 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The structural characteristics and mechanisms of antimicrobial carbon dots: a mini review
Baoyan
Guo†
a,
Guo
Liu†
b,
Chaofan
Hu
 a,
Bingfu
Lei
a,
Bingfu
Lei
 a and
Yingliang
Liu
a and
Yingliang
Liu
 *a
*a
aKey Laboratory for Biobased Materials and Energy of Ministry of Education/Guangdong Provincial Engineering Technology Research Center for Optical Agriculture, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China. E-mail: tliuyl@scau.edu.cn; Tel: +86 20-85283313
bCollege of Horticulture, South China Agricultural University, Guangzhou, 510642, China
First published on 31st August 2022
Abstract
Overuse of antibiotics in agricultural production and medical practices has led to the rapid development of drug-resistant bacteria worldwide, and the emergence of superbacteria has increased the mortality rate of diseases and seriously threatened human life and health. The development of new antibiotics is slow, and resistant bacteria will soon emerge. The substitution of antibiotics with other antimicrobials is, therefore, strongly encouraged. Vaccine development for emerging viruses is slow and has low efficacy. As a new member of the nanomaterial family, carbon dots (CDs) have the advantages of photoluminescence, easy surface functionalization modification, simple preparation, low toxicity, low side effects, and lower probability to develop resistance, showing great antibacterial and antiviral potential. This review summarizes the structural characteristics of CDs with antimicrobial properties and the mechanism of CDs against different types of microorganisms, expecting to provide valuable information for the purposeful synthesis of antimicrobial CDs effective against specific microorganisms in the future.
1. Introduction
Antibacterial agents and pesticides are widely used in medicine, food packaging, agriculture, and other aspects, but the side effects of antibacterial agent residues on the body and environmental pollution are still a problem that cannot be ignored.1 Overuse of antibiotics and pesticides has led to resistance of microorganisms.2 Alexander Fleming discovered penicillin in 1928. After a few years, about 50% of Staphylococcus aureus (S. aureus) infections were refractory to penicillin. Under ideal conditions, it only takes ten days for bacteria to develop resistance, and the discovery of a new antibiotic can take as little as a year or two or as long as a decade or two (Fig. 1).3 Since 1990, humans have rarely discovered new antibiotic species. The world health organization (WHO) announced in September 2017 that the world's antibiotics were on the verge of depletion.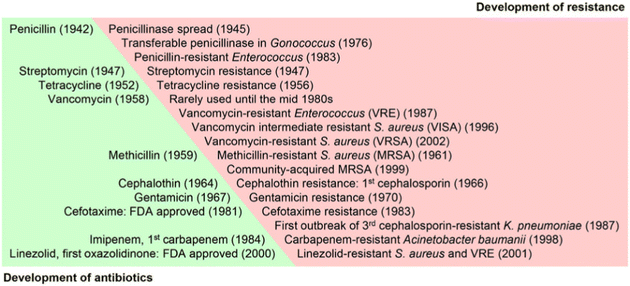 | ||
| Fig. 1 The timeline of antibiotics development and the subsequent emergence of resistant microorganisms (reprinted from ref. 3, with permission from Elsevier). | ||
The drug resistance of pathogenic bacteria is a serious biological and environmental problem, threatening the healthy survival of human beings. People have made unremitting efforts to seek new antimicrobial materials. Nanomaterials do not easily develop drug resistance due to: (1) high membrane permeability, (2) efflux pump inhibitors, and (3) multiple antibacterial action.4,5 Graphene-based nanomaterials have bactericidal properties,6–8 but they can affect the DNA and mitochondrial activity of cells, leading to apoptosis,9 and some nanomaterials exhibit biological toxicity toward bacteria and human cells.10
Carbon dots (CDs) are 0-dimensional spherical luminescent nanoparticles with a size of <10 nm. The structure of CDs is usually composed of an sp2/sp3 carbon core and a shell with a large number of functional groups/polymer chains on the surface. The existence of surface functional groups makes CDs easily functionalized and have good solubility. CDs are mainly divided into carbon nanodots (CNDs), carbon quantum dots (CQDs), graphene quantum dots (GQDs), and carbonized polymer dots (CPDs) according to their different structures. The core of CNDs has a graphite-like structure or amorphous form. The core of CQDs consists of a small number of graphene layers, and the layer spacing is 0.34 nm, corresponding to the (002) plane. The number of graphene layers in GQDs is smaller, and the atomic spacing is 0.24 nm, corresponding to the (100) plane. The structure of CPDs can be formed by cross-linked polymers or by functional groups/polymer chains linked to a spherical carbon core.11–13 Since CDs were discovered in 2004, due to their small size, biocompatibility, photoluminescence and easy functionalization and modification, they have been widely studied in biosensing,14 bioimaging,15,16 photothermal and photodynamic diagnosis and treatment,17–20 antibacterial nanomaterials,21,22 targeted drug delivery,23–26 and other aspects. In addition to top-down and bottom-up synthesis, researchers have isolated CDs from systems where Maillard reactions occurred in coffee, barbecue, and beer.27 The antimicrobial properties of CDs continue to attract the attention of researchers. The antibacterial mechanism of CDs is different from traditional antibiotics, and CDs are not prone to drug resistance. The different antimicrobial effects of CDs are mainly related to their physical and chemical properties. Due to the different preparation methods such as precursors and synthetic conditions, CDs with different structures have different antimicrobial properties.28 CDs combined with precious metal ions (silver, gold) have a strong antimicrobial ability,29,30 but after antimicrobial action, the metal ions are released, which are toxic to the target. Also, the cost of precious metals is very high. Hydrogen peroxide and light-assisted CDs also exhibit antimicrobial activities17 but require the help of external forces, resulting in limited conditions and scope of use. Generally, the antimicrobial ability of commonly synthesized CDs is limited.
Generally, the physicochemical properties are adjusted by changing the synthetic precursor, purification method, and surface functionalization, thereby improving the antimicrobial performance.21 Researchers have reviewed synthetic precursors of antimicrobial CDs and the structure of CDs, but there have been no discussions on the relationship between the structural characteristics and antimicrobial activity.31 So, what structure of CDs exhibits a better antimicrobial effect? Do CDs have specific antimicrobial effects on different types of microorganisms? What is the antimicrobial mechanism of CDs for different types of microorganisms? In view of the above questions, this paper will review the previous work of researchers and summarize the relationship between the structural characteristics of pure CDs and the antimicrobial effect, as well as the antimicrobial mechanism of different microbial types, so as to make contributions to the preparation of antimicrobial CDs in the future and its application in agricultural products.
2. Structural characteristics of CDs with antimicrobial properties
2.1. Size and shape
The size distribution of antimicrobial CDs is mostly less than 5 nm. Research results showed that the smaller size of the CDs was conducive to entering and exiting the cell and exhibited their antimicrobial ability. Positively charged large (5.3 ± 0.5 nm), medium (3.9 ± 0.6 nm), and small (2.0 ± 0.3 nm) CDs were synthesized using chlorhexidine gluconate as a precursor.32 They exhibited significant antibacterial activity against the Gram-negative bacteria Escherichia coli (E. coli) and Gram-positive bacteria S. aureus. The antibacterial mechanism was to increase the cell permeability and destroy the integrity of the plasma membrane. It was found that CDs with a smaller size exhibited a stronger antibacterial activity (Fig. 2A). The authors believed that the reason for this may be the difference in the distribution or entry of CDs on the plasma membrane or the interaction between the surface functional groups of the CDs and DNA molecules.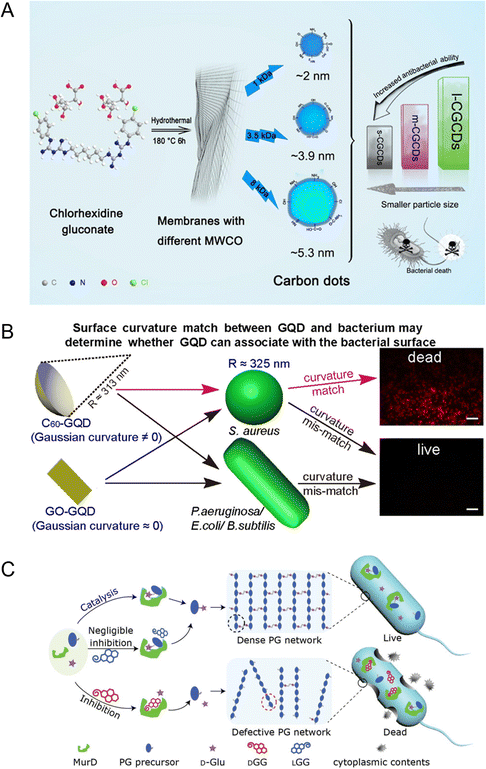 | ||
| Fig. 2 Antibacterial diagram of CDs with different sizes and shapes. (A) Schematic diagram of the antibacterial effect of CDs with different sizes (reprinted from ref. 32, with the permission of Elsevier). (B) Schematic diagram of how the surface curvature matching between GQD and the target bacterial surface correlates with the antibacterial performance of GQD (reprinted from ref. 6, with the permission of American Chemical Society). (C) The antibacterial strategies of chiral nanoparticles (DGG) (reprinted from ref. 37, with the permission of Wiley-VCH). | ||
The thickness of the passivation layer on the surface of the CDs had an effect on the antibacterial effect. Rabe et al. prepared CDs with different surface functionalities to study their photodynamic antibacterial activity.33 CDs with different surface passivation layer thicknesses were prepared with PEI of different molecular weights. The results showed that smaller molecular weight resulted in thinner CDs and stronger antibacterial activity. The authors speculated that a thinner surface passivation layer might allow the photogenerated reactive oxygen species (ROS) to act more efficiently on bacterial cells. In the above two references, although the author has adjusted the size of the CDs and the thickness of the passivation layer, the research on the specific antibacterial mechanism of CDs needs to be strengthened.
The shape of the CDs was also an important factor of its antibacterial activity. By controlling the surface Gaussian curvature of the CDs, Hui et al. prepared CDs matching the curvature of S. aureus using the C60 cage as a precursor.6 The obtained CDs could specifically kill S. aureus and its antibiotic-tolerant persisters but could not kill other bacterial species and mammalian cells (Fig. 2B). The surface Gaussian curvature matching between the CDs and the target bacteria played a key role in destroying the integrity of the bacterial cell membrane, thus exhibiting the antibacterial ability.
The chiral molecule D-glutamate (D-Glu) is the basic substance for the synthesis of peptidoglycan (PG), which is catalyzed by MurD ligase. PG is an important component of bacterial cell walls, but not mammalian cells, and protects them from damage caused by a high internal osmotic pressure.34 Chirality can be transferred from chiral molecules to achiral nanomaterials.35,36 Through the synthesis of chiral CDs (DGG), which compete with D-Glu to bind MurD, the formation of an intact cell wall could be inhibited to achieve the specific elimination of Gram-positive and Gram-negative bacteria while maintaining the integrity of mammalian cells (Fig. 2C).37 The van der Waals and hydrogen bond interaction between DGG and MurD was stronger than that between LGG and MurD, which resulted in a higher inhibitory effect of DGG.
2.2. Surface charge
Different surface groups of the CDs result in different surface charges and, thus, different antimicrobial properties. Generally, as the charge on the surface of the CDs (measured by a zeta potentiometer) becomes more positive, the antimicrobial ability becomes stronger.38,39 Because the cell membrane was negatively charged, the antimicrobial mechanism of the positively charged nanoparticles was the destruction of the cell membrane integrity.40,41Bing et al. prepared positively charged SC-CDs (27.6 mV), negatively charged CC-CDs (−19.5 mV), and neutrally charged GC-CDs (0.946 mV) to compare the effects of the different charge of the CDs on the antibacterial activity directly.38 The SC-CDs showed the best resistance to E. coli at 300 μg mL−1. The antibacterial mechanism was to induce ROS aggregation in vivo, induce cell apoptosis, and destroy the integrity of the bacterial cell membrane, which leads to programmed bacterial death (Fig. 3A). It is worth noting that the same CDs have different zeta potentials under different solution environmental conditions. Also, when the zeta potential of the system is below ±30 mV, it is unstable.
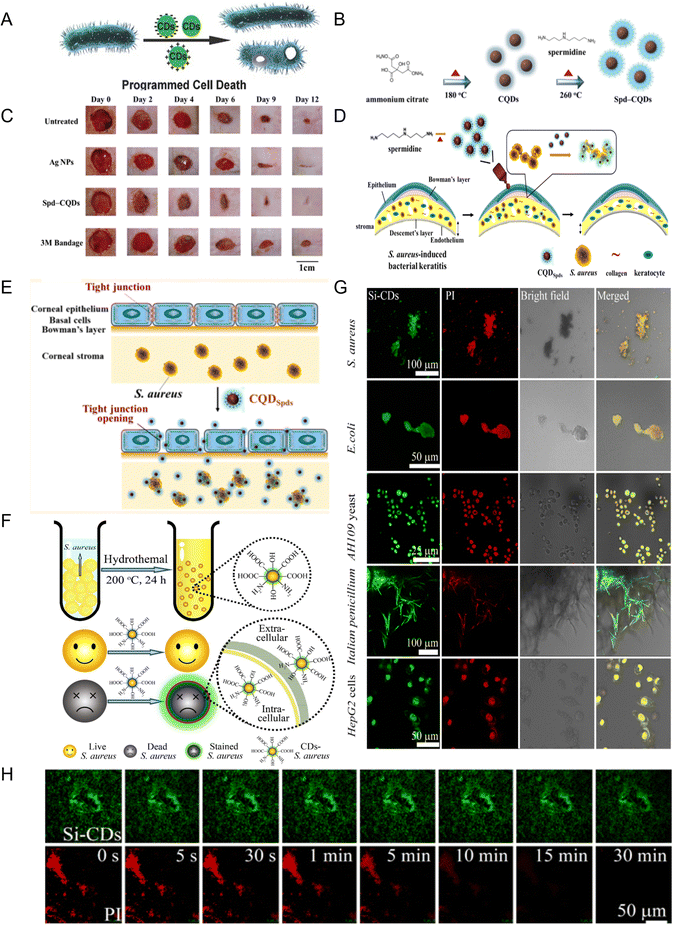 | ||
| Fig. 3 (A) Schematic diagram of programmed bacterial cell death induced by CDs with different surface charges (reprinted from ref. 38, with the permission of Wiley-VCH). (B) Schematic diagram of Spd-CQD synthesis. (C) Representative images of MRSA infected wounds treated on different days (B and C reprinted from ref. 42, with the permission of Wiley-VCH). (D) Schematic diagram of CQDSpds synthesis and treatment of S. aureus-induced bacterial keratitis. (E) Schematic diagram of CQDSpds inducing the opening of tight junctions of corneal epithelial cells for cell side transport ((D and E) reprinted from ref. 39, with the permission of American Chemical Society). (F) Synthesis of CDs and selective staining for dead S. aureus (reprinted from ref. 47, with the permission of Royal Society of Chemistry). (G) Co-located fluorescence images of Si-CDs and PI in different types of dead cells at a concentration of 20 μg mL−1. (H) Imaging stability of Si-CDs and PI in S. aureus cells ((G and H) reprinted from ref. 51, with the permission of Elsevier). | ||
Li et al. prepared CDs with a high positive charge (+60.6 mV) using ammonium citrate and spermidine as precursors in a two-step method (Fig. 3B). They had antibacterial activity against non-multidrug resistant bacteria (E. coli, S. aureus, Bacillus subtilis, Pseudomonas aeruginosa) and multidrug resistant bacteria (methicillin-resistant S. aureus (MRSA)).42 The minimum inhibitory concentration (MIC) was lower (>25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold) than that of spermidine. Further studies showed that the CDs were antibacterial not by inducing ROS production but due to the high positive charge on the surface of CDs, binding with proteins, porins, or peptidoglycan on the cell membrane through hydrogen bonds and electrostatic interaction, resulting in synergistic cell membrane damage or inhibition of membrane synthesis. The CDs could promote wound healing in rats infected with MRSA, with better epithelialization and collagen fiber formation (Fig. 3C).
000-fold) than that of spermidine. Further studies showed that the CDs were antibacterial not by inducing ROS production but due to the high positive charge on the surface of CDs, binding with proteins, porins, or peptidoglycan on the cell membrane through hydrogen bonds and electrostatic interaction, resulting in synergistic cell membrane damage or inhibition of membrane synthesis. The CDs could promote wound healing in rats infected with MRSA, with better epithelialization and collagen fiber formation (Fig. 3C).
Subsequently, their research group in the above research example further improved the preparation method and obtained super-cationic CQDSpds with a small size (6 nm) and high positive charge (+45 mV) by a one-step pyrolysis method using spermidine as the precursor.39 The CQDSpds also showed antibacterial activity against both non-multidrug resistant bacteria and multidrug resistant bacteria (Fig. 3D). The CQDSpds had a strong disintegration effect on bacterial membranes. In addition, in the BK rabbit model, the formulation of topical eye drops made by CQDSpds was shown to be more effective than Ag NPs in treating S. aureus eye infections because the super-cationic CQDSpds induced the opening of tight junctions in corneal epithelial cells for cellular side transport (Fig. 3E).
CDs with a high positive charge can fight viruses as well as bacteria. Researchers synthesized positively charged (15.6 ± 2.05 mV) CDs with curcumin as the precursor to resist porcine epidemic diarrhea virus (PEDV) infection, and the positively charged CDs may lead to viral aggregation through electrostatic interaction, thus reducing viral infectivity.43
However, it is not just the positively charged CDs that are antibacterial. Some negatively charged CDs have been reported to have antimicrobial activity. When CDs with a high negative surface charge (−75 ± 4 mV) were used to treat S. aureus and MRSA, they showed better antibacterial activity under laser irradiation.44 According to the report, it was because the van der Waals force between the highly negatively charged CDs and the bacteria could overcome the weak repulsive force,45 which made the CDs adhere to the cell surface, resulting in the production of ROS and cell wall damage, protein structure and function changes, and subsequently the death of the bacteria. However, some reports differ from these mechanisms. Zhu et al. prepared antibacterial CDs with a negative charge (−30 mV) by the electrolytic method using a vitamin C solution as the electrolyte.46 The CDs prevented the formation of the bacterial biofilm through electrostatic repulsion, and it might cover the outer surface of bacterial cells. Due to biological isolation, bacterial cells could neither increase in value nor consume nutrients after separation, thus damaging the cell wall or cell membrane to achieve the antibacterial effect.
Other negatively charged CDs showed a different result; that is, as the CDs become more negatively charged, the imaging ability is better, which makes them useful as an alternative dye to identify dead and live cells.47–50 Hua et al. for the first time used bacteria as the precursor to prepare CDs with a high negative charge (−42 mV) for differentiating live/dead cells (bacteria and fungi) (Fig. 3F).47 Compared with the conventional commercial dye propidium iodide (PI), CDs show advantages such as low toxicity, polychromatic imaging, and stability. This broadens the selection of raw materials for the preparation of CDs. Subsequently, using yeast extract as the precursor, CDs (−41.9 mV) with the ability to selectively stain dead bacteria were prepared, and when co-cultured with living bacteria, the bacterial activity could be monitored in real time with the increase in temperature.48 Similar results have also been reported.49 Silanized CDs with a small size (∼2.8 nm) and high fluorescence quantum yield (∼93.23%) were prepared by our group.51 They can stain bacterial, fungal, and mammalian dead cells in a passive diffusion manner (Fig. 3G) and have a lower cytotoxicity and better photostability than commercial staining (PI) (Fig. 3H).
2.3. Heteroatomic doping
Generally, N-doped CDs exhibit a strong antibacterial ability because N doping may endow CDs with positively charged properties52 or a lower band gap energy,53 which can exert antibacterial effects.The effect of S and N-doped CDs prepared by Travlou et al. was better on Gram-positive bacteria than on Gram-negative bacteria, and the minimum inhibitory concentration (MIC) was equal to or smaller than some antibiotics or silver nanoparticles.54 The antibacterial activity of N-CQDs was stronger than that of S-CQDs, mainly because the protonation of the nitrogen functional group of N-CQDs acted with the negatively charged cell membrane to generate ROS, which induced bacterial cell death. The negatively charged S-CQDs showed inhibition of bacterial growth due to the small size rather than the chemical dependence.
Wang et al. synthesized Cl-doped CDs with dual characteristics of anti-oxidation and pro-oxidation by the electrochemical method, and their free radical scavenging and free radical generation abilities were seven times and three times higher than those of undoped CDs, respectively.55 This was attributed to the high content of defect sites caused by Cl doping, which enabled them to produce ROS under visible light irradiation. This was considered a promising antibacterial application (Fig. 4A). However, the study would be more complete if there were comparisons with the antimicrobial activity of CDs doped with other elements.
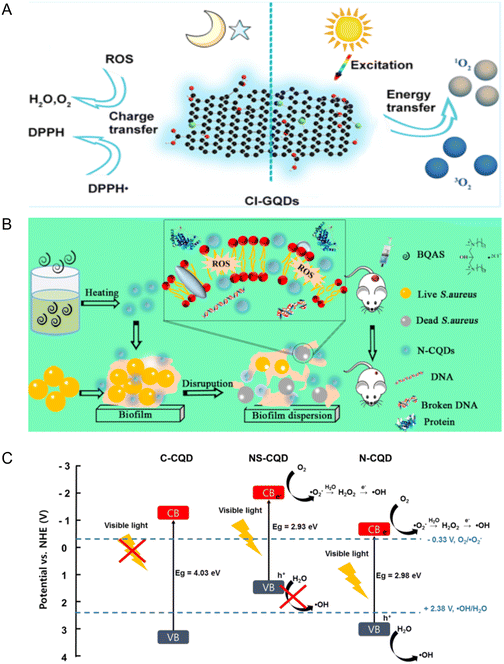 | ||
| Fig. 4 (A) Schematic diagram of Cl-GQDs with both anti-oxidation and pro-oxidation properties (reprinted from ref. 55, with the permission of American Chemical Society). (B) Schematic diagram of N-CQDs for antibacterial and disperse bacterial biofilms (reprinted from ref. 52, with the permission of American Chemical Society). (C) Schematic diagram of the photocatalytic mechanism of CQD doped with different elements. CB and VB are the conduction band and valence band, respectively (reprinted from ref. 53, with the permission of Elsevier). | ||
N-Doped CDs prepared with bis-quaternary ammonium salt as the precursor had a high antibacterial activity toward drug resistant bacteria.52 It can kill MRSA without producing drug resistance, prevent the formation of a biofilm, and eliminate the formed biofilm and persistent cells. The N-CD treatment fought bacterial infection and sped up the wound healing. Because N-CDs have a positive charge, they could cause cell membrane damage and increase the permeability through electrostatic interactions, induce the generation of ROS, and further affect bacterial metabolism, leading to death (Fig. 4B).
Doping CDs can change their structure and composition, essentially change their electronic properties or energy state, and then improve their optical properties and application performance.56,57 Recent studies have shown that CDs doped with N and/or S exhibited a vision-driven antimicrobial activity53 because doped N and/or S reduced the gap between the valence band and the conduction band of CDs compared with undoped CDs (Fig. 4C) and increased the electron density near the conduction band by supplying electrons to CDs through lone pair electrons of N or S.58–60 However, N-doping could improve the antibacterial performance of CDs better than NS-doping because with the increasing ratio of S, the CDs became not conducive to ROS generation and the specific surface area increased, resulting in the reduction in the reaction sites. The decrease in the fluorescence quantum yield led to adverse radiation recombination, and the decrease in the fluorescence lifetime made the photoexcitation state unable to be maintained well.53
2.4. Surface functionalization
CDs prepared with glucose and PEI as precursors and subsequently quaternized with benzyl bromide have an inhibitory effect on Gram-positive and Gram-negative bacteria.65 This was due to the adsorption of quaternized CDs on cell membranes, causing their destruction. Quaternized CDs were prepared by conjugating lauryl betaine (BS-12) containing a quaternary ammonium group onto the surface of CDs.64 The quaternized CDs have the ability to resist Gram-positive bacteria and selectively stain Gram-positive bacteria. Compared with BS-12, the local positive charge density and hydrophobic chain number of the quaternary ammonium CDs increased, which enhanced the antibacterial ability (Fig. 5A).
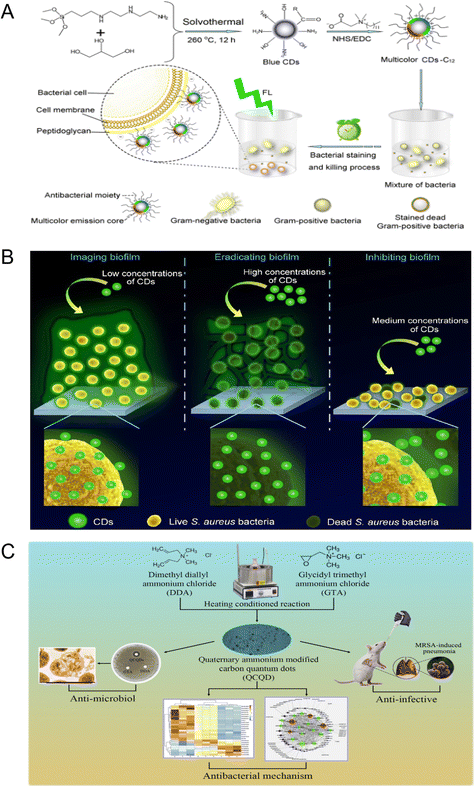 | ||
| Fig. 5 (A) Schematic diagram of CDs-C12 preparation and selective killing and staining of Gram-positive bacteria (reprinted from ref. 64, with the permission of American Chemical Society). (B) Schematic diagram of quaternary ammonium CDs in the imaging, eradication, and inhibition of S. aureus biofilms (reprinted from ref. 68, with the permission of Royal Society of Chemistry). (C) Preparation, antibacterial properties, and antibacterial mechanism of quaternary ammonium QCQD and its application in MRSA induced pneumonia in mice (reprinted from ref. 69, with the permission of Elsevier). | ||
The QACs can not only bind to the surface of CDs and play an antibacterial effect but can also be used as a precursor to undergo a solvothermal reaction with chloroform and diethylamine to generate CDs with a positive surface charge.66 Then, the as-prepared CDs interacted with the negatively charged cell membrane to resist Gram-positive and Gram-negative bacteria. Yang et al. prepared quaternized CDs by the solvent-thermal method with glycerol and dimethyloctadecyl [3-(trimethoxysilyl) propy] ammonium chloride (Si-QAC) as the precursor.67 The as-prepared CDs can selectively interact with Gram-positive bacteria through electrostatic and hydrophobic interactions, inserting long alkyl chains into the bacterial surface and eventually inactivating the Gram-positive bacteria. Subsequently, they synthesized quaternary ammonium CDs without long alkyl chains and verified that the long alkyl chain enhanced the hydrophobic adhesion between CDs and Gram-positive bacteria. They further showed that cationic CDs inhibited the formation of Gram-positive bacteria biofilms (Fig. 5B).68
Zhao et al. prepared quaternary ammonium CDs that had good antibacterial activity against Gram-positive bacteria including MRSA and could promote inflammation subside in pneumonia mice infected with MRSA.69 Further proteomic studies showed that the antimicrobial mechanism of these CDs was action on ribosomes and upregulation of RNA degradation related proteins, thus interfering with protein translation and modification in bacterial cells (Fig. 5C). Then, the quaternary ammonium CDs prepared by a one-step solvothermal method using p-phenylenediamine as the precursor were reported to have antibacterial activity against Gram-positive bacteria (S. aureus) and Gram-negative bacteria (E. coli), and the minimum inhibitory concentrations were 2 and 30 μg mL−1, respectively.70 The results showed that a large number of –NH3+ groups on the surface of the CDs enhanced their antibacterial activity. It is worth noting that quaternary ammonium salts themselves have anti-viral and antibacterial effects, so attention should be paid to the purification of CDs when preparing such CDs.
2.5. Other antibacterial CDs
With natural biomass as the precursor, CDs with antibacterial activity have been prepared. Zhao et al. prepared CDs with banana as the precursor and combined with chitosan to form a coating solution, which could prolong the shelf life of soy milk and significantly reduce the total bacterial count after 4 days of storage at room temperature.77 Using the natural biomass Artemisia argyi leaves, Wang et al. prepared CDs with 100% antibacterial activity against Gram-negative bacteria at a concentration of 150 μg mL−1 by a smoking simulation method.78 The prepared CDs could affect the secondary structure of the enzyme related to the cell wall synthesis of bacteria and, thus, inhibit about 50% of the enzyme activity, thereby selectively resisting the activity of Gram-negative bacteria.CDs were reported to have broad-spectrum antibacterial activity against Gram-positive (S. aureus and B. subtilis), Gram-negative (Bacillus sp. WL-6 and E. coli), fungi (R. solani and P. grisea), and drug-resistant bacteria (the ampicillin-resistant E. coli).79 The optimal inhibitory concentration was 100 μg mL−1 for bacteria and drug-resistant bacteria and 300 μg mL−1 for fungi. The research group then used cigarette smoke to prepare biodegradable CDs that were also broad-spectrum resistant to Gram-positive and Gram-negative bacteria, as well as drug-resistant bacteria.80
3. The antimicrobial mechanism of CDs
Although some CDs have a broad spectrum of antimicrobial properties, they can fight various types of bacteria, fungi, drug-resistant bacteria, multi-resistant bacteria, and even viruses. But overall, bactericidal targeting of specific bacteria is a promising treatment for infections, as it minimizes side effects and enhances antimicrobial activity.81 Therefore, it is necessary to summarize the types of microorganisms and the antimicrobial mechanism of CDs, so as to guide the purposeful synthesis of CDs.3.1. The mechanism of CDs against bacteria
The cell wall of bacteria can maintain the cell shape, improve mechanical strength, inhibit damage, assist cell movement and growth, and provide sensitivity to antibiotics. According to its form and function, it can be divided into Gram-positive and Gram-negative bacteria. The cell wall of Gram-positive bacteria consists mainly of thick layers of peptidoglycan and acidic polysaccharides including teichoic acid (Fig. 6A), and the overall negative charge on the cell surface is partly due to teichoic acid.82 The cell wall of Gram-negative bacteria, on the other hand, is more complex, consisting of thin monolayers of peptidoglycan and an outer membrane that includes porin and lipopolysaccharide (Fig. 6B).83 Different types of bacteria have different mechanisms of drug resistance. Gram-negative bacteria (E. coli) can change the nature of their cell walls so that they are not recognized by antibiotics, thus limiting the entry of antibiotics into their outer membranes.84 Gram-positive bacteria (K. pneumoniae and S. aureus) produce enzymes that destroy antibiotic molecules.85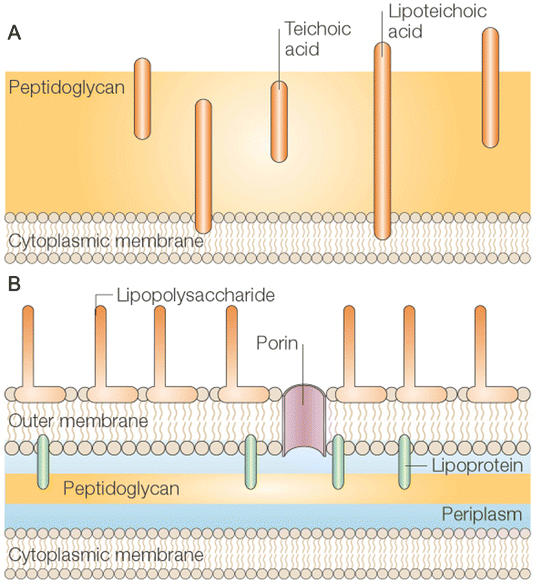 | ||
| Fig. 6 Cell walls of bacterial. The cell wall of Gram-positive (A) bacteria consists of multiple layers of thick polypeptidoglycan (PG) sheaths. Teichoic acid was embedded in PG, while lipoteichoic acid extended into the cytoplasmic membrane. The cell wall of Gram-negative (B) bacteria consists of an outer membrane connected by lipoproteins to a thin monolayer of PG. PG is placed in the periplasmic space formed by the outer and inner membranes. The outer membrane contains pores and lipopolysaccharide molecules ((A and B) reprinted from ref. 83, with the permission of Springer Nature). | ||
Quaternary ammonium polymers and compounds have strong bactericidal activity against Gram-positive bacteria. Generally, CDs with a positive surface charge approach bacteria with a negative surface charge through electrostatic interactions. In most cases, these are Gram-positive bacteria. They induce the bacteria to produce ROS, thus causing mechanical damage to the cell membrane, destroying the integrity of the cell membrane, and inducing cytoplasm leakage and cell apoptosis. However, some studies have observed that positively charged CDs can induce Gram-negative bacteria to produce endogenous ROS, destroy the integrity of the cell membrane, and lead to cell apoptosis along with DNA fragmentation.38 It is worth noting that each type of bacteria was sensitive to different free radicals. E. coli O157:H7 was more sensitive to ˙OH and 1O2, while L. monocytogenes was more sensitive to H2O2 and 1O2.53 According to a report from Huang et al., Gram-positive bacteria were more sensitive to 1O2, while Gram-negative bacteria were more sensitive to ˙OH.86
The antibacterial mechanism of CDs against bacteria involves multiple aspects: (1) causing physical and mechanical damage to the cell membrane, (2) affecting the activity of bacterial cell wall synthetases, (3) inducing cellular production of ROS, (4) disrupting cell membrane electron transport, (5) entering into the cell, affecting DNA and protein synthesis, (6) inhibiting the synthesis of bacterial biofilm, etc. When CDs exert antibacterial effects, it may be a single mechanism of action, or there may be a coexistence of multiple mechanisms. It is the multi-mechanism that makes CDs less likely to develop drug resistance. The antibacterial mechanisms of CDs in photodynamic therapy or photothermal therapy have been extensively reviewed14,17 and will not be described here.
The structural characteristics of CDs cannot be uniformly matched with the corresponding types of resistant bacteria, mainly because most studies are based on the existing available CDs and bacterial types rather than on specific CDs and bacterial types.
3.2. The mechanism of CDs against fungi
The imaging application of CDs in fungal cells has been widely reported. Thakur et al. first combined ciprofloxacin with CDs to form a Cipro@C-dots conjugate, which could perform in vivo imaging of Saccharomyces cerevisiae (yeast) and emit bright green fluorescence.71 Similar imaging of CDs in living fungi was also observed in Valsa Mali Miyabe et Yamada,87Aspergillus aculeatus,88,89Saccharomyces cerevisiae,90Colletotrichum gloeosporioides,91 and Fusarium oxysporum.92 When CDs were prepared with bacteria as a precursor, the imaging application of CDs can be observed in dead fungal cells Saccharomyces cerevisiae (yeast) and Trichoderma reesei.47 Silanized CDs prepared by our group can also stain dead fungal cells (AH109 yeast, Penicillium italicum).51 These results suggest that CDs can enter dead or living fungal cells.Rhizoctonia solani and Pyricularia grisea are two kinds of fungi causing plant diseases. Li et al. prepared biodegradable CDs with antifungal activity against the above two fungi at the concentration of 300 μg mL−1.79 Further studies showed that CDs could directly enter the fungal nucleus and destroy it, inhibiting the growth of fungal cells. Moreover, CDs could significantly inhibit the expression of the non-ribosomal peptide synthase gene in Rhizoctonia solani. The CDs prepared with tamarind as a precursor showed antifungal activity against Candida albicans.93 Confocal microscopy was used to observe the morphology of Candida albicans. The results showed that CDs could interact with fungal cells, but the antifungal mechanism was not further studied. Bagheri et al. reported that the prepared CDs inhibited the growth of Pichia pastoris in a dose-dependent manner.94 At high concentrations, the CDs can significantly change the morphology of yeast cells and stimulate the production of toxic levels of ROS to inhibit the growth of yeast cells. The multi-functional CDs prepared with dried seaweed as the precursor had anti-fungal cucumber downy mildew (Pseudoperonospora cubensis) activity.95 At the concentration of 40 mg L−1, the CDs could inhibit 20% fungal activity. With the increase in the concentration of CDs, there was no enhanced antibacterial activity. CDs loaded with flumorph exhibited better antifungal activity than CDs and flumorph alone. The authors suggest that the oxygen-containing groups on the surface of the CDs might adsorb on the fungal cell walls and destroy the integrity of the cell membrane, leading to cytoplasm leakage. However, there were no further mechanistic tests. Fungi are more stubborn than bacteria, so timeliness and dosage should also be taken into account when evaluating the activity of antifungal CDs.
3.3. The mechanism of CDs against virus
Most viruses can cause illness and even death in humans. The best way to prevent infection is to get vaccinated. However, there are currently no effective vaccines against the various viruses and their variants. Nanotechnology has shown powerful capabilities in antiviral strategies, and some researchers have reviewed relevant studies on the antiviral effects of functional nanoparticles and their corresponding antiviral mechanisms.96 As a new star in nanomaterials, CDs have also been studies in antiviral field. Especially since the outbreak of the novel Coronavirus (COVID-19) pandemic in 2019, more researchers have begun to focus on the study of CDs resistance toward novel Coronaviruses, and six relevant review papers have been published.97–102 We have summarized thirteen published research papers about antiviral CDs, including the precursor, antiviral types, and antiviral mechanism (Table 1), in order to provide a reference for future research on antiviral CDs.| Year | Precursor | Synthesis method | Virus species | Antiviral mechanism | Concentration |
|---|---|---|---|---|---|
| 2016106 | PEG-diamine and ascorbic acid | Solid-phase thermal reaction | Pseudorabies virus, porcine reproductive and respiratory syndrome virus | Inhibiting viral replication | 0.125 mg mL−1 |
| 2016103 | 4-Aminophenylboronic acid hydrochloride | Hydrothermal | Herpes simplex virus type 1 (HSV-1) | Inhibiting virus entry | EC50 = 80 and 145 ng mL−1 on Vero and A549 cells, respectively |
| 2017107 | Barley young leaves, citric acid anhydrous and urea | Solid pyrolysis | Pseudorabies virus | Inhibiting viral replication | 0.13 mg mL−1 |
| 2017108 | Carbon nano-powders, 3-ethoxypropylamine and 2,2′-(ethylenedioxy)bis(ethylamine) | Nitric acid reflux and thermal mixing | Human norovirus virus-like-particles | Inhibiting virus entry | 5 μg mL−1 |
| 201843 | Curcumin and citric acid | Solid pyrolysis | Enteric coronavirus | Various inhibitory effects including inhibition of viral entry, viral negative-strand RNA synthesis, viral budding, accumulation of ROS, and inhibition of viral replication | 125 μg mL−1 |
| 2019112 | Curcumin | Dry heating | Enterovirus 71 | Inhibiting the virus entry, replication, and preventing the virus-induced termination of host translation | EC50 (0.2 μg mL−1) and CC50 (452.2 μg mL−1) |
| 2019104 | 4-Aminophenylboronic acid | Hydrothermal | Human coronavirus | Inhibiting virus entry into receptor and virus replication | EC50 = 5.2 ± 0.7 μg mL−1 |
| 2019110 | Benzoxazine | Hydrothermal | Flaviviruses (Japanese encephalitis, Zika, and dengue viruses) and non-enveloped viruses (porcine parvovirus and adenovirus-associated virus) | Preventing the virion from entering the host cell | 75 μg mL−1 |
| 2019111 | Humic acid | Hydrothermal | Avian leukosis virus subgroup J | As an excellent adjuvant of the gp85 protein vaccine against chicken anti-avian leukosis virus subgroup J | 400 μg mL−1 |
| 2020114 | Glycyrrhizic acid | Hydrothermal | Porcine reproductive and respiratory syndrome virus (PRRSV), pseudorabies virus (PRV), and porcine epidemic diarrhea virus (PEDV) | Inhibiting virus invasion and replication, stimulating the production of interferons, inhibiting the accumulation of intracellular ROS, regulating the expression of some host-restricted factors | 0.30 mg mL−1 |
| 2020109 | Carbon nano-powders, 2,20-(ethylenedioxy)bis(ethylamine) | Nitric acid reflux and thermal mixing | Bacteriophages MS2 | Viral protein carbonylation and degradation of viral genomic RNA | 20 μg mL−1 |
| 2020113 | Spermidine trihydrochloride | Pyrolysis | White spot syndrome virus | Sticking to virus envelope and inhibiting virus infection, upregulating immune genes and reducing the mortality rate of virus infection | 10 ppm |
| 2020105 | Anhydrous citric acid and 2-amino phenylboronic acid | Pyrolysis | HIV-1 | Preventing the virus from entering the host cell | 50 μg mL−1 |
Barras et al. prepared boronic acid- and amine-functionalized CDs with phenylboronic acid derivatives as precursors.103 The prepared CDs could prevent herpes simplex virus type 1 infection. The EC50 was 80 ng mL−1 in Vero and 145 ng mL−1 in A549 cells, respectively. The antiviral mechanism of CDs was the inhibition of viral entry through the interaction with the viruses. Subsequently, their group prepared seven different CDs, and the results showed that the EC50 of CDs prepared with ethylenediamine and citric acid as precursors and modified with boronic acid was 52 ± 8 μg mL−1, although the presence of boronic acid was essential for antiviral activity, but CQDs-6 did not carry a large amount of boronic acid and was more antiviral with an EC50 of 5.2 ± 0.7 μg mL−1, indicating the complexity of the antiviral mechanism.104 The prepared CDs exerted antiviral effects by inhibiting viral entry into receptors and inhibiting viral replication.
Amino phenyboronic acid-modified CDs prepared by Aung et al. showed antiviral activity against the HIV-1 virus.105 The antiviral mechanism of CDs was similar to that previously reported by Barras et al. because CDs had boron and nitrogen on their surface, which can bind to the gp120 glycoprotein on the envelope of the HIV-1 virus, thus preventing the virus from entering the host cell.
Du et al. found that CDs induced the production of interferon-α and the expression of the IFN stimulating gene.106 Thus, it can inhibit the replication of the Pseudorabies virus and Porcine reproductive and respiratory syndrome virus. Subsequently, the same authors synthesized curcumin-based CDs that antiviral enteric coronavirus through multiple inhibitory effects, including inhibition of viral entry, synthesis of viral negative strand RNA, viral budding, and accumulation of ROS.43 In addition, the CDs were found to inhibit viral replication by enabling the production of pro-inflammatory cytokines and stimulating interferon-stimulated genes.43 Between the two studies, the research group synthesized blue-CDs and cyan-CDs with young barley leaves and citric acid anhydrous without or with urea.107 It was found that blue-CDs had a better antiviral effect than cyan-CDs because most of the factors involved in the interferon induction pathway were located in the cytoplasm, while the amount of blue-CDs in the cytoplasm was higher than that of cyan-CDs. Both of them could induce the expression of interferon and its stimulating genes.
The CDs prepared by Dong et al. using a two-step method showed resistance to human norovirus virus-like particles (VLPs).108 The positively charged EDA-CDots are much more effective than the negatively charged EPA-CDots in inhibiting the binding of VLPs to HBGA receptors because the binding of EDA-CDots to negatively charged VLPs is more favorable. The main antiviral mechanism of CDs is to effectively inhibit the binding of VLPs to HBGA receptors and moderately inhibit the binding of VLPs to antibodies without affecting the integrity of viral capsid proteins and virus particles. Then, their group studied the photodynamic antiviral activity of EDA-Cdots under visible light irradiation using bacteriophages MS2 as a virus model.109 The results showed that the CDs caused carbonylation of viral proteins and degradation of genomic RNA.
CDs synthesized by benzoxazine as a precursor were reported to have spectral antiviral activity.110 The antibacterial mechanism was the direct interaction of CDs with viruses to prevent viruses from entering host cells and to limit the spread of the viruses. It was reported that CDs prepared with humic acid as a precursor can be used as an excellent adjuvant of the chicken gp85 protein vaccine against avian leukosis virus subgroup J.111 Antibody levels in the gp85-CDs group were higher than those in the gp85-Freund adjuvant group.
CDs prepared using curcumin as a precursor by the dry heating method had antiviral activity against enterovirus 71.112 The results showed that CDs inhibited the virus by binding to the virus, prevented the virus from attaching to the host cell membrane, inhibited viral replication, and prevented the virus-induced termination of host translation. The polyamine CDs mentioned above can destroy bacterial cell membranes due to its high positive charge, thus having antibacterial activity, and can treat bacterial infection in rabbit eyes.39 The authors then treated the white spot syndrome virus, which is an envelope virus, with the same CDs and found that the CDs could disrupt the envelope of the virus, thus affecting the invasion of the virus. In addition, the CDs also upregulated immune genes and reduced the mortality of infected mice.113 CDs synthesized from glycyrrhizic acid, the active ingredient of Chinese herbal medicine, has been reported to have anti-porcine reproductive and respiratory syndrome virus (PRRSV) activity.114 The antiviral mechanisms of CDs were to inhibit the invasion and replication of PRRSV, stimulate the production of interferon, inhibit the accumulation of intracellular ROS caused by PRRSV infection, and regulate the expression of some host-restricted factors directly related to the proliferation of PRRSV, including DDX53 and NOS3. It can be seen from the above results that CDs prepared from natural substances have high biocompatibility, and the prepared CDs can inherit the antiviral properties of the precursor and have better effects and fewer side effects.
4. Concluding remarks
This paper reviews the current literature on CDs against bacteria, fungi, and viruses and summarizes the structural characteristics of CDs with antimicrobial effects. Although antimicrobial CDs are a developing field, the summary of this paper lays a foundation for future studies on the synthesis or optimization of antimicrobial CDs. Due to the unclear structure and chemical formula of CDs, their controllable and efficient synthesis still needs to be worked on. However, compared with other carbon materials, CDs possess advantages such as good biocompatibility, inexpensive and readily available synthetic materials, simple synthetic methods, and easy large-scale synthesis. At present, there are commercial products of CQDs on the market (https://www.pkuchemqd.com/c/35.html). It is believed that with the advancement of technology, the commercial production of antimicrobial CDs will soon be realized. By adjusting the size, surface charge, and doping atoms of CDs, the prepared CDs can have strong antibacterial properties. Compared with broad-spectrum resistance, precise antimicrobial properties have more advantages in reducing drug side effects and reducing drug-resistant bacteria. Therefore, we summarize the antimicrobial mechanisms of CDs for different types of microorganisms and look forward to helping the useful synthesis of antimicrobial CDs.CDs with a small size, that can easily enter and exit cells, show better antimicrobial ability. A thinner surface passivation layer of CDs might allow photogenerated ROS to act more efficiently on bacterial cells. The surface Gaussian curvature matching between the CDs and the target bacteria plays a key role in destroying the integrity of the bacterial cell membrane. Through the synthesis of chiral CDs, which compete with D-Glu to bind MurD, the formation of the intact cell wall could be inhibited to achieve the specific elimination of Gram-positive and Gram-negative bacteria while maintaining the integrity of mammalian cells. Because the bacterial cell membrane is negatively charged, CDs with a high positive charge easily adhere to the bacterial cell membrane and generate ROS, thus destroying the integrity of the cell membrane, leading to cytoplasmic leakage and bacterial cell death. N doping may endow CDs with positively charged properties or a lower band gap energy, which can exert antibacterial effects. Quaternized CDs have good antibacterial effects, while negatively charged CDs can be used to stain dead cells. The antibacterial properties of CDs doped with other different elements require more extensive and in-depth studies. CDs prepared with antibiotics as precursors or CDs grafted with antibiotics have good antibacterial effects, but it is necessary to pay attention to the purification steps in the preparation of CDs to exclude the antibacterial effects of the precursors themselves. CDs appear to have greater antiviral potential. The antiviral mechanisms of CDs are as follows: (1) inhibition of viral entry, (2) inhibition of viral negative-strand RNA synthesis, (3) inhibition of viral budding, (4) accumulation of ROS, (5) inhibition of viral replication, (6) upregulation of immune genes and reduction of the mortality rate of virus infection, and (7) prevention of the virus from entering the host cell. However, the high standards of the experimental conditions for infectious viruses limit their research. Future research needs to focus on the purposeful synthesis of CDs targeting specific types of microorganisms. The long-term biological toxicity of CDs has not been systematically studied. The antifungal and anti-phytopathogenic aspects of CDs are understudied.
There are currently no data on the clinical application and toxicity of CDs as antibiotics themselves. Before antimicrobial products of CDs can be used clinically, some challenges must be addressed, such as: (1) achieving targeted delivery and preventing shielding by the human/animal immune system or digestive system before reaching the site of action, (2) side effects on the human/animal body after passing through the blood–brain barrier, (3) toxicology test, (4) drug resistance test against bacteria, etc. In the era of antibiotic resistance, the development of safe, effective, low-side effect, and specific antibacterial agents requires interdisciplinary knowledge and tools in materials science, biology, immunology, pathology, and pharmacology. The summary of this paper is conducive to the purposeful synthesis of CDs with antimicrobial properties in the future and also lays a foundation for the application of CDs in the fields of medical materials, agricultural production, and food preservation.
Author contributions
Baoyan Guo and Guo Liu conceived the idea and initiated the project. Baoyan Guo and Guo Liu mainly wrote the manuscript and produced the figures. Chaofan Hu and Bingfu Lei contributed to parts of the manuscript. Yingliang Liu supervised the project and edited the manuscript. All the authors contributed to the discussion.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The present work was financially supported by the National Natural Science Foundation of China (Grant No. 12174119 and 52172142), and the Science and Technology Program of Guangzhou, China (Grant No. 202103000059).References
- T. Yu, G. Jiang, R. Gao, G. Chen, Y. Ren, J. Liu, H. C. van der Mei and H. J. Busscher, Expert Opin. Drug Delivery, 2020, 17, 1151–1164 CrossRef CAS PubMed.
- D. I. Andersson and D. Hughes, FEMS Microbiol. Rev., 2011, 35, 901–911 CrossRef CAS PubMed.
- A. J. Huh and Y. J. Kwon, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS PubMed.
- M. J. Hajipour, K. M. Fromm, A. Akbar Ashkarran, D. Jimenez De Aberasturi, I. R. D. Larramendi, T. Rojo, V. Serpooshan, W. J. Parak and M. Mahmoudi, Trends Biotechnol., 2012, 30, 499–511 CrossRef CAS PubMed.
- Q. Xin, H. Shah, A. Nawaz, W. Xie, M. Z. Akram, A. Batool, L. Tian, S. U. Jan, R. Boddula, B. Guo, Q. Liu and J. R. Gong, Adv. Mater., 2019, 31, 1804838 CrossRef CAS.
- L. Hui, J. Huang, G. Chen, Y. Zhu and L. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 20–25 CrossRef CAS PubMed.
- W. Hu, C. Peng, W. Luo, M. Lv, X. Li, D. Li, Q. Huang and C. Fan, ACS Nano, 2010, 4, 4317–4323 CrossRef CAS PubMed.
- H. Ji, H. Sun and X. Qu, Adv. Drug Delivery Rev., 2016, 105, 176–189 CrossRef CAS PubMed.
- T. Lammel, P. Boisseaux, M. Fernández-Cruz and J. M. Navas, Part. Fibre Toxicol., 2013, 10, 27 CrossRef CAS PubMed.
- O. Bondarenko, K. Juganson, A. Ivask, K. Kasemets, M. Mortimer and A. Kahru, Arch. Toxicol., 2013, 87, 1181–1200 CrossRef CAS PubMed.
- C. Xia, S. Zhu, T. Feng, M. Yang and B. Yang, Adv. Sci., 2019, 6, 1901316 CrossRef CAS PubMed.
- P. Koutsogiannis, E. Thomou, H. Stamatis, D. Gournis and P. Rudolf, Adv. Phys.: X, 2020, 5, 1758592 CAS.
- M. Varghese and M. Balachandran, J. Environ. Chem. Eng., 2021, 9, 106821 CrossRef CAS.
- F. Lin, Y. Bao and F. Wu, C-J Carbon Res., 2019, 5, 33 CrossRef CAS.
- G. Gao, Y. Jiang, W. Sun and F. Wu, Chin. Chem. Lett., 2018, 29, 1475–1485 CrossRef CAS.
- L. Ansari, S. Hallaj, T. Hallaj and M. Amjadi, Colloids Surf., B, 2021, 203, 111743 CrossRef CAS PubMed.
- B. Wang, H. Song, X. Qu, J. Chang, B. Yang and S. Lu, Coordin. Chem. Rev., 2021, 442, 214010 CrossRef CAS.
- X. Dong, W. Liang, M. J. Meziani, Y. Sun and L. Yang, Theranostics, 2020, 10, 671–686 CrossRef CAS PubMed.
- M. Kováčová, E. Apitalská, Z. Markovic and Z. Apitálský, Part. Part. Syst. Charact., 2020, 37, 1900348 CrossRef.
- R. Knoblauch and C. D. Geddes, Materials, 2020, 13, 4004 CrossRef CAS PubMed.
- M. Varghese and M. Balachandran, J. Environ. Chem. Eng., 2021, 9, 106821 CrossRef CAS.
- M. Alavi, E. Jabari and E. Jabbari, Expert Rev. Anti-Infect. Ther., 2021, 19, 35–44 CrossRef CAS PubMed.
- P. Namdari, B. Negahdari and A. Eatemadi, Biomed. Pharmacother., 2017, 87, 209–222 CrossRef CAS PubMed.
- M. Farshbaf, S. Davaran, F. Rahimi, N. Annabi, R. Salehi and A. Akbarzadeh, Artif. Cells, Nanomed. Biotechnol., 2018, 46, 1331–1348 CrossRef CAS PubMed.
- Z. Peng, X. Han, S. Li, A. O. Al-Youbi, A. S. Bashammakh, M. S. El-Shahawi and R. M. Leblanc, Coordin. Chem. Rev., 2017, 343, 256–277 CrossRef CAS.
- Z. Wang, Z. Wang and F. Wu, ChemMedChem, 2022, 17, e202200003 CAS.
- H. Wang, W. Su and M. Tan, Innovation, 2020, 1, 100009 Search PubMed.
- P. Li, L. Sun, S. Xue, D. Qu, L. An, X. Wang and Z. Sun, SmartMat, 2022, 3, 226–248 CrossRef.
- M. Hu, X. Gu, Y. Hu, Y. Deng and C. Wang, Macromol. Mater. Eng., 2016, 301, 1352–1362 CrossRef CAS.
- D. Gao, P. Zhao, B. Lyu, Y. Li, Y. Hou and J. Ma, Appl. Organomet. Chem., 2020, 34, e5665 CAS.
- Y. Wu, C. Li, H. C. van der Mei, H. J. Busscher and Y. Ren, Antibiotics, 2021, 10, 623 CrossRef CAS PubMed.
- B. Sun, F. Wu, Q. Zhang, X. Chu, Z. Wang, X. Huang, J. Li, C. Yao, N. Zhou and J. Shen, J. Colloid Interface Sci., 2021, 584, 505–519 CrossRef CAS.
- D. I. Abu Rabe, M. M. Al Awak, F. Yang, P. A. Okonjo, X. Dong, L. R. Teisl, P. Wang, Y. Tang, N. Pan, Y. Sun and L. Yang, Int. J. Nanomed., 2019, 14, 2655–2665 CrossRef CAS.
- A. L. Lovering, S. S. Safadi and N. C. J. Strynadka, Annu. Rev. Biochem., 2012, 81, 451–478 CrossRef CAS PubMed.
- S. Ostovar Pour, L. Rocks, K. Faulds, D. Graham, V. Parchaňský, P. Bouř and E. W. Blanch, Nat. Chem., 2015, 7, 591–596 CrossRef CAS.
- N. Suzuki, Y. Wang, P. Elvati, Z. Qu, K. Kim, S. Jiang, E. Baumeister, J. Lee, B. Yeom, J. H. Bahng, J. Lee, A. Violi and N. A. Kotov, ACS Nano, 2016, 10, 1744–1755 CrossRef CAS PubMed.
- Q. Xin, Q. Liu, L. Geng, Q. Fang and J. R. Gong, Adv. Healthcare Mater., 2017, 6, 1601011 CrossRef PubMed.
- W. Bing, H. Sun, Z. Yan, J. Ren and X. Qu, Small, 2016, 12, 4713–4718 CrossRef CAS PubMed.
- H. Jian, R. Wu, T. Lin, Y. Li, H. Lin, S. G. Harroun, J. Lai and C. Huang, ACS Nano, 2017, 11, 6703–6716 CrossRef CAS PubMed.
- P. Li, Y. F. Poon, W. Li, H. Zhu, S. H. Yeap, Y. Cao, X. Qi, C. Zhou, M. Lamrani, R. W. Beuerman, E. Kang, Y. Mu, C. M. Li, M. W. Chang, S. S. Jan Leong and M. B. Chan-Park, Nat. Mater., 2011, 10, 149–156 CrossRef CAS PubMed.
- Y. Zhao, Z. Chen, Y. Chen, J. Xu, J. Li and X. Jiang, J. Am. Chem. Soc., 2013, 135, 12940–12943 CrossRef CAS PubMed.
- Y. Li, S. G. Harroun, Y. Su, C. Huang, B. Unnikrishnan, H. Lin, C. Lin and C. Huang, Adv. Healthcare Mater., 2016, 5, 2545–2554 CrossRef CAS PubMed.
- D. Ting, N. Dong, L. Fang, J. Lu, J. Bi, S. Xiao and H. Han, ACS Appl. Nano Mater., 2018, 1, 5451–5459 CrossRef.
- N. Sattarahmady, M. Rezaie-Yazdi, G. H. Tondro and N. Akbari, J. Photochem. Photobiol., B, 2017, 166, 323–332 CrossRef CAS PubMed.
- M. C. van Loosdrecht, J. Lyklema, W. Norde, G. Schraa and A. J. Zehnder, Appl. Environ. Microb., 1987, 53, 1893–1897 CrossRef CAS PubMed.
- C. Zhu, H. Li, H. Wang, B. Yao, H. Huang, Y. Liu and Z. Kang, Small, 2019, 15, 1900007 CrossRef PubMed.
- X. Hua, Y. Bao, H. Wang, Z. Chen and F. Wu, Nanoscale, 2017, 9, 2150–2161 RSC.
- Y. Song, H. Li, F. Lu, H. Wang, M. Zhang, J. Yang, J. Huang, H. Huang, Y. Liu and Z. Kang, J. Mater. Chem. B, 2017, 5, 6008–6015 RSC.
- F. Lu, Y. Song, H. Huang, Y. Liu, Y. Fu, J. Huang, H. Li, H. Qu and Z. Kang, Carbon, 2017, 120, 95–102 CrossRef CAS.
- F. Lin, C. Li and Z. Chen, Front. Microbiol., 2018, 9, 2627 CrossRef PubMed.
- J. Chen, W. Liu, Y. Li, X. Zou, W. Li, J. Liang, H. Zhang, Y. Liu, X. Zhang, C. Hu and B. Lei, Chem. Eng. J., 2022, 428, 131168 CrossRef CAS.
- H. Wang, Z. Song, J. Gu, S. Li, Y. Wu and H. Han, ACS Biomater. Sci. Eng., 2019, 5, 4739–4749 CrossRef CAS.
- J. Kang and D. Kang, Chem. Eng. J., 2021, 420, 129990 CrossRef CAS.
- N. A. Travlou, D. A. Giannakoudakis, M. Algarra, A. M. Labella, E. Rodríguez-Castellón and T. J. Bandosz, Carbon, 2018, 135, 104–111 CrossRef CAS.
- L. Wang, Y. Li, Y. Wang, W. Kong, Q. Lu, X. Liu, D. Zhang and L. Qu, ACS Appl. Mater. Interfaces, 2019, 11, 21822–21829 CrossRef CAS PubMed.
- Y. Sun, C. Shen, J. Wang and Y. Lu, RSC Adv., 2015, 5, 16368–16375 RSC.
- J. Liu, X. Liu, H. Luo and Y. Gao, RSC Adv., 2014, 4, 7648–7654 RSC.
- H. Wang, J. Xiao, Z. Yang, H. Tang, Z. Zhu, M. Zhao, Y. Liu, C. Zhang and H. Zhang, J. Mater. Chem. A, 2015, 3, 11287–11293 RSC.
- T. K. Mondal and S. K. Saha, ACS Sustainable Chem. Eng., 2019, 7, 19669–19678 CrossRef CAS.
- S. H. Jin, D. H. Kim, G. H. Jun, S. H. Hong and S. Jeon, ACS Nano, 2013, 7, 1239–1245 CrossRef CAS PubMed.
- C. Zhu, Q. Yang, L. Liu, F. Lv, S. Li, G. Yang and S. Wang, Adv. Mater., 2011, 23, 4805–4810 CrossRef CAS PubMed.
- X. Li, S. M. Robinson, A. Gupta, K. Saha, Z. Jiang, D. F. Moyano, A. Sahar, M. A. Riley and V. M. Rotello, ACS Nano, 2014, 8, 10682–10686 CrossRef CAS PubMed.
- H. Wang, X. Hua, F. Wu, B. Li, P. Liu, N. Gu, Z. Wang and Z. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 7082–7092 CrossRef CAS PubMed.
- J. Yang, X. Zhang, Y. Ma, G. Gao, X. Chen, H. Jia, Y. Li, Z. Chen and F. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 32170–32181 CrossRef CAS PubMed.
- Q. Dou, X. Fang, S. Jiang, P. L. Chee, T. Lee and X. J. Loh, RSC Adv., 2015, 5, 46817–46822 RSC.
- B. Ju, H. Nie, X. Zhang, Q. Chen, X. Guo, Z. Xing, M. Li and S. X. Zhang, ACS Appl. Nano Mater., 2018, 1, 6131–6138 CrossRef CAS.
- J. Yang, G. Gao, X. Zhang, Y. Ma, X. Chen and F. Wu, Carbon, 2019, 146, 827–839 CrossRef CAS.
- H. Ran, X. Cheng, Y. Bao, X. Hua, G. Gao, X. Zhang, Y. Jiang, Y. Zhu and F. Wu, J. Mater. Chem. B, 2019, 7, 5104–5114 RSC.
- C. Zhao, L. Wu, X. Wang, S. Weng, Z. Ruan, Q. Liu, L. Lin and X. Lin, Carbon, 2020, 163, 70–84 CrossRef CAS.
- Z. Ye, G. Li, J. Lei, M. Liu, Y. Jin and B. Li, ACS Appl. Bio Mater., 2020, 3, 7095–7102 CrossRef CAS PubMed.
- M. Thakur, S. Pandey, A. Mewada, V. Patil, M. Khade, E. Goshi, M. Sharon and R. Cavalli, J. Drug Delivery, 2014, 2014, 282193 Search PubMed.
- P. Hou, T. Yang, H. Liu, Y. F. Li and C. Z. Huang, Nanoscale, 2017, 9, 17334–17341 RSC.
- J. S. Sidhu, Mayank, T. Pandiyan, N. Kaur and N. Singh, ChemistrySelect, 2017, 2, 9277–9283 CrossRef CAS.
- R. Jijie, A. Barras, J. Bouckaert, N. Dumitrascu, S. Szunerits and R. Boukherroub, Colloids Surf., B, 2018, 170, 347–354 CrossRef CAS PubMed.
- J. Liu, S. Lu, Q. Tang, K. Zhang, W. Yu, H. Sun and B. Yang, Nanoscale, 2017, 9, 7135–7142 RSC.
- X. Dong, M. A. Awak, N. Tomlinson, Y. Tang, Y. Sun and L. Yang, PLoS One, 2017, 12, e185324 Search PubMed.
- L. Zhao, M. Zhang, H. Wang and S. Devahastin, Int. J. Food Microbiol., 2020, 326, 108650 CrossRef CAS PubMed.
- H. Wang, M. Zhang, Y. Ma, B. Wang, M. Shao, H. Huang, Y. Liu and Z. Kang, J. Mater. Chem. B, 2020, 8, 2666–2672 RSC.
- H. Li, J. Huang, Y. Song, M. Zhang, H. Wang, F. Lu, H. Huang, Y. Liu, X. Dai, Z. Gu, Z. Yang, Z. Ruhong and Z. H. Kang, ACS Appl. Mater. Interfaces, 2018, 10, 26936–26946 CrossRef CAS PubMed.
- Y. Song, F. Lu, H. Li, H. Wang, M. Zhang, Y. Liu and Z. Kang, ACS Appl. Bio Mater., 2018, 1, 1871–1879 CrossRef CAS PubMed.
- M. Debono and R. S. Gordee, Annu. Rev. Microbiol., 1994, 48, 471–497 CrossRef CAS.
- J. R. Scott and T. C. Barnett, Annu. Rev. Microbiol., 2006, 60, 397–423 CrossRef CAS PubMed.
- M. T. Cabeen and C. Jacobs-Wagner, Nat. Rev. Microbiol., 2005, 3, 601–610 CrossRef CAS PubMed.
- H. S. Hayden, S. Matamouros, K. R. Hager, M. J. Brittnacher, L. Rohmer, M. C. Radey, E. J. Weiss, K. B. Kim, M. A. Jacobs, E. H. Sims-Day, M. Yue, M. B. Zaidi, D. M. Schifferli, S. D. Manning, J. L. Walson, S. I. Miller, B. B. Finlay, W. D. Hardt and C. A. Aranda, mBio, 2016, 7, e116–e154 CrossRef PubMed.
- S. Santajit and N. Indrawattana, Biomed Res. Int., 2016, 2016, 2475067 Search PubMed.
- L. Huang, Y. Xuan, Y. Koide, T. Zhiyentayev, M. Tanaka and M. R. Hamblin, Lasers Surg. Med., 2012, 44, 490–499 CrossRef PubMed.
- X. Jin, X. Sun, G. Chen, L. Ding, Y. Li, Z. Liu, Z. Wang, W. Pan, C. Hu and J. Wang, Carbon, 2015, 81, 388–395 CrossRef CAS.
- B. S. B. Kasibabu, S. L. D. Souza, S. Jha and S. K. Kailasa, J. Fluoresc., 2015, 25, 803–810 CrossRef CAS PubMed.
- J. R. Bhamore, S. Jha, T. J. Park and S. K. Kailasa, J. Photochem. Photobiol., B, 2019, 191, 150–155 CrossRef CAS PubMed.
- Y. Ma, Z. Ma, X. Huo, M. Gu, S. Ma, Y. Jing, Y. Wang, Y. Yue, Z. Feng and B. Tian, J. Agric. Food Chem., 2020, 68, 10223–10231 CrossRef CAS PubMed.
- Y. Chen, X. Sun, W. Pan, G. Yu and J. Wang, Front. Chem., 2020, 7, 911 CrossRef PubMed.
- Y. Chen, X. Sun, X. Wang, W. Pan, G. Yu and J. Wang, Spectrochim. Acta, Part A, 2020, 233, 118230 CrossRef CAS.
- M. A. Jhonsi, D. A. Ananth, G. Nambirajan, T. Sivasudha, R. Yamini, S. Bera and A. Kathiravan, Spectrochim. Acta, Part A, 2018, 196, 295–302 CrossRef CAS.
- Z. Bagheri, H. Ehtesabi, Z. Hallaji, N. Aminoroaya, H. Tavana, E. Behroodi, M. Rahimifard, M. Abdollahi and H. Latifi, Anal. Chim. Acta, 2018, 1033, 119–127 CrossRef CAS.
- S. Zhao, L. Huang, Y. Xie, B. Wang, F. Wang and M. Lan, Chin. J. Chem. Eng., 2021, 37, 97–104 CrossRef.
- L. Chen and J. Liang, Mater. Sci. Eng., C, 2020, 112, 110924 CrossRef CAS PubMed.
- P. Garg, S. Sangam, D. Kochhar, S. Pahari, C. Kar and M. Mukherjee, Nano Today, 2020, 35, 101001 CrossRef CAS PubMed.
- P. Innocenzi and L. Stagi, Chem. Sci., 2020, 11, 6606–6622 RSC.
- S. Kotta, H. M. Aldawsari, S. M. Badr-Eldin, N. A. Alhakamy, S. Md, A. B. Nair and P. K. Deb, Front. Mol. Biosci., 2020, 7, 616575 CrossRef CAS PubMed.
- S. Manivannan and K. Ponnuchamy, Appl. Organomet. Chem., 2020, 34, e5887 CrossRef CAS PubMed.
- A. H. Da Silva Júnior, D. L. Pier Macuvele, H. G. Riella, C. Soares and N. Padoin, J. Mater. Res. Technol., 2021, 12, 688–716 CrossRef.
- G. Camlika, E. K. Akkolb, Z. Degima and I. T. Degima, Iran. J. Pharm. Res., 2021, 20, 9–20 CrossRef.
- A. Barras, Q. Pagneux, F. Sane, Q. Wang, R. Boukherroub, D. Hober and S. Szunerits, ACS Appl. Mater. Interfaces, 2016, 8, 9004–9013 CrossRef CAS.
- A. Boczechin, K. Séron, A. Barras, E. Giovanelli, S. Belouzard, Y. Chen, N. Metzler-Nolte, R. Boukherroub, J. Dubuisson and S. Szunerits, ACS Appl. Mater. Interfaces, 2019, 11, 42964–42974 CrossRef PubMed.
- Y. Y. Aung, A. N. Kristanti, S. Q. Khairunisa, N. Nasronudin and M. Z. Fahmi, ACS Biomater. Sci. Eng., 2020, 6, 4490–4501 CrossRef CAS.
- T. Du, J. Liang, N. Dong, L. Liu, L. Fang, S. Xiao and H. Han, Carbon, 2016, 110, 278–285 CrossRef CAS.
- H. Liu, Y. Bai, Y. Zhou, C. Feng, L. Liu, L. Fang, J. Liang and S. Xiao, RSC Adv., 2017, 7, 28016–28023 RSC.
- X. Dong, M. M. Moyer, F. Yang, Y. Sun and L. Yang, Sci. Rep., 2017, 7, 519 CrossRef PubMed.
- X. Dong, R. Edmondson, F. Yang, Y. Tang, P. Wang, Y. Sun and L. Yang, RSC Adv., 2020, 10, 33944–33954 RSC.
- S. Huang, J. Gu, J. Ye, B. Fang, S. Wan, C. Wang, U. Ashraf, Q. Li, X. Wang, L. Shao, Y. Song, X. Zheng, F. Cao and S. Cao, J. Colloid Interface Sci., 2019, 542, 198–206 CrossRef CAS.
- J. Cheng, Y. Xu, D. Zhou, K. Liu, N. Geng, J. Lu, Y. Liu and J. Liu, Poultry Sci., 2019, 98, 5315–5320 CrossRef CAS PubMed.
- C. Lin, L. Chang, H. Chu, H. Lin, P. Chang, R. Y. L. Wang, B. Unnikrishnan, J. Mao, S. Chen and C. Huang, Small, 2019, 15, 1902641 CrossRef CAS PubMed.
- H. Huang, H. Lin, H. Huang, C. Huang, J. H. Lin and L. Chen, Sci. Rep., 2020, 10, 7343 CrossRef CAS PubMed.
- T. Tong, H. Hu, J. Zhou, S. Deng, X. Zhang, W. Tang, L. Fang, S. Xiao and J. Liang, Small, 2020, 16, 1906206 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |





