Active and durable copper phosphate catalysts modified with metal oxides for methane oxidation with oxygen into formaldehyde†
Mana
Shimakawa
 and
Sakae
Takenaka
*
and
Sakae
Takenaka
*
Faculty of Science and Engineering, Doshisha University, Tatara-Miyakodani 1-3, Kyotanabe, Kyoto 610-0321, Japan. E-mail: stakenak@mail.doshisha.ac.jp
First published on 19th June 2023
Abstract
Copper phosphates as active catalysts for methane oxidation with O2 into formaldehyde were deposited on silica supports or dispersed with aluminum oxides in order to enhance their catalytic performance. Deposition of copper phosphates on silica led to the formation of α-Cu2P2O7 crystallites with small sizes, which improved the formaldehyde yield in the methane oxidation with O2. The addition of aluminum oxides into copper phosphates resulted in the formation of Cu3(PO4)2 and α-Cu2P2O7. These copper phosphates diluted with aluminum oxides showed high durability for the formaldehyde formation in the methane oxidation with O2 at 923 K. These modifications of the catalysts with silica supports or alumina additives resulted in the formation of small copper phosphates crystallites and the modification of the redox performance of copper phosphates.
1. Introduction
Methane is an abundant and inexpensive resource on earth. Therefore, it is of economic and sustainable importance to find efficient ways to utilize methane.1,2 In a conventional chemical process, methane has been converted by steam reforming into synthesis gas which is a mixture of CO and H2. Many chemicals such as methanol and hydrocarbons have been produced from synthesis gas.3,4 However, the steam reforming process requires a large energy input and high temperatures. Additionally, multiple steps are inevitably needed in the chemical processes for the production of methanol and hydrocarbons utilizing the synthesis gas as a feedstock. Therefore, direct conversion of methane into valuable chemicals is required, and many researchers develop catalysts effective for the partial oxidation of methane to methanol, formaldehyde, ethylene and so on.5–7 Methane, which has strong C–H bonds (414 kJ mol−1), is a chemically inert molecule and thus requires high temperatures and high pressures for its activation. Even if oxygenates such as methanol and formaldehyde were formed in the oxidation of methane with O2 over catalysts, these products are easily oxidized into carbon monoxide and carbon dioxide over catalysts due to severe conditions such as high temperatures and high pressures, which makes it difficult to develop effective catalysts for the partial oxidation of methane.8,9 It was reported that metal oxides of Co, V, Mo and Fe supported on silica are effective for the partial oxidation of methane.10–13 For example, high yields of formaldehyde with 1–2% have been observed in the methane oxidation with O2 over VOx/SiO2 and MoOx/SiO2 catalysts at 903 K.14 It is generally accepted that active species for partial oxidation of methane into formaldehyde are highly dispersed metal oxide clusters such as tetrahedral VO4 on silica, while aggregated metal oxides catalyse the total oxidation of methane into CO2. Bulk crystallized FePO4 was also reported as active catalysts for the formaldehyde formation, showing a formaldehyde yield of 0.2% in methane oxidation at 673–723 K.15 Some research groups investigated the catalytic performance of Cu oxide clusters for the partial oxidation of methane. CuOx with low loading on the mesoporous silica SBA-15 catalysed the formaldehyde formation at 1% yield in the methane oxidation with O2 at 898 K.16 CuOx stabilized in zeolites has been also attracting attention as catalysts capable of selective conversion of methane into formaldehyde and methanol at temperatures as low as 573 K.17 The catalysts formed methanol and formaldehyde selectively at low temperatures, but treatment of the catalysts with water vapor was required for the desorption of oxygenate products from the catalysts after being in contact with fresh catalysts with methane.18 As described earlier, many catalysts have been developed for the direct conversion of methane into valuable chemicals by oxidation with O2, but further improvement of catalytic performance should be required for the utilization of methane as chemical feedstock.We have also focused on copper oxides as catalytically active components for the partial oxidation of methane with O2. We reported that crystallized Cu3Mo2O9 in copper–molybdenum complex oxides and α-Cu2P2O7 in copper phosphate catalysts are particularly effective for formaldehyde formation in the oxidation of methane with O2.19,20 However, these catalysts showed low activity for the reaction, which would be due to their low surface areas. Additionally, high durability of these Cu-based crystallites for sintering should be required because methane oxidation with O2 is performed at high temperatures and the active components for the reaction are repeatedly reduced with methane and oxidized with O2 during the reaction. In the present study, copper phosphates were stabilized on silica supports or dispersed with aluminum oxides in order to increase their surface areas and improve their stability at high temperatures. This modification of the copper phosphates enhanced their catalytic activity and durability for the partial oxidation of methane into formaldehyde.
2. Experimental
2.1 Preparation of silica-supported CuPOx catalysts
Silica-supported CuPOx catalysts (CuPOx/SiO2) were prepared by an impregnation method. Silica powder was impregnated into an aqueous solution containing copper nitrate, ammonium dihydrogen phosphate and malic acid and dried up at 353 K. The samples thus obtained were calcined in air at 973 K. Two types of silicas (JRC-SIO12 and JRC-SIO9A, specific surface area of 79 and 333 m2 g−1, respectively; reference catalysts of The Catalysis Society of Japan) were used as supports for copper phosphates. CuPOx/SiO2 catalysts with different CuPOx loadings and different molar ratios of Cu to P were prepared. Bulk copper phosphates (α-Cu2P2O7 and Cu(PO3)2) were also prepared as control samples with a similar method to the preparation of CuPOx/SiO2.2.2 Preparation of copper phosphates modified with aluminum oxides
Copper phosphates modified with aluminum oxides (AlOx) (AlOx–CuPOx) were prepared by a coprecipitation method. Copper nitrate, ammonium dihydrogen phosphate, and aluminum nitrate were dissolved in pure water (0.5 M), and then aqueous ammonia was added into the solution to obtain precipitates. The precipitates thus obtained were calcined at 973 K in air to obtain AlOx–CuPOx.2.3 Oxidation of methane with O2
Oxidation of methane with O2 was performed in a flow reactor with a fixed catalyst bed. Catalyst powder (0.050 g) diluted with quartz sands was packed in the catalyst bed of a quartz reactor. The catalyst was treated at 973 K in O2 diluted with He prior to the oxidation of methane. For the oxidation of methane, a mixed gas composed of methane, O2 and He (CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He = 1
He = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) was in contact with the fresh catalysts at 823 K. During the reaction, the temperatures of the catalyst bed were changed to 873, 923 and 973 K. The effluent gases from the catalyst bed were passed through the traps cooled at 200 K to condense oxygenate products such as formaldehyde and methanol. The gases through the cold traps were analyzed by gas chromatographs with a TCD and FID. O2, CO and CO2 in the effluent gases were analyzed using columns packed with activated carbon and molecular sieve 5A. CH4, C2H4 and C2H6 were separated using a column packed with Porapak Q. The concentration of formaldehyde contained in the cold traps was evaluated with UV-vis spectroscopy. The products in the cold traps were added to mixed aqueous solutions of ammonium acetate, acetic acid and acetylacetone at 333 K, to form 3,5-diacetyl-1,4-dihydrolutidine.21 The concentration of formaldehyde was evaluated from the absorption at 413 nm due to 3,5-diacetyl-1,4-dihydrolutidine formed from formaldehyde in the UV-vis spectra for the solutions.
5) was in contact with the fresh catalysts at 823 K. During the reaction, the temperatures of the catalyst bed were changed to 873, 923 and 973 K. The effluent gases from the catalyst bed were passed through the traps cooled at 200 K to condense oxygenate products such as formaldehyde and methanol. The gases through the cold traps were analyzed by gas chromatographs with a TCD and FID. O2, CO and CO2 in the effluent gases were analyzed using columns packed with activated carbon and molecular sieve 5A. CH4, C2H4 and C2H6 were separated using a column packed with Porapak Q. The concentration of formaldehyde contained in the cold traps was evaluated with UV-vis spectroscopy. The products in the cold traps were added to mixed aqueous solutions of ammonium acetate, acetic acid and acetylacetone at 333 K, to form 3,5-diacetyl-1,4-dihydrolutidine.21 The concentration of formaldehyde was evaluated from the absorption at 413 nm due to 3,5-diacetyl-1,4-dihydrolutidine formed from formaldehyde in the UV-vis spectra for the solutions.
2.4 Characterization of the catalysts
X-ray diffraction (XRD) patterns of the catalysts were collected at room temperature (Rigaku MiniFlex 600, Cu Kα radiation, λ = 1.54 Å). The crystal phases of the catalysts were identified using the powder diffraction file (PDF) database of the International Centre for Diffraction Data (ICDD). The profiles of temperature-programmed reduction (TPR) with H2 for the catalysts were obtained by heating the catalyst samples from room temperature up to 873 K at a heating rate of 10 K min−1 in the stream of H2 diluted with Ar. Prior to the measurement of the TPR profiles, the catalyst samples were treated with O2 at 973 K. The consumption of H2 during the TPR experiments was monitored using a TCD.3. Results and discussion
3.1 Catalytic performance of CuPOx/SiO2
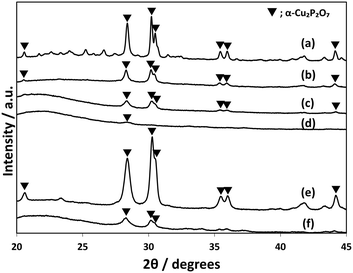
CuPOx/SiO2 catalysts with various molar ratios of Cu/(Cu + P) were prepared, while the total loading of Cu and P was fixed to 10 wt% for the catalysts. The CuPOx/SiO2 catalysts with molar ratio of Cu/(Cu + P) = 0.25, 0.33, 0.50 and 0.60 were denoted as Cu(25)-POx/SiO2, Cu(33)-POx/SiO2, Cu(50)-POx/SiO2, and Cu(60)-POx/SiO2, respectively. Fig. 1 shows the XRD patterns for the CuPOx/SiO2 catalysts. Only diffraction peaks assignable to α-Cu2P2O7 were observed at around 2θ = 28 and 30 degrees in the XRD patterns for Cu(25)-POx/SiO2, Cu(33)-POx/SiO2 and Cu(50)-POx/SiO2 catalysts (PDF No. 01-071-2177), whereas the peaks corresponding to CuO were also found in addition to those corresponding to α-Cu2P2O7 in the XRD pattern for Cu(60)-POx/SiO2. The crystallized compounds confirmed in the XRD patterns and specific surface areas evaluated by nitrogen adsorption at 77 K for the catalysts are summarized in Table 1. The formation of α-Cu2P2O7 only was confirmed as copper phosphates in the XRD patterns for all the CuPOx/SiO2 catalysts in spite of the molar ratios of Cu/(Cu + P). In contrast, various copper phosphates such as Cu(PO3)2, α-Cu2P2O7 and Cu3(PO4)2 were formed according to the molar ratio in the bulk copper phosphates without silica.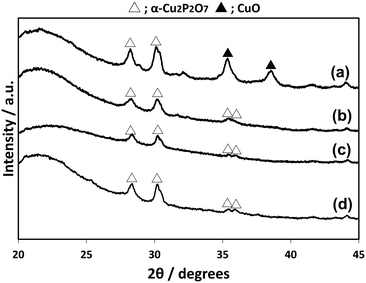 | ||
| Fig. 1 XRD patterns of CuPOx/SiO2 catalysts with different molar ratios of Cu/(Cu + P). a) Cu(60)-POx/SiO2, b) Cu(50)-POx/SiO2, c) Cu(33)-POx/SiO2, and d) Cu(25)-POx/SiO2. | ||
| Catalyst | Surface area [m2 g−1] | Main phase |
|---|---|---|
| Cu(25)-POx/SiO2 | 120 | α-Cu2P2O7 |
| Cu(33)-POx/SiO2 | 55 | α-Cu2P2O7 |
| Cu(50)-POx/SiO2 | 55 | α-Cu2P2O7 |
| Cu(60)-POx/SiO2 | 64 | α-Cu2P2O7·CuO |
| POx/SiO2 | 59 | Amorphous |
| CuOx/SiO2 | 69 | CuO |
| SiO2 | 79 | Amorphous |
| Cu(PO3)2 | 1 | Cu(PO3)2 |
| α-Cu2P2O7 | 11 | α-Cu2P2O7 |
| Cu3(PO4)2 | 6 | Cu3(PO4)2 |
Table 2 and Fig. S1† show the results of the oxidation of methane over CuPOx/SiO2 catalysts with different molar ratios of Cu/(Cu + P). Formaldehyde was selectively formed in the methane oxidation over silica and silica-supported POx catalysts (POx/SiO2), but their catalytic activity was very low. Thus, the formaldehyde yield was also quite low (0.1%) for both the catalysts. On the other hand, silica-supported CuOx catalysts (CuOx/SiO2) showed higher activity for methane oxidation than silica and POx/SiO2, but the selectivity to formaldehyde was quite low, and instead, CO2 was selectively formed. Methane conversion and selectivity to each product in the methane oxidation over CuPOx/SiO2 catalysts strongly depended on the molar ratio of Cu/(Cu + P). Generally, the catalytic activity became higher, and the selectivity to formaldehyde became lower as the Cu/(Cu + P) ratio in the CuPOx/SiO2 catalysts was higher. It should be noted that formaldehyde yield attained 0.9% in the methane oxidation over Cu(33)-POx/SiO2 catalysts at 923 K. The formaldehyde yield for Cu(33)-POx/SiO2 was higher than that for bulk α-Cu2P2O7 catalysts without silica supports as shown in Fig. S1,† although the weight of copper phosphates packed in the catalyst bed was very low in the reaction over Cu(33)POx/SiO2 compared with that over bulk α-Cu2P2O7, since the weight of the catalyst powder packed in the reactors was the same for both the catalysts. As shown in Table 2 and Fig. S1,† α-Cu2P2O7 is the most active catalyst for the formation of formaldehyde in the methane oxidation among all the bulk copper phosphate catalysts tested in the present study.20 These results indicated that copper phosphates α-Cu2P2O7 dispersed on the silica support also work as catalytically active sites for the formation of formaldehyde.
| Catalyst | Temp. [K] | Conversion [%] | Selectivity [%] | HCHO yield [%] | ||
|---|---|---|---|---|---|---|
| HCHO | CO | CO2 | ||||
| Cu(25)-POx/SiO2 | 873 | 0.6 | 72 | 0 | 28 | 0.4 |
| 923 | 2.0 | 48 | 2 | 50 | 1.0 | |
| Cu(33)-POx/SiO2 | 873 | 0.8 | 53 | 0 | 47 | 0.4 |
| 923 | 3.3 | 28 | 55 | 17 | 0.9 | |
| Cu(50)-POx/SiO2 | 873 | 1.5 | 34 | 0 | 66 | 0.5 |
| 923 | 3.6 | 23 | 0 | 78 | 0.8 | |
| Cu(60)-POx/SiO2 | 873 | 1.4 | 18 | 0 | 82 | 0.2 |
| 923 | 3.3 | 13 | 4 | 83 | 0.4 | |
| POx/SiO2 | 873 | 0.0 | 100 | 0 | 0 | 0.0 |
| 923 | 0.1 | 100 | 0 | 0 | 0.1 | |
| CuOx/SiO2 | 873 | 0.5 | 13 | 0 | 87 | 0.1 |
| 923 | 1.0 | 11 | 0 | 89 | 0.1 | |
| SiO2 | 873 | 0.04 | 100 | 0 | 0 | 0.04 |
| 923 | 0.1 | 100 | 0 | 0 | 0.1 | |
| Cu(PO3)2 | 873 | 0.03 | 100 | 0 | 0 | 0.03 |
| 923 | 0.06 | 100 | 0 | 0 | 0.06 | |
| α-Cu2P2O7 | 873 | 1.2 | 38 | 55 | 7 | 0.5 |
| 923 | 4.1 | 19 | 68 | 13 | 0.8 | |
| Cu3(PO4)2 | 873 | 1.8 | 4 | 1 | 95 | 0.06 |
| 923 | 1.8 | 11 | 6 | 83 | 0.2 | |
The catalytic performance of CuPOx/SiO2 with different loadings for methane oxidation was evaluated. Two types of silicas (specific surface area of 79 and 333 m2 g−1) were used as supports for copper phosphates. CuPOx of 6, 10, 60, and 80 wt% was loaded on silica with a low surface area, and these catalysts were denoted as CuPOx(Y wt%)/l-SiO2 (Y stands for the CuPOx loading). On the other hand, CuPOx of 20 and 60 wt% were supported on silica with high surface area, and the catalysts were denoted as CuPOx(Y wt%)/h-SiO2. For all CuPOx/SiO2 with different loadings, the molar ratio of Cu/(Cu + P) was fixed to 0.33. Fig. 2 shows the XRD patterns of CuPOx/SiO2 with different loading. In the XRD patterns for all the CuPOx/SiO2 catalysts in Fig. 2, only the diffraction peaks corresponding to α-Cu2P2O7 were observed, and these peaks became sharper with the CuPOx loading. Table 3 shows the specific surface area of each catalyst and an average crystallite size of α-Cu2P2O7 evaluated from the width at half-maxima of their diffraction lines. The results clearly showed that the average size of the α-Cu2P2O7 crystallite became larger with the CuPOx loading in the catalysts.
| Catalyst | Main phase | Crystallite size [Å] | Surface area [m2 g−1] |
|---|---|---|---|
| CuPOx(6 wt%)/l-SiO2 | α-Cu2P2O7 | 97 | 66 |
| CuPOx(10 wt%)/l-SiO2 | α-Cu2P2O7 | 113 | 55 |
| CuPOx(60 wt%)/l-SiO2 | α-Cu2P2O7 | 227 | 16 |
| CuPOx(80 wt%)/l-SiO2 | α-Cu2P2O7 | 340 | 2 |
| CuPOx(20 wt%)/h-SiO2 | α-Cu2P2O7 | 113 | 48 |
| CuPOx(60 wt%)/h-SiO2 | α-Cu2P2O7 | 227 | 25 |
| α-Cu2P2O7 | α-Cu2P2O7 | 205 | 11 |
| Cu(PO3)2 | Cu(PO3)2 | 466 | 1 |
Table 4 and Fig. S2† show the results of the methane oxidation over CuPOx/SiO2 catalysts with different loadings. Methane conversion gradually increased, and the selectivity to formaldehyde decreased as the CuPOx loading became higher. As described earlier, α-Cu2P2O7 with a larger crystallite size was supported on silica with the loading in the CuPOx/SiO2 catalysts. Thus, the difference in the catalytic performance of CuPOx/SiO2 with different loadings would result from the crystallite size of α-Cu2P2O7. It is interesting that the formaldehyde yield in the reaction over CuPOx/SiO2 catalysts with low loadings, for example, CuPOx(10 wt%)/l-SiO2 and CuPOx(20 wt%)/h-SiO2 was higher than that in the reaction over the bulk α-Cu2P2O7 catalysts although the amount of CuPOx packed in the catalyst bed for the former catalyst was only 10–20% of that for the latter one, as clarified from Table 4 and Fig. S2.† Thus, we conclude that deposition of small α-Cu2P2O7 crystallites on silica supports is effective for the design of CuPOx catalysts active for methane oxidation into formaldehyde.
| Catalyst | Temp. [K] | Conversion [%] | Selectivity [%] | HCHO yield [%] | ||
|---|---|---|---|---|---|---|
| HCHO | CO | CO2 | ||||
| CuPOx(6 wt%)/l-SiO2 | 873 | 1.5 | 33 | 11 | 56 | 0.5 |
| 923 | 4.5 | 19 | 63 | 18 | 0.8 | |
| CuPOx(10 wt%)/l-SiO2 | 873 | 0.8 | 53 | 0 | 47 | 0.4 |
| 923 | 3.3 | 28 | 55 | 17 | 0.9 | |
| CuPOx(60 wt%)/l-SiO2 | 873 | 0.1 | 100 | 0 | 0 | 0.1 |
| 923 | 0.9 | 86 | 1 | 13 | 0.8 | |
| CuPOx(80 wt%)/l-SiO2 | 873 | 0.1 | 100 | 0 | 0 | 0.1 |
| 923 | 0.2 | 59 | 23 | 19 | 0.2 | |
| CuPOx(20 wt%)/h-SiO2 | 873 | 1.3 | 40 | 0 | 60 | 0.5 |
| 923 | 3.7 | 24 | 0 | 77 | 0.9 | |
| CuPOx(60 wt%)/h-SiO2 | 873 | 0.5 | 69 | 0 | 31 | 0.3 |
| 923 | 1.7 | 48 | 6 | 47 | 0.8 | |
| α-Cu2P2O7 | 873 | 1.2 | 38 | 55 | 7 | 0.5 |
| 923 | 4.1 | 19 | 68 | 13 | 0.8 | |
| Cu(PO3)2 | 873 | 0.03 | 100 | 0 | 0 | 0.03 |
| 923 | 0.06 | 100 | 0 | 0 | 0.06 | |
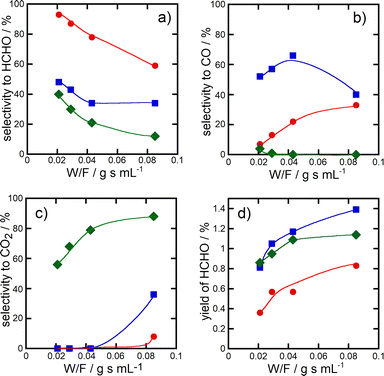
Fig. 3 shows the change of the selectivity to formaldehyde (panel a), CO (panel b) and CO2 (panel c) and the formaldehyde yield (panel d) as a function of the W/F value (W, weight of catalysts in the catalyst bed; F, flow rate of the reactant gas) in the methane oxidation over CuPOx (20 and 60 wt%)/h-SiO2 and bulk α-Cu2P2O7 catalysts at 923 K. As the W/F value became lower, selectivity to formaldehyde increased, and instead, the selectivity to CO2 decreased in the methane oxidation over all the catalysts shown in Fig. 3. In particular, formaldehyde was selectively formed in the reaction over CuPOx(60 wt%)/h-SiO2 catalysts at a low W/F value, which means short contact time of the reactant gases with the catalysts. These results suggest that formaldehyde is the primary product in methane oxidation over the CuPOx/SiO2 catalysts. Successive oxidation of oxygenate products during the methane oxidation should be inhibited by the deposition of small α-Cu2P2O7 crystallites onto the silica supports.
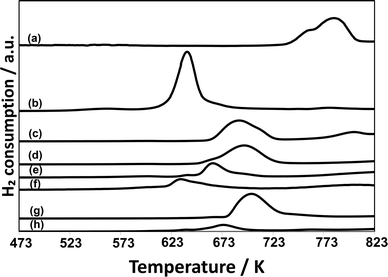
Temperature-programed reduction (TPR) with H2 for CuPOx/SiO2 catalysts was performed to clarify the difference in the catalytic performance between CuPOx stabilized on silica supports and bulk copper phosphates. Fig. 4 shows the TPR profiles for various CuPOx/SiO2 catalysts and bulk copper phosphates (Cu(PO3)2 and α-Cu2P2O7). In the TPR profiles for the CuPOx/SiO2 catalysts, a peak due to the reduction of copper phosphates with hydrogen was observed in the temperature range from 700 to 950 K, and its position was shifted toward higher temperatures with the CuPOx loadings. These peaks should be assignable to the reduction of copper oxides surrounded with phosphates.22,23 As described earlier, α-Cu2P2O7 crystallites were present on silica for all the CuPOx/SiO2 shown in Fig. 2, and their average crystallite size became larger with CuPOx loadings. Thus, smaller α-Cu2P2O7 crystallites should be reduced with hydrogen at lower temperatures.24 It should be noted that bulk α-Cu2P2O7 is reduced with hydrogen at lower temperatures than CuPOx(60 w%)/l-SiO2 and CuPOx(60 w%)/h-SiO2, although the average crystallite size of α-Cu2P2O7 was very similar for these catalysts. Chemical interaction between α-Cu2P2O7 and silica supports should cause the change in the redox properties of CuPOx. The formation of small α-Cu2P2O7 crystallites and their chemical interaction with silica would lead to the selective formation of formaldehyde, as well as the inhibition of its successive oxidation into CO and CO2 in the methane oxidation with O2.
3.2 Catalytic performance of AlOx-CuPOx
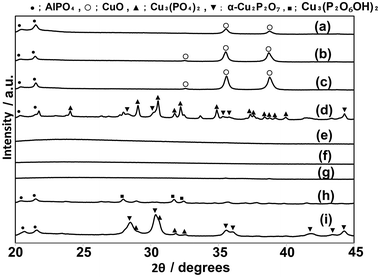
CuPOx catalysts were modified with different metal oxides (MgOx, AlOx, CrOx, MnOx and SrOx) to enhance their catalytic performance. The results are shown in Table S1 (ESI†). The loading of metal oxides added in CuPOx catalysts (molar ratio of Cu/(Cu + P) = 0.5) was fixed to 10 mol% for all the catalysts shown in Table S1.† The addition of AlOx enhanced the catalytic activity of CuPOx for the methane oxidation, while the selectivity to formaldehyde was not changed significantly by the addition of AlOx. In contrast, the modification with MgOx, CrOx, MnOx and SrOx decreased the catalytic activity of CuPOx for methane oxidation. Thus, the formaldehyde yields in the reaction over CuPOx catalysts with these additives were lower than that over the catalysts without additives. It was reported that the addition of AlOx into metal oxides suppresses their sintering during the catalytic reactions at high temperatures.25,26 Thus, we evaluated the catalytic performance of CuPOx catalysts modified with aluminum oxides (AlOx) in detail.AlOx of different amounts was added into copper phosphates to increase their specific surface areas. The molar ratios of Cu, Al and P in AlOx–CuPOx catalysts are denoted as Cu(x)-Al(y)-P(z) (x, y and z are mol% of Cu, Al, and P, respectively). Fig. 5 shows XRD patterns of different AlOx–CuPOx catalysts. Based on the results of these XRD patterns, AlOx–CuPOx catalysts were classified into three types. In the XRD patterns for Cu(25)-P(25)-Al(50), Cu(10)-P(10)-Al(80), and Cu(45)-P(10)-Al(45), any diffraction lines were not observed, indicating that structures of these catalysts were amorphous. In the XRD patterns for Cu(10)-P(45)-Al(45), Cu(50)-P(25)-Al(25), and Cu(80)-P(10)-Al(10), the diffraction peaks corresponding to any crystallized copper phosphates were not observed, but those corresponding to AlPO4 and CuO were observed. In contrast, Cu(45)-P(45)-Al(10), Cu(25)-P(50)-Al(25), and Cu(10)-P(80)-Al(10) catalysts were composed of crystallized copper phosphates. Cu(45)-P(45)-Al(10) catalysts contained crystallized Cu3(PO4)2 in addition to small amounts of α-Cu2P2O7 and AlPO4. The main component in Cu(25)-P(50)-Al(25) catalysts was Cu3(P2O6OH)2. On the other hand, crystallized α-Cu2P2O7 was mainly present in addition to a small amount of Cu3(PO4)2 and AlPO4 in Cu(10)-P(80)-Al(10) catalysts. As clarified in Table 5, the specific surface areas for AlOx–CuPOx with amorphous structures were relatively higher than those for the other catalysts. Although the surface areas of AlOx–CuPOx catalysts with crystallized compounds were low, their surface areas were slightly larger than those for bulk copper phosphates without AlOx.
| Catalyst | Surface area [m2 g−1] | Main phase |
|---|---|---|
| Cu(10)-P(45)-Al(45) | 80 | CuO |
| Cu(50)-P(25)-Al(25) | 26 | CuO |
| Cu(80)-P(10)-Al(10) | 8 | CuO |
| Cu(45)-P(45)-Al(10) | 15 | Cu3(PO4)2 |
| Cu(25)-P(25)-Al(50) | 81 | Amorphous |
| Cu(10)-P(10)-Al(80) | 286 | Amorphous |
| Cu(45)-P(10)-Al(45) | 105 | Amorphous |
| Cu(25)-P(50)-Al(25) | 39 | Cu3(P2O6OH)2 |
| Cu(10)-P(80)-Al(10) | 11 | α-Cu2P2O7 |
| α-Cu2P2O7 | 11 | α-Cu2P2O7 |
| Cu3(PO4)2 | 3 | Cu3(PO4)2 |
| CuO | 4 | CuO |
Table 6 and Fig. S3† show the results of the methane oxidation over AlOx–CuPOx catalysts containing crystallized copper phosphates in addition to the results on the bulk α-Cu2P2O7, Cu3(PO4)2 and CuO catalysts. Cu(25)-P(50)-Al(25) catalysts showed the highest activity for methane oxidation among all the catalysts in Table 6, but methane was completely oxidized with O2 into CO2 over the catalysts. It is likely that Cu3(P2O6OH)2 in the catalysts catalyzes the total oxidation of methane into CO2. In contrast, the selectivity to formaldehyde in the reaction over the other AlOx–CuPOx catalysts was significantly higher than that over Cu(25)-P(50)-Al(25) catalysts. The catalytic performance of Cu(10)-P(80)-Al(10) for the reaction was very similar to that of bulk α-Cu2P2O7 catalysts, which showed the highest yield of formaldehyde in the methane oxidation among all the bulk copper phosphate catalysts tested in the present study. High yield of formaldehyde should be obtained in the reaction over Cu(10)-P(80)-Al(10) catalysts since the catalysts were composed of α-Cu2P2O7 active for the partial oxidation of methane into formaldehyde. It is interesting that the Cu(45)-P(45)-Al(10) also showed higher activity for the reaction compared to the bulk copper phosphate catalysts, and the selectivity to formaldehyde was also relatively high. Thus, a formaldehyde yield of 1.0% was obtained in the reaction over the catalysts at 923 K. As described earlier, the Cu(45)-P(45)-Al(10) catalysts are composed of Cu3(PO4)2. The methane conversion was very low in the methane oxidation over bulk Cu3(PO4)2 catalysts without AlOx additives at 873 and 923 K. This would be due to the low surface area of bulk Cu3(PO4)2. AlOx additives would prevent the sintering of Cu3(PO4)2 crystallites during the preparation of the catalysts and the methane oxidation at high temperatures.
| Catalyst | Temp. [K] | Conversion [%] | Selectivity [%] | HCHO yield [%] | ||
|---|---|---|---|---|---|---|
| HCHO | CO | CO2 | ||||
| Cu(45)-P(45)-Al(10) | 873 | 2.4 | 30 | 14 | 56 | 0.7 |
| 923 | 6.6 | 16 | 19 | 65 | 1.0 | |
| Cu(25)-P(50)-Al(25) | 873 | 9.9 | 0 | 0 | 100 | 0.2 |
| 923 | 21.8 | 0 | 0 | 100 | 0.2 | |
| Cu(10)-P(80)-Al(10) | 873 | 1.2 | 43 | 5 | 52 | 0.5 |
| 923 | 3.5 | 22 | 9 | 69 | 0.8 | |
| α-Cu2P2O7 | 873 | 1.2 | 38 | 55 | 7 | 0.5 |
| 923 | 4.1 | 19 | 68 | 13 | 0.8 | |
| CuO | 873 | 2.1 | 1 | 0 | 99 | 0.0 |
| 923 | 2.7 | 2 | 0 | 98 | 0.1 | |
| Cu3(PO4)2 | 873 | 0.3 | 25 | 6 | 75 | 0.1 |
| 923 | 1.0 | 20 | 10 | 75 | 0.2 | |
Table S2 (ESI†) shows the results of the oxidation of methane over other AlOx–CuPOx catalysts in addition to the catalysts shown in Table 6. These catalysts are composed of amorphous compounds or mixtures of CuO and AlPO4. As clarified from the results in Table S2,† these catalysts showed high activity for methane oxidation, but selectivity to formaldehyde and its yield were quite low. Thus, we conclude that α-Cu2P2O7 and Cu3(PO4)2 work as catalytically active components for methane oxidation into formaldehyde.
Fig. 6 shows change of the selectivity to formaldehyde, CO and CO2 (panel a) and the formaldehyde yield (panel b) as a function of W/F value in the methane oxidation over Cu(45)-P(45)-Al(10) catalysts at 923 K. The selectivity to formaldehyde became lower and instead that to CO2 became higher with W/F values, suggesting that formaldehyde was the primary product in the methane oxidation over the catalysts. Formaldehyde was successively oxidized with O2 into CO2 over the catalysts.
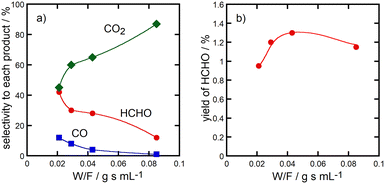 | ||
| Fig. 6 Change of the selectivity to formaldehyde, CO and CO2 (panel a) and the HCHO yield (panel b) as a function of W/F value in the methane oxidation over Cu(45)-Al(45)-P(10) catalysts at 923 K. | ||
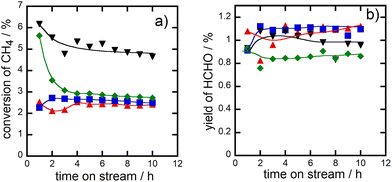
The addition of AlOx into CuPOx also improved the durability of the catalysts during the methane oxidation. Fig. 7 showed the change of methane conversion (panel a) and formaldehyde yield (panel b) as a function of time on stream in the methane oxidation over α-Cu2P2O7 and Cu(45)-P(45)-Al(10) catalysts at 923 K, in addition to the results of the reaction over CuPOx(10 wt%)/l-SiO2 and CuPOx(20 wt%)/h-SiO2 catalysts. Methane conversion in the reaction over bulk α-Cu2P2O7 catalyst was rapidly decreased for 2 h after the reactants were contacted with the fresh catalyst and then it was kept to 3% for 10 h. Both CuPOx/SiO2 catalysts showed high durability for the reaction although the methane conversion over these catalysts was not so high compared to those over the other catalysts. AlOx–CuPOx (Cu(45)-P(45)-Al(10)) was also deactivated slightly at the early period of the reaction, but the methane conversion over the catalysts was always higher than those over the other catalysts. Thus, formaldehyde yields in the reaction over CuPOx(10 wt%)/l-SiO2, CuPOx(20 wt%)/h-SiO2 and Cu(45)-P(45)-Al(10) are always higher than that in the reaction over bulk α-Cu2P2O7 catalyst. The deactivation of the catalyst should result from the sintering of copper phosphates which worked as active components for the reaction. AlOx additives in copper phosphates suppress the contact between copper phosphate crystallites during the methane oxidation at 923 K, which improves the durability of the catalysts.
Fig. 8 shows TPR profiles for AlOx–CuPOx catalysts and the reference samples. Cu(25)-P(50)-Al(25) catalysts, which showed the highest activity for the total oxidation of methane into CO2 among all the AlOx–CuPOx catalysts in Table 6, were reduced with H2 at lower temperatures. The high redox property of copper phosphates in Cu(25)-P(50)-Al(25) catalysts would lead to the high selectivity to the total oxidation in the methane oxidation with O2. In contrast, Cu(45)-P(45)-Al(10) and Cu(10)-P(80)-Al(10) catalysts, which showed high selectivity to formaldehyde in the methane oxidation, were reduced with H2 at higher temperatures than the bulk copper phosphates shown in Fig. 8. As described earlier, the deposition of CuPOx on silica decreased their activity for the reduction with H2. The control of the redox property of CuPOx by the addition of AlOx or by the deposition onto silica supports is effective for the development of active CuPOx catalysts for the partial oxidation of methane with O2 into formaldehyde. The deposition onto silica supports or the addition of AlOx should result in the formation of copper phosphates with smaller crystallite sizes as well as chemical interaction with silica or aluminum oxide. The interaction should prevent the total oxidation of methane into CO2 and the sintering of copper phosphates in the catalysts during the methane oxidation at high temperatures.
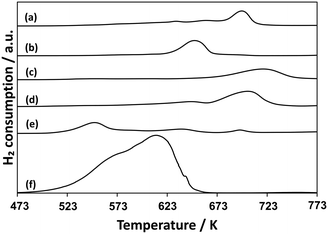 | ||
| Fig. 8 TPR profiles for AlOx–CuPOx catalysts. a) Cu3(PO4)2, b) α-Cu2P2O7, c) Cu(10)-P(80)-Al(10), d) Cu(45)-P(45)-Al(10), e) Cu(25)-P(50)-Al(25), and f) CuO. | ||
Conclusion
Copper phosphates as catalysts for the methane oxidation with O2 into formaldehyde were supported on silica or were modified with aluminum oxide (AlOx) to improve the catalytic activity and durability. Deposition of copper phosphates on silica supports led to the formation of α-C2P2O7 crystallites with small sizes while crystallized Cu3(PO4)2 with a high surface area was stabilized in the copper phosphate catalysts modified with AlOx. Copper phosphate catalysts supported on silica or modified with AlOx formed formaldehyde with a yield of 1.1–1.4% by methane oxidation. These modifications with silica or AlOx resulted in the control of the redox properties of copper phosphates.Author contributions
M. Shimakawa carried out and interpreted the experimental part. S. Takenaka conceived the idea and supervised the whole project. Both authors have contributed and agreed with the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers 20H02526 and 23H01766.Notes and references
- A. Demirbas, Energy Convers. Manage., 2010, 51, 1547–1561 CrossRef CAS.
- M. J. G. Fait, A. Ricci, M. Holena, J. Rabeah, M.-M. Pohl, D. Linke and E. V. Kondratenko, Catal. Sci. Technol., 2019, 9, 5111–5121 RSC.
- P. Schwach, X. Pan and X. Bao, Chem. Rev., 2017, 117, 8497–8520 CrossRef CAS PubMed.
- M. J. G. Fait, A. Ricci, M. Holena, J. Rabeah, M.-M. Pohl, D. Linke and E. V. Kondratenko, Catal. Sci. Technol., 2019, 9, 5111–5121 RSC.
- J. T. Grant, J. M. Venegas, W. P. McDermott and I. Hermans, Chem. Rev., 2018, 118, 2769–2815 CrossRef CAS PubMed.
- E. V. Kondratenko, T. Peppel, D. Seeburg, V. A. Kondratenko, N. Kalevaru, A. Martin and S. Wohlrab, Catal. Sci. Technol., 2017, 7, 366–381 RSC.
- Z. Guo, B. Liu, Q. Zhang, W. Deng, Y. Wang and Y. Wang, Chem. Rev., 2014, 43, 3480–3524 CAS.
- A. Matsuda, H. Tateno, K. Kamata and M. Hara, Catal. Sci. Technol., 2021, 11, 6987–6998 RSC.
- M. J. G. Fait, A. Ricci, M. Holena, J. Rabeah, M.-M. Pohl, D. Linke and E. V. Kondratenko, Catal. Sci. Technol., 2019, 9, 5111–5121 RSC.
- L. D. Nguyen, S. Loridant, H. Launay, A. Pigamo, J. L. Dubois and J. M. M. Millet, J. Catal., 2006, 237, 38–48 CrossRef CAS.
- J. Ohyama, D. Abe, A. Hirayama, H. Iwai, Y. Tsuchimura, K. Sakamoto, M. Irikura, Y. Nakamura, H. Yoshida, M. Machida, S. Nishimura, T. Yamamoto and K. Takahashi, J. Phys. Chem. C, 2022, 126, 1785–1792 CrossRef CAS.
- V. Foréns, C. López, H. H. López and A. Martínez, Appl. Catal., A, 2003, 249, 345–354 CrossRef.
- J. He, Y. Li, D. An, Q. Zhang and Y. Wang, J. Nat. Gas Chem., 2009, 18, 288–294 CrossRef CAS.
- R. G. Herman, Q. Sun, C. Shi, K. Klier, C. Wang, H. Hu, I. E. Wachs and M. M. Bhasin, Catal. Today, 1997, 37, 1–14 CrossRef CAS.
- Y. Wang and K. Otsuka, J. Catal., 1995, 155, 256–267 CrossRef CAS.
- L. Yang, A. Dongli, Q. Zhang and Y. Wang, J. Phys. Chem. C, 2008, 112, 13700–13708 CrossRef.
- M. H. Groothaert, P. J. Smeets, B. F. Sels, P. A. Jacobs and R. A. Schoonheydt, J. Am. Chem. Soc., 2005, 127, 1394–1395 CrossRef CAS PubMed.
- J. Ohyama, A. Hirayama, Y. Tsuchimura, N. Kondou, H. Yoshida, M. Machida, S. Nishimura, K. Kato, I. Miyazato and K. Takahashi, Catal. Sci. Technol., 2021, 11, 3437–3446 RSC.
- T. Akiyama, R. Sei and S. Takenaka, Catal. Sci. Technol., 2021, 51, 5273–5281 RSC.
- T. Akiyama, M. Shimakawa and S. Takenaka, Chem. Lett., 2022, 51, 511–514 CrossRef CAS.
- T. Nash, Biochem. J., 1953, 55, 416–421 CrossRef CAS PubMed.
- C. Sepúlveda, L. Delgado, R. García, M. Melendrez, J. L. G. Fierro, I. T. Ghampson and N. Escalona, Catal. Today, 2017, 279, 217–223 CrossRef.
- H. Li, F. Wang, W. Cai, J. Zhang and X. Zhang, Catal. Sci. Technol., 2015, 5, 5174–5184 RSC.
- S. Takenaka, T. Kaburagi, C. Yamada, K. Nomura and K. Otsuka, J. Catal., 2004, 228, 66–74 CrossRef CAS.
- S. Takenaka, M. Serizawa and K. Otsuka, J. Catal., 2004, 222, 520–531 CrossRef CAS.
- S. Takenaka, K. Nomura, N. Hanaizumi and K. Otsuka, Appl. Catal., A, 2005, 282, 333–341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cy00573a |
| This journal is © The Royal Society of Chemistry 2023 |