Are deep eutectic solvents really green?: A life-cycle perspective†
Qammer
Zaib
 a,
Matthew J.
Eckelman
b,
Yi
Yang
*cde and
Daeseung
Kyung
a,
Matthew J.
Eckelman
b,
Yi
Yang
*cde and
Daeseung
Kyung
 *a
*a
aSchool of Civil and Environmental Engineering, University of Ulsan, Daehak-ro 93, Nam-gu, Ulsan 44610, Republic of Korea. E-mail: dkyung@ulsan.ac.kr; Fax: +82-52-259-2629; Tel: +82-52-259-2259
bDepartment of Civil and Environmental Engineering, Northeastern University, 400 Snell Engineering Center, 360 Huntington Ave., Boston, MA 02115, USA
cKey Laboratory of the Three Gorges Reservoir Region's Eco-Environment, Ministry of Education, Chongqing University, Chongqing, 400045, PR China. E-mail: yi.yang@cqu.edu.cn
dThe National Centre for International Research of Low-carbon & Green Buildings (Ministry of Science & Technology), Chongqing University, Chongqing, 40045, PR China
eThe Joint International Research Laboratory of Green Buildings and Built Environments (Ministry of Education), Chongqing University, Chongqing, 40045, PR China
First published on 28th July 2022
Abstract
Deep eutectic solvents (DESs) have generated great interest as promising green alternatives to replace conventional solvents due to their application-specific tunability, nonflamability, variable viscosity, low vapor pressure, and chemical (and thermal) stability. However, their greenness or sustainability remains unclear and requires rigorous quantification. This work uses life-cycle assessment (LCA) to evaluate the potential environmental impacts incurred from the production of a representative choline chloride (ChCl)/urea DES, reline. The environmental profile of reline is compared with that of common organic solvents (methanol, ethanol, dichloromethane (DCM), and ethyl acetate) on the basis of their utilization as a solvent for the oxidation of alcohol to the ketone; acetophenone. The results indicate that, in general, the DES imparts lower environmental impacts than DCM and ethyl acetate but has higher impacts than methanol and ethanol. Chemical constituent materials (urea, trimethylamine, hydrochloric acid, and ethylene oxide) required for the synthesis of reline DES contribute significantly to its life-cycle environmental impacts when compared to auxiliary processes. In addition to reline, the LCA of four other ChCl-based DESs is performed by substituting urea (hydrogen bond donor in the reline) with ethylene glycol, glycerol, citric acid, or glucose. ChCl/citric acid DES, the so-called natural deep eutectic solvent, imposes the highest environmental impacts among the studied ChCl-based DESs. This is partly due to the high water consumption and carbon dioxide emission during fermentation to synthesize the citric acid. Our study challenges the greennesses of DESs but more research with better data is required to corroborate our findings as some key inputs were modeled from a commercial patent.
Introduction
Deep eutectic solvents (DESs) are eutectic mixtures formed from Lewis/Brønsted acids and bases.1 They have demonstrated utility in a wide range of industrial uses including metallurgy, electrodeposition, gas separation, gas capture, power systems, battery technology, bio-catalysis, biomass processing, pharmaceutical and medical research, organic synthesis, and nanomaterials’ synthesis and functionalization.2–7 DESs are emerging as a new class of sustainable solvents and are generally considered renewable, inexpensive, and green “designer” solvents, owing to their tunability to optimize solubility, viscosity, selectivity, and other physicochemical properties for a particular application.2,8 They are being promoted as environmental-friendly alternatives to a wide variety of conventional solvents.1,2,8,9DESs are generally classified into four types based on composition. Types I, II, and IV contain metal salts and are, therefore, considered toxic and less sustainable when compared with type III DESs.1,2,9,10 Type III DESs are frequently synthesized from readily biodegradable and regenerable raw materials such as animal feed additive (choline chloride, ChCl), fertilizer (urea), antifreeze (ethylene glycol), sweetener (glycerol), and plant metabolites (sugars, sugar alcohols, and organic acids).8,10 The ecological footprint and hazardousness of type III DESs are reported to be comparatively lower than their conventional counterparts.1,11 Their greenness is attributed to their environmental benignness, biodegradability, low vapor pressure, and the natural origin of their components.12
However, the greenness of type III DESs has been lately questioned. Some studies have raised concerns about their toxicity and environmental impacts.13–17 De Morais et al. classified them as “moderately toxic” and affirmed their ecotoxicities to be higher than their corresponding ionic liquids.16 Moreover, a cytotoxicity study on brine shrimp hatches ratified them as more toxic than their respective components.15 To better understand the greenness of type III DESs requires rigorous assessment using quantitative and systematic methods that evaluate not only the process of DES production but also how constitutes of DES are produced. Life cycle assessment (LCA) is such a method that quantifies the various environmental impacts of a product throughout its entire supply chain from resource extraction to manufacture and distribution to use and disposal.18,19 It is standardized and widely used to assess the relative greenness or sustainability of, e.g., emerging technologies and novel materials.20 LCA has been applied to compare emerging and conventional solvents.21,22 Xia et al.23 and Luo et al.24 performed some basic LCA of the DESs but their analyses were limited by the unavailability of data about the chemical constituents of DESs. Therefore, they approximated the data from alternative compounds. The steady growth in the applications of DESs requires a comprehensive LCA of the most widely used DESs.
In this study, we performed the LCA of a choline chloride/urea DES (reline). We selected reline because it is, arguably, the most popular and extensively studied DES.1,2,25 In addition, reline production requires choline chloride (ChCl), which is the most widely used hydrogen bond acceptor in the type III DESs.1,8 The objective of this study is to assess the cradle-to-gate environmental impacts of the reline (ChCl-based DES) production and its functional comparison with conventional organic solvents across a range of indicators. Reline DES is also compared with other ChCl-based DESs including natural deep eutectic solvents (NADES) synthesized from plant metabolites. This comparison will determine the environmental advantages of substituting a hydrogen bond donor in a DES, if any. Performing an LCA on DESs has been challenging mainly because of the lack of relevant data, a challenge that is true for emerging chemicals in general. Compared with previous studies, we filled the data gaps by using a commercial patent and an industrial scale-up framework, which constitutes a contribution of our work. Overall, our study sheds light on the environmental sustainability of DESs and will hopefully stimulate more studies of the sort.
Methods and modeling
LCA is performed according to ISO standard 14040:2006 – Environmental management—Life cycle assessment—Principles and framework.18 The standard requires goal and scope definition, inventory analysis, impact assessment, and interpretation phases.Goal and scope
This study aims to estimate the main environmental burdens associated with the production of reline; the most popular ChCl-based DES. Additionally, the environmental impacts of reline are compared with other conventional organic solvents (on a functional basis) and with ChCl-based DESs (on a mass basis). Reline has been proposed to potentially replace organic solvents – as an alternative reaction media – for organic oxidation reactions.26,27 Therefore, environmental impacts from the utilization of reline are assessed against four conventional organic solvents (methanol, ethanol, dichloromethane (DCM), and ethyl acetate) for the synthesis of acetophenone; one of the simplest aromatic ketones which is a precursor (and/or constituent) of a wide variety of industrially important compounds such as fragrances, specialty solvents, resins, and pharmaceuticals, etc.28 The functional unit is the mass of solvent (reline or conventional) required to synthesize 1.67 moles (0.2 kg) acetophenone via oxidation of 1-phenylethanol (alcohol) using N-bromosuccinimide as an oxidizing agent.27 1.0 kg of reline is required instead of 1.0 kg methanol, 1.0 kg ethanol, 2.4 kg DCM, and 1.2 kg ethyl acetate to accomplish the oxidation reaction.29,30 Finally, the reline is compared with four similar ChCl-based DESs on a mass basis. One mole of reline is prepared from one mole of ChCl (hydrogen bond acceptor, HBA) and two moles of urea (hydrogen bond donor, HBD). To synthesize similar DESs, the urea is replaced with respective HBDs: ethylene glycol (EG), glycerol (Gly), citric acid (CA), or glucose (Glu).1The system boundary includes the upstream processes to obtain the solvents. The construction, installation, and end-of-life phases are reported to insignificantly (<5%) contribute towards the overall life cycle impacts of a chemical plant and, therefore, only operational phase impacts are accounted.31 This study does not include the reuse of solvents because the reuse of a solvent often requires recycling and regeneration which needs additional resources (energy, makeup solvent, etc.). Also, the regeneration efficiency further complicates the comparative analysis among the solvents. Since this is the cradle-to-gate study where solvents are the main reference flow, we expect the end-user to compare the environmental and economic costs of recycling to select the competitive solvent. For instance, Xia et al.,23 recycled (ChCl/oxalic acid) DES nine times to justify its use in synthesizing bioplastics. According to them, the environmental impacts of the lignocellulose bioplastic synthesis process can be reduced further by recycling DES more than nine times.
Life cycle inventory
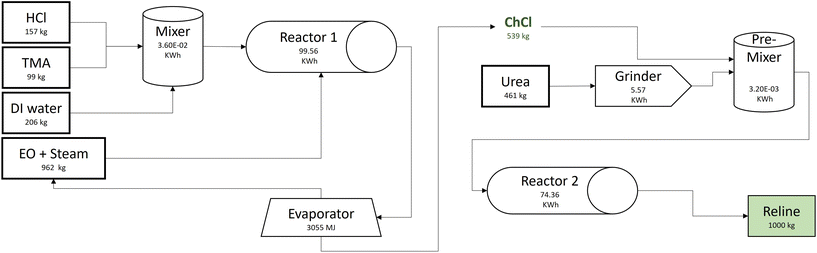
The life cycle inventory (LCI) data for the foreground processes are either obtained directly (where available) or calculated from background unit processes in the Ecoinvent 3.7 database (Table S1 in ESI†). For instance, the reline production process requires chemicals (ChCl and urea), electricity, and heat as shown in Fig. 1. The data for urea production, electricity, and heat are directly obtained from representative data from the database. The electricity production mix is used where the electricity is generated from coal (28.7%), natural gas (32.1%), nuclear (9.5%), hydropower (22.8%), wind (3.0%), and other renewables (3.3%).32 However, since LCI data for the ChCl production process is neither available in the database nor in the literature,23 the process is modeled according to the commercial patent,33 following an industrial scale-up framework.34 The calculations for auxiliary processes are provided in Table S2 in ESI.†The synthesis of ChCl is modeled from cradle to the DES production plant gate. The calculation basis is the production of one ton of reline per cycle. It includes the utilization of hydrochloric acid (HCl), trimethylamine (TMA), deionized water (DI water), ethylene oxide (EO), electricity, and steam. HCl is produced by combusting chlorine and hydrogen in a gas combustion chamber. The hydrogen chloride gas, thus formed, is passed through a cooler and ultimately absorbed in water to obtain aqueous 30% HCl. TMA is co-synthesized with di-methylamine and mono-methylamine by vaporizing methanol and ammonia. The calculations are based of stoichiometric calculations and 95% process yield is assumed. The TMA is recovered from a distillation column and the excess ammonia and amine are recycled in a continuous process. EO is obtained by directly oxidizing ethylene in the presence of a catalyst.35
ChCl production is a semi-continuous process and each cycle produces 539 kg ChCl required for one metric ton of reline (Fig. 1). The process line includes a mixer, reactor, evaporator, and product storage tank. Initial mixing is carried out in a mixing tank (V = 500 dm3) where 157 kg of 30% (w/w) HCl is mixed with 99 kg of 30% (w/w) TMA in the presence of 206 kg of DI water. The mixture (HCl-TMA) is fed to the lower end of a gas–liquid reactor (reactor 1) where it is mixed with 962 kg of 8% EO/steam mixture (w/w). The HCl-TMA and EO react to synthesize ChCl after two hours of mixing and heating at 65 °C and standard pressure (1 atm). The aqueous ChCl is fed to the evaporator where it is heated to vaporize water and the 539 kg dry ChCl is obtained.33,34 The dry ChCl is pre-mixed with finely ground urea before feeding to the twin-screw extruder (reactor 2) for thermal mixing.36 The high purity reline is obtained at the end of reactor 2.
Life-cycle impact assessment
Life cycle impact assessment estimates the environmental impacts of a product by linking emissions and resource flows from the inventory to the physical changes or damages that each flow causes in the environment, represented by characterization factors.37,38 Characterization factors are derived from environmental and toxicological models that describe the fate and transport, physical effects, exposures, and potential damages for each category of impact. There are several methods to assess the characterization factors. ReCiPe midpoint, first developed in 2008 and later updated in 2016,37 is selected for this study and includes eighteen indicators: climate change, ozone depletion, ionizing radiation, fine particulate matter formation, photochemical ozone formation, terrestrial acidification, freshwater eutrophication, human toxicity: cancer, human toxicity: non-cancer, terrestrial ecotoxicity, freshwater ecotoxicity, marine ecotoxicity, marine eutrophication, land use, water use, mineral resource scarcity, and fossil resource scarcity. This study focuses on seven of the eighteen ReCiPe midpoint indicators: global warming potential (GWP), freshwater eutrophication potential (FEP), terrestrial acidification potential (TAP), metal depletion potential (MDP), water depletion potential (WDP), freshwater ecotoxicity potential (FETP), and human toxicity potential (HTP). These indicators are selected to comparatively assess the sustainability (GWP, FEP, TAP, MDP, WDP) and toxicity (FETP and HTP) of DESs in order to appraise popular assertions that DESs are “greener and benign” alternatives to conventional solvents.2,39,40Sensitivity analysis is performed by replacing the HBD of reline (i.e., urea) with other commonly used HBDs. Two moles of urea are substituted with one mole of either EG, Gly, CA, or Glu to prepare two popular DESs (ChCl:EG and ChCl:Gly) and two NADES (ChCl:CA and ChCl:Glu).1,11
Results and discussion
LCA results for reline DES production
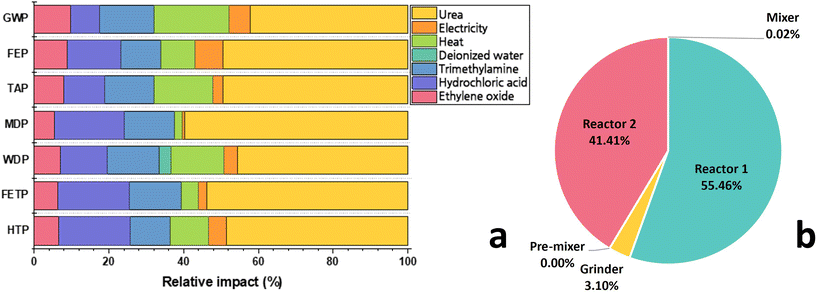
The proportion of environmental impacts caused by the materials and energies utilized in reline production are presented in Fig. 2 (numerical values are provided in Table S3 in ESI†). The respective contributions of ChCl and urea toward GWP are 52% and 42%, as shown in Fig. 2a. The heat energy utilized in the evaporator, for simultaneously drying ChCl and generating steam for ethylene oxide/steam mixture, is the highest contributor towards GWP in the ChCl synthesis process (∼20% overall). After steam, TMA constitutes only 2.6% (w/w%) of the reline but contributes over 15% to the overall GWP (0.27 kg CO2-eq per kg reline). The TMA synthesis process emits 2.6 kg CO2-eq per kg TMA produced, mainly due to ammonia consumption which releases 2.9 tons CO2 per ton ammonia produced.35 The MDP of ChCl (contributed mainly by HCl) and urea are primarily caused by copper metal depletion in the earth's crust.35 The toxicities (FETP and HTP) are nearly equally contributed by ChCl and urea. In ChCl synthesis, the HCl is mainly responsible for the toxicities. During HCl production, the manganese discharge to the groundwater has the largest allowance towards HTP followed by the chlorine gas emission to air and arsenic ions released to surface water. FETP of HCl is mainly caused by hazardous copper ions emission to groundwater.35 All the heat energy and approximately 55% of the total electrical energy is consumed in ChCl synthesis (Table S2 in ESI†). The remaining 45% of the total required electrical energy is utilized for grinding, pre-mixing, and thermal mixing ChCl and urea to produce reline (Fig. 2b).Comparison of the environmental impacts of reline vs. conventional organic solvents
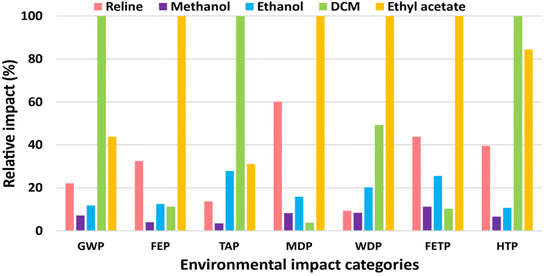
The LCA results of the reline versus conventional organic solvents required to oxidize 1.7 mole 1-phenylethanol to acetophenone (0.2 kg) are presented in Fig. 3. The figure compares the environmental impacts of the reline, methanol, ethanol, DCM, and ethyl acetate with the maximum value of each indicator set to cent-percent. The actual values are presented in Table S4 in ESI.† The environmental impacts of reline are not the highest in any of the studied categories. The impacts of DCM are the highest in GWP, TAP, and HTP categories, whereas ethyl acetate is the most impactful in FEP, MDP, WDP, and FETP categories.GWP of the DCM is the highest (8.23 kg CO2-eq) and that of methanol (0.59 kg CO2-eq) is the lowest among conventional solvents, followed by ethanol (0.97 kg CO2-eq), and ethyl acetate (3.60 kg CO2-eq). The GWP of reline is only 22% of that of DCM, primarily because of the lower mass of reline required for the process. Also, the production of reline is associated with 1.8 kg CO2-eq (per kg reline) when compared with 3.4 kg CO2-eq (per kg DCM) (Table S4 in ESI†). DCM is synthesized by chlorination of methane which releases high quantities of carbon dioxide during its production as a fossil fuel.35,41 The TAP and HTP of the DCM are the highest as well, followed by the ethyl acetate. Over 99% of TAP is caused by sulfur dioxide and nitrogen oxides emissions during DCM production.35 Whereas, mercury and chlorinated hydrocarbons’ emissions to air during DCM synthesis are chiefly (>80%) responsible for HTP.35
Ethyl acetate, besides following DCM in three of the seven studied categories, imposes the highest adverse impacts in the remaining four categories namely FEP, MDP, WDP, and FETP. Ethyl acetate is synthesized by mixing acetic acid and methanol followed by the addition of sulfuric acid. The mixture is preheated before discharging into a series of esterification columns where it is refluxed, diluted with water, distilled, and purified to obtain over ≥ 99% pure ethyl acetate.42
In essence, the comparative LCA of reline versus conventional organic solvents could not qualify the reline as a “greener and sustainable” alternative. Reline is not the worst choice; however, it is not the best either. DCM and ethyl acetate impose the highest adverse environmental impacts, yet methanol performs better than reline in all the studied categories. Ethanol, too, would be a more environmental-friendly choice than reline when considering GWP, FEP, MDP, FETP, and HTP categories for catalyst-free acetophenone synthesis. Therefore, substituting DES as a greener alternative to conventional solvent requires careful consideration.
These LCA results (Fig. 3) are based on one-time-use of solvents. DESs are known for their excellent reusability.1,23,43 They can be efficiently recovered, recycled, and reused using a wide range of techniques such as membrane filtration, liquid–liquid extraction, solid–liquid extraction, supercritical fluid extraction, short-path distillation, anti-solvent addition, density separation, and crystallization.26,43 The effectiveness of DES recovery for reuse is highly specific and depends on several factors including properties of DES (dictated by its constituents and their relative proportion), features of the targeted product (or process) for reuse, energy required, cost, and nature of extraction (synthesis or conversion) process.43 A process-specific assessment is recommended to determine the potential of DES for reuse.
LCA is a relatively holistic approach. It considers the entire life-cycle of a product with the aim to avoid burden shifting – reducing the environmental impact of one stage at the cost of the other stage(s). It helps identify hotspots and provides the opportunity to improve the entire process instead of optimizing one indicator. However, LCA has its limitations. LCA studies are based on assumptions and models and, therefore, vary considerably with respect to scope, assumptions, and scenarios. A solvent, with the lowest environmental impacts – such as methanol in our study – may or may not be the best option for a particular application. The end-user should also consider recyclability, cost, flammability, hazardousness, volatility, explosivity, toxicity, and other factors along with the life-cycle impacts to decide on a solvent. In particular, the toxicity of a solvent should be a priority concern of the user.
The toxicity can be classified into direct (acute and/or chronic) toxicity and life-cycle toxicity. Unlike life-cycle toxicity which spans from cradle to gate and might not impact the health of an end-user. The direct acute toxicity of a solvent may immediately pose a health risk. Therefore, we compared the acute toxicities – median lethal dose, LD50 – of studied solvents (Table S5 in ESI†). The acute toxicities of reline and other DESs are debatable;44,45 therefore, the LD50 of the constituents of common DESs is provided for comparison with conventional solvents. We observed that the ChCl (HBA of reline) is more acutely toxic than three of the four studied conventional solvents (methanol, ethanol, and ethyl acetate) except DCM. Therefore, caution should be exercised when working with reline and other ChCl-based DESs.
Sensitivity analysis: comparison of the environmental impacts of various choline chloride-based DESs
This section explores the impacts of HBDs (EG, Gly, CA, and Glu) on the life-cycle environmental impacts of DESs. The comparative analysis of ChCl-based DESs is shown in Fig. 4 and the actual values are presented in Table S6 in ESI.† One kg of each of the four DESs (ChCl:EG, ChCl:Gly, ChCl:CA, and ChCl:Glu) is synthesized by substituting urea in reline with the respective HBD. The comparison is drawn with respect to the maximum value set to a hundred percent as shown in Fig. 4. The ChCl:CA appears to confer the highest impacts across all the studied categories followed by ChCl:Gly and ChCl:Glu. Reline and ChCl:EG imparted <50% impacts when compared with ChCl:CA. In case of WDP, their impacts were negligibly small (<3%).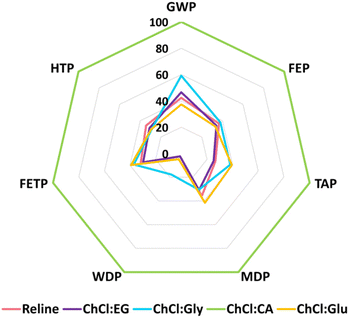 | ||
| Fig. 4 Life-cycle environmental impacts of one kg of reline DES compared with other choline chloride-based deep eutectic solvents, including natural deep eutectic solvents (NADES). | ||
Citric acid, identified as the most impactful among the studied HBDs, is produced via fermentation where carbohydrates are preheated to inoculate using aspergillus niger fungus. The synthesized citric acid is separated from biological solids in the fermented broth and purified using ion exchange and carbon treatment processes.35 Citric acid production emits 6.02 kg CO2-eq per kg citric acid which is 3.7 times higher than an equal mass of urea production (1.62 kg CO2-eq per kg urea). The GWP of ChCl:CA is calculated to be 4.3 kg CO2-eq per kg ChCl:CA, which is 58% higher than reline. The GWP of reline is comparable with ChCl:EG and ChCl:Glu (±5%) but lower than ChCl:Gly (17%). The WDP of ChCl:CA is enormous (200 L kg−1 ChCl:CA) when compared with other DESs including reline (5 liters per kg reline). It is because citric acid production utilizes 153 L kg−1 of water during the fermentation process.35
The sensitivity analysis shows that the environmental impacts of reline are comparable with various ChCl-based DESs (ChCl:EG, ChCl:Gly, ChCl:Glu) for most impact categories (on a mass per unit basis). However, in the case of ChCl:CA NADES, the choice of citric acid as a HBD remarkably enhances the environmental impacts of a DES. Contrary to statements that NADES “fully represent green chemistry principles”,11 they too require careful selection of constituents to qualify as green and sustainable solvents. NADES, like other type III DESs, can also lead to significant adverse environmental impacts.
Conclusions
Cradle-to-gate LCA analysis of the reline DES production process is carried out. The results indicate that ChCl (HBA) and urea (HBD) commensurably impacted the studied environmental categories: GWP, FEP, TAP, MDP, WDP, FETP, and HTP. The comparison between reline and conventional organic solvents was performed based on their use as a solvent in a chemical oxidation reaction. Reline might be an environmental-friendly solvent when compared with some conventional organic solvents such as DCM and ethyl acetate. However, it poses higher adverse impacts when compared with the other conventional solvents such as methanol and ethanol. Therefore, its promotion as a “greener and sustainable” alternative to conventional solvents requires careful consideration. Finally, the HBD of reline (i.e. urea) was substituted with other popular HBDs including natural ones (citric acid and glucose). The citric acid containing NADES was the most environmentally burdensome when compared with the urea, ethylene glycol, glycerol, and glucose containing ChCl-based DESs. Therefore, neither the reline DES qualifies as the “greener and sustainable” alternatives to conventional solvents nor does the NADES justifies as environmentally preferable among the ChCl-based DESs. These LCA results reveal additional environmental information about DESs that can aid in green solvent selection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is partly supported by the Korea Agency for Infrastructure Technology Advancement (KAIA) grant funded by the Ministry of Land, Infrastructure and Transport (Grant 22UMRG-C158194-03), the “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE)(2021RIS-003), and the 111 project (Grant Number B13041).References
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- A. Söldner, J. Zach and B. König, Green Chem., 2019, 21, 321–328 RSC.
- A. P. Abbott, K. El Ttaib, G. Frisch, K. S. Ryder and D. Weston, Phys. Chem. Chem. Phys., 2012, 14, 2443–2449 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, J. Chem. Eng. Data, 2006, 51, 1280–1282 CrossRef CAS.
- Y. P. Mbous, M. Hayyan, A. Hayyan, W. F. Wong, M. A. Hashim and C. Y. Looi, Biotechnol. Adv., 2017, 35, 105–134 CrossRef CAS PubMed.
- X. Ge, C. Gu, X. Wang and J. Tu, J. Mater. Chem. A, 2017, 5, 8209–8229 RSC.
- F. M. Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustainable Chem., 2020, 21, 27–33 CrossRef.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- T. Zhekenov, N. Toksanbayev, Z. Kazakbayeva, D. Shah and F. S. Mjalli, Fluid Phase Equilib., 2017, 441, 43–48 CrossRef CAS.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- M. Bystrzanowska and M. Tobiszewski, J. Mol. Liq., 2021, 321, 114878 CrossRef CAS.
- C. J. Clarke, W. C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- L. Soh and M. J. Eckelman, ACS Sustainable Chem. Eng., 2016, 4, 5821–5837 CrossRef CAS.
- M. Hayyan, M. A. Hashim, A. Hayyan, M. A. Al-Saadi, I. M. AlNashef, M. E. S. Mirghani and O. K. Saheed, Chemosphere, 2013, 90, 2193–2195 CrossRef CAS PubMed.
- P. De Morais, F. Gonçalves, J. A. P. Coutinho and S. P. M. Ventura, ACS Sustainable Chem. Eng., 2015, 3, 3398–3404 CrossRef CAS.
- M. D. Murugan, L. H. Tee and K. S. Oh, J. Phys.: Conf. Ser., 2021, 2120, 012005 CrossRef.
- ISO 14040, 2006.
- P. Nuss and M. J. Eckelman, PLoS One, 2014, 9, e101298 CrossRef PubMed.
- M. Cossutta, J. McKechnie and S. J. Pickering, Green Chem., 2017, 19, 5874–5884 RSC.
- H. Baaqel, I. Díaz, V. Tulus, B. Chachuat, G. Guillén-Gosálbez and J. P. Hallett, Green Chem., 2020, 22, 3132–3140 RSC.
- Y. Zhang, B. R. Bakshi and E. S. Demessie, Environ. Sci. Technol., 2008, 42, 1724–1730 CrossRef CAS PubMed.
- Q. Xia, C. Chen, Y. Yao, J. Li, S. He, Y. Zhou, T. Li, X. Pan, Y. Yao and L. Hu, Nat. Sustain., 2021, 1–9 CAS.
- F. Luo, X. Liu, S. Chen, Y. Song, X. Yi, C. Xue, L. Sun and J. Li, ACS Sustainable Chem. Eng., 2021, 9, 10250–10265 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 1, 70–71 RSC.
- G. D. Carmine, A. P. Abbott and C. D'Agostino, React. Chem. Eng., 2021, 6, 582–598 RSC.
- N. Azizi, M. Khajeh and M. Alipour, Ind. Eng. Chem. Res., 2014, 53, 15561–15565 CrossRef CAS.
- H. J. Sanders, H. F. Keag and H. S. McCullough, Ind. Eng. Chem., 1953, 45, 2–14 CrossRef CAS.
- V. Agieienko and R. Buchner, J. Chem. Eng. Data, 2019, 64, 4763–4774 CrossRef CAS.
- I. M. Smallwood, Handb. Org. Solvent Prop., 2012, 1–306 Search PubMed.
- F. Saunier, S. Fradette, F. Clerveaux, S. Lefebvre, É. Madore, G. Veilleux, C. Bulle and R. Surprenant, Int. J. Greenhouse Gas Control, 2019, 88, 134–155 CrossRef CAS.
- K. Treyer and C. Bauer, Int. J. Life Cycle Assess., 2016, 21, 1255–1268 CrossRef.
- Shanghai Petrochemical general plant, China Petrochemical Corporation, CN Patent 92108398, 1992 Search PubMed.
- F. Piccinno, R. Hischier, S. Seeger and C. Som, J. Cleaner Prod., 2016, 135, 1085–1097 CrossRef CAS.
- H. Althaus, M. Chudacoff, R. Hischier, N. Jungbluth, M. Osses, A. Primas and S. Hellweg, Life Cycle Inventories of Chemicals, 2007 Search PubMed.
- D. E. Crawford, L. A. Wright, S. L. James and A. P. Abbott, Chem. Commun., 2016, 52, 4215–4218 RSC.
- M. A. J. Huijbregts, Z. J. N. Steinmann, P. M. F. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- A. M. De Schryver, K. W. Brakkee, M. J. Goedkoop and M. A. J. Huijbregts, Environ. Sci. Technol., 2009, 43, 1689–1695 CrossRef CAS PubMed.
- S. Das, A. Mondal and S. Balasubramanian, Curr. Opin. Green Sustainable Chem., 2017, 5, 37–43 CrossRef.
- S. Sarmad, Y. Xie, J. P. Mikkola and X. Ji, New J. Chem., 2016, 41, 290–301 RSC.
- H. H. Khoo and R. B. H. Tan, Energy Fuels, 2006, 20(5), 1914–1924 CrossRef CAS.
- G. M. Wells, Handbook of Petrochemical Processes, 1991, vol. 1 Search PubMed.
- A. Isci and M. Kaltschmitt, Biomass Convers. Biorefin., 2021, 1, 1–30 Search PubMed.
- M. Hayyan, M. A. Hashim , M. A. Al-Saadi, A. Hayyan, I. M. AlNashef and M. E. S. Mirghani, Chemosphere, 2013, 93, 455–459 CrossRef CAS PubMed.
- M. Hayyan, C. Y. Looi, A. Hayyan, W. F. Wong and M. A. Hashim, PLoS One, 2015, 10, e0117934 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc01752k |
| This journal is © The Royal Society of Chemistry 2022 |