Mechanical milling processed highly luminescent Cs–Pb–Br perovskite emitters†
Teng
Zhang
 *a,
Youru
Bai
a,
Shaohuan
Feng
a,
Qifan
Xue
*a,
Youru
Bai
a,
Shaohuan
Feng
a,
Qifan
Xue
 *bc,
Xiaotian
Hu
*bc,
Xiaotian
Hu
 *d,
Xueqing
Xu
*c,
Heyuan
Liu
*d,
Xueqing
Xu
*c,
Heyuan
Liu
 a,
Yuriy N.
Luponosov
a,
Yuriy N.
Luponosov
 e,
Muhammad Bilal Khan
Niazi
f and
Xiyou
Li
e,
Muhammad Bilal Khan
Niazi
f and
Xiyou
Li
 *a
*a
aSchool of Materials Science and Engineering, China University of Petroleum (East China), Qingdao, 266580, P. R. China. E-mail: tzhangae@connect.ust.hk; xiyouli@upc.edu.cn
bState Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, School of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, P. R. China
cKey Laboratory of New and Renewable Energy Research and Development, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, P. R. China
dCollege of Chemistry and Chemical Engineering/Institute of Polymers and Energy Chemistry (IPEC)/Jiangxi Provincial Key Laboratory of New Energy Chemistry, Nanchang University, 999 Xuefu Avenue, Nanchang 330031, China
eEnikolopov Institute of Synthetic Polymeric Materials of the Russian Academy of Sciences, Moscow 117393, Russia
fSchool of Chemical & Materials Engineering, National University of Sciences & Technology, Islamabad, Pakistan
First published on 5th September 2023
Abstract
We report well-dispersed highly emitting perovskite emitters synthesized via the surfactant-assisted ball-milling method. Both the emitting peaks and the colour purity of the synthesized perovskite emitters can be effectively tuned through additive functionalization and precursor engineering.
In the past decade, lead halide perovskites with the ABX3 structure have shown great potential in many optoelectronic fields including solar cells,1–3 light-emitting diodes4–7 and photodetectors.8,9 In terms of light-emitting and displays, all inorganic CsPbX3 (X = Cl, Br, I) nanocrystals (NCs) are most attractive due to their low exciton binding energy and colour tuning features.10 To be more specific, different research groups have reported the successful synthesis of CsPbX3 (X = Cl, Br, I) NCs with a near-unity photoluminescence quantum yield (PLQY).11,12 The full width at half maxima (FWHM) of the emission spectra were less than 20 nm,13,14 thus guaranteeing the colour purity of the synthesized emitters. The emission peak of the synthesized CsPbX3 (X = Cl, Br, I) NCs can be tuned from 410–700 nm via compositional engineering, covering the whole visible light spectrum.11
Hot injection (HI),15–17 ligand-assisted precipitation (LARP)18–20 and mechanical milling21,22 are widely used to prepare high-quality perovskite NCs. Different from the HI and LARP methods, mechanical milling avoided the massive use of organic solvents. Besides, the strong repeatability and energy-saving features make mechanical milling promising for industrialization. S. Eslava reported the gram-scale synthesis of CsPbBr3 NCs through mechanical milling.23 M. V. Kovalenko suggested that the solvents and capping ligands were essential during the preparation of highly emissive mechanical milling processed perovskite NCs.24 H. Zhang introduced the facial synthesis of compositional engineered cesium lead halide perovskite NCs.22
The phase complexity of the synthesized Cs–Pb–Br component was also a problem. To be more specific, CsPbBr3 with an emission wavelength near 510 nm has been widely investigated for light-emitting applications.22–24 However, the synthesized Cs–Pb–Br emitters are frequently composed of a mixture of zero-dimensional (0D) Cs4PbBr6, two-dimensional (2D) CsPb2Br5 and three-dimensional (3D) CsPbBr3. It has been reported that the three phases can be transformed reversibly with the assistance of solvent and ligand.25–27 Besides, the emitting characteristics of the synthesized Cs–Pb–Br compound were structure and composition sensitive.28 Instead of green emitting, J. Tian reported the blue emission of core–shell structured CsPbBr3@amorphous CsPbBrx.17 Blue emission has also been observed in CsPb2Br5 nanocrystals.29 It has been reported that a CsPbBr3@Cs4PbBr6 core–shell structure can reduce the aggregation tendency of the emitting CsPbBr3 NCs.28,30 A similar situation can be expected for the CsPbBr3–CsPb2Br5 nanostructures.31,32 Very recently, via precursor engineering, T. Lee reported ball-milled CsPbBr3-decorated Cs4PbBr6 heterostructures for light emitting applications.33 Compared with the HI and LARP methods, it seems that mechanical milling is advantageous in the controlled synthesis of highly luminescent structure-varied Cs–Pb–Br emitters.
In this study, the ball milling method has been selected to synthesize Cs–Pb–Br perovskite emitters. To start with, the influence of different surfactants has been investigated in detail. The oleic acid (OA) functionalized Cs–Pb–Br powders were not emissive, while the octylamine (OLA), butyl amine (BLA) and oleylamine (OAM) passivation resulted in blue, green and cyan emission. Detailed investigations showed that the OAM functionalized Cs–Pb–Br NCs exhibited the highest PLQY among the three long-chain amine surfactants. Shown in the steady-state photoluminescence (PL) spectrum, two distant peaks at 453 nm and 515 nm can be observed in the OAM passivated Cs–Pb–Br emitters, mainly due to the coexistence of CsPbBr3 and CsPb2Br5. Finally, via precursor engineering, monochromatic green emitting NCs (PLQY 86.11%) with sharp emission characteristics (full width at half maximum (FWHM) of 21 nm) were synthesized. This research opened a new avenue for the facial and large-scale synthesis of high-quality colour-tuning perovskite emitters.
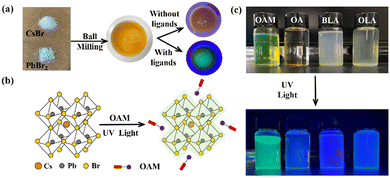
Fig. 1a shows the detailed description of the ball-milling processed Cs–Pb–Br emitters. PbBr2 and CsBr have been mixed in the milling cans and the milling time has been set to 0.5 h. To start with, the number of milling balls has been optimized (Fig. S1, ESI†). It should be noted that the dry-milling processed Cs–Pb–Br powders were not emissive under ultraviolet (UV) light illumination. This is possibly due to the large number of defects brought by the strong mechanical shearing force, which led to severe non-radiative recombinations.21 So, surfactants have been introduced to improve the fluorescence properties of the products (Fig. 1b). Here, 4 kinds of widely used surfactants, namely OA, OAM, BLA and OLA, have been added to the milling cans. Shown by Fourier Transform Infrared Spectroscopy (FTIR) (Fig. S2, ESI†), the signal at 1750 cm−1 was due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and the signal that showed up near 1500 cm−1 belonged to the N–H blending. Thus, the successful anchoring of the surfactant on the perovskite NCs can be confirmed. Photographs of the surfactant dependent Cs–Pb–Br emitters dispersed in toluene are provided in Fig. 1c. The fluorescence properties of the synthesized powder were surfactant-dependent. According to Fig. 1c, the OAM-functionalized Cs–Pb–Br powders showed cyan emmission (R 1, G 162, B 147), while the rest exhibited blue.
O stretching vibration, and the signal that showed up near 1500 cm−1 belonged to the N–H blending. Thus, the successful anchoring of the surfactant on the perovskite NCs can be confirmed. Photographs of the surfactant dependent Cs–Pb–Br emitters dispersed in toluene are provided in Fig. 1c. The fluorescence properties of the synthesized powder were surfactant-dependent. According to Fig. 1c, the OAM-functionalized Cs–Pb–Br powders showed cyan emmission (R 1, G 162, B 147), while the rest exhibited blue.
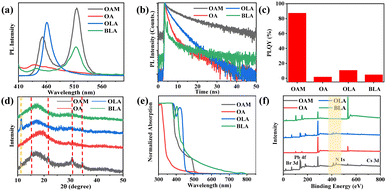
The steady-state PL spectrum of the surfactant functionalized Cs–Pb–Br NCs are listed in Fig. 2a. The PL intensity peaked at 453 nm and 515 nm in the OAM-functionalized NCs, corresponding with the cyan emission shown in Fig. 1c. Due to the lack of strong coordinative species, OA-functionalized NCs were not emissive. The PL spectrum of the OLA-functionalized Cs–Pb–Br NCs peaked at 461 nm, in agreement with the blue emission shown in Fig. 1c. Different from the photographs recorded in Fig. 1c, the BLA-functionalized NCs peaked at 513 nm, pointing to a green emission. This controversy will be discussed later coupled with the photoluminescence quantum yield (PLQY) analysis.
The transient resolved photoluminescence spectra (TRPL) curves of our synthesized NCs can be fitted with a biexponential decay mode (Fig. 2b and Table S1, ESI†). A prolonged average time constant (tave) has been observed in OAM-functionalized NCs, corresponding with a high PLQY (87.53%). This can be explained by the strong anchoring effect of the OAM surfactant. This PLQY has been reduced to 10.72% of OLA-functionalized NCs and quenched to almost zero in OA and BLA-functionalized NCs (Fig. 2c). The controversy between the photographs of UV light illumination (blue) and the steady state PL spectrum (green) was possibly due to the relatively low PLQY of the BLA-functionalized NCs.
Next came the structural characterization of the synthesized Cs–Pb–Br NCs. Here, the dispersed Cs–Pb–Br NCs solution has been mixed with polymethyl methacrylate (PMMA) and drop-coated on glass substrates for XRD characterization. The relatively strong background signals on the XRD patterns were attributed to the amorphous PMMA matrices (Fig. 2d). Interestingly, the background intensity of the OAM-functionalized emitters almost vanished, implying the high yield of the OAM-functionalized NCs. Besides, the OA showed hardly detectable signals beside the background information, this possibly suggested that OA was not effective to convert the PbBr2 and CsBr reactant into the Cs–Pb–Br perovskite product during the ball-milling process. This hypothesis was further supported by the absorption spectrum (Fig. 2e). The absorption peaking at ∼310 nm for the OA-functionalized NCs basically rules out the existence of the Cs–Pb–Br phase. The relatively low product yield also explained the poor PLQY of the OA-functionalized NCs (Fig. 2c).
The OLA-functionalized NCs were mainly composed of CsPb2Br5, as the sharp diffraction peak at 11.6° was due to the (002) facet of CsPb2Br5 (JCPDF card # 25-0211). The XRD pattern peaked at 30.7° in the BLA-functionalized NCs, which could be indexed to the (200) facet of CsPbBr3 (JCPDF card #18-0364), while CsPbBr3 and CsPb2Br5 phase coexisted in the prepared OAM-functionalized NCs (Fig. 2d). As a two-dimensional (2D) inorganic perovskite halide, CsPb2Br5 exhibited an indirect band gap of 3.06 eV, larger than that of CsPbBr3 (2.30 eV).34 Listed in Fig. 2e, the absorption peak of the OLA-functionalized NCs has been blue-shifted compared with that of the OAM-functionalized samples, supporting the dominant formation of CsPb2Br5 in the OLA-functionalized NCs. As for the emission characteristics, previous studies reported the blue emission of the CsPb2Br5 NCs,29 and a similar case was observed in our OLA-functionalized NCs. The CsPbBr3 (green emitting) and CsPb2Br5 (blue emitting) mixed-phase contributed to the dual emission feature of the OAM-functionalized NCs (Fig. 2a). Special attention has been given to the signals near 400 eV in the XPS spectrum (Fig. 2f), which can be assigned to the N characteristics of the organic amine. This signal turned out to be the strongest in the OAM-functionalized Cs–Pb–Br emitters, highlighting the strong anchoring potential of the OAM surfactant.
The transmission electron microscope (TEM) characterizations have been presented in Fig. S3 (ESI†). Since the chemical exfoliation effect of OA was not effective, we especially focused on the amine-functionalized NCs. The OAM-functionalized Cs–Pb–Br emitters can be roughly divided into two kinds of nanostructures, (a) large NCs exceeding 10 nm and (b) small NCs with an average size of 5 nm. From the high-resolution TEM (HRTEM) images, a lattice spacing of 0.29 nm has been observed in the large NCs, corresponding with the (200) facet of CsPbBr3 (JCPDF card #18-0364). Besides, we identified a lattice spacing of 0.76 nm in the small crystals, which could be indexed to the (002) facet of CsPb2Br5. (JCPDF card # 25-0211). The TEM characterization supported the coexistence of CsPbBr3 and CsPb2Br5 in the OAM-functionalized emitters (Fig. S3e, ESI†). Distinct shuttle-shaped crystals can be observed in the OLA-functionalized NCs (Fig. S3c, ESI†), and the formation of CsPb2Br5 can be confirmed from HRTEM analysis (Fig. S3g, ESI†). The BLA-functionalized NCs were composed of CsPbBr3 (Fig. S3d and h, ESI†). It should be noted that the size of the prepared OLA and BLA-functionalized NCs was much larger than that of the OAM-functionalized NCs, highlighting the excellent chemical exfoliation effect of the OAM surfactant.
The above investigations showed that the coexistence of the CsPbBr3 and CsPb2Br5 phases was the reason for the dual emission. We next consider if the compositional engineering method can be used to tune the emission characteristics of the synthesized Cs–Pb–Br NCs. The following three conditions with PbBr2:CsBr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 and 1
1.25 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
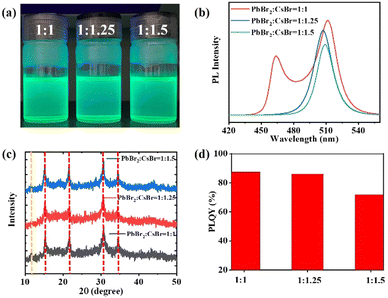
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 were considered for further optimization. Despite the photographs of the synthesized emitters under UV illumination showing a negligible difference (Fig. 3a), the steady-state PL spectrum suggested that the emission was largely influenced by the ratio of the precursors. Different from the Cs/Pb = 1 condition, the blue emission peak at 453 nm vanished after adding excess CsBr in the milling tank (Fig. 3b). Besides, the green emission peaked at 512 nm (Cs/Pb = 1) and has been blue-shifted with excess CsBr (Cs/Br = 1.25, Cs/Br = 1.5), pointing to a reduced defect density.35,36 The FWHM of the synthesized Cs–Pb–Br NCs has been reduced from 25 nm (Cs/Pb = 1) to 21 nm (Cs/Pb = 1.25) and 19 nm (Cs/Pb = 1.5), verifying the high colour purity of the synthesized emitters. This reported FWHM was also among the smallest FWHM values reported by other researchers.13,37,38
1.5 were considered for further optimization. Despite the photographs of the synthesized emitters under UV illumination showing a negligible difference (Fig. 3a), the steady-state PL spectrum suggested that the emission was largely influenced by the ratio of the precursors. Different from the Cs/Pb = 1 condition, the blue emission peak at 453 nm vanished after adding excess CsBr in the milling tank (Fig. 3b). Besides, the green emission peaked at 512 nm (Cs/Pb = 1) and has been blue-shifted with excess CsBr (Cs/Br = 1.25, Cs/Br = 1.5), pointing to a reduced defect density.35,36 The FWHM of the synthesized Cs–Pb–Br NCs has been reduced from 25 nm (Cs/Pb = 1) to 21 nm (Cs/Pb = 1.25) and 19 nm (Cs/Pb = 1.5), verifying the high colour purity of the synthesized emitters. This reported FWHM was also among the smallest FWHM values reported by other researchers.13,37,38
Listed in Fig. 3c, with the increased ratio of Cs/Pb, the peak at 11.6°, which can be indexed to CsPb2Br5, disappeared. This suggested that the Cs excess processed NCs were mainly composed of CsPbBr3. TEM analysis was also performed to verify this argument. Small CsPb2Br5 nanoflakes can hardly be detected in Cs/Pb = 1.25 (Fig. 4b) and Cs/Pb = 1.5 (Fig. 4c) processed NCs, confirming the phase purity of the synthesized product. It should also be noted that the PLQY reduced with the increased ratio of Cs/Pb (Fig. 3d). As for the stability concerns, the Cs–Pb–Br NCs were mixed with PMMA toluene solution and dropped coated on the glass substrates. The substrates remained highly emitting during a 2-month evaluation (Fig. S4, ESI†). Further still, we have also considered compositional varied CsPbCl3 and CsPbI3 emitters. Distinct blue emission can be observed from our CsPbCl3 emitters, while the attempt for CsPbI3 was not successful (Fig. S5 and S6, ESI†), possibly due to the transformation from the highly emitting cubic phase to the non-emitting orthorhombic polymorph.11
To conclude, highly emitting Cs–Pb–Br NCs have been synthesized via the mechanical milling method. To start with, the emitting characteristics of the synthesized NCs were largely influenced by the surfactant used in the wet-ball milling process. Compared with OA, long-chain amines such as OAM, OLA and BLA can produce emissive Cs–Pb–Br NCs. Besides, the emitting can be tuned from blue (OLA-functionalized) to cyan (OAM-functionalized) and green (BLA-functionalized) depending on the surfactant. To solve the dual emission characteristics observed in OAM-functionalized NCs, we tuned the ratio of Cs/Pb precursor added into the milling tank. Finally, with a ratio of Cs/Pb = 1.25, we received sharp (FWTH = 21 nm) green-emitting Cs–Pb–Br NCs with relatively high PLQY (86.11%). The current research demonstrated the effectiveness of mechanical milling to synthesize highly emitting compositional and structural tuning Cs–Pb–Br emitters.
Q. Xue acknowledges the open research fund of Songshan Lake Materials Laboratory (No. 2021SLABFN17), Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development (No. E239kf0901), Guangdong Basic and Applied Basic Research Foundation for Distinguished Young Scholar (No. 2021B1515020028), National Natural Science Foundation of China (No. 22379045) and TCL Young Scholars Program. X. Li and T. Zhang acknowledge the financial support from the Key Program of the National Natural Science Foundation of China (22133006), the National Natural Science Foundation of China (ZX20210286, 62074060), and the Fundamental Research Funds for the Central Universities (20CX06004A). The Taishan Scholar Program of Shandong Province (ts201712019, tsnq201909069) is also acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Bai, Z. Huang, X. Zhang, J. Z. Lu, X. X. Niu, Z. W. He, C. Zhu, M. Q. Xiao, Q. Z. Song, X. Y. Wei, C. Y. Wang, Z. H. Cui, J. Dou, Y. H. Chen, F. T. Pei, H. C. Zai, W. Wang, T. L. Song, P. F. An, J. Zhang, J. C. Dong, Y. M. Li, J. J. Shi, H. B. Jin, P. W. Chen, Y. C. Sun, Y. J. Li, H. N. Chen, Z. M. Wei, H. P. Zhou and Q. Chen, Science, 2022, 378(6621), 747–754 CrossRef CAS PubMed.
- L. Liu, A. Najar, K. Wang, M. Y. Du and S. Z. Liu, Adv. Sci., 2022, 9(7), 2104577 CrossRef CAS PubMed.
- J. Yang, Q. Bao, L. Shen and L. Ding, Nano Energy, 2020, 76, 105019 CrossRef CAS.
- K. Lin, J. Xing, L. N. Quan, F. P. G. de Arquer, X. W. Gong, J. X. Lu, L. Q. Xie, W. J. Zhao, D. Zhang, C. Z. Yan, W. Q. Li, X. Y. Liu, Y. Lu, J. Kirman, E. H. Sargent, Q. H. Xiong and Z. H. Wei, Nature, 2018, 562(7726), 245–248 CrossRef CAS PubMed.
- X. B. Tang, N. L. Kothalawala, Y. L. Zhang, D. L. Qian, D. Y. Kim and F. Q. Yang, Chem. Eng. J., 2021, 425, 131456 CrossRef CAS.
- H. Huang, C. Zuo, H. Zeng and L. Ding, J. Semicond., 2021, 42(3), 030202 CrossRef.
- L. Zhang, X. Pan, L. Liu and L. Ding, J. Semicond., 2022, 42(3), 030203 CrossRef.
- L. T. Dou, Y. Yang, J. B. You, Z. R. Hong, W. H. Chang, G. Li and Y. Yang, Nat. Commun., 2014, 5(1), 5404 CrossRef CAS PubMed.
- J. G. Feng, X. X. Yan, Y. Liu, H. F. Gao, Y. C. Wu, B. Su and L. Jiang, Adv. Mater., 2017, 29(16), 1605993 CrossRef PubMed.
- F. Yan and H. V. Demir, Nanoscale, 2019, 11(24), 11402–11412 RSC.
- Q. X. Zhong, M. H. Cao, Y. F. Xu, P. L. Li, Y. Zhang, H. C. Hu, D. Yang, Y. Xu, L. Wang, Y. Y. Li, X. H. Zhang and Q. Zhang, Nano Lett., 2019, 19(6), 4151–4157 CrossRef CAS PubMed.
- Q. G. Zhang, W. L. Zheng, Q. Wan, M. M. Liu, X. P. Feng, L. Kong and L. Li, Adv. Opt. Mater., 2021, 9(11), 2002130 CrossRef CAS.
- E. Erol, O. Kibrisli, M. C. Ersundu and A. E. Ersundu, Chem. Eng. J., 2020, 401, 126053 CrossRef CAS.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15(6), 3692–3696 CrossRef CAS PubMed.
- D. D. Yan, Q. H. Mo, S. Y. Zhao, W. S. Cai and Z. G. Zang, Nanoscale, 2021, 13(21), 9740–9746 RSC.
- J. F. Liao, Y. F. Xu, X. D. Wang, H. Y. Chen and D. B. Kuang, ACS Appl. Mater. Interfaces, 2018, 10(49), 42301–42309 CrossRef CAS PubMed.
- S. X. Wang, C. H. Bi, J. F. Yuan, L. X. Zhang and J. Tian, ACS Energy Lett., 2018, 3(1), 245–251 CrossRef CAS.
- J. F. Cao, Z. D. Yin, Q. Pang, Y. X. Lu, X. Q. Nong and J. Z. Zhang, J. Chem. Phys., 2021, 155(23), 234701 CrossRef CAS PubMed.
- Y. T. Cai, P. J. Zhang, W. H. Bai, L. Lu, L. Wang, X. Chen and R. J. Xie, ACS Sustainable Chem. Eng., 2022, 10(22), 7385–7393 CrossRef CAS.
- J. K. Park, J. H. Heo, B. W. Kim and S. H. Im, J. Ind. Eng. Chem., 2020, 92(25), 167–173 CrossRef CAS.
- L. Wang, D. C. Ma, C. Guo, X. Jiang, M. L. Li, T. T. Xu, J. P. Zhu, B. B. Fan, W. Liu, G. Shao, H. L. Xu, H. L. Wang, R. Zhang and H. X. Lu, Appl. Surf. Sci., 2021, 543(30), 148782 CrossRef CAS.
- Z. Y. Zhu, Q. Q. Yang, L. F. Gao, L. Zhang, A. Y. Shi, C. L. Sun, Q. Wang and H. L. Zhang, J. Phys. Chem. Lett., 2017, 8(7), 1610–1614 CrossRef CAS PubMed.
- S. Kumar, M. Regue, M. A. Isaacs, E. Freeman and S. Eslava, ACS Appl. Energy Mater., 2020, 3(5), 4509–4522 CrossRef CAS.
- L. Protesescu, S. Yakunin, O. Nazarenko, D. N. Dirin and M. V. Kovalenko, ACS Appl. Nano Mater., 2018, 1(3), 1300–1308 CrossRef CAS PubMed.
- Y. Li, H. Huang, Y. Xiong, S. V. Kershaw and A. L. Rogach, CrystEngComm, 2018, 20(34), 4900–4904 RSC.
- G. Maity and S. K. Pradhan, J. Alloys Compd., 2020, 816(5), 152612 CrossRef CAS.
- S. K. Balakrishnan and P. V. Kamat, Chem. Mater., 2018, 30(1), 74–78 CrossRef CAS.
- A. F. V. da Fonseca, B. R. C. Vale, T. A. D. S. Carvalho, J. Bettini, A. C. Pereira and M. A. Schiavon, J. Phys. Chem. C, 2021, 125(49), 27363–27371 CrossRef.
- C. Collantes, V. G. Pedro, M. J. Bañuls and Á. Maquieira, ACS Appl. Nano Mater., 2021, 4(2), 2011–2018 CrossRef CAS PubMed.
- Q. Jing, Y. Xu, Y. C. Su, X. Xing and Z. D. Lu, Nanoscale, 2019, 11(4), 1784–1789 RSC.
- B. S. Zhu, H. Z. Li, J. Ge, H. D. Li, Y. C. Yin, K. H. Wang, C. Chen, J. S. Yao, Q. Zhang and H. B. Yao, Nanoscale, 2018, 10(41), 19262–19271 RSC.
- G. C. Jiang, C. Guhrenz, A. Kirch, L. Sonntag, C. Bauer, X. L. Fan, J. Wang, S. Reineke, N. Gaponik and A. Eychmüller, ACS Nano, 2019, 13(9), 10386–10396 CrossRef CAS PubMed.
- K. Y. Baek, W. Lee, J. Lee, J. Kim, H. Ahn, J. I. Kim, J. Kim, H. Lim, J. Shin, Y. J. Ko, H. D. Lee, R. H. Friend, T. W. Lee, J. Lee, K. Kang and T. Lee, Nat. Commun., 2022, 13(1), 4263 CrossRef CAS PubMed.
- P. Acharyya, P. Pal, P. K. Samanta, A. Sarkar, S. K. Pati and K. Biswas, Nanoscale, 2019, 11(9), 4001–4007 RSC.
- S. Yang, J. Dai, Z. H. Yu, Y. C. Shao, Y. Zhou, X. Xiao, X. C. Zeng and J. S. Huang, J. Am. Chem. Soc., 2019, 141(14), 5781–5787 CrossRef CAS PubMed.
- B. H. Zhao, X. Y. Yan, T. Zhang, X. T. Ma, C. B. Liu, H. Y. Liu, K. Y. Yan, Y. L. Chen and X. Y. Li, ACS Appl. Mater. Interfaces, 2020, 12(8), 9300–9306 CrossRef CAS PubMed.
- Z. C. Li, L. Kong, S. Q. Huang and L. Li, Angew. Chem., Int. Ed., 2017, 56(28), 8134–8138 CrossRef CAS PubMed.
- Z. T. Wang, R. Fu, F. Li, H. D. Xie, P. W. He, Q. Sha, Z. B. Tang, N. Wang and H. Z. Zhong, Adv. Funct. Mater., 2021, 31(22), 2010009 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Milling ball dependent PL characteristics (Fig. S1), FTIR spectrum of surfactant functionalized Cs–Pb–Br NCs (Fig. S2), TEM characterizations (Fig. S3) and stability evaluation (Fig. S4). See DOI: https://doi.org/10.1039/d3cc01345f |
| This journal is © The Royal Society of Chemistry 2023 |