnSiS4 (3.9 eV, 1.1 × AgGaS2), Pb4SeBr6 (2.62 eV, 1.3 × AgGaS2), Pb18O8Cl15I5 (2.82 eV, 1.05 × AgGaS2) and Li[LiCs2Cl][Ga3S6] (4.18 eV, 0.7 × AgGaS2).15–18 Recently, polymorphous modification has been regarded as an effective strategy for exploring new IR NLO crystals, which has been attributed to the following reasons: (i) polymorphism is a common phenomenon in crystalline materials; and (ii) the arrangement of the building units in the structure can be further optimized, which might enhance the SHG effect, for example, [Ga4Se11] C2-type supertetrahedra in α-BaGa4Se7 can be reconstituted to T2-type [Ga4Se10] supertetrahedra in β-BaGa4Se7 with a stronger SHG response.19 Until now, during the process of exploring new NLO polymorphism, most attention has been focused on borates and Pb-based compounds because the variable architectures of [BxOy] and the flexible coordination environment of the Pb2+ cation (2–10) were found to be favorable in forming polymorphs.20,21 Thus, many polymorphous borates and Pb-based compounds were reported, such as α-BaB4O5F4, α-BaBOF3, α-LiPbB9O15 and β-PbGa2S4.22–25 Moreover, using additives and adjusting temperatures were also helpful to obtain new polymorphs, for instance, β-Sc(IO3)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 can be obtained with Li2CO3 as an additive. Besides, β- and γ-BaGa4S7, β-BaGa4Se7 and β-BaGa2Se4
26 can be obtained with Li2CO3 as an additive. Besides, β- and γ-BaGa4S7, β-BaGa4Se7 and β-BaGa2Se4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27,28 can be synthesized at differing appropriate temperatures. In this study, we proposed thiophosphates as a new system to explore NLO polymorphism.
27,28 can be synthesized at differing appropriate temperatures. In this study, we proposed thiophosphates as a new system to explore NLO polymorphism.
Thiophosphates are a very promising system for mid-IR NLO applications because the existence of strong covalent P–S bonds not only improves the NLO response but can also drive the blue shift of the short-wave absorption edge;29 for example, Hg3P2S8 (2.72 eV, 4.2 × AgGaS2), Eu2P2S6 (2.54 eV, 0.9 × AgGaS2), RbBiP2S6 (2.10 eV, 11.9 × AgGaS2) and CuHgPS4 (2.03 eV, 6.5 × AgGaS2) exhibit good IR-NLO performances.30–33 In addition, positive valence P atoms have a flexible coordination environment (2–4), which is favorable in forming polymorphs. For example, the polymorphism of P2S7 (α: P21/c, β: P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) )34 exhibits two different crystal structures. Moreover, until now, there have been no studies on the NLO properties of metal-free thiophosphates, and thus, we have a strong interest in studying the title compound. Therefore, in this work, we have focused on the P–S–I system and a new non-centrosymmetric polymorph of P4S3I2 was discovered and named γ-P4S3I2, with the previously reported phases named as α-P4S3I2 (P
)34 exhibits two different crystal structures. Moreover, until now, there have been no studies on the NLO properties of metal-free thiophosphates, and thus, we have a strong interest in studying the title compound. Therefore, in this work, we have focused on the P–S–I system and a new non-centrosymmetric polymorph of P4S3I2 was discovered and named γ-P4S3I2, with the previously reported phases named as α-P4S3I2 (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and β-P4S3I2 (Pnma).35,36 γ-P4S3I2 was successfully synthesized via solution evaporation at low temperature, and its linear and nonlinear optical properties were studied experimentally and theoretically. Results show that this compound exhibits a suitable SHG response (0.5 × AgGaS2), wide transparency window, and large birefringence (0.118@2050 nm), indicating that γ-P4S3I2 is a promising mid-IR NLO candidate.
) and β-P4S3I2 (Pnma).35,36 γ-P4S3I2 was successfully synthesized via solution evaporation at low temperature, and its linear and nonlinear optical properties were studied experimentally and theoretically. Results show that this compound exhibits a suitable SHG response (0.5 × AgGaS2), wide transparency window, and large birefringence (0.118@2050 nm), indicating that γ-P4S3I2 is a promising mid-IR NLO candidate.
Experimental section
Synthesis and crystal growth
All of the chemicals, carbon disulfide CS2 (99.9%), S (99.99%), P (99.99%) and I2 (99.99%), were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd and used without further purification. The synthesis of γ-P4S3I2 was operated in two steps. First, P4S3 was prepared by heating a stoichiometric mixture of P and S in an evacuated silica tube at 300 °C for 24 h. Second, P4S3 and I2 were evenly mixed with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and loaded into an evacuated silica tube and then sealed under vacuum. After that, the mixture was heated to 180 °C in a drying oven, held for about 2 days, and then cooled to room temperature naturally, whereupon molten P4S3I2 was obtained. γ-P4S3I2 was prepared by recrystallizing amorphous P4S3I2 in CS2 at −10 °C. The millimeter-sized crystal of γ-P4S3I2 was grown directly by an evaporation method. The recrystallized γ-P4S3I2 (1.5 g) was dissolved in dried CS2 (12 ml) in a round bottom centrifuge tube with a cover, and then the centrifuge tube was put into a refrigerator at −30 °C and held for about 5 days; the products were pure deep yellow block-shaped single crystals without byproducts.
1 and loaded into an evacuated silica tube and then sealed under vacuum. After that, the mixture was heated to 180 °C in a drying oven, held for about 2 days, and then cooled to room temperature naturally, whereupon molten P4S3I2 was obtained. γ-P4S3I2 was prepared by recrystallizing amorphous P4S3I2 in CS2 at −10 °C. The millimeter-sized crystal of γ-P4S3I2 was grown directly by an evaporation method. The recrystallized γ-P4S3I2 (1.5 g) was dissolved in dried CS2 (12 ml) in a round bottom centrifuge tube with a cover, and then the centrifuge tube was put into a refrigerator at −30 °C and held for about 5 days; the products were pure deep yellow block-shaped single crystals without byproducts.
Single crystal structure determination
Single crystal X-ray diffraction data for γ-P4S3I2 were collected on a Rigaku Mercury CCD diffractometer with graphite-monochromatic Mo Kα radiation (λ = 0.71073 Å) at room temperature. Some γ-P4S3I2 crystals were cut into appropriate sizes on a slide; for measurements, a high-quality single crystal was selected and mounted on top of a glass fiber using epoxy. The intensity data were corrected using a narrow-frame method in ω-scan mode. All data were integrated based on the CrystalClear program. The intensities were corrected for Lorentz polarization, air absorption, and absorption attributable to variation in the path length through the detector faceplate. Absorption corrections were also achieved by using a multi-scan technique. The crystal structures were determined by means of the direct methods and refined by full-matrix least-squares fitting on F2 using SHELXL.37,38 All the atoms were refined with anisotropic displacement parameters. The ADDSYM algorithm from the PLATON program39 was used to monitor the correctness of the structures and no higher symmetry was found. Relevant crystallographic data and details of the experimental conditions are listed in Table S1.† Besides, the data of atomic coordinates and equivalent isotropic displacement parameters, selected bond lengths and angles, and anisotropic displacement parameters are summarized in Tables S2–S4.†Powder X-ray diffraction
The powder X-ray diffraction analysis of compound γ-P4S3I2 was implemented on a Miniflex-600 diffractometer with Cu Kα radiation (λ = 1.540598 Å) at room temperature. The angular range was 2θ = 10–70° with a scan step width of 0.02° and a fixed time of 0.2 s. The powder XRD pattern of polycrystalline materials matched with the calculated XRD pattern from the single crystal model (Fig. S1†), indicating that pure samples could be used for subsequent measurements.Energy dispersive X-ray spectroscopy (EDS) analysis
The EDS analysis of γ-P4S3I2 crystals was carried out on a field emission scanning electron microscope (FESEM, SU-8010) equipped with an energy dispersive X-ray spectrometer. The shaped crystals were rinsed using carbon disulfide and absolute ethyl alcohol, and then were affixed on the copper sample stage with a carbon conductive tape. Different regions on the crystals were tested with a focused beam, accelerating voltage of 20 kV and emission current of 12 μA (Fig. S2†).Thermal analysis
The thermogravimetric (TG) analysis of γ-P4S3I2 was conducted on a Netzsch STA449F3 simultaneous analyzer. The reference (Al2O3) and crystal samples (5–10 mg) were enclosed in an Al2O3 crucible, heated from 30 to 1000 °C at a rate of 10 °C min−1 under a constant flow of nitrogen gas, and then cooled to room temperature naturally.UV-vis-NIR diffuse reflectance spectroscopy
The UV-vis-NIR diffuse reflection data of γ-P4S3I2 were collected on a PerkinElmer Lamda-950 ultraviolet/visible/near-infrared spectrophotometer at room temperature in the range of 400–800 nm with BaSO4 as the standard of 100% reflectance. The reflectance values were converted to absorbance based on the Kubelka–Munk function F(R) = (1 − R)2/(2R) = K/S, where R is the reflectance, K is the absorption, and S is the scattering.40,41Fourier-transform infrared (FT-IR) spectroscopy
The Fourier-transform infrared (FT-IR) transmittance spectra were measured on a Bruker VERTEX 70 FT-IR spectrophotometer in the range of 4000–400 cm−1. Dry KBr was ground into fine powder and then pressed into a transparent wafer as the reference. The powder sample and dry KBr were mixed and pressed into the same wafer for the measurements.Powder SHG and LDT measurements
Polycrystalline SHG responses were measured using the Kurtz–Perry method42 with a Q-switched Nd:YAG solid-state laser at a laser radiation wavelength of 2050 nm. Polycrystalline γ-P4S3I2 was ground and sieved into several different particle size ranges of 25–45, 45–63, 63–74, 74–106, 106–150, and 150–210 μm. Polycrystalline AgGaS2 was also ground and sieved into the same particle size ranges as the reference. After that, samples were added into aluminous holders and pressed into 1 mm thick slides between two glass sheets bound with a 2 mm thick rubber ring, containing an 8 mm diameter hole in the center. Subsequently, the samples were then placed in a light-tight box and under the irradiation of a pulsed laser. The intensities of the SHG signals were measured with a photomultiplier tube attached to a RIGOL DS1052E 50 MHz oscilloscope. As a result, the ratio of the intensity of the SHG signals between the samples and the reference can eventually be calculated. The laser damage threshold (LDT) measurement was executed at room temperature using microcrystal samples of γ-P4S3I2 and AgGaS2 samples of similar sizes (150–212 μm) under a pulsed Nd:YAG laser (1064 nm, 1 Hz, 10 ns). The LDT was evaluated through increasing the energy of the laser gradually until the damage spot was observed. The value of the LDT can be calculated based on eqn (1):| I(threshold) = E/(πr2t) | (1) |
Computational methods
The first-principles calculations for the physical properties of γ-P4S3I2 were performed by using CASTEP,43 a plane-wave pseudopotential total energy package based on density functional theory (DFT).44 The exchange and correlative potential of electron–electron interactions were represented by generalized gradient approximation (GGA) in the scheme of Perdew–Burke–Ernzerhof (PBE).45 Furthermore, the interaction of the electrons with ion cores was represented by the norm-conserving pseudopotentials, and the valence electrons were expressed as P: 3s2 3p3, S: 3s2 3p3 and I: 5s2 5p5. The k-point of the first Brillouin zone for γ-P4S3I2 was sampled as the 2 × 2 × 1 Monkhorst–Pack scheme,46 which was used to calculate the optical properties and density of states (DOS). The cut-off energy was set to be 650 eV and the self-consistent convergence of the total energy was 1.0 × 10−5 eV per atom. The scissor operation was adopted in the dielectric function calculation owing to the inherent underestimation of the band gap by the DFT method. In addition, the “velocity-gauge”47,48 formula was employed to calculate the SHG coefficients of γ-P4S3I2, and the SHG density of d33 was calculated by the band-resolved method.49Results and discussion
Structural description and comparison
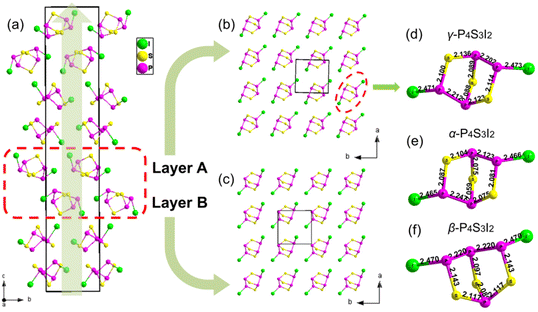
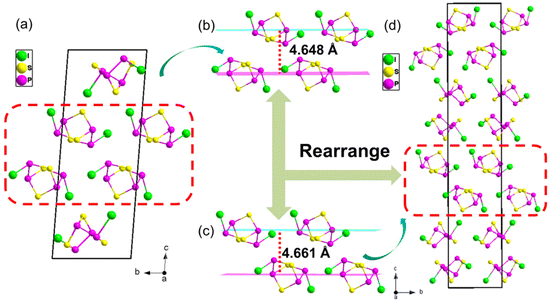
γ-P4S3I2 crystallized in the tetragonal crystal system with an asymmetric space group of P43 (no. 78), with a = 7.3415(2) Å, b = 7.3415(2) Å, and c = 39.1942(19) Å (crystallographic details are shown in Tables S1–S4†). As shown in Fig. 1a, the basic building unit (BBU) of γ-P4S3I2 was a unique molecular cluster (P4S3I2)n (n = 4) constructed from P4S3I2 molecules with two-dimensional (2D) layers (layer A and layer B). The BBUs in γ-P4S3I2 were arranged along the c direction with C4 symmetry and the 43 screw axis is paralleled to the c-axis. In addition, layers A and B in the (P4S3I2)n molecular cluster were interconnected through weak van der Waals interactions between the two layers, and both layers were constructed by using the paralleled P4S3I2 molecules in the a–b plane, where adjacent P4S3I2 molecules are connected with each other also via van der Waals interactions (Fig. 1b and c). The boat-like P4S3I2 molecule in γ-P4S3I2 contained four P atoms, three S atoms and two I atoms with the I–P bonds ranging from 2.471(3) to 2.480(3) Å, the P–S bonds ranging from 2.082(5) to 2.136(5) Å and the P–P bonds ranging from 2.202(5) to 2.212(5) Å, which are comparable to I–P bonds, P–S bonds and P–P bonds in α-P4S3I2 and β-P4S3I2.35,36 Moreover, the structural feature of the boat-like P4S3I2 molecules in γ-P4S3I2 was similar to that in α-P4S3I2, yet quite different from that in β-P4S3I2 (Fig. 1d, c and e). The P4S3I2 molecules in γ-P4S3I2 and α-P4S3I2 both showed C2 local symmetry, while the molecules in β-P4S3I2 exhibited Cs local symmetry.To better understand the structure of γ-P4S3I2, α-P4S3I2 was chosen to compare with γ-P4S3I2 (Fig. 2) because their P4S3I2 molecules have the same local symmetry. The structural evolution of γ-P4S3I2 could be regarded as that where P4S3I2 molecules in α-P4S3I2 (Fig. 2a) were partially picked out and regularly rearranged according to a new symmetry, leading to γ-P4S3I2, which crystallizes in another space group (P43). However, as shown in Fig. 2b and c, the distance between the two layers in the (P4S3I2)n (n = 4) molecular cluster of γ-P4S3I2 (4.661 Å) is greater than that of α-P4S3I2 (4.648 Å). In addition, the literature values of the van der Waals radii for these atoms are 2.15 Å for I and 1.90 Å for P, which give a sum of 4.05 Å, while the experimental I⋯P distance in the (P4S3I2)n (n = 4) molecular cluster of γ-P4S3I2 (3.82 Å) and α-P4S3I2 (3.87 Å) was considerably less than the sum of their van der Waals radii (Fig. S3†), indicating the existence of intermolecular van der Waals interactions between their P4S3I2 molecules.
Thermal analysis
Thermogravimetric (TG) and differential thermal analysis (DTA) curves revealed that γ-P4S3I2 was decomposed at 120–300 °C with an exothermic peak at 120 °C (Fig. S5†), indicating that this compound was incongruent. Solid γ-P4S3I2 was transformed into liquid amorphous P4S3I2 at 120 °C and began to decompose, and γ-P4S3I2 can be obtained by recrystallizing the liquid amorphous P4S3I2 in CS2 (Fig. S4†). γ-P4S3I2 exhibited considerable thermal stability compared to some metal halide IR NLO crystals, such as HgBr2 (100 °C), Cs2Hg3I8–H2O (110 °C) and RbHgI3 (120 °C).50–53Optical properties
The optical band gap of γ-P4S3I2 was determined to be 2.38 eV (Fig. S6†), which is consistent with the deep yellow colour of its crystals. The band gap of γ-P4S3I2 is larger than those of some IR NLO thiophosphates, such as SnPS3 (2.35 eV), RbBiP2S6 (2.10 eV), CuHgPS4 (2.03 eV) and AgHg3PS6 (1.85 eV).32,33,54,55 Powder laser damage threshold (LDT) measurements suggested that the LDT of γ-P4S3I2 was 11.97 MW cm−2, which was about 2.8 times that of AgGaS2 (4.25 MW cm−2) under the same conditions (Table S5†). In addition, no obvious absorption from 3 to 20 μm was observed in the IR transmittance spectra of γ-P4S3I2 (Fig. S7†), indicating that this compound could potentially be applied in the IR region.NLO properties
According to the anionic group theory, the orientated arrangement of NLO-active structural units is beneficial for achieving a large SHG reponse.56 Thus, γ-P4S3I2 may be expected to exhibit suitable SHG responses because the (P4S3I2)n (n = 4) molecular clusters were arranged in parallel along the same direction (Fig. 1a). The size-dependent SHG measurements of the polycrystalline samples of γ-P4S3I2 and standard AgGaS2 were performed using a 2.05 μm laser. The intensities of the SHG signals were gradually increased along with an increase of particle sizes (Fig. 3b), indicating that the required type-I phase-matching behavior could be realized in γ-P4S3I2. Furthermore, the SHG response of γ-P4S3I2 was about 0.5 times that of AGS within the same particle size range of 150–210 μm, which was considerable among some reported IR NLO chalcogenides, such as SnI4·S8 (0.5 × AgGaS2), CH3I·S8 (0.7 × AgGaS2), LiGa2PS6 (0.5 × AgGaS2), Rb2GaP2S9 (0.1 × AgGaS2) and AgHg3PS6 (0.5 × AgGaS2).55,57–59 These results demonstrated that γ-P4S3I2 had great potential as an IR NLO candidate.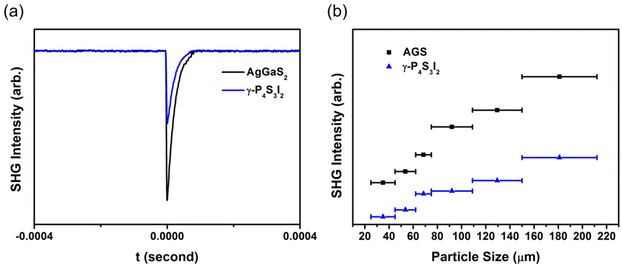 | ||
| Fig. 3 Measured SHG signals of γ-P4S3I2 and AgGaS2 with a 150–210 μm particle size (a). Measured SHG intensities of γ-P4S3I2 and AgGaS2 under 2.05 μm laser irradiation at room temperature (b). | ||
Theoretical calculations
To further understand the electronic structure and optical properties, systematic theory calculations for γ-P4S3I2 were performed based on density functional theory (DFT). The calculated band structure suggests that the direct band gap of γ-P4S3I2 was 2.123 eV as both the top of the valence band (VB) and the bottom of the conduction band (CB) were located at the X point (Fig. S8†). As can be seen in the partial density of state (PDOS) curves of γ-P4S3I2 (Fig. S9†), the top of the valence band was dominated by I 5p, P 3p and S 3p orbitals, and the bottom of the conduction band was also occupied by I 5p, P 3p and S 3p orbitals, indicating that the linear optical properties of γ-P4S3I2 were determined by the P4S3I2 molecules.The space group P43 of γ-P4S3I2 belonged to point group 4. According to the Kleinman symmetry, two independent nonzero SHG tensors, d31 and d33 remained (Fig. S10†). The largest tensor d33 value at 2.05 μm in γ-P4S3I2 was 9.60 pm V−1, which was about 0.63 times that of AgGaS2 (15.3 pm V−1), matching with the corresponding measured results. In addition, to clearly illustrate the origin of the contribution of the SHG effect in γ-P4S3I2, the SHG-weighted densities of the largest tensor d33 were calculated. As shown in Fig. 4, the I atoms, S atoms, P atoms and P–P bonds in the P4S3I2 molecule contributed to both occupied and unoccupied electronic states, which confirmed that the (P4S3I2)n molecular clusters determined the SHG response.
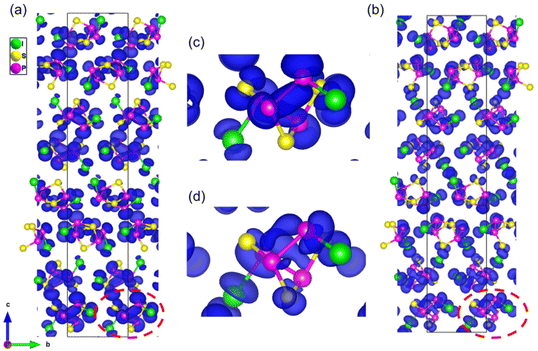 | ||
| Fig. 4 The SHG-weighted densities for occupied (a, c) and unoccupied (b, d) electronic states in γ-P4S3I2. | ||
Additionally, the calculated dispersion of the refractive indices curves of γ-P4S3I2 is displayed in Fig. S11.† The results were nc < na and the birefringence (nc − na) of γ-P4S3I2 was calculated to be 0.118 at 2050 nm, which contributed to the type-I phase-matching behavior over a wide IR region and matched the experimental results. These results indicated that the P4S3I2 molecules may be potential fundamental building units for birefringence materials.
Conclusions
In summary, a non-centrosymmetric metal-free thiophosphate, γ-P4S3I2, was successfully synthesized and investigated for NLO materials based on polymorphism strategy. The crystal structure of γ-P4S3I2 featured paralleled (P4S3I2)n molecular clusters connecting with each other through van der Waals interactions. γ-P4S3I2 also showed excellent nonlinear optical performances including a suitable SHG response (0.5 × AgGaS2), wide band gap (2.38 eV), large birefringence (0.118@2050 nm), and wide infrared transparency. More importantly, the polymorphism strategy in this work may provide new thoughts for exploring new NLO materials with enhanced properties.Author contributions
Xin Zhao performed the experiments, data analysis, and paper writing; Shunda Yang and Chao Wang offered help in synthesizing the compounds; Tao Yan and Jian Zhang offered help in analyzing the experimental data; Chensheng Lin and Haotian Tian performed the theoretical calculations; Bingxuan Li performed the powder laser damage threshold (LDT) measurements; Min Luo revised the manuscript. Ning Ye and Min Luo guided and supervised the experiments. All authors contributed to the general discussion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22222510, 21975255 and 21921001), Natural Science Foundation of Fujian Province (2021J01514), the Foundation of Fujian Science & Technology Innovation Laboratory (2021ZR202), Youth Innovation Promotion Association CAS (2019303) and Open Project of State Key Laboratory of Crystal Materials, Shandong University (KF1907).References
- C. J. Cui, F. Xue, W. J. Hu and L. J. Li, Two-dimensional Materials with Piezoelectric and Ferroelectric Functionalities, npj 2D Mater. Appl., 2018, 2, 1–14 CrossRef.
- L. Gao, J. B. Huang, S. R. Guo, Z. H. Yang and S. L. Pan, Structure-property Survey and Computer-assisted Screening of Mid-infrared Nonlinear Optical Chalcohalides, Coord. Chem. Rev., 2020, 421, 213379 CrossRef CAS.
- Z. X. Chen, W. L. Liu and S. P. Guo, A Review of Structures and Physical Properties of Rare Earth Chalcophosphates, Coord. Chem. Rev., 2023, 474, 214870 CrossRef CAS.
- W. F. Zhou, B. X. Li, W. L. Liu and S. P. Guo, AAg(2)PS(4) (A = K, Na/K): The First-type of Non-centrosymmetric Alkali Metal Ag-based Thiophosphates Exhibiting Excellent Second-order Nonlinear Optical Performances, Inorg. Chem. Front., 2022, 9, 4990–4998 RSC.
- Q. Wu, C. Yang, X. Liu, J. Ma, F. Liang and Y. S. Du, Dimensionality Reduction Made High-performance Mid-Infrared Nonlinear Halide Crystal, Mater. Today Phys., 2021, 21, 100569 CrossRef CAS.
- F. Xu, G. Zhang, M. Luo, G. Peng, Y. Chen, T. Yan and N. Ye, A Powder Method for The High-efficacy Evaluation of Electro-optic Crystals, Natl. Sci. Rev., 2020, 8, nwaa104 CrossRef PubMed.
- B. J. Guo, Y. Wang, C. Peng, H. L. Zhang, G. P. Luo, H. Q. Le, C. Gmachl, D. L. Sivco, M. L. Peabody and A. Y. Cho, Laser-based Mid-infrared Reflectance Imaging of Biological Tissues, Opt. Express, 2004, 12, 208–219 CrossRef PubMed.
- L. K. Cheng, W. R. Bosenberg and C. L. Tang, Growth and Characterization of Nonlinear Optical-crystals Suitable for Frequency-conversion, Prog. Cryst. Growth Charact. Mater., 1990, 20, 9–57 CrossRef CAS.
- C. T. Chen, Y. C. Wu, A. D. Jiang, B. C. Wu, G. M. You, R. K. Li and S. J. Lin, New Nonlinear-optical Crystal-LiB3O5, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef CAS.
- T. Sasaki, Y. Mori, I. Kuroda, S. Nakajima, K. Yamaguchi, S. Watanabe and S. Nakai, Cesium Lithium Borate-a New Nonlinear-optical Crystal, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1995, 51, 2222–2224 CrossRef.
- J. D. Bierlein and H. Vanherzeele, Potassium Titanyl Phosphate and New Applications, J. Opt. Soc. Am. B, 1989, 6, 622–633 CrossRef CAS.
- G. D. Boyd, K. Nassau, R. C. Miller, W. L. Bond and A. Savage, LiB3O6-efficient Phase Matchable Nonlinear Optical Material, Appl. Phys. Lett., 1964, 5, 234–236 CrossRef CAS.
- D. N. Nikogosyan, Nonlinear Optical Crystals: a Complete Survey, Springer-Science and Business Media, New York, 2005 Search PubMed.
- Y. C. Liu, Y. Q. Li, Y. Zhou, Q. R. Ding, Y. X. Chen, S. G. Zhao and J. H. Luo, A New Nonlinear Optical Sulfate of Layered Structure: Cs2Zn2(SO4)3, Inorg. Chem. Commun., 2021, 124, 108390 CrossRef CAS.
- G. M. Li, Y. Chu and Z. X. Zhou, From AgGaS2 to Li2ZnSiS4: Realizing Impressive High Laser Damage Threshold Together with Large Second-harmonic Generation Response, Chem. Mater., 2018, 30, 602–606 CrossRef CAS.
- J. Wang, H. Wu, H. Yu, Z. Hu, J. Wang and Y. Wu, Pb4SeBr6: A Congruently Melting Mid-infrared Nonlinear Optical Material with Excellent Comprehensive Performance, Adv. Opt. Mater., 2022, 10, 2102673 CrossRef CAS.
- X. Chen, Q. Jing and K. M. Ok, Pb18O8Cl15I5: A Polar Iead Mixed Oxyhalide with Unprecedented Architecture and Excellent Infrared Nonlinear Optical Properties, Angew. Chem., Int. Ed., 2020, 59, 20323–20327 CrossRef CAS PubMed.
- B. W. Liu, X. M. Jiang, B. X. Li, H. Y. Zeng and G. C. Guo, Li[LiCs2Cl][Ga3S6] : A Nanoporous Framework of GaS4 Tetrahedra with Excellent Nonlinear Optical Performance, Angew. Chem., Int. Ed., 2020, 59, 4856–4859 CrossRef CAS PubMed.
- Z. Qian, Q. Bian, H. P. Wu, H. W. Yu, Z. S. Lin, Z. G. Hu, J. Y. Wang and Y. C. Wu, β-BaGa4Se7: A Promising IR Nonlinear Optical Crystal Designed by Predictable Structural Rearrangement, J. Mater. Chem. C, 2021, 10, 96–101 RSC.
- S. C. Wang, N. Ye, W. Li and D. Zhao, Alkaline Beryllium Borate NaBeB3O6 and ABe2B3O7 (A = K, Rb) as UV Nonlinear Optical Crystals, J. Am. Chem. Soc., 2010, 132, 8779–8786 CrossRef CAS PubMed.
- C. M. Huang, F. F. Zhang, S. C. Cheng, Z. H. Yang and S. L. Pan, α-, β-Pb4B2O7 and α-, β-Pb4B6O13: Polymorphism Drives Changes in Structure and Performance, Sci. China Mater., 2020, 63, 806–815 CrossRef CAS.
- W. B. Zhang, S. R. Guo, S. J. Han, L. Y. Wang, X. Zhou, Z. H. Yang and S. L. Pan, BaB4O5F4 with Reversible Phase Transition Featuring Unprecedented Fundamental Building Blocks of B16O21F16 in the α-phase and B4O6F4 in the β-phase, Chem. Commun., 2021, 57, 4182–4185 RSC.
- K. T. Liu, J. Han, T. Baiheti, F. M. Li, Z. L. Wei, Z. H. Yang, M. Mutailipu and S. L. Pan, Finding a Series of BaBOF3 Fluorooxoborate Polymorphs with Tunable Symmetries: A Simple but Flexible Case, Chem. Mater., 2021, 33, 7905–7913 CrossRef CAS.
- K. T. Liu, J. Han, F. M. Li, S. J. Han, Z. H. Yang, X. P. Wang and S. L. Pan, α-LiMB9O15 (M = Sr, Pb): Flexible B3O7 Units Leading to the Low Temperature Phase of β-LiMB9O15 (M = Sr, Pb), Inorg. Chem. Front., 2022, 9, 5371–5376 RSC.
- W. F. Chen, B. W. Liu, X. M. Jiang and G. C. Guo, Infrared Nonlinear Optical Performances of a New Sulfide β-PbGa2S4, J. Alloys Compd., 2022, 905, 164090 CrossRef CAS.
- C. Wu, G. F. Wei, X. X. Jiang, Q. K. Xu, Z. S. Lin, Z. P. Huang, M. G. Humphrey and C. Zhang, Additive-Triggered Polar Polymorph Formation: β-Sc(IO3)3, a Promising Next-Generation Mid-Infrared Nonlinear Optical Material, Angew. Chem., Int. Ed., 2022, 61, e202208514 CAS.
- Z. Qian, H. N. Liu, Y. J. Zhang, H. P. Wu, Z. G. Hu, J. Y. Wang, Y. C. Wu and H. W. Yu, The Exploration of New Infrared Nonlinear Optical Crystals Based on the Polymorphism of BaGa4S7, Inorg. Chem. Front., 2022, 9, 4632–4641 RSC.
- Y. J. Zhang, Q. Bian, H. P. Wu, H. W. Yu, Z. G. Hu, J. Y. Wang and Y. C. Wu, Designing a New Infrared Nonlinear Optical Material, β-BaGa2Se4 Inspired by the Phase Transition of the BaB2O4 (BBO) Crystal, Angew. Chem., Int. Ed., 2022, 61, e202115374 CAS.
- Z. Li, J. Y. Yao and Y. C. Wu, Chalcophosphates: A Treasure House of Infrared Nonlinear Optical Materials, Cryst. Growth Des., 2020, 20, 7550–7564 CrossRef CAS.
- W. H. Xing, F. Liang, C. L. Tang, E. Uykur, Z. S. Lin, J. Y. Yao, W. L. Yin and B. Kang, Highly Distorted HgS4 Motif-Driven Structural Symmetry Degradation and Strengthened Second-Harmonic Generation Response in the Defect Diamond-Like Chalcogenide Hg3P2S8, ACS Appl. Mater. Interfaces, 2021, 13, 37321–37328 Search PubMed.
- X. Huang, S. H. Yang, X. H. Li, W. L. Liu and S. P. Guo, Eu2P2S6: The First Rare-Earth Chalcogenophosphate Exhibiting Large Second-Harmonic Generation Response and High Laser-Induced Damage Threshold, Angew. Chem., Int. Ed., 2022, 61, e202206791 CAS.
- M. M. Chen, S. H. Zhou, W. B. Wei, M. Y. Ran, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, RbBiP2S6: A Promising IR Nonlinear Optical Material with a Giant Second-Harmonic Generation Response Designed by Aliovalent Substitution, ACS Mater. Lett., 2022, 4, 1264–1269 CrossRef CAS.
- M. Y. Li, Z. J. Ma, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, HgCuPS4: An Exceptional Infrared Nonlinear Optical Material with Defect Diamond-like Structure, Chem. Mater., 2020, 32, 4331–4339 CrossRef CAS.
- T. Rodl, R. Weihrich, J. Wack, J. Senker and A. Pfitzner, Rational Syntheses and Structural Characterization of Sulfur-Rich Phosphorus Polysulfides: α-P2S7 and β-P2S7, Angew. Chem., Int. Ed., 2011, 50, 10996–11000 CrossRef PubMed.
- D. A. Wright and B. R. Penfold, The Crystal and Molecular Structure of Phosphorus Thioiodide, Acta Crystallogr., Sect. A, 1959, 12, 455–460 CrossRef CAS.
- G. J. Penney and G. M. Sheldrick, Preparation and Molecular Structure of a New Isomer of P4S3I2: 3,5-Di-iodo-2,6,7-trithia-1,3,4,5-tetraphosphabicyclo [2,2,1] heptane, J. Chem. Soc. A, 1971, 9, 1100–1103 RSC.
- G. M. Sheldrick, A Short History of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- G. M. Sheldrick, Crystal Structure Refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- A. L. Spek, Single-crystal Structure Validation with The Program PLATON, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- J. Tauc, Absorption Edge and Internal Electric Fields in Amorphous Semiconductors, Mater. Res. Bull., 1970, 5, 721–730 CrossRef CAS.
- S. Landi, I. R. Segundo, E. Freitas, M. Vasilevskiy, J. Carneiro and C. J. Tavares, Use and misuse of the Kubelka-Munk Function to Obtain the Band Gap Energy From Diffuse Reflectance Measurements, Solid State Commun., 2022, 341, 114573 CrossRef CAS.
- S. K. Kurtz and T. T. Perry, A Powder Technique for the Evaluation of Nonlinear Optical Materials, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, First Principles Methods Using CASTEP, Z. Kristallogr., 2005, 220, 567–570 CAS.
- W. Kohn, Nobel Lecture: Electronic Structure of Matter-wave Functions and Density Functionals, Rev. Mod. Phys., 1999, 71, 1253–1266 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. L. Zhou and K. Burke, Restoring the Density-gradient Eexpansion for Exchange in Solids and Surfaces, Phys. Rev. Lett., 2008, 100, 136406 CrossRef PubMed.
- H. J. Monkhorst and J. D. Pack, Special Points for Brillouin-zone Integrations, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- D. J. Moss, E. Ghahramani, J. E. Sipe and H. M. Vandriel, Band-structure calculation of dispersion and anisotropy in χ(3) for third-harmonic generation in Si, Ge, and GaAs, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 1542–1560 CrossRef CAS PubMed.
- W. D. Cheng, C. S. Lin, H. Zhang and G. L. Chai, Theoretical Evaluation on Terahertz Source Generators from Ternary Metal Chalcogenides of PbM6Te10 (M = Ga, In), J. Phys. Chem. C, 2018, 122, 4557–4564 CrossRef CAS.
- M. H. Lee, C. H. Yang and J. H. Jan, Band-resolved Analysis of Nonlinear Optical Properties of Crystalline and Molecular Materials, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 235110 CrossRef.
- T. Liu, J. Qin, G. Zhang, T. Zhu, F. Niu, Y. Wu and C. Chen, Mercury Bromide (HgBr2): A Promising Nonlinear Optical Material in IR Region with a High Laser Damage Threshold, Appl. Phys. Lett., 2008, 93, 091102 CrossRef.
- Y. Li, Y. Ding, Y. Li, H. Liu, X. Meng, Y. Cong, J. Zhang, X. Li, X. Chen and J. Qin, Synthesis, Crystal Structure and Nonlinear Optical Property of RbHgI3, Crystals, 2017, 7, 148 CrossRef.
- Q. Wu, Y. Huang, X. G. Meng, C. Zhong, X. G. Chen and J. G. Qin, Exploration of New Decond-order Nonlinear Optical Materials of the Cs-Hg-Br-I system, Dalton Trans., 2014, 43, 8899–8904 RSC.
- Q. Wu, C. Yang, J. Ma, X. Liu and Y. J. Li, Halogen-Ion-Induced Structural Phase Transition Giving a Polymorph of HgBr2 with Balanced Nonlinear Optical Properties, Inorg. Chem., 2021, 60, 19297–19303 CrossRef CAS PubMed.
- Z.-H. Shi, M. Yang, W.-D. Yao, W. Liu and S.-P. Guo, SnPQ3 (Q=S, Se, S/Se): A Series of Lone-Pair Cationic Chalcogenophosphates Exhibiting Balanced NLO Activity Originating from SnQ8 Units, Inorg. Chem., 2021, 60, 14390–14398 CrossRef CAS PubMed.
- Y. Wang, Y. Q. Fang, Y. Z. Cao and F. Q. Huang, Two Nonlinear Optical Thiophosphates Cu5Hg0.5P2S8 and AgHg3PS6 Activated by Their Tetrahedra-Stacking Architecture, Inorg. Chem., 2022, 61, 1620–1626 CrossRef CAS PubMed.
- C. T. Chen, Y. C. Wu and R. K. Li, The Anionic Group Theory of the Non-linear Optical Effect and its Applications in the Development of New High-quality NLO Crystals in the Borate Series, Int. Rev. Phys. Chem., 1989, 8, 65–91 Search PubMed.
- S.-P. Guo, Y. Chi and H.-G. Xue, SnI4·(S8)2: A Novel Adduct-Type Infrared Second-Order Nonlinear Optical Crystal, Angew. Chem., Int. Ed., 2018, 57, 11540–11543 CrossRef CAS PubMed.
- J. H. Feng, C. L. Hu, B. X. Li and J. G. Mao, LiGa2PS6 and LiCd3PS6: Molecular Designs of Two New Mid-Infrared Nonlinear Optical Materials, Chem. Mater., 2018, 30, 3901–3908 CrossRef CAS.
- Q. C. Hu, K. B. Ruan, Y. Z. Wang, K. Ding and Y. Xu, Synthesis and Nonlinear Optical Properties of New Gallium Thiophosphate Rb2Ga2P2S9, New J. Chem., 2019, 43, 12468–12474 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, measurements of physical properties, and theoretical calculations. CCDC 2216006. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi02313j |
| This journal is © the Partner Organisations 2023 |