Open Access Article
Open Access ArticleCracking the immune fingerprint of metal–organic frameworks†
T.
Hidalgo
 ab,
R.
Simón-Vázquez
ab,
R.
Simón-Vázquez
 cd,
A.
González-Fernández
cd,
A.
González-Fernández
 cd and
P.
Horcajada
cd and
P.
Horcajada
 *a
*a
aAdvanced Porous Materials Unit (APMU), IMDEA Energy Institute, Av. Ramón de la Sagra 3, 28935 Móstoles-Madrid, Spain. E-mail: patricia.horcajada@imdea.org
bInstitut Lavoisier, UMR CNRS 8180, Université de Versailles Saint-Quentin-en-Yvelines, 45 Av. des Etats-Unis, 78035 Versailles Cedex, France
cCINBIO, Immunology Group, Universidade de Vigo, 36310 Vigo, Spain
dInstituto de Investigación Sanitaria Galicia Sur (IIS Galicia Sur), SERGAS-UVIGO, Spain
First published on 5th November 2021
Abstract
The human body is in a never-ending chess game against pathogens. When the immune system, our natural defence tool, is weakened, these organisms are able to escape, overcoming the body's contingency plan, which results in the body going into a pathological state. To overcome this checkmate status, emerging nanomedicines have been successfully employed as one of the best tactics for boosting the immune response, manipulating the body's defence tools for the specific recognition/elimination of pathological cells via the active ingredient delivery. However, the vast majority of these drug-delivery systems (DDS) are considered to be exclusively passive vehicles, with nanoscale metal–organic frameworks (nanoMOFs) attracting a great deal of attention due to their versatility and ability to carry and deliver exceptional drug payloads and to modulate their biological bypass. Nonetheless, their intrinsic immunogenicity character has been never addressed. Considering the immense possibilities that nanoMOFs offer as a treatment platform, the present study aimed to unveil the immunological fingerprint of MOFs, including an in-deep evaluation of the cellular oxidation balance, the inflammation and recruitment of immune cells and the precise Th1/Th2 cytokine profile that is triggered. This study aims to gain insights that will make more feasible the design of customized immune-active MOF nanoplatforms according to targeted diseases, as the next ace up immune system sleeve.
Introduction
The use of immunotherapy to trigger the adequate cornerstones of the immune system is a recent tactic to treat challenging illnesses (e.g. cancer, infection, autoimmune diseases) as it allows manipulating the body's defence tools like in a game of chess. Through the immune system's machinery, the specific recognition/targeting or elimination of pathological tumoral cells along with refining the immunological memory are feasible.1,2 While it is often associated with cancer, immunotherapy with monoclonal antibodies (mAbs) also serves as a benchmark to study other diseases (e.g. autoimmunity diseases, macular degeneration, allergies). Recently, the mAbs approach against immune-check point inhibitors was shown to offer real therapeutic success for many different cancer types. Moreover, cellular immunotherapy also offers appropriate responses, such as the adoptive therapies based on engineered T cells (e.g. chimeric antibody receptor T (CAR-T) cells, natural killer (NK) cells, tumour infiltrating lymphocytes, dendritic cells). In some instances, the disease progression (e.g. metastasis, relapse or critical therapeutic failure) manages to escape from its constant surveillance, which makes it an arduous challenge to treat.3 Thus, harnessing the immune response is a smart alternative to the current therapies (i.e. surgery, radiotherapy, chemotherapy).4,5The clinical and preclinical treatment trends are not just limited to a single strategy as combined multiple treatments have demonstrated superior efficacy to any monotherapy. Thus, the combination of immunotherapy with other conventional treatment modalities can magnify the immune response, thereby maximizing their therapeutic effect. In this context, emerging nanomedicines have arisen as an appealing approach, as they are able to transport the desired active pharmaceutical ingredient (API, e.g. adjuvants, antigens, chemodrugs) in a safe and effective manner to the target cells and/or tissues.6 However, the vast majority of such materials have been employed as passive vehicles, providing just API-protection against degradation and offering longer retention times in the body.7,8 Revealing the NP inherent impact on the immune response (in absence of any APIs) would provide meaningful inputs for their in vitro and in vivo performance, allowing more personalized nanotherapies.2
Among the large variety of engineered nanocarriers (e.g. nanoparticles (NPs), liposomes, micelles), a new class of crystalline hybrid materials known as nanoscale metal–organic frameworks (nanoMOFs) has recently attracted a great deal of attention in the biomedical domain.9,10 These hybrid NPs (composed of inorganic nodes and organic polydentate linkers assembled into multidimensional periodic lattices) can be precisely designed/manipulated at their molecular level, giving rise to multifunctional smart entities, which is known as multifunctional efficiency,11 offering several advantages as drug-delivery systems (DDS), including: (i) chemical and structural versatility, which permits a suitable biocompatibility upon appropriate chemical design and the potential control of their in vivo fate; (ii) an ideal amphiphilic internal microenvironment, conveniently adapted to host a very broad variety of APIs (biological gases, cosmetics, enzymes, nucleic acids, drugs, etc.), releasing them in a controlled manner under physiological conditions; (iii) easy and scalable synthesis, following green methods with high yields; (iv) a general trend of a high biocompatible profile (e.g. the lack of in vivo toxicity for the benchmarked mesoporous Fe trimesate MIL-100(Fe) or the microporous Zr carboxylates Uio-66(Zr)); (v) additional abilities, where the recent successful external surface modification in some prototypes has proven the capability to endow further multifunctional abilities, such as targeting, imaging or enhanced stability (chemical/structural or colloidal) under biorelevant conditions.9,10,12–14
The latest advances on MOF nanocarriers have been mainly focused on their targeting via external functionalization and/or formulation.9,13 However, their immunological impact has not been in the spotlight within the scientific community and still remains relatively unknown.
Most of the research reported so far on this topic is focused on cancer immunotherapy,3–5 showing significant features exclusively when used as a passive vehicle for effectively releasing adjuvants, immunomodulators or antigens.15–18 For instance, the first MOF-based vaccine using ZIF-8-loaded ovalbumin (OVA) with cytosine-phosphate-guanine oligodeoxynucleotide (CpG ODN) attached as an adjuvant prototype was able to activate a potent immune memory.19 In terms of exploring the intrinsic immunogenicity behaviour of MOFs, some researchers have preliminary evaluated the inflammatory response induced by iron-based MOFs modified on their surface with polymers.20,21 However, despite the biomedical progress made over the last five years, the potential for MOFs to have intrinsically active repercussions at the immune level has not been investigated in depth. This basic notion is crucial, as it could provide valuable information about the innate features of MOFs and their precursors and their aroused immune reaction, which is a critical factor for boosting multi-therapy with diverse APIs.
Bearing this in mind, we decided to evaluate the immunogenicity of a selection of three different nanoMOF platforms: (i) two cubic-zeotype mesoporous metal (Fe3+ or Al3+) trimesates, namely MIL-100(Fe, Al) (MIL stands for the Material of the Institut Lavoisier) with a very important mesoporosity (surface area SBET ∼ 2400 m2 g−1, pore volume Vp ∼ 1.2 cm3 g−1),22 which have been highlighted as efficient DDSs with a lack of in vitro and in vivo toxicity;23 and (ii) the cubic microporous zinc 2-methyl-imidazolate ZIF-8(Zn) (ZIF stands for zeolitic imidazolate framework), which can be described by a space-filling packing of a regular truncated octahedral (SBET ∼ 1800 m2 g−1, Vp ∼ 1.2 cm3 g−1),24 which has also been highly selected as a suitable MOF-based device for immunotherapy.17,18 In all instances, we characterized their ability to induce human cytokine production and complementary activation, together with their potential cytotoxicity and production of reactive oxygen species (ROS).
Considering the immense possibilities that MOFs offer as a therapeutic platform (e.g. high porosity, versatile structure and biosafe character), shedding light on the specific roles of the MOFs and their constituents on the cellular homeostasis could tip the balance towards the generation of a therapeutic effect according to a targeted pathological dysfunction (e.g. cancer, infections, allergies, autoimmune diseases). In other words, MOFs could be used as potential immunoactivators or immunomodulatory carriers, able to induce immune activation or tolerance under a pathological or undesirable activation (‘tunable immune response’). Therefore, unveiling the native MOF immunological fingerprint could provide insights to facilitate the design of a targeted immune-active MOF nanoplatform for an efficient combined therapy.
Results and discussion
NanoMOF characterization and biosafety profile
Despite the recent tendency to explore novel MOF applications in the biomedical field, where MOFs are mainly oriented as potential drug vehicles, the repercussion of their intrinsic impact on the immune system is still unknown. Since the physicochemical properties of nanocarriers highly impact their affinity to different biological structures (e.g. proteins, cells, tissues, nucleic acids), as well as their efficacy and/or biodistribution (in other words, their biomedical performance),25,26 we first fully characterized the nanoMOFs (XRPD, DLS, TEM, surface chemistry and colloidal stability, etc.; see Experimental section and the ESI,† for the synthetic and characterization details) prior to any immunological encounters.The resulting materials displayed a nanometric particle size in aqueous solution (hydrodynamic diameter from DLS ∼150, 220 and 110 nm for MIL-100(Al), MIL-100(Fe) and ZIF-8(Zn), respectively, see Table 1; which is in agreement with the microscopic observation acquired by TEM, see Fig. S1†), preserving in all cases their crystalline structure (Fig. S2†) and textural properties (Table 1), as previously reported.27,28 However, it should be pointed out that there was a slight increase in the hydrodynamic diameter in the case of MIL-100(Al) compared to MIL-100(Fe), which could be related to an aggregation effect due to the proximity to more neutral ζ-potential values (absence of enough electrostatic repulsions). This growth effect was also reflected in ZIF-8(Zn) NPs in comparison with a previously reported one (28 vs. 110 nm),24 which was mainly associated with the nature of the medium used for its dispersion (EtOH vs. H2O, respectively), maintaining also their polydispersity index (PdI ∼ 0.2).
| Media | MIL-100(Fe) | MIL-100(Al) | ZIF-8(Zn) | |
|---|---|---|---|---|
| a Brunauer–Emmett–Teller (BET) surface area. | ||||
| Composition | Metal | Fe | Al | Zn |
| Ligand |
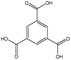
|
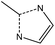
|
||
| Size (nm) (PdI) | H2O | 153 ± 53(0.3) | 218 ± 28(0.2) | 110 ± 48(0.2) |
| PBS | 177 ± 17(>0.3) | 209 ± 41(>0.3) | 227 ± 26(>0.3) | |
| RPMI | 145 ± 38(0.3) | 248 ± 50(>0.3) | 284 ± 22(>0.3) | |
| ξ-Potential (mV) | H2O | −25 ± 4 | −7 ± 3 | +96 ± 0 |
| PBS | −32 ± 0 | −16 ± 1 | −27 ± 1 | |
| RPMI | −31 ± 2 | −10 ± 2 | −9 ± 2 | |
| BET surface (m2 g−1)a | 1530 | 1510 | 1400 | |
Regarding the above-mentioned ζ-potential outcomes, the fluctuation of the nanoMOF surface charge observed here should be related to the diverse proportions of the partially coordinated cations vs. the linkers when exposed to the physiological media. For instance, the more negative ζ-potential values displayed by MIL-100(Fe) NPs compared to their aluminium analogue (−25 ± 4 vs. −7 ± 3 mV, respectively, Table 1) could be due to the higher amount of carboxylate/carboxylic acid vs. cation and/or the presence of elements on the surface of Fe–F (fluorine coming from a washing step in the MIL-100(Fe) preparation). Similarly, despite the contrary ζ-potential value obtained on the external ZIF-8(Zn) surface, the high positive charge (+96 ± 0 mV) could be explained by the same trend: a large proportion of cations or a higher presence of protonated ligands, as the pH of the aqueous solution was lower than the pKa of the imidazolate (6.0 vs. 7.0 and 14.9).29,30
Bearing in mind the high-impact of the surrounding media on the NP stability, and hence, on their biological affinities, biodistribution and efficacy,25,26 the nanoMOF particle size and ζ-potential were investigated under diverse simulated physiological conditions: from a simple phosphate buffer solution (PBS) to a more complex medium consisting of supplemented cell culture media (RPMI, Table 1). In all cases, the nanometric range was maintained, exhibiting an average size close to 160 and 225, and 207 nm for MIL-100(Fe & Al) and ZIF-8 NPs, respectively. However, the ZIF-8(Zn) NPs underwent a notable size increase in the presence of more complex media (from 110 ± 48 nm in H2O to 227 ± 26 or 284 ± 22 nm in PBS or RPMI, respectively, Table 1). This destabilizing effect was related to the tremendous ζ-potential fluctuation, shifting from a highly positive charge in H2O to a lower negative character in PBS and RPMI (+96 ± 0 vs. −27 ± 1 and −9 ± 2 mV, respectively). This dramatic conversion has been already observed in other nanoMOF prototypes due to the formation of a superficial corona composed by phosphate groups and/or other salts/proteins from the media.20,27 Overall, the colloidal stability of the tested nanoMOFs in these biorelevant media makes them suitable candidates for the assessment of their immunological recognition.
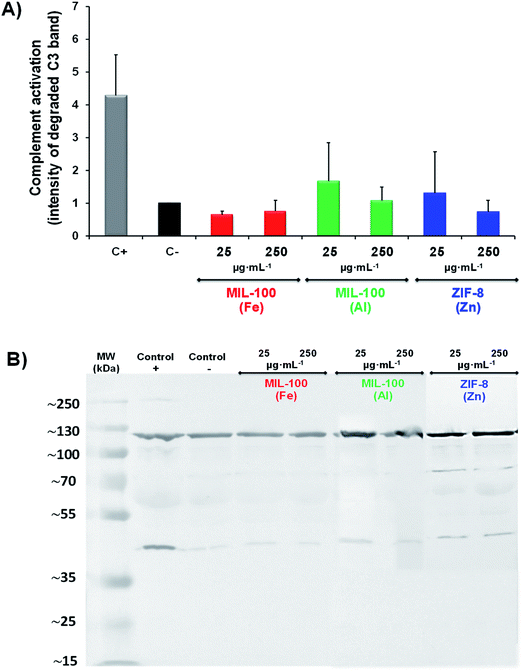
Prior to exploring the associated immune fingerprint of these nanoMOFs and their future implications, their toxicological character needed also to be evaluated. On this basis, a macrophage cell line (J774.A1) was selected as an appropriate model of the first defence line in the immune system against pathogens (those involved in the innate immune response).31,32 Remarkably, an absence of toxicity was observed by the MTT method33 for both MIL-100 (Fe & Al) and ZIF-8(Zn) NPs after 24 h incubation even at very high concentrations (1.2 mg mL−1, Fig. S3†). Despite previous data suggesting a higher cytoxicity tendency induced by ZIF-8(Zn) than MIL-100(Fe) NPs (maybe as a consequence of a potential competition between Zn2+vs. Fe2+vs. Ca2+ through ion channels and/or deoxyribonucleic acid (DNA) damage),28,34 as well as the often associated cytotoxicity effect of diverse cationic carriers,35 these outcomes were in good agreement with the lack of severe toxicity observed in other cell lines9,21,34 as well as with previous in vivo data.10,12 Therefore, the biofriendly profile obtained from these nanoMOFs could enable further investigations into their self-immunoactive activity. In other words, shedding light on the interaction between MOFs and/or its precursors with the immune constituents could support the generation of a specific therapeutic activity, providing valuable data starting from their particular affinities with the biological surrounding, type of internalization pathways according to the cellular source and their influence on specific chemical reactions, such as catalytic and oxidative processes. Thus, in the next section, the MOF recognition by essential actors of the innate immune system is addressed considering (i) the cellular oxidation balance via reactive oxidative stress production (ROS), (ii) the complementary activation and (iii) the cytokine secretion pattern.
NanoMOF immune fingerprint: innate and adaptive immunity
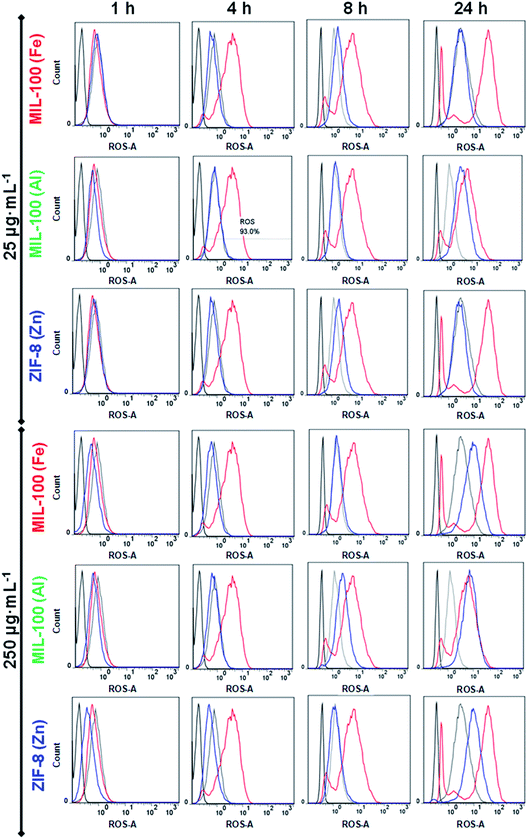
Given that we are proposing three nanoMOFs based on different cations (Fe3+, Al3+ and Zn2+), their repercussion on the cellular oxidation balance should be investigated. To address this point, a human promyelocytic leukaemia cell line (HL-60) was selected, as it has been shown to modulate ROS production in a dose-dependent response.21,39 Two different doses (25 and 250 μg mL−1) of MIL-100(Fe, Al) and ZIF-8(Zn) NPs were put in contact with the HL-60 cells at different time points (1, 4, 8 and 24 h), and the results compared with three different controls: (i) a positive control (C+), cells incubated with PMA (ROS inducer); (ii) the basal control (Cbasal), the intrinsic oxidation state of HL-60 cells (in the absence of ROS inducer but with the ROS reactant) and (iii) a negative control (C−), cells in media without any treatment (neither the ROS inducer or the ROS reactant; see the Experimental section). Remarkably, no ROS induction was detected at short times (≤8 h) regardless of the NPs' concentration, with the exception of the highest concentration of MIL-100(Al) NPs (250 μg mL−1), which exhibited a slight increase in oxidative stress (Fig. 1). On the contrary, at longer incubation times (24 h), ROS production rose in all cases at high dose (250 μg mL−1), being more prominent in the MIL-100(Al) NPs (even at the lowest concentration). This oxidative stress, promoted by the Al-trimesate, was higher than with its Fe-analogue or Zn based NPs, displaying an oxidative strength tendency of Al > Fe ∼ Zn. Therefore, all the tested nanoMOFs induced moderate ROS at 24 h, being stronger for the MIL-100(Al) NPs; this performance could be beneficial to enhancing their potential immunotherapeutic effect as the immune system can be smoothly activated (‘friendly warning’), as previously proposed for other nanoparticle systems.29,38 In fact, it is not the first occasion that ROS production by innate immune cells has been related to a good in vivo adjuvancy, as an immune activation mechanism is triggered.40
Consequently, the nature of the metal seems to be a crucial parameter as the redox homeostasis could be tampered with.36,41 Although the mechanism is not well-described, these metals favour superoxide radical formation [mainly the superoxide anion (O2˙−), hydrogen peroxide (H2O2), singlet oxygen (1O2) and hydroxyl radical (˙OH)].36 In our particular case, despite the non-redox character of Al compounds, MIL-100(Al) NPs have proven to be a powerful in vitro and in vivo pro-oxidant,42,43 promoting both iron auto-oxidation and ROS formation by their binding with superoxide radical anions.44,45 Most of the Al3+ present in the human organism is not free in solution, but forms stable complexes with low/high molecular mass biomolecules, with around 90% of the aluminium in the blood serum bounded to the transferrin protein.46 Concerning Fe3+, it is widely reported that iron oxides (e.g. Fe3O4, Fe2O3) can induce ROS production through the Fenton reaction (catalyzing the H2O2 reaction), showing high reactivity with biological molecules such as lipids, proteins and DNA.47,48 In fact, iron is generally bound to specific proteins, leaving few free iron cations available for the Fenton reaction (e.g. inducing ferroptosis).49 In our particular case, the moderate ROS levels of MIL-100(Fe) NPs were also in agreement with an increase in the in vitro34 and in vivo12 oxidative stress, as already previously described by some of us, demonstrating a totally reversible effect after 2 weeks of its intravenous administration with a high dose (up to 220 mg kg−1). Finally, the redox-inert Zn2+ is the most abundant metal in the brain, being also an essential component in various enzymes and transcription factors involved in the regulation of key cellular functions (DNA replication, repair of DNA damage, cell cycle progression and apoptosis).41 The depletion of zinc may enhance DNA damage by impairing DNA repair mechanisms, generating free radicals: its high solubility and easy nitrogen- or oxygen-coordination lead to the formation of chelates with many biomolecules that are involved in the oxidative balance homeostasis, resulting in their inactivation and then, the induction of ROS.50 For instance, Zn2+ is associated with the inhibition of the important antioxidant enzyme glutathione reductase.51,52 Besides, zinc competition with other redox active metals (such as copper or iron) may also play a role in oxidative stress-mediated damage, as Zn2+ may bind and protect sulfhydryl groups belonging to proteins. In contrast, other researchers have also proposed a possible antioxidant and anti-inflammatory effect of this cation associated with (i) potential activation of the antioxidant enzyme superoxide dismutase (SOD1 and 3), which possesses Zn and Cu in its active metal site and (ii) inhibition of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which involved in free radical production.53,54
Therefore, the moderate oxidative stress generated by ZIF-8(Zn) NPs would favour designing a dual functionality (oxidant and antioxidant behaviour) according to the immune system demand. In other words, the presence of additional metals and the potential action of the selected nanoMOFs will be determined by the particular cellular status and/or pathological environment to be treated. Thus, previous knowledge of each clinical condition would lead to more precise nanoMOF therapies.
Moreover, a lack of inflammatory signals or the presence of regulatory factors during antigen presentation can promote tolerogenic responses, suppressing immune reactions (e.g. modulating inflammation, restricting migration of self-reactive immune cells),61 which could be a great scenario for combined immuno- and chemo-therapeutic nanocarriers for autoimmune and allergic diseases, among others.
The transition from innate to adaptive immunity requires antigen processing and T cell presentation by antigen-presenting cells (APCs): dendritic cells (DC) are able to trigger naïve T cells that, with the already activated macrophages and B cells (effectors of the antibody production), can promote the activation of helper T cells (CD4+), which is crucial for a specific immune response and immunological memory.5,6
However, it should be mentioned that the immunological scenario and its consequent action rely on the type of disease. For instance, (i) in a tumoral environment, a high presence of immunocompromised cells is observed, where the therapeutic approach aims to reverse this immunosuppression by stimulating the immune system; or (ii) when facing viral pathogens (e.g. SARS-Cov-2), the current vaccine treatments aim to induce both B and T cell responses, either by the generation of neutralizing antibodies or anti-viral specific helper and/or cytotoxic T cells (CD8+) as well as long-live memory cells;62 (iii) on the contrary, for the autoreactivity and inflammatory processes concerned with autoimmune and autoinflammatory diseases, the induction of a tolerance response is required. Consequently, revealing the intrinsic immunogenicity of nanomaterials can be exploited to modulate the immune response; whereby understanding the molecular action mechanisms of different cytokines in the context of a specific disease could contribute to developing more targeted anti-cytokine/cytokine therapy (‘innovative nanotherapeutic immune-modulating strategies’).6
To shed light on the type of adaptive immune response elicited by the nanoMOF, and thereby its potential role as a therapeutic carrier of diverse diseases, two different concentrations (25 and 250 μg mL−1) were incubated with human peripheral blood mononuclear cells (PBMCs) from three voluntary donors (see Experimental section; Table 2) for the determination of their cytokine profile. It should be noted that the PBMC fraction was mainly composed of lymphocytes (70–90% including T, B and NK cells) and monocytes (10–30%), generally activated in response to external stimuli, such as the nanoMOFs or positive controls (selected here as C+: lipopolysaccharide –LPS– and lectin phytohemagglutinin –PHA–), which are also known as human cytokine activators. In particular, the cytokine profile was here represented as the average of three donors' values for each nanoMOF in comparison with the negative control (C−) together with the number of donors included within this variation (Table 2), for greater clarity.
| NPs dose (μg mL−1) | Positive control (LPS & PHA) | MIL-100(Fe) | MIL-100 (Al) | ZIF-8(Zn) | ||
|---|---|---|---|---|---|---|
| a Secretion of Th2 cytokines (IL-4 and IL-5) was not observed with the MIL-100(Fe), MIL-100(Al) and ZIF-8 (Zn) NPs. The values correspond to the variation of the cytokines concentration (in pg mL−1) compared with the negative control (10, 102, 103, 104 or >105 times) obtained from 3 different donors (1/3, 2/3 or 3/3), with the representation of the activation shown in the positive control (LPS and PHA, which acted as inducers of the cytokines production). | ||||||
| Th1 cytokines | IL-12p70 | 25 | 3/3(102 to 103) | 3/3(102 to 103) | 2/3(10 to 102) | 3/3(10 to 102) |
| 250 | ||||||
| INF-γ | 25 | 3/3(103 to 104) | 2/3(102 to 103) | 3/3(10 to 102) | 2/3(10 to 102) | |
| 250 | 3/3(102 to 103) | 3/3(102 to 103) | 2/3(102 to 103) | |||
| IL-2 | 25 | 2/3(102 to 103) | 2/3(10 to 102) | 2/3(10 to 102) | 3/3(10 to 102) | |
| 250 | 3/3(10 to 102) | 2/3(10 to 102) | ||||
| Anti-inflammatory cytokine | IL-10 | 25 | 2/3(103 to 104) | 2/3(103 to 104) | 2/3(103 to 104) | 2/3(102 to 103) |
| 250 | 2/3(102 to 103) | 2/3(103 to 104) | ||||
| Pro-inflammatory cytokines | IL-6 | 25 | 3/3(>105) | 3/3(102 to 103) | 3/3(>105) | 2/3(>105) |
| 250 | ||||||
| IL-8 | 25 | 2/3(103 to 104) | 2/3(102 to 103) | 2/3(103 to 104) | 3/3(103 to 104) | |
| 250 | 3/3(102 to 103) | |||||
| IL-1β | 25 | 3/3(102 to 103) | 3/3(103 to 104) | 2/3(103 to 104) | 2/3(103 to 104) | |
| 250 | 3/3(103 to 104) | |||||
| TNF-α | 25 | 3/3(103 to 104) | 2/3(>105) | 3/3(104 to 105) | 2/3(104 to 105) | |
| 250 | 3/3(>105) | 2/3(>105) | 3/3(104 to 105) | |||
| TNF-β | 25 | 3/3(103 to 104) | 2/3(102 to 103) | 3/3(10 to 102) | 2/3(10 to 102) | |
| 250 | 3/3(102 to 103) | 2/3(10 to 102) | ||||
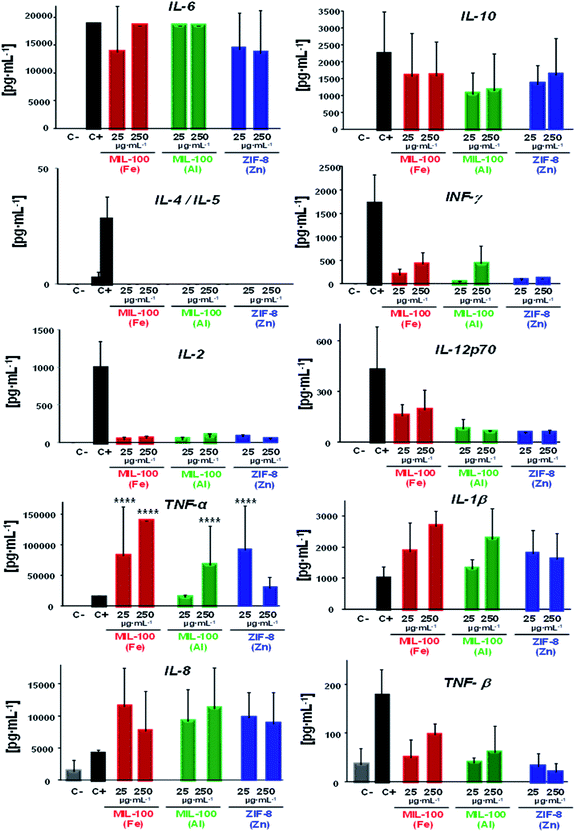
On the whole, a substantial immune response was evidenced in presence of the three nanoMOFs; whereby a diverse cytokine production 10 to >105 times higher than the negative control was obtained (see Table 2; Fig. 3), highlighting a general secretion of pro-inflammatory cytokines in all cases (mostly derived from the activated monocytes). One example was IL-6, secreted by the activated monocytes, which participate in diverse functions, such as the B cell growth or endocrine effects (e.g. induction of fever, production of reactive C protein in the liver). In this case, similar values were obtained for the positive control, being more than 105 times higher than the negative control. Other significant pro-inflammatory cytokines observed here were interleukin IL-1β (relevant cytokine for the activation of T and B lymphocytes) along with the tumour necrosis factor (TNF, α and β responsible for the signalling pathways for cell survival, apoptosis, inflammatory responses or cellular differentiation), displaying levels higher than for C−, from 103 to 104 for IL-1β and ∼104 to >105 in the case of TNFα (being even 10 to 102 times higher than for C+ in both cases), which suggest that the tested nanoMOFs significantly induced inflammation. On the contrary, high levels of the anti-inflammatory IL-10 were also observed within the same range than for the positive control with ∼103 to 104 times higher values than for the negative control. Although they can be produced by different cell types, such as Th2 cells or regulatory T and B cells, activated monocytes are also able to be secreted in large amounts.
However, this secretion is usually delayed in the presence of other pro-inflammatory factors, as shown here with the IL-6, tumour necrosis factor or INF-γ production (the main Th1 cytokine implicated in the inflammation and proliferation of the macrophages). Regarding the chemokine IL-8, which is a potent chemoattracting agent, its levels were also raised with values ∼103 to 104 higher than cells incubated with the media containing MIL-100(Al) and ZIF-8(Zn) NPs, being 10 times lower in the case of MIL-100(Fe) NPs. These findings reveal a great trend of those nanoMOFs to be recognized by the innate cells.
An expected optimal scenario should include a well-balanced Th1 and Th2 response, as this would be suited to a particular immune challenge. In view of unveiling the potential type of adaptive immune response induced by these nanoMOFs, the specific influence pursued by Th1–Th2 cytokines was investigated in depth. Related with Th1 stimulation, the interplay of interleukin 2 (IL-2, involved in T and NK cells proliferation), interferon gamma (IFNγ) and Th2 cytokine profiles (IL-4 and IL-5, which are the main markers of Th2 cells, promoting specific cellular differentiation) showed low levels of IL-2 regardless of the nature of the MOF (Table 2, Fig. 3), with levels slightly higher for those of IFNγ with MIL-100(Fe) and (Al) than with ZIF-8(Zn) NPs. In both cases, the levels produced by these Th1 cells were 10 to 102 times higher than those in the negative control. Similar to IL-2, IL12p70 production in the presence of MIL-100(Al) and ZIF-8(Zn) NPs was ∼10 to 102 times higher than in cells incubated with the media, with the exception of MIL-100(Fe) NPs, where the values reached the positive control levels. It should be noted that this last interleukin stimulates the Th1 profile and inhibits the Th2 response.63 In fact, looking deeper into the Th2 cell impact, no secretion of IL-4 or IL-5 were detected notwithstanding the MOF topology or composition. These outputs evidence the activation of mainly the cellular response vs. the humoral (antibody), which can be beneficial for vaccine purposes, for instance.57
Overall, all the nanoMOFs seemed to be very well-recognized by the innate monocyte population, eliciting a potent response with the secretion of pro-inflammatory cytokines together with the chemokine IL-8. Conversely, IL-10 release, produced by activated monocytes and other immune cells after exposure to nanoMOFs, could indicate the tendency of the cells to revert to this pro-inflammatory status, showing higher IL-10 levels that might be also associated with a slight INF-γ inhibition. This CK pattern, detected on human cells, reflects the type of immune response that one could expect if these nanoMOFs were to be used in vivo.
In a nutshell, the lack of IL-4 and IL-5 (the main markers of the Th2 profile), the presence of IL-2, IL12p70 and IFNγ (distinctive Th1 profile) and the induction of IL-6, IL1β and TNFα (involved in inflammatory processes) suggest that the presence of nanoMOFs could tip the balance to the Th1 responses (highly recommended for anti-tumoral, anti-viral and/or intracellular bacteria responses), promoting their specific differentiation.
Conclusions
Understanding the native immunological features of nanoMOFs could make it possible to customize the design of effective nanomedicines to prevent and/or treat specific pathological disorders. Each nanoMOF has a unique biological repercussion: their large versatility (type of metal/linker nature, topology, reactivity, etc.) requires specific safety profiles, considering not only the cellular but also the geno and/or immunological impact.The nanoMOFs studied here (i.e. MIL-100(Al), MIL-100(Fe) and ZIF-8(Zn)) showed a high biocompatible profile with a slight activation of the complement cascade along with ROS induction in innate cells, especially for the innate monocytes, displaying the production of both several pro-inflammatory (IL-6, TNFα and β, IL1β, IL-8) and anti-inflammatory (IL-10) cytokines. Despite all showing a very similar pattern, MIL-100(Fe) seemed to induce a higher Th1 immune response compared to MIL-100(Al) and ZIF-8(Zn) NPs, with a higher induction of INF-γ and IL12p70 cytokines. Moreover, the lack of Th2 response elicited by any nanoMOF was noteworthy, which could suggest a slight cellular response (antibody production).
Overall, the activation of innate and Th1 cells induced by these nanoMOFs make them promising adjuvant candidates for targeted immunotherapy. These findings should help to create more novel and effective immunoactive MOFs, opening new horizons not only in biomedicine (e.g. therapy, imaging, vaccines) but also in other economically and societally relevant fields, such as the environment, catalysis or sensing, in which the safety of the MOFs is a crucial parameter to their practical use.
Author contributions
TH and PH: synthesis, characterisation cellular toxicity; TH, RSV, AGF, and PH: immunological studies (ROS, Complement, CKs). The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Labex NanoSaclay financial support for the MSc studies (ANR-11-IDEX-0003-02) together with the CNRS, IMDEA Energy and Xunta de Galicia (GRC-ED431C 2020/02) funding. T. H. and P. H. acknowledge the Regional Madrid Founding (Talento 2018 Modality 2, (2018-T2/IND-11407), the Multifunctional Metallodrugs in Diagnosis and Therapy Network (MICIU, RED2018-102471-T) and the European Union's Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement No 897678. P. H. acknowledges the Spanish Ramón y Cajal Programme (grant agreement no. 2014 to 16823).References
- X. Zhong and X. Sun, Acta Pharmacol. Sin., 2020, 41, 928 CrossRef CAS.
- W. Sang, Z. Zhang, Y. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 3771 RSC.
- L. Scheetz, K. S. Park, Q. Li, P. R. Lowenstein, M. G. Castro, A. S. Schewendeman and J. J. Moon, Nat. Biomed. Eng., 2019, 3, 768 CrossRef CAS.
- N. Papaioannou, O. V. Beniata, P. Vitsos, O. Tsitsilonis and P. Samara, Ann. Transl. Med., 2016, 4, 261 CrossRef.
- M. L. Guevara, F. Persano and S. Persano, Semin. Cancer Biol., 2021, 69, 238–348 CrossRef CAS PubMed.
- K. Naran, T. Nundalall, S. Chetty and S. Barth, Front. Microbiol., 2018, 9, 3158 CrossRef.
- C. T. Perciani, L. Y. Lui, L. Wood and S. A. MacParland, ACS Nano, 2021, 15, 7 CrossRef CAS.
- C. D'Amico, F. Fontana, R. Cheng and H. A. Santos, Drug Deliv. Transl. Res., 2021, 11, 353 CrossRef PubMed.
- J. Yang and Y. W. Yang, Small, 2020, 16, 1906846 CrossRef CAS.
- S. Rojas, A. Arenas-Vivo and P. Horcajada, Coord. Chem. Rev., 2019, 388, 202 CrossRef CAS.
- R. Freund, U. Lächelt, T. Gruber, B. Rühle and S. Wuttke, ACS Nano, 2018, 12, 2094 CrossRef CAS PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. B. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS.
- S. Wuttke, M. Lismont, A. Escudero, B. Rungtaweevoranit and W. J. Parak, Biomaterials, 2017, 123, 172 CrossRef CAS.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosh, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H. C. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- Y. B. Miao, W. Y. Pan, K. H. Chen, H. J. Wei, F. L. Mi, M. Y. Lu, Y. Chang and H. W. Sung, Adv. Funct. Mater., 2019, 29(43), 1904828 CrossRef CAS.
- X. F. Zhong, Y. T. Zhang, L. Tan, T. Zheng, Y. Y. Hou, X. Y. Hong, G. Du, X. Chen, Y. Zhang and X. Sun, J. Control. Release, 2019, 300, 81 CrossRef CAS.
- Y. Yang, Q. Chen, J. P. Wu, T. B. Kirk, J. Xu, Z. Liu and W. Xue, ACS Appl. Mater. Interfaces, 2018, 10, 12463 CrossRef CAS.
- H. Zhang, J. Zhang, Q. Li, A. Song, H. Tian, J. Wang, Z. Li and Y. Luan, Biomaterials, 2020, 245, 119983 CrossRef CAS.
- Y. Zhang, F. M. Wang, E. G. Ju, Z. Liu, Z. W. Chen, J. S. Ren and X. Qu, Adv. Funct. Mater., 2016, 26, 6454 CrossRef CAS.
- E. Bellido, T. Hidalgo, M. V. Lozano, M. Guillevic, R. Simón-Vázquez, M. J. Santander-Ortega, Á. González-Fenández, C. Serre, M. J. Alonso and P. Horcajada, Adv. Healthc. Mater., 2015, 4, 1246 CrossRef CAS.
- T. Hidalgo, M. Giménez-Marqués, E. Bellido, J. Avila, M. C. Asensio, F. Salles, M. V. Lozano, M. Guillevic, R. Simón-Vázquez, Á. González-Fernández, C. Serre, M. J. Alonso and P. Horcajada, Sci. Rep., 2017, 7, 43099 CrossRef CAS.
- A. García-Márquez, A. Demessence, A. E. Platero-Prats, D. Heurtaux, P. Horcajada, C. Serre, J. S. Chang, G. Férey, V. A. Peña-O'Shea, C. Boissière, D. Grosso and C. Sanchez, Eur. J. Inorg. Chem., 2012, 32, 5165 CrossRef.
- M. Giménez-Marqués, T. Hidalgo and P. Horcajada, Coord. Chem. Rev., 2016, 307, 342 CrossRef.
- J. Cravillon, S. Munzer, S. J. Lohmeier, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2009, 21, 1410 CrossRef CAS.
- S. Mitragotri, P. A. Burkre and R. Langer, Nat. Rev. Drug Discovery, 2014, 13, 650 CrossRef PubMed.
- E. Fattal and N. Tsapis, Clin. Transl. Imaging, 2014, 2, 77 CrossRef.
- E. Bellido, M. Guillevic, T. Hidalgo, M. J. Santander-Ortega, C. Serre and P. Horcajada, Langmuir, 2014, 30, 5911 CrossRef CAS PubMed.
- R. Grall, T. Hidalgo, J. Delic, A. Garcia-Marquez, S. Chevillarda and P. Horcajada, J. Mater. Chem. B, 2015, 3, 8279 RSC.
- A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58 CrossRef CAS.
- S. Luo, P. G. Wang and J. P. Cheng, J. Org. Chem., 2004, 69, 555 CrossRef CAS.
- L. Franken, M. Schiwon and C. Kurts, Cell. Microbiol., 2016, 18, 475 CrossRef CAS PubMed.
- D. Hirayama, T. Iida and H. Nakase, Int. J. Mol. Sci., 2018, 19, 92 CrossRef.
- S. Arora, J. R. Rajwade and K. M. Paknikar, Toxicol. Appl. Pharmacol., 2012, 258, 151 CrossRef CAS.
- C. Tamames-Tabar, D. Cunha, E. Imbuluzqueta, F. Ragon, C. Serre, M. J. Blanco-Prieto and P. Horcajada, J. Mater. Chem. B, 2014, 2, 262 RSC.
- C. Teijeiro, A. McGlone, N. Csaba, M. Garcia-Fuentes and M. J. Alonso, Handbook of Nanobiomedical Research, World Scientific Ed., 2014 Search PubMed.
- B. Yang, Y. Chen and J. Shi, Chem. Rev., 2019, 119, 4881 CrossRef CAS.
- B. Fadeel, Front. Immunol., 2019, 133, 1 Search PubMed.
- V. Mallikarjun, D. J. Clarke and C. J. Campbell, Free Radic. Biol. Med., 2012, 53, 280 CrossRef CAS PubMed.
- M. D. Ferrer, A. Sureda, A. Mestre, J. A. Tur and A. Pons, Cell. Physiol. Biochem., 2010, 25, 241 CrossRef CAS PubMed.
- M. Peleteiro, E. Presas, J. V. González-Aramundiz, B. Sánchez-Correa, R. Simón-Vázquez, N. Csaba, M. J. Alonso and A. González-Fernández, Front. Immunol., 2018, 9, 791 CrossRef.
- M. Valko, K. Jomova, C. J. Rhodes, K. Kuča and K. Musílek, Arch. Toxicol., 2016, 90, 1 CrossRef CAS PubMed.
- C. Exley, Free Radic. Biol. Med., 2004, 36, 380 CrossRef CAS PubMed.
- J. I. Mujika, F. Ruipérez, I. Infante, J. M. Ugalde, C. Exley and X. Lopez, J. Phys. Chem. A, 2011, 115, 6717 CrossRef CAS.
- C. Exley, P. Siesjo and H. Eriksson, Cell Press, 2010, 31, 103 CAS.
- F. Ruipérez, J. Mujika, J. Ugalde, C. Exley and X. Lopez, J. Inorg. Biochem., 2012, 117, 118 CrossRef.
- J. Mujika, G. Dalla Torre and X. Lopez, Phys. Chem. Chem. Phys., 2018, 20, 16256 RSC.
- H. J. H. Fenton, J. Chem. Soc. Trans., 1894, 65, 899 RSC.
- M. Wlaschek, K. Singh, A. Sindrilaru, D. Crisan and K. Scharffetter-Kochanek, Free Radic. Biol. Med., 2019, 133, 262 CrossRef CAS.
- X. Qiana, J. Zhangb, Z. Gud and Y. Chenc, Biomaterials, 2019, 211, 1 CrossRef.
- Y. Chang, M. Zhang, L. Xia, J. Zhang and G. Xing, Materials, 2012, 5, 2850 CrossRef CAS.
- M. G. Bishop, R. Dringen and S. R. Robinson, Free Radic. Biol. Med., 2007, 42, 1222 CrossRef.
- R. Ryu, Y. Shin, J. W. Choi, W. Min, H. Ryu, C. R. Choi and H. Ko, Exp. Brain Res., 2002, 143, 257 CrossRef PubMed.
- A. S. Prasad, Curr. Opin. Clin. Nutr., 2009, 12, 646 CrossRef CAS.
- Y. Song, S. W. Leonard, M. G. Traber and E. Ho, J. Nutr., 2009, 139, 1626 CrossRef CAS PubMed.
- M. G. Netea, A. Schlitzer, K. Placek, L. A. B. Joosten and J. L. Schultze, Cell Host Microbe, 2019, 25, 13 CrossRef CAS.
- M. A. Dobrovolskaia and S. E. McNeil, Handbook of Inmunological properties of Engineered Nanomaterials, World Scientific Publishing Co. Inc., 2012 Search PubMed.
- J. Szebeni, D. Simberg, Á. González-Fernández, Y. Barenholz and M. A. Dobrovolskaia, Nat. Nanotechnol., 2018, 13, 1100 CrossRef CAS.
- T. R. Ghimire, SpringerPlus, 2015, 4, 181 CrossRef.
- R. S. Oakes, E. Froimchuk and C. M. Jewell, Adv. Ther., 2019, 2, 1800150 Search PubMed.
- A. Iwasaki and R. Medzhitov, Nat. Immunol., 2015, 16, 343 CrossRef CAS.
- J. M. Gammon and C. M. Jewell, Adv. Healthc. Mater., 2019, 8, 1801401 Search PubMed.
- G. Canedo-Marroquín, F. Saavedra, C. A. Andrade, R. V. Berrios, L. Rodríguez-Guilarte, M. C. Opazo, C. Riedel and A. M. Kalergis, Front. Immunol., 2020, 11, 569760 CrossRef.
- M. Elsabahy and K. L. Wooley, Chem. Soc. Rev., 2013, 42, 5552 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc04112f |
| This journal is © The Royal Society of Chemistry 2022 |