Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The way to AI-controlled synthesis: how far do we need to go?
Wei
Wang†
a,
Yingwei
Liu†
b,
Zheng
Wang
a,
Gefei
Hao
 *a and
Baoan
Song
*a and
Baoan
Song
 a
a
aState Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Research and Development Center for Fine Chemicals, Guizhou University, Guiyang 550025, P. R. China. E-mail: gefei_hao@foxmail.com
bState Key Laboratory of Public Big Data, Guizhou University, Guiyang 550025, P. R. China
First published on 27th September 2022
Abstract
Chemical synthesis always plays an irreplaceable role in chemical, materials, and pharmacological fields. Meanwhile, artificial intelligence (AI) is causing a rapid technological revolution in many fields by replacing manual chemical synthesis and has exhibited a much more economical and time-efficient manner. However, the rate-determining step of AI-controlled synthesis systems is rarely mentioned, which makes it difficult to apply them in general laboratories. Here, the history of developing AI-aided synthesis has been overviewed and summarized. We propose that the hardware of AI-controlled synthesis systems should be more adaptive to execute reactions with different phase reagents and under different reaction conditions, and the software of AI-controlled synthesis systems should have richer kinds of reaction prediction modules. An updated system will better address more different kinds of syntheses. Our viewpoint could help scientists advance the revolution that combines AI and synthesis to achieve more progress in complicated systems.
1 Introduction
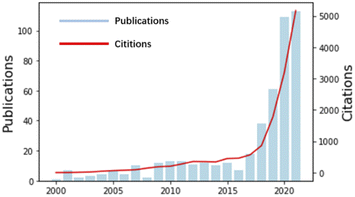
After decades of pioneering research in academia, it has been shown that chemical synthesis has long been an integral part of human life.1 Synthesis is everywhere; everything that can be heard, seen, smelled, tasted, and touched involves synthesis. However, completing the chemical synthesis process faster, safer, more economically and more efficiently is still an issue of concern all over the world. Nonetheless, traditional research methods may not be sufficiently efficient.2,3 Thus, people propose using AI techniques to assist with chemical synthesis.AI is based on computational machines, and the theory and application of computational machines have a long history and have been applied gradually in many fields since the last century. In 1948, Claude Shannon reported that information can be encoded by binary systems, which launched the field of information theory and set the stage for the merger of data science and chemical synthesis.4 With years of developing electronic techniques, more AI algorithms have been developed; therefore, the applications are not restricted to the development of simple tools.5–9 As shown in Fig. 1, from 2000 to 2021, studies combining AI and chemical synthesis were increasingly carried out. Especially in this five-year period, regardless of publications or citations, they are increasing exponentially. Currently, AI applications can be realized by different methods (e.g., support vector machines (SVMs),10 random forests (RFs),11 artificial neural networks (ANNs),12 convolutional neural networks (CNNs),13 recurrent neural networks (RNNs),14 and graph neural networks (GNNs)15). As shown in Table 1, AI algorithms are causing a rapid technological revolution in many fields of science, especially in the chemical field, with high process speed and high accuracy.16–20
| Methods | Classification | Case | Main work of case | Ref. |
|---|---|---|---|---|
| SVM | Kernel learning | Granda et al. | The reactivity of about 1000 combinations was predicted and the accuracy was more than 80% | 16 |
| RF | Decision tree | Ahneman et al. | The performance of C–N cross-coupling reactions was predicted by a RF module | 17 |
| CNN | ANN | Staker et al. | A structure prediction model was based on SMILES strings with more than 80% accuracy | 18 |
| RNN | ANN | Schwaller et al. | An attention-based model which is like the learning process of the human brain determined a better connection style to solve problems | 19 |
| GNN | ANN | Coley et al. | With the information on solvents, reactants and reagents, the model could fast predict the products of organic reactions in 100 ms | 20 |
It is not a new concept that AI is used as a chemical assist-tool. Corey et al. employed AI techniques to generate chemical pathways for complex molecules in 1969.21 AI as an assist-tool is also a promising field of research with the potential to have a tremendous impact on drug discovery, industrial chemistry, and materials science.22–25 For example, Li et al. used a unified and fully automated process to complete the synthesis of 14 different small molecules.26 Mijalis et al. reported an automated method for synthesizing polypeptides. The amide bond formation time was only seven seconds, and the total synthesis of each amino acid residue only required 40 seconds.27 Coley et al. combined AI and a series of hardware, making synthesis automation take a leap forward. They predicted and automated the synthesis of 15 drugs or drug-like molecules in 96 hours with an AI-based model.28 Clayton et al. reported a multistep reaction automatic optimization platform that could provide substantial savings in time and resources. This AI-based method had the ability to simultaneously optimize multistep processes for multiple goals.29 Gómez-Bombarelli et al. used a computational method to guide all stages of a materials synthesis workflow. They successfully synthesized efficient molecular organic light-emitting diodes.30 Minato et al. utilized a robotic workflow modular wheel platform (MWP) to generate new heteromultinuclear metal clusters with high energy barriers for magnetization reversal. With the assistance of the MWP, the time cycle of discovering new clusters is shortened.31 Chemical synthesis combined with AI could produce revolutionary achievements. Moreover, AI has also been widely applied in the chemical synthesis field due to the following advantages: (1) increasing the efficiency of chemical synthesis; (2) saving time and manpower; (3) avoiding errors from manual operation; and (4) increasing personal security. Here, the concept of “AI-controlled synthesis” is proposed. An AI-controlled synthesis system is dependent on multiple AI-aided synthesis platforms; that is, the technique of AI-controlled synthesis is a combination of several techniques of AI-aided synthesis. Therefore, a mature AI-controlled synthesis system should be made from at least two parts (Fig. 2): software as a controller in the control room and hardware as an executor in the laboratory. However, AI-aided synthesis systems are only applicable in some specific laboratories that have developed their own AI-aided synthesis systems.32–34 Many general laboratories still use manual operations to complete synthesis. Thus, we are facing an issue. How long do we need to go to extend the applications of AI-controlled synthesis into general laboratories?
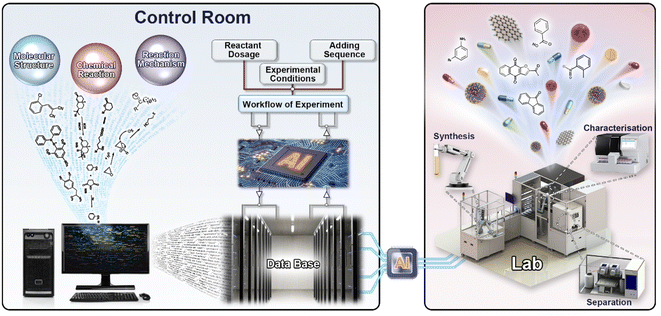 | ||
| Fig. 2 A mature AI-controlled chemical automatic synthesis laboratory (the software in the control room; the hardware in the lab; automation control components connecting them). | ||
In this work, we overviewed the current workflow of AI-aided synthesis and summarized current applications. We found the key restrictive processes for AI-controlled synthesis in general laboratories, and we also proposed some perspectives to overcome these limiting factors. We believe that this work could help overcome the difficulties that hinder AI-controlled synthesis in general laboratories.
2 Hardware: executor of AI-controlled synthesis
The hardware of the AI-controlled synthesis system is the equivalent of chemists' hands during manual operation. It plays the role of an executor and liberates chemists' hands. Its configuration can be as simple as a machine arm or be quite complex as a large automation platform.2.1 The configuration of the hardware
Traditionally, chemical syntheses are completed by manual operation. Nothing is more common than this. However, people, as traditional executors, have some disadvantages: (1) they incur large amounts of time and manpower costs; (2) they make errors; and (3) they pose security risks. Therefore, the traditional chemical synthesis laboratory is encouraged to do something to promote change. AI-controlled synthesis can be much safer and much faster and could greatly reduce human errors and guarantee the repeatability of experiments. In our opinion, the configuration of automated systems as hardware should have five modules (Fig. 3): reagent storage modules, reactors, the reaction analytic module, the purification module, and the device customization module.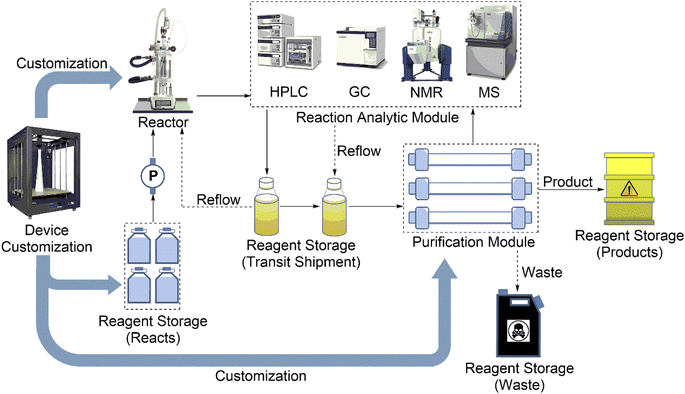 | ||
| Fig. 3 The configuration of the hardware includes five modules: reagent storage modules, reactors, the reaction analytic module, the purification module, and the device customization module. | ||
| Modules | Compositions | Techniques/methods | Maturity | Flaws |
|---|---|---|---|---|
| Reagent storage | (1) Laboratory bottles | (1) Anhydrous | Poor adaptability to solid reagents | (1) Reagent transport pipe or pump blockage |
| (2) Gas/liquid tanks | (2) Oxygen-free | (2) Solid reagents easily clump | ||
| (3) Injectors | (3) Difficult for grinding and precise weighing of solid reagents | |||
| (4) Waste tanks | ||||
| Reactor | (1) Reaction kettles | (1) Heater | Poor adaptability to different reagents | (1) Reagent transport pipe or pump blockage |
| (2) Condition makers | (2) Dehumidifier | (2) Blockage hindering or stopping the reaction | ||
| (3) Pumps and pipe | (3) Deaerator | (3) General reactors are not suited to specific reactions | ||
| (4) Reflux unit | ||||
| Analytic module | (1) Spectrometers | (1) IR, Raman, UV-vis | Low performance for real-time tracking | (1) Application of real-time tracking is lacking |
| (2) GC, HPLC | ||||
| (3) MS | ||||
| (4) NMR | ||||
| Purification module | (1) Chromatographic column | (1) Chromatographic | Automatic technique is not mature | (1) Reagent transport pipe or pump blockage |
| (2) Filter | (2) Purification processes sometimes need manual operation | |||
| (3) Fractionation tube | ||||
| Device customization module | (1) 3D printer | (1) Printing | Optional methods are limited | (1) Optional methods are lacking |
| (2) Machining | (2) Application promotion remains at a low stage, almost just for customization of specific reactors |
For the first function, the reactor, as the area of reaction, conducts synthesis reactions. At this time, the reactor is similar to a reagent storage module and provides a place where the starting materials react, and the reaction environment needs to be adjusted according to the requirements of the reactions. However, there are some points that differ between the reactor as a place of reaction and reagent storage. The reactor as a place of reaction can be deemed a kind of reagent storage module, but it always needs some attachments (e.g., a heater, dehumidifier, deaerator, and reflux unit) to achieve different reaction conditions.
For the second function, the reactor is used as a connector connecting multistep reactions. When a synthesis reaction is completed in the reactor, the products should be moved into the next area (it can be the final reagent storage module or the next reactor). For example, Angelone et al. employed the same reactors and reused them multiple times to perform multistep synthesis reactions.35 At this time, the reactor acts as a connector. Therefore, the connector can also be deemed an important part of the reactor.
2.2 Applications and flaws of current hardware
The hardware of the AI-controlled synthesis system should be made from the five parts discussed above. There are some applications that have been published. Perera et al. reported an automated flow-based synthesis platform for nanomole-scale reaction screening and micromole-scale synthesis.33 Taking into account the potential susceptibility of the assessed chemical to air and moisture, the reactor was configured in a glovebox (<20 ppm O2, <20 ppm H2O). A convenient and efficient purification module could greatly reduce manual work. Rougeot et al. described a device that enabled automatic sampling of soluble components (solutions only) and slurries (solids and solutions) in parallel.46 Liu et al. developed an automatic platform “SPS-flow” for processing solid-phase synthesis. By employing the attachment high-pressure gas pump, SPS-flow completed the solid-phase synthesis reaction in six steps in 32 h and obtained a good yield (65%). This work opened a door for the subsequent development of AI-controlled solid-phase synthesis.47However, existing systems are not perfect. There are some problems with the hardware (Table 2). Table 2 shows that the main flaw is the blockage of pipes or pumps with slurry. Flow system clogging includes several types: (1) crystallization of dissolved salts; (2) oil phase of the multiliquid phase; (3) particle deposits/bubbles present in the fluid; (4) chemical reaction (such as polymerization or decomposition) with wall materials or reactants, leading to solid precipitation. Therefore, precipitates formed during the reaction lead to irreversible blockage of reactor microchannels. This flaw has become a main barrier to the applications of AI-aided synthesis in general laboratories. Other flaws are mainly from devices with imperfect technologies. For example, some general reaction devices are not suited to specific reactions that need customized devices, the real-time tracking technique of the reaction analytics module needs to be improved, and manual operations of the purification module need to be resolved. Regardless of the flaw, they have reduced the performance of the hardware. Future hardware should deal better with different phases (especially solid), and require the seamless integration of sampling, reaction operations, purification separations, and analysis procedures.
3 Software: controller of AI-controlled synthesis
The software is the most important part of the AI-controlled synthesis system. As the center, the software not only stores and processes various data but also controls the behaviors of the hardware. This means that the AI-controlled synthesis system will be a lifeless system if there is no software. Therefore, the software should have the functions of recording, storing, processing and invoking data.3.1 The configuration of the software
The software is the core/brain of the system and the prerequisite for automation to become a reality. Generally, the software in automated systems for synthesis has three main functional categories: (1) controlling the hardware operating the synthesis reaction, (2) monitoring/analyzing the synthesis process, and (3) designing the synthesis strategy. To date, chemical synthesis, especially organic synthesis, is still a labor-intensive experimental subject. Robotic systems for the chemical domain are becoming increasingly popular due to the lack of general chemical programming languages and the ability to directly access natural language documents. A series of powerful AI algorithms are emerging quickly. They will perhaps make chemical synthesis no longer dependent on excessive human intervention. The rapid development of AI technology has brought rapid progress in experimental automation. The software should be made from four software modules (Fig. 4): (1) the reaction information module; (2) the reaction prediction module; (3) the reaction operation information module; and (4) the feedback recording module.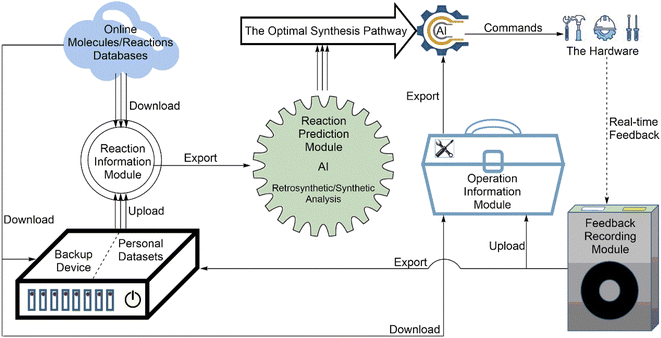 | ||
| Fig. 4 The configuration of the software includes four parts: the reaction information module, reaction prediction module, reaction operation information module, and feedback recording module. | ||
Moreover, the reaction information module should have a backup device as an attachment. When a network failure happens, the backup device can upload data to the reaction information module. If the main body of the reaction information module does not work, the backup device can also get data directly from online databases.
Retrosynthetic analysis is more frequently used by current programs46 because chemists often know products more than reactants. Many retrosynthetic analysis programs based on AI are available. For example, Automated System for Knowledge-based Continuous Organic Synthesis (ASKCOS) is a frequently used AI retrosynthesis prediction program.55 Other programs such as IBM RXN,56 Chemical.AI,57 and Molecule.one58 are famous programs. IBM RXN is also the first free AI web service for predicting chemical reactions. Forward-synthetic analysis is actually a reaction mechanism inference and is commonly used in ab initio or quantum-based prediction.59,60 AI-based forward-synthetic prediction is less common than retrosynthetic analysis. Although forward-synthetic analysis programs are few, it does not mean that there is no program to perform forward-synthetic analysis in the AI field. ASKCOS can also perform forward-synthetic analysis. New reaction prediction programs have also been developed recently. For example, researchers from AstraZeneca developed two AI-based open-source prediction programs REINVENT61,62 and GraphINVENT63–65 for guiding new drug molecule synthesis and exploring new chemical places of new drug molecules.
The feedback recording module first acts as a scout base for the software. It can be based on real-time feedback data from the hardware and help the software modify old commands or send new commands to the hardware. Therefore, the software can also use the feedback data from the hardware to improve its performance. Therefore, the feedback recording module plays a key role in supervising machine learning and improving software strategies.
3.2 Applications and flaws of current software
Recently, a series of powerful functions of AI algorithms have been rapidly developed in parallel, and they could greatly facilitate the development of platforms that could automate custom general synthesis routes. There are many applications for the software to be reported. For example, Burger et al. developed an AI robot named the mobile robotic chemist and proposed the concept “AI Chemist”. AI Chemist can complete research on approximately 1000 precatalyst compounds a week, which is a burdensome task that a PhD student needs 4 years to complete.66 Cronin et al. developed a general system for chemical digitization and automatic synthesis that could automatically read the literature.67 This is another epoch-making achievement in the field of AI-based chemistry following the Chemputer.68 By combining Chemical Description Language (χDL) and the easy-to-use interface ChemIDE, ChemIDE allows users to program chemical synthesis without any coding knowledge. This “paper in, product out” AI chemist took synthesis automation to a new level. Surprisingly, Stuyver et al. reported this year that they combined QM with GNN learning to develop an ml-QM-GNN module for forward-synthetic analysis. The ml-QM-GNN module can not only predict the activation energy, reactivity, and chemoselectivity of reactants but also find the preferred reaction mode.69 This work provided a new way of developing forward-synthetic analysis methods with a combination of ab initio and machine learning. Applications of software are becoming increasingly widespread. Software is the brain of automated platforms that help chemists control the entire synthesis process. Existing software has much room for improvement in designing reaction routes.There are still some flaws in the software that need to be considered. In Table 3, we find that the current software as a controller of AI-controlled synthesis systems has two main flaws: (1) AI-based forward-synthetic analysis programs are few; (2) weakly integrating computer-aided synthesis planning (CASP) tools; and (3) the data of failed syntheses are not attended to. Many chemists want to know the synthesis route for a product. Retrosynthetic analysis has been developed and used widely. However, many laboratories focus on developing new synthesis methods and generating new compounds. Traditional forward-synthetic analysis is mainly based on QM calculations but not AI calculations. This hinders AI-controlled synthesis systems in laboratories that are interested in finding new synthesis methods and new compounds. Because pure retrosynthetic analysis can obtain many synthesis pathways with many different reactions, many of the predicted synthesis pathways are unhelpful for some general chemosynthesis laboratories. Therefore, a mature forward-synthetic analysis with special reactants can guide these general chemosynthesis laboratories to generate new compounds or to explore new synthesis methods. This is more helpful than retrosynthetic analysis to increase the success rate of synthesis experiments in wet laboratories. Some software weakly integrates CASP tools. This makes the software rely too much on the internet (e.g., online CASP tools and online databases). Moreover, the data of failed syntheses as feedback can make the software avoid previous mistakes. Thus, software with more kinds of synthetic analysis, including CASP tools, should receive more attention in the future.
| Modules | Data source | Main jobs | Maturity | Flaws |
|---|---|---|---|---|
| Reaction information module/operation information module | (1) Online databases | (1) Obtaining and processing data | The information modules are overly dependent on online databases, but the backup device is not valued | (1) Many databases are not open source |
| (2) Personal datasets | (2) Storing data | (2) The software integrates online databases weakly | ||
| (3) Invoking data | (3) The backup device is not valued | |||
| (4) User-friendliness is insufficient | ||||
| Reaction prediction module | (1) Machine learning | (1) Synthetic analysis | Retrosynthetic analysis is used widely, but AI-based forward-synthetic analysis is lacking | (1) Few AI-based forward-synthetic analysis programs are used |
| (2) QM calculations | (2) Finding an optical synthesis path out | (2) The software integrates CASP tools weakly | ||
| (3) Ab initio calculations | (3) User-friendliness is insufficient | |||
| Feedback recording module | (1) The hardware | (1) Obtaining real-time feedback data from the hardware | The data of failed syntheses are usually discarded | (1) The data of failed syntheses are not valued |
| (2) Sending data to information modules |
4 Automation control component: the bridge between software and hardware
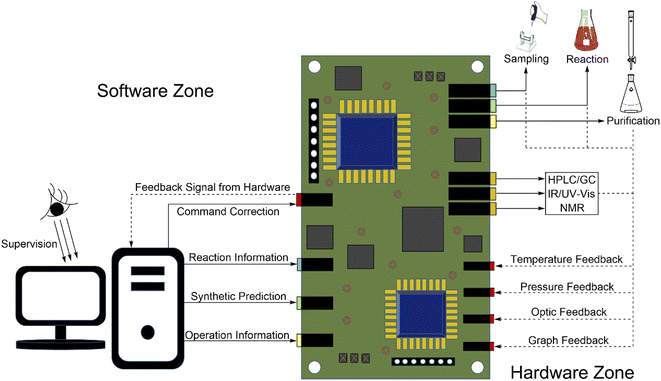
We have pointed out that the software controls every behavior of the hardware to perform AI-controlled synthesis. Chemists need a/some device(s) to connect the software and the hardware (Fig. 2). For a control system, the automation control component (also called the digitization control component) is usually the first choice. There are some advantages to the automation control component: (1) mature technology; (2) various implementation methods; and (3) running stably. The automation control component is generally embedded. This means that the automation control component does not need to occupy a larger space. Currently, people can directly use microcontroller units or employ integrated chips or even integrated programs into the software.As shown in Fig. 5, we can directly know the working principle of the automation control component. First, we introduced that the software controls the hardware to execute synthesis reactions in the AI-controlled synthesis system. As shown in Fig. 5, the software sends commands that include reaction and operation information and synthetic prediction information to the automation control component. Then, the automation control component processes them and hands them to the hardware to perform sampling, reaction, purification, etc. Second, we note that the software should have a feedback information module to obtain feedback information from the hardware. Actually, the automation control component also plays the role of a sensor. The different original feedback signals mainly come from the hardware and are input to different sensor units of the automation control component. Then, the automation control component processes these feedback signals and sends them to the feedback information module of the software so that the software can more flexibly correct the controlling strategies. Overall, the automation control component is a connected hub that connects the software and the hardware.
After introducing the working principle of the automation control component, we obtain a question. Which type of automation control component has been used for connecting the software and the hardware? Hammer et al. reported that chemists mainly use microcontroller units with Python or C++ codes to control the hardware for simple synthesis.70 Prabhu et al. pointed out that chemical researchers can choose multiple types of microcontroller units to make the software control the hardware (Fig. 5). Chemists can directly use microcontroller boards (e.g., Arduino 101, Ardbox PLC, chipKIT Uno32, and Particle Argon) or choose single-board computers (e.g., Raspberry Pi 3 and BeagleBone Black).71 Wilbraham et al. reported that they utilized χDL to drive the microcontroller unit “chemical processing unit (ChemPU)” for controlling, optimizing, and exploring synthesis reactions.72 In this year, Cronin et al. improved the compatibility between χDL and ChemPU. They employed this more mature automatic platform to process 53 kinds of chemical reactions, which are among the 103 kinds of reactions that are encoded by χDL, and obtained good results and high yields. This work makes it possible to replicate chemical synthesis experiments from previous literature, and also greatly improves the performance of current AI-controlled synthesis systems.73 The real jobs of chemists are just to rewrite or to modify codes in C++, Python, or even in their own machine languages.
According to these reports above, we find that an automation control system can already be executed on a personal computer. But there are still two unfavorable factors around the automation control system in current AI-aided synthesis platforms: (1) the technical barrier is high for general chemists; (2) open-source application programming interfaces (APIs) of commercial programs on third-party platforms are lacking. Although applications of the automation control system may have been sufficient for the automation control component of AI-aided synthesis, the development of a more intelligentized automation control component of AI-aided synthesis should become chemists' focus in the future.
5 Perspective and outlook
From the contents above, we can find that AI-aided synthesis systems are increasingly widely used in the chemical synthesis field. However, the rate-determining step of AI-controlled synthesis systems is rarely mentioned, which makes it difficult to apply in general laboratories. Recently, many chemists have been developing new devices for AI-controlled synthesis and trying to improve the performance of AI-controlled synthesis systems. Therefore, we have some perspectives for the subsequent development of AI-controlled synthesis systems.We propose some viewpoints for the hardware of AI-controlled synthesis systems:
(1) More adaptable. The hardware should execute the synthesis reaction in different phases. It needs to add a new main module as a cleaner to clean blockage from solid or multiliquid reagents. The module can be an ultrasonic generator or a high-pressure air/liquid pump.
(2) More customization devices. General devices are sometimes not suited to some specific synthesis reactions. This is a hindrance to AI-controlled synthesis in general laboratories. In addition to 3D printing techniques, more methods of updating or even building specific devices should be developed.
(3) More automatic. Manual operations of the purification module reduce the automatic performance of the hardware. The purification module should load flow controllers and automatic replacement devices to simulate and replace chemists' hands.
(4) Quicker. Real-time tracking is important for the process of a reaction. The reaction analytic module should be quicker to analyze the situation of products.
(5) More convenient. An automated chemical operation platform should save the time cost of chemical experts. The system should not be too complicated such that it would require much training for operators to operate the equipment.
(6) Lower economic investment. Fund-rich laboratories are not everywhere. Thus, lower cost is helpful to make AI-controlled synthesis migrate into general laboratories.
We propose some viewpoints for the software of AI-controlled synthesis systems:
(1) AI-based forward-synthetic analysis. The development of forward-synthetic analysis programs should be attended to. Many general laboratories focus on exploring new synthesis methods and new products from specific reactants. Predicting possible products from known reactants is necessary. On the other hand, the success rate of choosing the right synthesis reaction path is higher with forward-synthetic analysis. Hence, they need forward-synthetic analysis rather than retrosynthetic analysis. Forward-synthetic analysis has some advantages that retrosynthesis analysis does not have. It is helpful to extend AI-controlled synthesis systems into laboratories that focus on developing new synthesis methods or discovering new compounds.
(2) Paying attention to negative data. Chemists generally pay attention to the positive data that they obtain, but the negative data (e.g., infeasible reaction data and low yield reaction data) are always skipped. Therefore, the reaction information module of the software should add the information on negative data. Negative data can offer failed examples for the AI-controlled synthesis system so that the AI-controlled synthesis system can avoid unnecessary mistakes.
(3) Combined with CASP tools. At present, chemical automation studies focusing on synthesis planning are rarely reported, and they are much more complicated than the automation of the synthesis process. Thus, an automated system that includes CASP tools should receive more attention in the future.
(4) Greater interactivity. The software should be able to intervene in the entire reaction process in a timely manner while monitoring the reaction process to ensure the synthesis effect and avoid potential safety hazards.
(5) User-friendliness of the CASP programs integrated into automated systems. The existing command line programs may be suitable for chemo-informatics, but some chemists use graphical user interfaces (GUIs).
6 Conclusion
A series of powerful advancements in the hardware and software of AI-controlled synthesis systems are emerging quickly and in parallel. They have the potential to make synthesizer development possible and automate custom and general synthesis routes. AI-controlled synthesis has the following advantages: (1) safety; (2) high speed; (3) reproducibility; and (4) low cost of synthesizing compounds. We have pointed out some limiting factors that mean AI-controlled synthesis systems cannot be extended to general laboratories. The hardware of the AI-controlled synthesis system should include a cleaner module to keep the hardware channels open. Regardless of solid or multiliquid reagents, the improved hardware can use the cleaning module to clean blockages in channels. For the software of the AI-controlled synthesis system, its reaction prediction module should have more mature AI-based forward-synthetic analysis functions. It is helpful to extend the AI-controlled synthesis system into general laboratories that are interested in developing new organic synthesis methods. In summary, an AI-controlled synthesis system could quickly and accurately control reaction conditions such as temperature and pressure to handle sensitive chemicals and dangerous reactions more safely.74–76 Fortunately, some groups are trying to overcome these challenges from the flaws of current AI-controlled synthesis systems and have made some achievements.35,47,69,73 Beyond all doubt, with the development of AI algorithms of the software and engineering technology of the hardware, we believe that chemists will quickly move toward the goal of AI-controlled synthesis. Therefore, extending the method of AI-controlled synthesis into general laboratories should not take much longer.Funding
This work was supported by the National Natural Science Foundation of China (32125033) and the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023).Author contributions
Mr Wei Wang wrote mainly the manuscript. Mr Yingwei Liu and Prof Gefei Hao provided guidance on knowledge of machine learning algorithms. Mr Wei Wang, Mr Zheng Wang and Prof. Baoan Song provided support on knowledge of chemical synthesis. Prof. Gefei Hao and Prof. Baoan Song provided suggestions to improve and modify the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Prof. Dr Connor W. Coley for providing worthy suggestions which helped us to improve this manuscript.References
- Beauties of synthesis, Nature, 2006, 443(7107) DOI:10.1038/443001b.
- I. W. Davies, The digitization of organic synthesis, Nature, 2019, 570(7760), 175 CrossRef CAS PubMed.
- J. Vamathevan, D. Clark, P. Czodrowski, I. Dunham, E. Ferran, G. Lee, B. Li, A. Madabhushi, P. Shah and M. Spitzer, Applications of machine learning in drug discovery and development, Nat. Rev. Drug Discovery, 2019, 18(6), 463 CrossRef CAS PubMed.
- C. E. Shannon, A mathematical theory of communication, Bell Syst. Tech. J., 1948, 27, 379 CrossRef.
- G. F. Hao, W. Jiang, Y.-N. Ye, F.-X. Wu, X.-L. Zhu, F.-B. Guo and G. Yang, ACFIS: a web server for fragment-based drug discovery, Nucleic Acids Res., 2016, 44, W550 CrossRef CAS PubMed.
- M.-X. Chen, L.-C. Mei, F. Wang, I. K. W. Boyagane Dewayalage, J.-F. Yang, L. Dai, G.-F. Yang, B. Gao, C.-L. Cheng and Y.-G. Liu, PlantSPEAD: a web resource towards comparatively analysing stress-responsive expression of splicing-related proteins in plant, Plant Biotechnol. J., 2021, 19(2), 227 CrossRef CAS PubMed.
- Y.-L. Wang, F. Wang, X.-X. Shi, C.-Y. Jia, F.-X. Wu, G.-F. Hao and G.-F. Yang, Cloud 3D-QSAR: a web tool for the development of quantitative structure–activity relationship models in drug discovery, Briefings Bioinf., 2020, 22(4), bbaa276 CrossRef PubMed.
- F.-X. Wu, F. Wang, J.-F. Yang, W. Jiang, M.-Y. Wang, C.-Y. Jia, G.-F. Hao and G.-F. Yang, AIMMS suite: a web server dedicated for prediction of drug resistance on protein mutation, Briefings Bioinf., 2018, 21(1), 318 Search PubMed.
- F. Wang, J. F. Yang, M. Y. Wang, C. Y. Jia, X. X. Shi, G. F. Hao and G. F. Yang, Graph attention convolutional neural network model for chemical poisoning of honey bees? Prediction, Sci. Bull., 2020, 65(14), 1184 CrossRef CAS.
- C. Cortes and V. N. Vapnik, Support-vector networks, Mach. Learn., 1995, 20(3), 273 Search PubMed.
- L. Breiman, Random Forests, Mach. Learn., 2001, 45(1), 5 CrossRef.
- I. N. d. Silva, D. H. Spatti, R. A. Flauzino, L. H. B. Liboni and S. F. d. R. Alves, Artificial Neural Networks, Springer, 2017 Search PubMed.
- Q. R. Zhang, M. Zhang, T. H. Chen, Z. F. Sun, Y. Z. Ma and B. Yu, Recent advances in convolutional neural network acceleration, Neurocomputing, 2019, 323, 37 CrossRef.
- L. Medsker and L. C. Jain, Recurrent Neural Networks: Design and Applications, CRC Press, 1999 Search PubMed.
- Z. H. Wu, S. R. Pan, F. W. Chen, G. D. Long, C. Q. Zhang and P. S. Yu, A Comprehensive Survey on Graph Neural Networks, IEEE Transactions on Neural Networks and Learning Systems, 2021, 32(1), 4 Search PubMed.
- J. M. Granda, L. Donina, V. Dragone, D. L. Long and L. Cronin, Controlling an organic synthesis robot with machine learning to search for new reactivity, Nature, 2018, 562(7728), E26 CrossRef CAS.
- D. T. Ahneman, J. G. Estrada, S. S. Lin, S. D. Dreher and A. G. Doyle, Predicting reaction performance in C-N cross-coupling using machine learning, Science, 2018, 360(6385), 186 CrossRef CAS PubMed.
- J. Staker, K. Marshall, R. Abel and C. M. McQuaw, Molecular Structure Extraction from Documents Using Deep Learning, J. Chem. Inf. Model., 2019, 59(3), 1017 CrossRef CAS PubMed.
- P. Schwaller, T. Gaudin, D. Lanyi, C. Bekas and T. Laino, “Found in translation”: predicting outcomes of complex organic chemistry reactions using neural sequence-to-sequence models, Chem. Sci., 2018, 9, 6091 RSC.
- C. W. Coley, W. G. Jin, L. Rogers, T. F. Jamison, T. S. Jaakkola, W. H. Green, R. Barzilay and K. F. Jensen, A graph-convolutional neural network model for the prediction of chemical reactivity, Chem. Sci., 2019, 10(2), 370 RSC.
- E. J. Corey and W. T. Wipke, Computer-Assisted Design of Complex Organic Syntheses, Science, 1969, 166(3902), 178 CrossRef CAS PubMed.
- Y. Shen, J. E. Borowski, M. A. Hardy, R. Sarpong, A. G. Doyle and T. Cernak, Automation and computer-assisted planning for chemical synthesis, Nat. Rev. Methods Primers, 2021, 1(1), 23 CrossRef CAS.
- Z. Wang, W. Zhao, G. F. Hao and B. A. Song, Automated synthesis: current platforms and further needs, Drug Discovery Today, 2020, 25(11), 2006 CrossRef CAS PubMed.
- Z. Wang, W. Zhao, G. Hao and B. Song, Mapping the resources and approaches facilitating computer-aided synthesis planning, Org. Chem. Front., 2021, 8(4), 812 RSC.
- Y. L. Wang, J. Y. Li, X. X. Shi, Z. Wang, G. F. Hao and G. F. Yang, Web-Based Quantitative Structure-Activity Relationship Resources Facilitate Effective Drug Discovery, Top. Curr. Chem., 2021, 379(6), 37 CrossRef CAS.
- J. Q. Li, S. G. Ballmer, E. P. Gillis, S. Fujii, M. J. Schmidt, A. M. E. Palazzolo, J. W. Lehmann, G. F. Morehouse and M. D. Burke, Synthesis of many different types of organic small molecules using one automated process, Science, 2015, 347(6227), 1221 CrossRef CAS PubMed.
- A. J. Mijalis, D. A. Thoma, M. D. Simon, A. Adamo, R. Beaumont, K. F. Jensen and B. L. Pentelute, A fully automated flow-based approach for accelerated peptide synthesis, Nat. Chem. Biol., 2017, 13(5), 464 CrossRef CAS PubMed.
- C. W. Coley, D. A. Thomas, J. A. M. Lummiss, J. N. Jaworski, C. P. Breen, V. Schultz, T. Hart, J. S. Fishman, L. Rogers and H. Y. Gao, A robotic platform for flow synthesis of organic compounds informed by AI planning, Science, 2019, 365(6453), 557 CrossRef.
- A. D. Clayton, A. M. Schweidtmann, G. Clemens, J. A. Manson, C. J. Taylor, C. G. Nino, T. W. Chamberlain, N. Kapur, A. J. Blacker and A. A. Lapkin, Automated self-optimisation of multi-step reaction and separation processes using machine learning, Chem. Eng. J., 2020, 384, 123340 CrossRef CAS.
- R. Gomez-Bombarelli, J. Aguilera-Iparraguirre, T. D. Hirzel, D. Duvenaud, D. Maclaurin, M. A. Blood-Forsythe, H. S. Chae, M. Einzinger, D. G. Ha and T. Wu, Design of efficient molecular organic light-emitting diodes by a high-throughput virtual screening and experimental approach, Nat. Mater., 2016, 15(10), 1120 CrossRef CAS PubMed.
- T. Minato, D. Salley, N. Mizuno, K. Yamaguchi, L. Cronin and K. Suzuki, Robotic Stepwise Synthesis of Hetero-Multinuclear Metal Oxo Clusters as Single-Molecule Magnets, J. Am. Chem. Soc., 2021, 143(32), 12809 CrossRef CAS PubMed.
- J. Li, S. G. Ballmer, E. P. Gillis, S. Fujii, M. J. Schmidt, A. M. E. Palazzolo, J. W. Lehmann, G. F. Morehouse and M. D. Burke, Synthesis of many different types of organic small molecules using one automated process, Science, 2015, 347(6227), 1221 CrossRef CAS PubMed.
- D. Perera, J. W. Tucker, S. Brahmbhatt, C. J. Helal, A. Chong, W. Farrell, P. Richardson and N. W. Sach, A platform for automated nanomole-scale reaction screening and micromole-scale synthesis in flow, Science, 2018, 359(6374), 429 CrossRef CAS PubMed.
- S. Steiner, J. Wolf, S. Glatzel, A. Andreou, J. M. Granda, G. Keenan, T. Hinkley, G. Aragon-Camarasa, P. J. Kitson and D. Angelone, Organic synthesis in a modular robotic system driven by a chemical programming language, Science, 2019, 363(6423), eaav2211 CrossRef CAS PubMed.
- D. Angelone, A. J. S. Hammer, S. Rohrbach, S. Krambeck, J. M. Granda, J. Wolf, S. Zalesskiy, G. Chisholm and L. Cronin, Convergence of multiple synthetic paradigms in a universally programmable chemical synthesis machine, Nat. Chem., 2021, 13(1), 63 CrossRef CAS.
- M. T. Drexler, D. A. Foley, H. W. Ward and H. J. Clarke, IR and NMR Reaction Monitoring Techniques for Nucleophilic Addition Reactions: In Situ Monitoring of the Addition of Benzimidazole to a Pyridinium Salt, Org. Process Res. Dev., 2015, 19(9), 1119 CrossRef CAS.
- K. Grabow and U. Bentrup, Homogeneous Catalytic Processes Monitored by Combined in Situ ATR-IR, UV-Vis, and Raman Spectroscopy, ACS Catal., 2014, 4(7), 2153 CrossRef CAS.
- G. Q. Chen, P. Y. Cao and R. J. Liu, A multi-residue method for fast determination of pesticides in tea by ultra performance liquid chromatography-electrospray tandem mass spectrometry combined with modified QuEChERS sample preparation procedure, Food Chem., 2011, 125(4), 1406 CrossRef CAS.
- M. Vinaixa, E. L. Schymanski, S. Neumann, M. Navarro, R. M. Salek and O. Yanes, Mass spectral databases for LC/MS- and GC/MS-based metabolomics: state of the field and future prospects, TrAC, Trends Anal. Chem., 2016, 78, 23 CrossRef CAS.
- D. A. Foley, J. Wang, B. Maranzano, M. T. Zell, B. L. Marquez, Y. Q. Xiang and G. L. Reid, Online NMR and HPLC as a Reaction Monitoring Platform for Pharmaceutical Process Development, Anal. Chem., 2013, 85(19), 8928 CrossRef CAS.
- L. Cronin, S. H. M. Mehr and J. M. Granda, Catalyst: The Metaphysics of Chemical Reactivity, Chem, 2018, 4(8), 1759 CAS.
- M. Bornemann-Pfeiffer, J. Wolf, K. Meyer, S. Kern, D. Angelone, A. Leonov, L. Cronin and F. Emmerling, Standardization and Control of Grignard Reactions in a Universal Chemical Synthesis Machine Using Online NMR, Angew. Chem., Int. Ed., 2021, 60(43), 23202 CrossRef CAS.
- J. E. Grob, M. A. Dechantsreiter, R. B. Tichkule, M. K. Connolly, A. Honda, R. C. Tomlinson and L. G. Hamann, One-Pot C-N/C-C Cross-Coupling of Methyliminodiacetic Acid Boronyl Arenes Enabled by Protective Enolization, Org. Lett., 2012, 14(21), 5578 CrossRef CAS PubMed.
- S. O'Neill, AI-Driven Robotic Laboratories Show Promise, Engineering, 2021, 7(10), 1351 CrossRef.
- A. Bubliauskas, D. J. Blair, H. Powel-Davies, P. J. Kitson, M. D. Burke and L. Cronin, Digitizing Chemical Synthesis in 3D Printed Reactionware, Angew. Chem., Int. Ed., 2022, 61, e202116108 ( Angew. Chem. , 2022 , 134 , e202116108 ) CrossRef CAS PubMed.
- C. Rougeot, H. Situ, B. H. Cao, V. Vlachos and J. E. Hein, Automated reaction progress monitoring of heterogeneous reactions: crystallization-induced stereoselectivity in amine-catalyzed aldol reactions, React. Chem. Eng., 2017, 2(2), 226 RSC.
- C. G. Liu, J. X. Xie, W. B. Wu, M. Wang, W. H. Chen, S. B. Idres, J. W. Rong, L. W. Deng, S. A. Khan and J. Wu, Automated synthesis of prexasertib and derivatives enabled by continuous-flow solid-phase synthesis, Nat. Chem., 2021, 13(5), 451 CrossRef CAS PubMed.
- W. A. A. Warr, Short Review of Chemical Reaction Database Systems, Computer-Aided Synthesis Design, Reaction Prediction and Synthetic Feasibility, Mol. Inf., 2014, 33(6–7), 469 CrossRef CAS PubMed.
- J. Blake and R. Dana, CASREACT: more than a million reactions, J. Chem. Inf. Comput. Sci., 1990, 30, 394 CrossRef CAS.
- J. S. Schreck, C. W. Coley and K. J. M. Bishop, Learning Retrosynthetic Planning through Simulated Experience, ACS Cent. Sci., 2019, 5(6), 970 CrossRef CAS PubMed.
- G. Marcou, J. A. de Sousa, D. A. R. S. Latino, A. de Luca, D. Horvath, V. Rietsch and A. Varnek, Expert System for Predicting Reaction Conditions: The Michael Reaction Case, J. Chem. Inf. Model., 2015, 55(2), 239 CrossRef CAS.
- D. Lowe, Chemical reactions from US patents (1976-Sep 2016), figshare, 2017 DOI:10.6084/m9.figshare.5104873.v1.
- J. Guo, A. S. Ibanez-Lopez, H. Y. Gao, V. Quach, C. W. Coley, K. F. Jensen and R. Barzilay, Automated Chemical Reaction Extraction from Scientific Literature, J. Chem. Inf. Model., 2021, 61(8), 4124, DOI:10.1021/acs.jcim.1c00284.
- S. M. Kearnes, M. R. Maser, M. Wleklinski, A. Kast, A. G. Doyle, S. D. Dreher, J. M. Hawkins, K. F. Jensen and C. W. Coley, The Open Reaction Database, J. Am. Chem. Soc., 2021, 143(45), 18820 CrossRef CAS PubMed.
- Available from: https://askcos.mit.edu.
- Available from: https://rxn.res.ibm.com.
- Available from: https://chemical.ai.
- Available from: https://molecule.one.
- B. Huang and O. A. von Lilienfeld, Ab Initio Machine Learning in Chemical Compound Space, Chem. Rev., 2021, 121(16), 10001 CrossRef CAS.
- O. A. von Lilienfeld, K. R. Muller and A. Tkatchenko, Exploring chemical compound space with quantum-based machine learning, Nat. Rev. Chem., 2020, 4(7), 347 CrossRef.
- T. Blaschke, J. Arús-Pous, H. Chen, C. Margreitter, C. Tyrchan and O. Engkvist, REINVENT 2.0 – An AI Tool for De Novo Drug Design, ChemRxiv, Cambridge Open Engage, Cambridge, 2020.
- Available from: https://github.com/MolecularAI/Reinvent.
- R. Mercado, T. Rastemo, E. Lindelof, G. Klambauer, O. Engkvist, H. M. Chen and E. J. Bjerrum, Graph networks for molecular design, Mach. Learn., 2021, 2(2), 025023 Search PubMed.
- R. Mercado, T. Rastemo, E. Lindelöf, G. Klambauer, O. Engkvist, H. Chen, E. J. Bjerrum, Practical notes on building molecular graph generative models, ChemRxiv, Cambridge: Cambridge Open Engage, 2020, This content is a preprint and has not been peer-reviewed Search PubMed.
- Available from: https://github.com/MolecularAI/GraphINVENT.
- B. Burger, P. M. Maffettone, V. V. Gusev, C. M. Aitchison, Y. Bai, X. Wang, X. Li, B. M. Alston, B. Li and R. Clowes, A mobile robotic chemist, Nature, 2020, 583(7815), 237 CrossRef CAS PubMed.
- S. H. M. Mehr, M. Craven, A. I. Leonov, G. Keenan and L. Cronin, A universal system for digitization and automatic execution of the chemical synthesis literature, Science, 2020, 370(6512), 101 CrossRef CAS PubMed.
- S. Steiner, J. Wolf, S. Glatzel, A. Andreou, J. M. Granda, G. Keenan, T. Hinkley, G. Aragon-Camarasa, P. J. Kitson and D. Angelone, Organic synthesis in a modular robotic system driven by a chemical programming language, Science, 2019, 363(6423), 144 CrossRef PubMed.
- T. Stuyver and C. W. Coley, Quantum chemistry-augmented neural networks for reactivity prediction: performance, generalizability, and explainability, J. Chem. Phys., 2022, 156(8), 084104 CrossRef CAS.
- A. J. S. Hammer, A. I. Leonov, N. L. Bell and L. Cronin, Chemputation and the Standardization of Chemical Informatics, JACS Au, 2021, 1(10), 1572 CrossRef CAS.
- G. R. D. Prabhu, T. H. Yang, C. Y. Hsu, C. P. Shih, C. M. Chang, P. H. Liao, H. T. Ni and P. L. Urban, Facilitating chemical and biochemical experiments with electronic microcontrollers and single-board computers, Nat. Protoc., 2020, 15(3), 925 CrossRef CAS.
- L. Wilbraham, S. H. M. Mehr and L. Cronin, Digitizing Chemistry Using the Chemical Processing Unit: From Synthesis to Discovery, Acc. Chem. Res., 2021, 54(2), 253 CrossRef CAS.
- S. Rohrbach, M. Šiaučiulis, G. Chisholm, P.-A. Pirvan, M. Saleeb, S. H. M. Mehr, E. Trushina, A. I. Leonov, G. Keenan and A. Khan, Digitization and validation of a chemical synthesis literature database in the ChemPU, Science, 2022, 377(6602), 172 CrossRef CAS PubMed.
- K. P. Cole, J. M. Groh, M. D. Johnson, C. L. Burcham, B. M. Campbell, W. D. Diseroad, M. R. Heller, J. R. Howell, N. J. Kallman and T. M. Koenig, Kilogram-scale prexasertib monolactate monohydrate synthesis under continuous-flow CGMP conditions, Science, 2017, 356(6343), 1144 CrossRef CAS PubMed.
- D. R. Snead and T. F. Jamison, A Three-Minute Synthesis and Purification of Ibuprofen: Pushing the Limits of Continuous-Flow Processing, Angew. Chem., Int. Ed., 2015, 54(3), 983 CrossRef CAS PubMed.
- M. Trobe and M. D. Burke, The Molecular Industrial Revolution: Automated Synthesis of Small Molecules, Angew. Chem., Int. Ed., 2018, 57(16), 4192 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |