Open Access Article
Open Access ArticleLimonene as a natural product extraction solvent
Mario
Pagliaro
 a,
Anne-Sylvie
Fabiano-Tixier
a,
Anne-Sylvie
Fabiano-Tixier
 b and
Rosaria
Ciriminna
b and
Rosaria
Ciriminna
 *a
*a
aIstituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146 Palermo, Italy. E-mail: rosaria.ciriminna@cnr.it
bAvignon University, INRA, UMR 408, GREEN Team Extraction, 84000 Avignon, France. E-mail: anne-sylvie.fabiano@univ-avignon.fr
First published on 11th July 2023
Abstract
First reported in 2004, and subsequently advanced by Chemat's team and other research teams in the following two decades, the use of D-limonene as a natural product extraction solvent offers multiple benefits that go beyond its environmentally benign nature. Following a review of the main research achievements concerning the use of this biosolvent in the extraction of widely different natural products, we offer a comprehensive perspective including usually neglected economic and safety aspects. The study concludes by identifying three main research outcomes and providing two guidelines to bioeconomy practitioners willing to use this terpene in new natural production extraction and production processes.
1 Introduction
Limonene (1-methyl-4-isopropenylcyclohex-1-ene), namely the monocyclic terpene abundant in the essential oils isolated from the peel of nearly all citrus fruits as the dextrorotatory isomer D-limonene (or (+)-limonene), is a key bioproduct of the emerging bioeconomy.1 The history of its identification and ongoing misconceptions of the odour–structure relationship are instructive also for today's chemistry educators and researchers. In 1884, Wallach and Brass reported that boiling cineol with hydrogen chloride gave a hydrocarbon, C10H16, with lemon-like odour.2 Continuing his research studies on terpenes, for which he was eventually awarded the 1910 Nobel Prize in chemistry, Wallach identified both limonene isomers, dextro-limonene from citrus fruits and laevo-limonene from Pinus (pine needle oils), and their racemate, which he called “dipentene” having identified a rule in which terpenes had the general formula (C5H8)n. In his monumental book Terpenes und Campher (1914), the chapter about limonene reads:“Under the name limonene and dipentene in 1884 I distinguished components of essential oils by the following properties:
“Limonene. The boiling point is between 175 and 177°. The terpene has a characteristic lemon odor, gives a nitroso derivative melting at 71°, combines with bromine to form a tetrabromide melting at 104–105° forming rhombic-hemiedric crystals, and with hydrochloric acid in ethereal solution to form dichlorohydrate of dipentene melting at 50 °C. At high temperature it turns into dipentene. From now on, the following are to be designated as limonene: Hesperiden, citren, carven, etc.”3
As recently reported by Kvittingen and co-workers, numerous organic chemistry textbooks (and scientific articles) of primary relevance in use today (in the 2020s) claim that “(+) limonene is primarily responsible for the smell of oranges and (−) limonene of lemons”.4 The only limonene isomer present in all citrus fruits (orange, lemon, grapefruit, mandarin, citron, etc.), however, is the dextrorotary isomer (Fig. 1). In 2014, Sell explained that the misconception, originating from a research paper published in 1971 in Science, was due to the more abundant presence of citral in the essential oil extracted from lemons used in the 1971 investigation.5
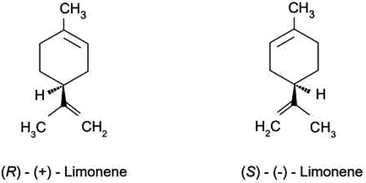 | ||
| Fig. 1 Stereoisomers of limonene (reproduced from ref. 6, with kind permission from the Royal Society of Chemistry, Copyright 2023). | ||
Being edible (with a sweet taste), fragrant (with an exquisite citrus scent) and safe7 for human consumption, D-limonene is a widely employed food additive (flavouring agent) and cosmetic ingredient added to numerous perfumes and personal care products.1 The only adverse effects of limonene on human health are due to its oxidation products acting as skin (contact allergen)8 and non-specific airway irritants.9 Simultaneous occurrence of contact dermatitis and bronchial asthma in workers due to oxidised limonene present in the air of citrus fruit processing plants has been reported.10 In the presence of water and air molecules, such as in many beverages and cosmetics containing limonene, water molecules promote the oxidative degradation of limonene after prolonged exposure to UV irradiation (via three reaction branches involving epoxidation, C–O bond homolysis, and addition of hydrogen radicals and oxygen in a subsequent cascade of reactions).11
The use of D-limonene as industrial solvent dates back to the early 1990s, when the terpene started to be used as a replacement for highly toxic trichloroethylene, for example in microelectronics. No degradation of the electrical performance of all parameters measured, even under prolonged exposure of electronic assemblies to a saturated D-limonene atmosphere, indeed occurs.12 Limonene is also used as a degreasing agent for contaminated oilfield equipment.13 In the late 1990s, the use of limonene for the treatment of oil spills on land to dissolve the more recalcitrant petroleum fractions (after the volatile fraction had evaporated) and make it available for bacterial degradation (bioremediation) was reported.14 Limonene, therefore, was used in massive amounts both as a degreasing agent for boat hulls and as a bioremediation aid during the Deepwater Horizon oil spill accident in the Gulf of Mexico in 2010 (causing a spike in limonene price due to the sudden, huge demand for the solvent).15
In a “greenness” ranking published in 2016 listing biobased solvents chiefly for use as solvents to carry out chemical reactions, D-limonene (along with turpentine) was classified as “problematic” due to its relatively high boiling point, aquatic toxicity, and ease of oxidation.16
To the best of our knowledge, the first to report in the literature the use of limonene as a natural product extraction solvent were Liu and Mamidipally in the USA in 2004–2005.17,18 The team described the successful use of the biosolvent replacing n-hexane in the extraction of rice bran oil. In the subsequent two decades, the use of citrus-derived terpene as a natural product extraction solvent was substantially advanced by the team of Chemat19 and by other research teams from across the world.
Today, the practical utilization of this multifunctional solvent in the natural product industry is rapidly approaching. Its use as a natural product extraction solvent, indeed, offers multiple benefits that go beyond its environmentally benign nature. Following a review of the main research achievements concerning the use of this biosolvent in the extraction of different natural products, this study offers a comprehensive perspective that includes usually neglected, but practically relevant, economic and safety aspects. The study concludes by identifying three main research outcomes and providing two guidelines to bioeconomy practitioners willing to use terpene in new natural production extraction and production processes.
2 Research achievements
Table 1 presents selected research achievements in the extraction of natural products using limonene as an extraction biosolvent.| Achievement | Year | Team | Study (ref.) |
|---|---|---|---|
| Extraction of rice bran oil | 2004 | Liu and Mamidipally | First approach to rice bran oil extraction using limonene (17) |
| Demonstration of comparable quality rice bran oil extracted with limonene and hexane and full separation of the extracted oil from the limonene biosolvent | 2005 | Liu and Mamidipally | Quality comparison of rice bran oil extracted with D-limonene and hexane (18) |
| Extraction of olive oil from olive seeds using microwaves as a heat source | 2007 | Chemat and Visinoni | New microwave-integrated Soxhlet extraction. An advantageous tool for the extraction of lipids from food products (20) |
| Extraction of lycopene from fresh tomato fruits | 2010 | Chemat | Carotenoid extraction from tomato using a green solvent resulting from orange processing waste (23) |
| Extraction of palm oil from oil palm decanter cake | 2015 | Sahad and Sulaiman | Recovery of Residual Crude Palm Oil (RCPO) from Oil Palm Decanter Cake (OPDC) Using D-limonene (22) |
| Extraction of carotenoids, aromas, and rapeseed oil from carrots, rapeseed and caraway seeds. | 2017 | Chemat and Fabiano-Tixier | Limonene as an agro-chemical building block for the synthesis and extraction of bioactive compounds (25) |
| Extraction of fish oil from anchovy fillet leftovers | 2019 | Ciriminna and Pagliaro | A circular economy approach to fish oil extraction (29) |
| Extraction of shrimp oil rich in omega-3 and natural astaxanthin | 2020 | Pagliaro and Chemat | High yields of shrimp oil rich in omega-3 and natural astaxanthin from shrimp waste (31) |
| Extraction of Gutta-Percha from leaves, samara, and bark of Eucommia ulmoides | 2022 | Yang and Gu | Potential use of limonene as an alternative solvent for the extraction of Gutta-Percha from Eucommia ulmoides (47) |
| High yield extraction of nuciferine from lotus leaf | 2023 | Shi | D-Limonene as an alternative for the extraction and purification of nuciferine from lotus leaf via multi-stage vortex assisted two-phase solvent extraction integrated with solid phase extraction using the mesoporous material SBA-15 as an adsorbent (27) |
2.1 Extraction under reflux
In their pioneering study, Liu and Mamidipally demonstrated that limonene at its boiling point (wrongly reported as 162.78 °C, when D-limonene under atmospheric pressure boils at 176 °C)3 can replace n-hexane at its boiling point (68 °C) in the extraction of edible rice bran oil under reflux.17 Using limonene, crude rice bran oil was extracted in substantially higher yield under any applied experimental conditions (solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bran ratio and extraction time). The optimum solvent-to-bran ratio and extraction time required for the extraction of oil, based on the crude rice bran oil yield and color, were found to be 5
bran ratio and extraction time). The optimum solvent-to-bran ratio and extraction time required for the extraction of oil, based on the crude rice bran oil yield and color, were found to be 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.5 h for D-limonene and 3
1 and 0.5 h for D-limonene and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 h for n-hexane, affording respectively, 20.73 and 18.20 wt% yields. Reducing the extraction time from 1 to 0.5 h resulted in a significant reduction in the color of the extracted oil due to the reduced formation of oxidative polymers and other oil-soluble dark products via the Maillard reaction. The researchers ascribed the higher yield to the larger dissolving ability of limonene for triglycerides (and of free fatty acids) due to the substantially higher extraction temperature, and to the higher diffusion rate of solutes from the solid phase.18
1 and 1 h for n-hexane, affording respectively, 20.73 and 18.20 wt% yields. Reducing the extraction time from 1 to 0.5 h resulted in a significant reduction in the color of the extracted oil due to the reduced formation of oxidative polymers and other oil-soluble dark products via the Maillard reaction. The researchers ascribed the higher yield to the larger dissolving ability of limonene for triglycerides (and of free fatty acids) due to the substantially higher extraction temperature, and to the higher diffusion rate of solutes from the solid phase.18
Remarkably, furthermore, the team demonstrated that regardless of the absence of antioxidants during the limonene recovery steps via vacuum evaporation (first at 90 °C under 40 mbar, and then at 95 °C under 40 mbar to remove residual limonene),17 the amount of the oxidation products in the recovered limonene was <1 wt% of the original amount of solvent. “Recovered D-limonene could be suitable for reuse as a solvent in the subsequent cycle”, the scholars concluded, “potentially eliminating the safety, environmental, and health issues associated with the use of hexane”.18
Two years later, in 2007, Chemat and Visinoni, a chemist owner of a biotechnology and chemical company later to become one of the world's first manufacturers of microwave-assisted natural product extractors, reported the outcomes of using D-limonene to extract olive oil from olive seeds using a microwave-integrated Soxhlet (MIS) alongside a microwave-heated Clevenger distillation unit.20 The yield in olive oil with an MIS employing limonene (44.9%) was higher than using a conventional Soxhlet with n-hexane (40.3%) as well as using a MIS system with hexane (39.1%). The extraction time went from 8 h of conventional Soxhlet extraction to 32 min. The composition of the extracted olive oil in terms of saturated, mono-, and polyunsaturated fatty acids was similar to that reported in the literature, showing evidence that the use of microwave energy and limonene as solvents did not alter the composition of the extracted olive oil. Finally, about 90% of limonene could be recovered and made available for a subsequent extraction cycle carrying out azeotropic distillation to less than 100 °C rather than at a limonene boiling temperature of ∼176 °C.
In any case, the Soxhlet extraction of vegetable oils from corn, olive oil, olive pomace and nuts (almonds, cashews, walnuts, and peanuts) using limonene systematically affords oils with higher yields when compared to hexane, with the oil triglyceride composition similar to that reported in the literature using the latter petrochemical solvent.21
In 2015, Sahad and co-workers in Malaysia demonstrated that limonene is an excellent solvent to recover under reflux during Soxhlet extraction all the residual crude palm oil contained in the oil palm decanter cake obtained from the palm oil mill (12–13% oil, dry basis).22 Similar to n-hexane, D-limonene was able to recover 100% of the oil successfully with a slight variation in the fatty acid composition of the extracted oil, and a 23% higher content of the carotene content in the oil extracted using limonene when compared to the oil obtained using hexane (756 ppm vs. 615 ppm). However, whereas only 70% of hexane could be recovered and reused, limonene could be recovered at a rate of 90% (by azeotropic distillation adding water to distil limonene at ∼100 °C). Traces of the terpene remaining in the oil are observed. This oil, the team concluded, could be used in non-food applications in the pharmaceutical, cosmetic and plastic industries, from drug and soap making through plasticizing and many other uses.
2.2 Extraction at room temperature
In 2010, Chemat and co-workers reported the extraction of lycopene from fresh tomato fruit with D-limonene as a solvent at room temperature compared to extraction with dichloromethane (DCM).23 The lycopene yield obtained using DCM was higher (19.2%) than that obtained using the biosolvent (13.1%) due to the better solubility of lycopene in the chlorinated solvent. The team obtained limonene used for extraction by steam distillation of 500 g of orange peel derived from oranges locally cultivated in Algeria, followed by “deterpenation”. On the other hand, DCM is produced by the petrochemical industry upon reacting methane with chlorine at 400–500 °C in huge petrochemical plants using a large amount of energy. On the other hand, the heat needed for steam distillation of orange peels, as shown in 2019 by Chemat and collaborators from Morocco, can be readily supplied by concentrating sunlight with a parabola, into a solid–liquid mixture containing only orange peels and water.24Two years before, Chemat and Fabiano-Tixier evaluated the ability of limonene as a solvent using Hansen solubility parameters (HSP) and the Conductor-like Screening Model for Real Solvents (COSMO-RS) predictive model based on quantum chemistry, dielectric continuum models, electrostatic surface interactions, and statistical thermodynamics.25 We briefly present HSP calculations that afford the relative energy difference (RED), which is used to estimate the capacity of a solvent to dissolve a solute. RED values <1 point to good solubility (the compound has similar properties and will dissolve), while medium and poor solvents, respectively, have REDs ranging from 1 to 3, and >3. The outcomes of the simulations were compared with those of experiments in the extraction of carotenoids, aromas, and rapeseed oil.
The results in Table 2 (including the three Hansen solubility parameters δD, δP and δH, corresponding to dispersion, polar and hydrogen bonding forces)26 clearly show that limonene is a suitable alternative solvent for the extraction of valued biological compounds such as carotenoids. For example, in the extraction of carrots by maceration in D-limonene or in n-hexane at room temperature for 1 h, limonene was able to extract 94.8% of the maximum carotenoid content while n-hexane only extracted 78.1% of available carotenoids, with the solubility of β-carotene in limonene being nearly 1.5 higher than in hexane.25
| Compound | δD | δP | δH | RED | |
|---|---|---|---|---|---|
| Limonene | Hexane | ||||
| α-Carotene | 17.4 | 0.0 | 1.5 | 0.70 | 1.31 |
| β-Carotene | 17.4 | 0.8 | 1.7 | 0.55 | 1.34 |
| Lutein | 17.8 | 1.6 | 5.1 | 0.74 | 1.97 |
| Lycopene | 17.3 | 0.0 | 1.7 | 0.64 | 1.27 |
Similarly, the excellent solvent capacity of limonene was recently exploited by Shi and co-workers in China. The team replaced petroleum ether with terpene in the two-phase extraction of nuciferine from lotus leaf followed by solid phase extraction and pH-dependent liquid–liquid extraction (both with ethyl acetate) and crystallization, and eventually isolated nuciferine in 57% (with a purity of 95.9%) rather than in the previous 50% yield.27 In detail, limonene was used to form a two-phase solvent mixture with 5% ammonia aqueous solution and used to extract nuciferine. Considerable solvent saving could be achieved when compared to ether. In detail, only a 10-fold (v/w) volume excess of limonene with respect to the raw material (10 mL mg−1 of leaf powder) was sufficient to reach an extraction efficiency of 97.4% (Fig. 2), which is much lower than the 75 mL mg−1 ratio needed when using petroleum ether as an extraction solvent to extract 95.3% nuciferine only.
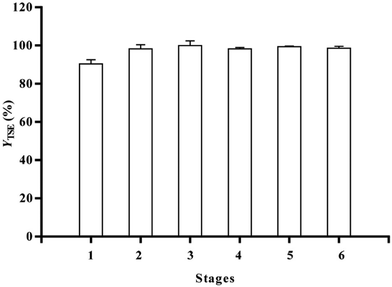 | ||
| Fig. 2 Nuciferine yields in the multi stage-vortex assisted two-phase solvent extraction of nuciferine from a lotus leaf. [Reproduced from ref. 27, with kind permission from Elsevier, Copyright 2023]. | ||
Furthermore, the team was able to reuse D-limonene without further purification five times. “In other words”, the researchers concluded “it is not costly as much as people tend to think to replace petroleum ether with D-limonene” and its use “as an extraction solvent for the large scale extraction of nuciferine is fully feasible”.27
An alkaloid with remarkably broad bioactivity (anti-hyperlipidemic, anti-obesity, antidiabetic, antitumor, anti-inflammatory, etc.) nuciferine is currently being investigated to develop new drugs for different diseases.28
2.3 Extraction at low temperature
In 2019, Pagliaro and co-workers reported the successful use of D-limonene at a low temperature (4 °C) to extract fish oil from anchovy leftovers.29 A whole fish oil rich dubbed “AnchoisOil” rich in polyunsaturated omega-3 fatty acids (PUFAs) in natural triglyceride form, and in vitamin D in its most bioavailable cholecalciferol (vitamin D3) form,30 can now be extracted in high yield from the fillet by-products of the world's most caught fish species. After extraction, ∼90% of limonene could be recovered by simple solvent evaporation at 90 °C under reduced pressure.Closing the materials cycle, the method establishes a circular economy process to obtain high quality fish oil from biowaste available worldwide in several million t per year. Indeed shortly afterwards, the approach was extended in collaboration with Chemat to shrimp biowaste,31 namely another by-product produced in several million t per year (global shrimp production >5 million t in 2022). A marine oil rich in omega-3 lipids in the natural triglyceride form and in natural astaxanthin was readily obtained, while the biosolvent was again recovered in a nearly ∼90% amount via evaporation under reduced pressure at 90 °C. The teams thus conducted HSP and COSMO-RS computations to assess the solubility in limonene and hexane of the most representative triacylglycerols (TAGs) present in anchovy oil.32 Invariably, the results showed that according both to HSP and COSMO-RS models, D-limonene is a better solvent than n-hexane for extraction of all main anchovy oil TAGs.
Fig. 3 simulates the chemical structure of TAG 1 with oleic acid bound to all residues of the glycerol molecule.
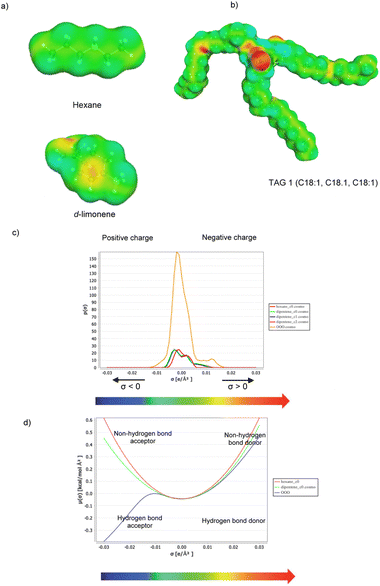 | ||
| Fig. 3 Step calculation with COSMO-RS: (a) D-limonene and n-hexane σ-surface; (b) oleic acid triglyceride (TAG 1) σ-surface; (c) energies of local surface interactions between σ-profiles of TAG 1 and the two solvents; (d) σ-potentials of TAG 1 and solvents. [Reproduced from ref. 32 with kind permission from American Chemical Society, Copyright 2019]. | ||
The process is feasible on a large scale because the capital investment in the low-energy extraction setup (including the biobased solvent and a solar dryer) is relatively modest, and the operational costs are mostly due to labor and electricity to separate the oil from the agro solvent.32 The method indeed eliminates the need for the conventional energy-intensive fish oil extraction and purification processes, including the production of commercial fish oil in ethyl ester form typically containing about 30% omega-3 fatty acids (18% in eicosapentaenoic acid and 12% in docosahexaenoic acid) via several consecutive steps.33
The conventional fish oil production process, furthermore, removes important antioxidant compounds from the refined oil, such as carotenoids and biophenols, which protect chemically labile PUFAs from oxidation and auto-oxidation (requiring manufacturers to omega-3 food supplements to add synthetic or biobased antioxidants).34 Antibacterial and antioxidant limonene, furthermore, protects the solid residue of anchovy fillet leftovers from rapid microbial spoilage, affording instead a new organic fertilizer rich in proteins, minerals, and flavonoids of exceptional efficacy.35 The use of limonene as an extraction and stabilization solvent (the LimoFish process), in other words, allows to convert anchovy fillet leftovers into a precious fish oil (AnchoisOil) and a fertilizer (“AnchoisFert”) whose essential, quasi-essential and non-essential amino acids would otherwise be lost in the environment.36 This substantially improves the overall sustainability of anchovy fishing, processing and consumption. Accordingly, the Life Cycle Assessment (LCA) methodology used to evaluate the environmental burdens associated with the production of AnchoisFert and AnchoisOil at the industrial scale reveals that in the extraction of AnchoiSoil followed by mild drying of the solid residue the main contributor to the environmental impacts of the process would be the production of virgin limonene.37 The LCA also suggests that limonene production by cold pressing extraction from orange peel would lead to approximately 70% lower direct environmental impacts.
3 Economic aspects
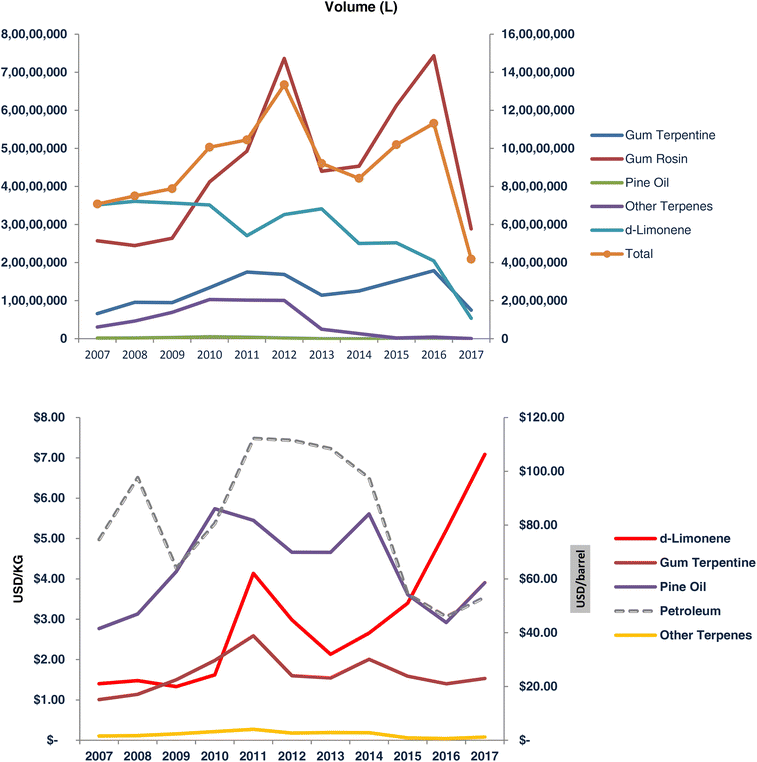
The citrus juice industry, chiefly based in Brazil and Florida (but also significant in South Africa, Spain, Italy, Mexico, China and India), has long been isolating orange and lemon essential oils prior to squeezing the fruits to extract the juice. As is known since the 1920s that it is “best to store oil in a cool dark place and as soon after preparation as possible”,38 the industry today typically stores freshly extracted orange oil in metal drums (with a nitrogen blanket to minimize oxidation prior to tight closure) kept at −20 °C in large refrigerated rooms from where the oil is delivered to customers.Oil makes up an increasing share of the industry's overall revenues. Only in the 2007–2017 decade, the price of limonene rose by 406% from $1.4 kg−1 to $7.09 kg−1 (Fig. 4).39 The 14-year average supply of orange oil has been around 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons, with a 9000 ton shortage in 2016/2017 due to citrus greening disease lowering Florida's oil production. Since then, production has increased again. The market price of limonene was about $4.5 kg−1 in late 2020.40
000 tons, with a 9000 ton shortage in 2016/2017 due to citrus greening disease lowering Florida's oil production. Since then, production has increased again. The market price of limonene was about $4.5 kg−1 in late 2020.40
Reporting a price of around $4 kg−1 in Brazil by late 2022, Martinez and co-workers in Brazil recently concluded the use of D-limonene to extract waxes from sugar cane in place of n-hexane peels is not convenient.41 In detail, the team ascribed the lack of economic viability to the 30% higher amount of energy and 10 times larger amount of acetone in the purification stage required to separate the biobased solvent from the wax and to dry the wax, due to the need to purify a mass of extract 8–9 times larger than that obtained with hexane requiring. These outcomes, regardless of lack of toxicity, lower flammability and safety risks, would prevent its use in place of low cost and easily evaporated n-hexane. We briefly remind that hexane, in August 2021, reached the highest price in nine years at €1.05 kg−1 (€1050 t−1), driven by increasing demand from the polymer (it is a solvent for polymerization reactions) and vegetable oil industries (where hexane is used to extract vegetable oils from crops like soy, rapeseed and sunflower beans, and agrifood residues such as olive pomace).42
However, the use of limonene as an extraction solvent affords far better sugarcane wax, because limonene is a much better solvent than hexane, being able to extract also relatively polar molecules. Indeed, the wax extracted with limonene showed a broader melting peak over the 69–73.83 °C range vs. the 76.84 °C of the purified wax extracted with hexane, requiring substantially less heat to melt (−111.24 vs. −145.60 J g−1). Indeed, the use of limonene produced a wax with a higher content of fatty acids and fatty alcohols, including octacosanol (27.8% with limonene vs. 22.2% with hexane). Plant sterols such as octacosanol exert numerous health beneficial effects.43 This means that sugarcane wax extracted with limonene would have a significantly higher economic value. The higher product price paid by nutraceutical and cosmetic industrial customers would be largely sufficient to cover the 30% extra energy and extra solvent (acetone) costs incurred by the wax manufacturer to dry and purify the sugar cane wax when using the biosolvent.
Limonene, furthermore, is nearly entirely recovered after each extraction cycle whereas, like in any other natural product extraction carried out with n-hexane, huge losses of the oil-derived solvent in the atmosphere and in the food chain are recorded every year.44 In closer detail, the vegetable oil and natural product industries use approximately 1.1 million tonnes of hexane annually (650 kt for oilseed extraction and 450 kt for the production of natural extracts) to compensate for solvent losses during processing.44 Of this, two-thirds (730 kt) are released into the atmosphere, and one-third (370 kt) remain in the oils and natural extracts.44,45 For example, only in the oil extraction from oilseeds losses amount to about 0.3–0.55 kg hexane per tonne of crushed seeds for soybeans and 0–2–0.7 kg t−1 for rape and sunflower seeds (when using the best available technologies, much more with older plants).45
A similar conclusion can be reached for valued Gutta-Percha extracted with limonene from leaves, samara, and bark of Eucommia ulmoides, a tree whose excellent natural rubber chiefly composed of trans-1,4-polyisoprene holds large potential application.46 Recently, a joint team in China led by Gu reported that using D-limonene at extraction temperatures ranging from 128 °C (for the samara) to 138 °C (for the leaves), excellent yields of high quality Gutta-Percha, ranging from 81.14 for the samara to 22.15 mg g−1 for the leaves and 55.32 mg g−1 for the bark, are readily obtained.47 For each tree's gummiferous part, the trans-1,4-polyisoprene content of Gutta-Percha obtained by limonene extraction (92.67% from leaves, 93.65% from samara, and 92.59% from the bark) was higher than that obtained with petroleum ether (boiling range 90–120 °C) as an extraction solvent. Furthermore, extraction with limonene is faster, with the extraction of Gutta-Percha for example from the samara nearly complete within 1.5 h vs. 2.5 h required when using petroleum ether. The filtrate obtained following precipitation of Gutta-Percha by simple addition of a strongly polar solvent such as ethanol, the team concluded, “can be reused in the next extraction by distillation, which greatly decreases the extraction cost and may reduce environmental damage”.47 Hence the high boiling point of limonene (176 °C) is twice beneficial, because it (i) allows to carry out the extraction at higher temperatures increasing the amount of Gutta-Percha extracted, and (ii) limits solvent evaporation during the extraction, allowing substantial solvent recovery for reuse.
4 Safety aspects
Table 3 presents selected chemical and physical properties of limonene. Since considerable differences exist in the literature amid published values, for each value, the corresponding experimental work in which the parameter was measured is also given.| Property | Value | Ref. |
|---|---|---|
| Melting point | −73.97 °C | 48 |
| Boiling point | 175.05 °C | 49 |
| Density (25 °C) | 0.841 g cm−3 | 49 |
| Vapor pressure (25 °C) | 200 Pa | 50 |
| Water solubility (25 °C) | 7.5 mg L−1 | 51 |
| Henry's law constant (25 °C) | 2851 Pa m3 mol−1 | 52 |
| Enthalpy of volatilization ΔHvol | 37.8 kJ mol−1 | 52 |
| Octanol/water partition coefficient KOW (25 °C) | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 770 |
52 |
| Flash point | 47 °C | 53 |
The vapour pressure at 25 °C is relatively high (200 Pa),50 and the solubility in water at room temperature is low (7.5 mg L−1),51 significantly lower than previously reported values. The enthalpy of volatilization ΔHvol is low (37.8 kJ mol−1, again significantly lower than that reported in the past), suggesting a relatively high rate of vaporization of terpene.52 The flash point (the lowest temperature at which the vapour phase of a liquid material will ignite in air in the presence of a spark or a flame) of 47 °C is low,53 though significantly higher than that of hexane (−22 °C, but the hexane vapor is heavier than air, and explodes when mixed with air and can travel long distances).54
Getting to safety, limonene is an edible food ingredient widely employed by the food and beverage industries as a sweetener and a fragrance, as well as by the cosmetic industry as a key ingredient of personal care products, and “top-note” fragrance in several perfumes, and in several domestic and occupational fragrant products.55 Due to its antibacterial, antifungal, antioxidant, anticancer, vasorelaxant, hypotensive, and antispasmodic health-beneficial effects, the terpene is also widely used in aromatherapy.56 Finally, in the environment, D-limonene is the major monoterpene emitted from conifers, citrus trees, and herbs, with a concentration in forests that can be as high or higher than the α-pinene concentration.57 Its use on a large scale to extract natural products, however, needs to take into account its flammability, toxicity at high dosages for the airways (and eyes), as well as the adverse effects of the main products formed upon contact with air's oxygen in the presence of sunlight: cis- and trans-limonene-1,2-oxide, cis- and trans-carveol, carvone and a number of hydroperoxy derivatives.58 The latter hydroperoxides act as potent contact allergens,7 and readily irritate the skin and airways.8,59 Indeed, the simultaneous occurrence of contact dermatitis and bronchial asthma of workers at a citrus fruit processing plant has been reported,10 along with contact dermatitis of a worker using limonene (in place of xylene) to dissolve paraffin in histological analyses.60 The industrial use of the chemical, in other words, is hazardous and accordingly regulated. For example in EU countries its use requires the display of four hazard pictograms in the workplace (Fig. 5), indicating five hazards: flammable liquid and vapour; may be fatal if swallowed and entered airways; causes skin irritation; may cause an allergic skin reaction; and very toxic to aquatic life (with long lasting effects).61
 | ||
| Fig. 5 Hazard pictograms for limonene in European Union countries (from left to right): GH502 (flammable); GH508 (serious health hazard); GH507 (health hazard); GH509 (hazardous to the environment). | ||
Said otherwise, even though we are dealing with a health-beneficial substance56 possessing immunomodulatory activity (it modulates T lymphocyte activity and viability),62 its handling and use on a massive scale requires following appropriate regulatory guidelines to prevent accidents and occupational disease, as well as to mitigate risk in the case of accidents. Companies planning to use limonene as a natural product extraction solvent will therefore use extractors and solvent storage units intrinsically capable of preventing the accidental release of the terpene. The extraction and storage units containing the solvent will be kept in tightly closed containers placed in a dry, cool and well-ventilated place, away from heat, sparks and flame, strong acids and bases, oxygen, and other oxidizing agents. Workers working at the maintenance of the extraction and storage units will use protective gloves, eyeglasses, and clothing to reduce the risk of dermal and eye irritation in the case of accidental contact.
Both the oil and citrus juice industries have plentiful experience in the safe utilization and handling of large amounts of limonene. In the oil industry, where limonene is also used as hydrocarbon waste cleaner for both extraction and search equipment, the terpene is used as a uniquely powerful solvent that enhances oil recovery from sand and rocks63 (with most recent results pointing to great efficacy in increasing the oil recovery even of 3% D-limonene diluted in brine),64 as well as in the case of oil spills at land14 or at sea.15 The citrus juice industry has a substantial and increasing fraction of its revenues in orange oil. The industry usually stores the oil in tightly closed drums under a nitrogen atmosphere with minimum headspace, placing the drums in a dry, aerated place usually kept at low temperatures (up to −20 °C) to retain all fragrance and flavour properties for which the oil is sold at a high price to customers in their home country and abroad (especially in the case of the world's largest orange juice makers, based in Brazil).
5 Outlook and conclusions
Taking into account the usually neglected (but crucially relevant from the practical viewpoint) economic and safety aspects and, upon reviewing the use of D-limonene as a biosolvent in the extraction of natural products in place of oil-derived solvents, this study meets Chemat's plea for “information of costs and economical and ecological constraints”.65 Three main research outcomes and two guidelines for bioeconomy practitioners emerge from the present analysis.The first research outcome is that, from fish oil29 to sugarcane wax41 to Gutta-Percha,47 the use of limonene as a natural product extraction solvent offers multiple benefits that go beyond its environmentally benign nature, including superior bioproduct quality and higher extraction yields.
Second, the higher product quality translates into a significantly higher natural extract price which compensates for the higher energy consumption to separate the extract from limonene due to the high boiling point of terpene.
Third, limonene can be nearly entirely (90–92%, in one step),17,22,29 or entirely (in two cycles, first at 90 °C under 40 mbar, and then at 95 °C under 40 mbar to remove residual limonene),18 recovered and reused in subsequent extraction cycles with modest oxidation of the terpene, thereby affording a truly circular economy production process. On the other hand, volatile organic solvents such as hexane are readily lost in the atmosphere to such an extent that the vegetable oil and natural product industries need 1.1 million tonnes of fresh hexane every year to replace solvent losses.44,45
Two important guidelines concern bioeconomy companies evaluating the use of limonene as a recyclable extraction solvent for natural products. First, even though limonene is an edible food ingredient and health-beneficial substance56,62 widely employed by the food, beverage and cosmetic industries,66 when used in massive amounts such as in natural production extraction, limonene is a hazardous chemical whose safe handling and utilization need to follow proper regulatory guidelines to prevent accidents and occupational disease.61 Such a safe use and handling of large amounts of flammable limonene have regularly been taking place since decades in the citrus juice and petroleum industries.
Second, the main obstacles to be overcome for bioeconomy companies willing to commercialize highly valued substances via circular economy processes relying on biowaste extraction with limonene are of organizational and management nature. For example, in the production of sugarcane wax,41 fish oil,32 Gutta-Percha47 or organic fertilizer67 with citrus-derived limonene, new partnerships should be established with agri-food companies for collection and delivery of perishable by-products under reciprocally advantageous conditions.68 Looking back to the past, in conclusion, it might be interesting to investigate the reasons why the use of limonene as a natural product extraction solvent had to wait until the 2000s to record the first research articles in the scientific literature,17,18,20 even though Thomas and Bessière wrote in 1989 in a leading natural product chemistry journal that the use of limonene as a solvent was “increasing on account of the low toxicity, pleasant odour and biodegradability”.69
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This study is dedicated to the memory of Professor Farid Chemat (1968–2023), a giant of chemistry and of life. M. P. and R. C. thank Professor Francesco Mauriello, Università Mediterranea di Reggio Calabria, for the joint research studies on the valorization of fishery biowaste using limonene, and Professor Francesco Parrino, Università di Trento, for collaboration on limonene catalytic green conversion into valued derivatives. R.C. and M.P. thank Salvatore Romeo and Giovanna Bellanti, Istituto per lo Studio dei Materiali Nanostrutturati, CNR, for many years of helpful assistance in administrative matters.References
- R. Ciriminna, M. Lomelli, P. Demma Carà, J. Lopez-Sanchez and M. Pagliaro, Limonene: a versatile chemical of the bioeconomy, Chem. Commun., 2014, 50, 15288–15296, 10.1039/c4cc06147k.
- O. Wallach and W. Brass, Ueber das oleum cynae; ein beitrag zur kenntniss der terpene, Justus Liebigs Ann. Chem., 1884, 225, 291–314, DOI:10.1002/jlac.18842250304.
- O. Wallach III, Limonen. Allgemeines über Limonen und Dipenten, in Terpene und Campher, De Gruyter, Berlin, 1914, p. 268. DOI:10.1515/9783112674123-014.
- L. Kvittingen, B. J. Sjursnes and R. Schmid, Limonene in Citrus: a string of unchecked literature citings?, J. Chem. Educ., 2021, 98, 3600–3607, DOI:10.1021/acs.jchemed.1c00363.
- C. S. Sell, Chemistry and the Sense of Smell, John Wiley & Sons, New York, 2014, p. 191. DOI:10.1002/9781118522981.ch3.
- Royal Society of Chemistry, Education - Properties of stereoisomers, https://edu.rsc.org/experiments/properties-of-stereoisomers/544.article (accessed July 5, 2023).
- Y. W. Kim, M. J. Kim, B. Y. Chung, D. Y. Bang, S. K. Lim, S. Choi, D. S. Lim, M. C. Cho, K. Yoon, H. S. Kim, K. B. Kim, Y. S. Kim, S. J. Kwack and B.-M. Lee, Safety evaluation and risk assessment of D-limonene, J. Toxicol. Environ. Health, Part B, 2013, 16, 17–38, DOI:10.1080/10937404.2013.769418.
- A. T. Karlberg, L. P. Shao, U. Nilsson, E. Gäfvert and J. L. G. Nilsson, Hydroperoxides in oxidized D-limonene identified as potent contact allergens, Arch. Dermatol. Res., 1994, 286, 97–103, DOI:10.1007/bf00370734.
- A. C. Rohr, C. K. Wilkins, P. A. Clausen, M. Hammer, G. D. Nielsen, P. Wolkoff and J. D. Spengler, Upper airway and pulmonary effects of oxidation products of (+)-(α)-pinene, D-limonene, and isoprene in BALB/c mice, Inhalation Toxicol., 2002, 14, 663–684, DOI:10.1080/08958370290084575.
- F. Guarneri, O. Barbuzza, M. Vaccaro and G. Galtieri, Allergic contact dermatitis and asthma caused by limonene in a labourer handling citrus fruits, Contact Dermatitis, 2008, 58, 315–316, DOI:10.1111/j.1600-0536.2007.01294.x.
- L. J. Li, P. Hong, Z. D. Jiang, Y. F. Yang, X. P. Du, H. Sun, L. Wu, H. Ni and F. Chen, Water accelerated transformation of D-limonene induced by ultraviolet irradiation and air exposure, Food Chem., 2018, 239, 434–441, DOI:10.1016/j.foodchem.2017.06.075.
- T. A. Somer, Aging of d-limonene-cleaned assemblies - Final report, U.S. Department of Energy Office of Scientific and Technical Information, 1995. DOI:10.2172/95178.
- W. B. Page and D. J. Chilton, An integrated approach to waste minimization, SPE Health, Safety and Environment in Oil and Gas Exploration and Production Conference, The Hague, Netherlands, 10–14 November 1991, SPE-23365-MS. DOI:10.2118/23365-ms.
- G. Gray, K. Chappell and V. K. Broscomb-Smith, Solvent enhanced bioremediation of weathered oil contamination, WIT Trans. Ecol. Environ., 1998, 20, 315–322, DOI:10.2495/oil980281.
- M. J. Cortez and H. G. Rowe, Alternative oil spill response technology: results from the Deepwater horizon response, J. Pet. Technol., 2012, 64, 60–68, DOI:10.2118/0912-0060-jpt.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical- and less classical-solvents, Green Chem., 2016, 18, 288–296, 10.1039/c5gc01008j.
- P. K. Mamidipally and S. X. Liu, First approach on rice bran oil extraction using limonene, Eur. J. Lipid Sci. Technol., 2004, 106, 122–125, DOI:10.1002/ejlt.200300891.
- S. X. Liu and P. K. Mamidipally, Quality comparison of rice bran oil extracted with D-limonene and hexane, Cereal Chem., 2005, 82, 209–215, DOI:10.1094/cc-82-0209.
- S. Chemat, V. Tomao and F. Chemat, Limonene as green solvent for extraction of natural products, in: Green Solvents I, ed. A. Mohammad, Springer, Dordrecht, 2012, pp. 175–186. DOI:10.1007/978-94-007-1712-1_5.
- M. Virot, V. Tomao, G. Colnagui, F. Visinoni and F. Chemat, New microwave-integrated Soxhlet extraction. An advantageous tool for the extraction of lipids from food products, J. Chromatogr. A, 2007, 1174, 138–144, DOI:10.1016/j.chroma.2007.09.067.
- S. Akretche-Kelfat, Z. Ferhat and M. Amiali, Vegetable and nut oils extraction by D-limonene as alternative solvent, Int. J. Agric. Res., 2017, 12, 82–87, DOI:10.3923/ijar.2017.82.87.
- N. Sahad, A. Md Som, A. S. Baharuddin, M. N. Mokhtar, Z. Busu and A. Sulaiman, Recovery of Residual crude palm oil (RCPO) from oil palm decanter cake (OPDC) using D-limonene, Adv. Mater. Res., 2015, 1113, 405–410, DOI:10.4028/www.scientific.net/amr.1113.405.
- Z. Chemat-Djenni, M. A. Ferhat, V. Tomao and F. Chemat, Carotenoid extraction from tomato using a green solvent resulting from orange processing waste, J. Essent.Oil Bear. Plants, 2010, 2, 139–147, DOI:10.1080/0972060x.2010.10643803.
- S. Hilali, A.-S. Fabiano-Tixier, K. Ruiz, A. Hejjaj, F. A. Nouh, A. Idlimam, A. Bily, L. Mandi and F. Chemat, Green extraction of essential oils, polyphenols, and pectins from orange peel employing solar energy: toward a zero-waste biorefinery, ACS Sustainable Chem. Eng., 2019, 7, 11815–11822, DOI:10.1021/acssuschemeng.9b02281.
- M. Aissou, Z. Chemat-Djenni, E. Yara-Varón, A.-S. Fabiano-Tixier and F. Chemat, Limonene as an agro-chemical building block for the synthesis and extraction of bioactive compounds, C. R. Chim., 2017, 20, 346–358, DOI:10.1016/j.crci.2016.05.018.
- M. Díaz de los Ríos and E. Hernández Ramos, Determination of the Hansen solubility parameters and the Hansen sphere radius with the aid of the solver add-in of Microsoft Excel, SN Appl. Sci., 2020, 2, 676, DOI:10.1007/s42452-020-2512-y.
- J. Xu, L. Peng, J. Chu, J. Shi, Q. Cui and Q. Shi, d-limonene as an alternative for the extraction and purification of nuciferine from lotus leaf via multi-stage vortex assisted two-phase solvent extraction integrated with solid phase extraction using mesoporous material SBA-15 as adsorbent, Sustainable Chem. Pharm., 2023, 32, 100997, DOI:10.1016/j.scp.2023.100997.
- X. B. Huang, N. Hao, G. Q. Chen, S. M. Liu and Z. P. Che, Chemistry and biology of nuciferine, Ind. Crops Prod., 2022, 179, 114694, DOI:10.1016/j.indcrop.2022.114694.
- R. Ciriminna, A. Scurria, G. Avellone and M. Pagliaro, A circular economy approach to fish oil extraction, ChemistrySelect, 2019, 4, 5106–5109, DOI:10.1002/slct.201900851.
- A. Scurria, C. Lino, R. Pitonzo, M. Pagliaro, G. Avellone and R. Ciriminna, Vitamin D3 in fish oil extracted with limonene from anchovy Leftovers, Chem. Data Collect., 2020, 25, 100311, DOI:10.1016/j.cdc.2019.10031.
- A. Scurria, A.-S. Fabiano Tixier, C. Lino, M. Pagliaro, G. Avellone, F. D'Agostino, F. Chemat and R. Ciriminna, High yields of shrimp oil rich in omega-3 and natural astaxanthin from shrimp waste, ACS Omega, 2020, 5, 17500–17505, DOI:10.1021/acsomega.0c01978.
- R. Ciriminna, A. Scurria, A. S. Fabiano-Tixier, C. Lino, G. Avellone, F. Chemat and M. Pagliaro, Omega-3 extraction from anchovy fillet leftovers with limonene: chemical, economic and technical Aspects, ACS Omega, 2019, 4, 15359–15363, DOI:10.1021/acsomega.9b01168.
- R. Ciriminna, F. Meneguzzo, R. Delisi and M. Pagliaro, Enhancing and improving the extraction of omega-3 from fish oil, Sustainable Chem. Pharm., 2017, 5, 54–59, DOI:10.1016/j.scp.2017.03.001.
- M. Pagliaro, D. M. Pizzone, A. Scurria, C. Lino, E. Paone, F. Mauriello and R. Ciriminna, Sustainably sourced olive polyphenols and omega-3 marine lipids: A synergy fostering public health, ACS Food Sci. Technol., 2021, 1, 139–145, DOI:10.1021/acsfoodscitech.0c00082.
- A. Muscolo, F. Mauriello, F. Marra, P. S. Calabrò, M. Russo, R. Ciriminna and M. Pagliaro, AnchoisFert: A new organic fertilizer from fish processing waste for sustainable agriculture, Glob. Chall., 2022, 6, 2100141, DOI:10.1002/gch2.202100141.
- M. Pagliaro, C. Lino, D. M. Pizzone, F. Mauriello, M. Russo, A. Muscolo, R. Ciriminna and G. Avellone, Amino acids in new organic fertilizer AnchoisFert, ChemistrySelect, 2022, 7, e202203665, DOI:10.1002/slct.202203665.
- F. Arfelli, D. M. Pizzone, D. Cespi, L. Ciacci, R. Ciriminna, P. S. Calabrò, M. Pagliaro, F. Mauriello and F. Passarini, Prospective life cycle assessment for the full valorization of anchovy fillet leftovers: The LimoFish process, Waste Manage., 2023, 168, 156–166, DOI:10.1016/j.wasman.2023.06.002.
- F. J. De Villiers, Citrus By-products Research: Orange Oil, in Farming in South Africa, 1930, 515. https://journals.co.za/doi/pdf/10.10520/AJA00148490_283 Search PubMed.
- F. Thomazelli , Citrosuco, personal communication to M.P., February 2018.
- Citrus oils and by-products – A current market overview, in Fruit Juice Focus, 2020. https://www.fruitjuicefocus.com/citrus-oils-and-bi-products-a-current-market-overview (accessed June 12, 2023) Search PubMed.
- R. M. A. Oliveira, J. D. O. Henriques, A. Sartoratto, M. R. W. Maciel and P. F. M. Martinez, Evaluation of Limonene in sugarcane wax extraction, Sustainable Chem. Pharm., 2022, 27, 100657, DOI:10.1016/j.scp.2022.100657.
- C. Sinclair, Hydrocarbon solvent hexane prices hit nine-year high on bullish fundamentals, S&P Global, 2021. https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/petrochemicals/081821-hydrocarbon-solvent-hexane-prices-hit-nine-year-high-on-bullish-fundamentals (accessed June 9, 2023) Search PubMed.
- Y. Zhou, F. Cao, F. Luo and Q. Lin, Octacosanol and health benefits: Biological functions and mechanisms of action, Food Biosci., 2022, 47, 101632, DOI:10.1016/j.fbio.2022.101632.
- C. Cravotto, A.-S. Fabiano-Tixier, O. Claux, M. Abert-Vian, S. Tabasso, G. Cravotto and F. Chemat, Towards substitution of hexane as extraction solvent of food products and ingredients with no regrets, Foods, 2022, 11, 3412, DOI:10.3390/foods11213412.
- G. Giner Santonja, P. Karlis, K. Raunkjær Stubdrup, T. Brinkmann and S. Roudier, in Best Available Techniques (BAT) Reference Document for the Food, Drink and Milk Industries: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control), Joint Research Centre of the European Commission, Publications Office of the European Union, Luxemburg, 2019. DOI:10.2760/243911.
- X. Wei, P. Peng, F. Peng and J. Dong, Natural polymer Eucommia Ulmoides rubber: a novel material, J. Agric. Food Chem., 2021, 69, 3797–3821, DOI:10.1021/acs.jafc.0c07560.
- G. Cui, X. Yang, Z. Liu, M. Wei, T. Liu, H. Gu and L. Yang, Potential use of limonene as an alternative solvent for extraction of Gutta-Percha from Eucommia ulmoides, ACS Sustainable Chem. Eng., 2022, 10, 11057–11068, DOI:10.1021/acssuschemeng.2c03898.
- H. E. Gallis, G. J. K. van den Berg and H. A. J. Oonk, Thermodynamic properties of crystalline D-limonene determined by adiabatic calorimetry, J. Chem. Eng. Data, 1996, 41, 1303–1306, DOI:10.1021/je960094l.
- R. A. Clará, A. C. Gómez Marigliano and H. N. Sólimo, Density, viscosity, and refractive index in the range (283.15 to 353.15) K and vapor pressure of α-pinene, D-limonene, (±)-linalool, and citral over the pressure range 1.0 kPa atmospheric pressure, J. Chem. Eng. Data, 2009, 54, 1087–1090, DOI:10.1021/je8007414.
- M. A. Espinosa Díaz, T. Guetachew, P. Landy, J. Jose and A. Voilley, Experimental and estimated saturated vapour pressures of aroma compounds, Fluid Phase Equilib., 1999, 157, 257–270, DOI:10.1016/S0378-3812(99)00016-3.
- D. J. Miller and S. B. Hawthorne, Solubility of liquid organic flavor and fragrance compounds in subcritical (hot/liquid) water from 298 K to 473 K, J. Chem. Eng. Data, 2000, 45, 315–318, DOI:10.1021/je990278a.
- L. O. Copolovici and Ü. Niinemets, Temperature dependencies of Henry's law constants and octanol/water partition coefficients for key plant volatile monoterpenoids, Chemosphere, 2005, 61, 1390–1400, DOI:10.1016/j.chemosphere.2005.05.003.
- D. Shcherbakov, S. Massebeuf and V. Normand, Flash-point prediction of fragrances or flavours accounting for non-ideality of the liquid phase, Flavour Fragrance J., 2019, 34, 63–69, DOI:10.1002/ffj.3485.
- J. A. Young, n-Hexane, J. Chem. Educ., 2001, 78, 587, DOI:10.1021/ed078p587.
- S. C. Rastogi, S. Heydorn, J. D. Johansen and D. A. Basketter, Fragrance chemicals in domestic and occupational products, Contact Dermatitis, 2001, 45, 221–225, DOI:10.1034/j.1600-0536.2001.450406.x.
- P. Anandakumar, S. Kamaraj and M. K. Vanitha, d-Limonene - a multifunctional compound with potent therapeutic effects, J. Food Biochem., 2021, 45, e13566, DOI:10.1111/jfbc.13566.
- S. Hayward, R. J. Muncey, A. E. James, C. J. Halsall and C. N. Hewitt, Monoterpene emissions from soil in a Sitka spruce forest, Atmos. Environ., 2001, 35, 4081–4087, DOI:10.1016/S1352-2310(01)00213-8.
- U. Nilsson, M. Bergh, L. P. Shao and A.-T. Karlberg, Analysis of contact allergenic compounds in oxidized D-limonene, Chromatographia, 1996, 42, 199–205, DOI:10.1007/bf02269653.
- J. B. Christensson, P. Forsström, A.-M. Wennberg, A.-T. Karlberg and M. Matura, Air oxidation increases skin irritation from fragrance terpenes, Contact Dermatitis, 2009, 60, 32–40, DOI:10.1111/j.1600-0536.2008.01471.x.
- C. Foti, C. G. Zambonin, A. Conserva, C. Casulli, L. D'Accolti and G. Angelini, Occupational contact dermatitis to a limonene-based solvent in a histopathology technician, Contact Dermatitis, 2007, 56, 109–112, DOI:10.1111/j.1600-0536.2007.00995.x.
- See limonene registration dossier on the European Chemicals Agency website: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/15256/2/1 (accessed June 12, 2023).
- C. M. Lappas and N. T. Lappas, d-Limonene modulates T lymphocyte activity and viability, Cell. Immunol., 2012, 279, 30–41, DOI:10.1016/j.cellimm.2012.09.002.
- H. Lazenby, US Oil Sands’ biosolvent extraction patent approved, Mining Weekly, 2014. https://www.miningweekly.com/article/us-oil-sandss-bio-solvent-extraction-patent-approved-2014-04-22/(accessed June 9, 2023) Search PubMed.
- M. Espinosa, M. Zavala-Arriaga and P. V. Ramírez-González, Enhanced oil recovery with D-limonene diluted in brine, J. Pet. Sci. Eng., 2022, 210, 110110, DOI:10.1016/j.petrol.2022.110110.
- Y. Li, W. Kunz and F. Chemat, From Petroleum to bio-based solvents: from academia to industry, in Plant Based “Green Chemistry 2.0”. Green Chemistry and Sustainable Technology, ed. Y. Li and F. Chemat, Springer, Singapore, 2019, pp. 51–87. DOI:10.1007/978-981-13-3810-6_3.
- C. Ravichandran, P. C. Badgujar, P. Gundev and A. Upadhyay, Review of toxicological assessment of D-limonene, a food and cosmetics additive, Food Chem. Toxicol., 2018, 120, 668–680, DOI:10.1016/j.fct.2018.07.052.
- R. Ciriminna, A. Scurria, D. M. Pizzone, P. S. Calabrò, A. Muscolo, F. Mauriello and M. Pagliaro, Economic and technical feasibility of AnchoisFert organic fertilizer production, Curr. Res. Green Sustainable Chem., 2022, 5, 100315, DOI:10.1016/j.crgsc.2022.100315.
- R. Ciriminna, L. Albanese, F. Meneguzzo and M. Pagliaro, Educating the managers of the bioeconomy, J. Cleaner Prod., 2022, 366, 132851, DOI:10.1016/j.jclepro.2022.132851.
- A. F. Thomas and Y. Bessière, Limonene, Nat. Prod. Rep., 1989, 6, 291–309, 10.1039/np9890600291 . The uses of limonene as a solvent mentioned therein are in the manufacture of children's balloons, in household cleaning fluids, as a solvent for cleaning paint brushes, and in varnishes for cleaning and restoring art objects.
| This journal is © The Royal Society of Chemistry 2023 |