Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An [FeIII8] molecular oxyhydroxide†
Daniel J.
Cutler
 a,
Marco
Coletta
a,
Marco
Coletta
 a,
Mukesh K.
Singh
a,
Mukesh K.
Singh
 a,
Angelos B.
Canaj
a,
Angelos B.
Canaj
 a,
Laura J.
McCormick
a,
Laura J.
McCormick
 b,
Simon J.
Coles
b,
Simon J.
Coles
 b,
Jürgen
Schnack
b,
Jürgen
Schnack
 *c and
Euan K.
Brechin
*c and
Euan K.
Brechin
 *a
*a
aEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, Scotland, UK. E-mail: E.Brechin@ed.ac.uk
bEPSRC National Crystallography Service, School of Chemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK
cUniversitat Bielefeld, Postfach 100131, D-33501 Bielefeld, Germany. E-mail: jschnack@uni-bielefeld.de
First published on 18th May 2022
Abstract
An [FeIII8] hexagonal bipyramid displays antiferromagnetic exchange between the two capping tetrahedral ions and the six ring octahedral ions resulting in a spin ground state of S = 10.
Polymetallic complexes of FeIII ions have always played a prominent role in the development molecular magnetism. [FeIII2] dimers,1 [FeIII3] triangles2 and [FeIII4] butterflies3 not only represented ideal systems for developing quantitative magneto-structural correlations, they were also employed synthetically as starting materials for constructing larger and more complex species.4 The latter often contain the same vertex-, edge-, face-sharing structural units as the reactants, linked via organic and inorganic bridging ligands. The large library of complexes produced therefrom allowed chemists to understand the processes by which larger clusters self-assemble, allowing some control over the resultant magnetic properties, targeting, for example, slow relaxation of the magnetisation,5 spin frustration,6 or an enhanced magnetocaloric effect.7
One interesting sub-set of species in this family are molecular iron oxides, an emerging class of materials whose structures, in the main, contain no bridging organic ligands and conform to mineral phases such as ferrihydrite and magnetite.8 As well as displaying fascinating magnetic behaviours, such species potentially have applications in a breadth of areas ranging from catalysis9 and battery technologies10 to biomedical imaging.11
The striking structural similarties between the molecular iron oxides [FeIII13],12 [FeIII17],8,13 [FeIII30],14 and [FeIII34]15 which all possess alternating “layers” or tetrahedral and octahedral FeIII ions has prompted us to speculate, and examine, whether very large molecular iron oxides, perhaps even rivalling the size and complexity of the polyoxometalates,16 can be isolated. The inability of FeIII to be stabilised by terminal oxide ions and the propensity of aqueous solutions of FeIII to produce mixtures of intractable/insoluble/amorphous solids suggests however that alternative synthetic pathways may have to be explored. The simplicity of the synthesis of [Fe17] represents a good starting point. It is made by dissolving anhydrous FeBr3 in wet pyridine.8,13 The latter acts as solvent, base, source of oxide/hydroxide, terminal ligand and charge balancing counter cation (pyH+). Analogous reactions replacing the pyridine with β-picoline, 4-ethylpyridine, isoquinolene, 3,4-lutidine, results in a series of isostructural species.13 Addition of different bases, templates and solvent combinations results in the formation of the related, but larger [Fe30] and [Fe34] clusters.14,15 Herein, we extend this methodology to the use of 4-methoxypyridine (MeO-py) and the synthesis of the smallest member of this molecular iron oxide family, an [Fe8] cage.
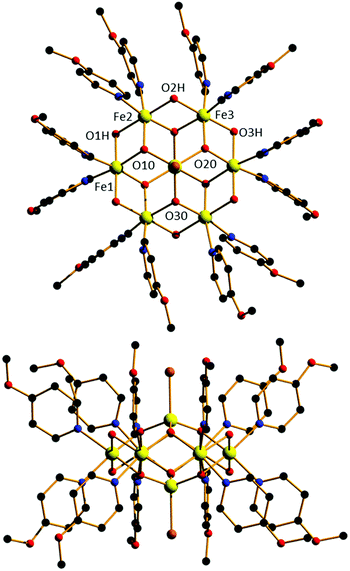
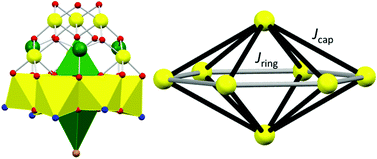
Dissolution of FeBr3 in MeO-py with stirring for 2.5 hours, followed by filtration and vapour diffusion with acetone results in the formation of orange plate-like crystals in 2 weeks. Crystals of [FeIII8O6(μ-OH)6(MeO-py)12Br2]Br4·3H2O·2MeO-py (1·3H2O·2MeO-py; Fig. 1) are in a monoclinic crystal system and structure solution was performed in the space group C2/c (Table S1 and Fig. S1†). The asymmetric unit contains half the formula. The metallic skeleton of 1 describes a hexagonal bipyramid, in which a ring of six octahedral FeIII ions (Fe1–3 and symmetry equivalent, s.e.) is capped top and bottom by a tetrahedral FeIII ion (Fe4 and s.e.). The tetrahedral FeIII ions are linked to the [Fe6] ring through three μ3-O2− ions (O10, O20, O30 and s.e.), which further bridge two octahedral Fe ions around the inner rim of the wheel. The outer rim is bridged by six μ-OH− ions (O1H, O2H, O3H and s.e.). The coordination sphere of the tetrahedral Fe ion is completed by the presence of a terminal Br− ion (Br3 and s.e.), and those of the octahedral Fe ions by two MeO-py molecules. Fe–O–Fe bond angles fall in the ranges Fe(tet)–O–Fe(oct), 122.62–123.42°, and Fe(oct)–O–Fe(oct), 95.25–98.38°; note that the former are very much bigger than the latter. The Br− counter anions (Br1, Br2 and s.e.) are H-bonded to the μ-OH ions (Br⋯O, 3.199–3.227 Å), as are the two MeO-py and three H2O molecules of crystallization (Br⋯O, 3.365–3.674 Å; N⋯O, 2.847 Å; Fig. S2†). Closest inter-cluster interactions are between neighbouring MeO-py molecules at C/O⋯C/O distances ≥3.45 Å (Fig. S3†). The structural similarity between 1 and [Fe17] can be seen in Fig. 2 in which the [Fe8] cation can be directly mapped onto half of the [Fe17] framework. The similarity between [Fe8] and [Fe13], [Fe17], [Fe30], [Fe34] and selected Fe minerals is shown in Fig. S4.† The hexagonal bipyramidal core is unique amongst the [Fe8] clusters reported in the Cambridge Structural Database, but the same unit exists in two [Fe14] clusters, [Fe14(bta)6O6(OMe)18Cl6] (where btaH = 1,2,3-benzotriazole) and [Gd12Fe14O12(OH)18(tea)6(CH3COO)16(H2O)8] (where H3tea = triethanolamine), whose Fe metallic skeletons both describe hexacapped hexagonal bipyramids, albeit with the Fe ions all being octahedral.7,17 Complex 1 also has some structural similarity to a family of [Fe7] clusters whose metallic skeletons describe a capped hexagon or ‘dome-like’ topology. Magnetic measurements of these species suggested the presence of significant spin frustration effects.18
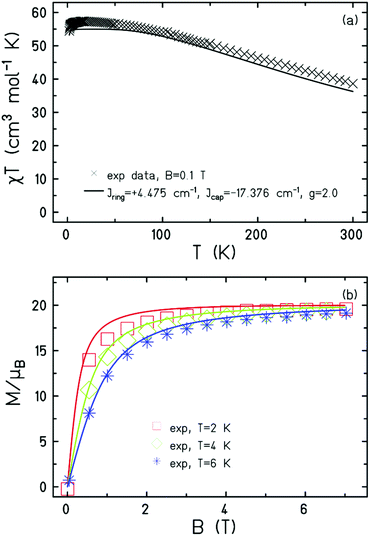
Magnetic measurements of 1 reveal strong, dominant antiferromagnetic interactions between the FeIII ions. The experimental dc susceptibility data (T = 2–300 K, B = 0.1 T) for 1 are plotted in Fig. 3 as the χT product versus T, where χ is the molar magnetic susceptibility, T is the temperature, and B the field. The value of χT at T = 300 K is ∼38 cm3 K mol−1, larger than that expected for the sum of the Curie constants for eight FeIII (S = 5/2) ions with gFe = 2.00 (35 cm3 K mol−1). As the temperature decreases, the magnitude of χT increases rapidly, reaching a maximum value of ∼57 cm3 K mol−1 at T = 16 K, where it then plateaus before decreasing slightly to a value of ∼55 cm3 K mol−1 at T = 2 K. The data are clearly indicative of competing ferro- and antiferromagnetic interactions, with the maximum in χT suggesting a ground state spin value of S = 10. This is corroborated by magnetisation data (T = 2–7 K, B = 0.5–7 T; Fig. 3) which rise rapidly with increasing field strength and saturate just below M = 20 μB. Given the large discrepancy in the Fe(tet)–O–Fe(oct) and Fe(oct)–O–Fe(oct) bond angles, the data suggest a strong antiferromagnetic interaction between the tetrahedral and octahedral Fe ions, analogous to that seen for [Fe17],8,13 and consistent with published magneto-structural studies of O-bridged FeIII compounds.1 The magnetic data can be simulated using exact diagonalisation19 and an isotropic spin-Hamiltonian 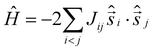 with a coupling scheme that assumes just two independent exchange interactions, Jring and Jcap, describing the interaction between the octahedral ions in the [Fe6] wheel and between the tetrahedral and octahedral Fe ions, respectively (Fig. 3). This affords Jring = +4.475 cm−1 and Jcap = −17.376 cm−1 with g = 2.00. This results in a spin ground state of S = 10.
with a coupling scheme that assumes just two independent exchange interactions, Jring and Jcap, describing the interaction between the octahedral ions in the [Fe6] wheel and between the tetrahedral and octahedral Fe ions, respectively (Fig. 3). This affords Jring = +4.475 cm−1 and Jcap = −17.376 cm−1 with g = 2.00. This results in a spin ground state of S = 10.
To further support the relative sign and magnitude of the exchange interactions obtained experimentally, we have performed DFT calculations on both dimeric and trimeric models derived from the full structure of 1 (Fig. S6 and 7; see ESI† for computational details).20 This affords Jring = +4.1 cm−1 and Jcap = −30.1 cm−1 for the dimeric model, and Jring = +2.1 cm−1 and Jcap = −30.4 cm−1 for the trimeric model. We have also performed overlap integral calculations between the singly occupied molecular orbitals of the FeIII ions.20,21 These suggest three moderate magnetic orbital overlaps for Jring resulting in small ferromagnetic interactions (Table S2 and Fig. S8a–c†), and one strong and ten moderate magnetic orbital overlaps for Jcap leading to strong antiferromagnetic interactions (Table S2 and Fig. S8d–n†). Spin density analysis indicates the presence of a strong spin delocalization mechanism, as seen previously for other polymetallic iron complexes (Fig. S9†).20
In conclusion, the simple reaction between anhydrous FeBr3 and MeO-py results in the formation of an [FeIII8] cluster whose metallic skeleton conforms to a hexagonal bipyramid. Magnetic measurements reveal antiferromagnetic exchange between the two capping tetrahedral FeIII ions and the six ring octahedral FeIII ions leading to an S = 10 ground state. While the single capping FeIII unit in the three [Fe7] systems was not sufficient to overcome the spin frustration and force the six spins in the ring parallel, the presence of two such caps in [Fe8], each strongly coupled to the six ring spins, has now overcome the frustration effects. The ease of synthesis and the striking structural similarity of [Fe8] to other molecular iron oxides such as [Fe13], [Fe17], [Fe30] and [Fe34] suggests that many more such species with much larger nuclearities remain undiscovered.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
E. K. B. thanks the EPSRC (EP/V010573/1). M. K. S. would like to thank Edinburgh Compute and Data Facility (ECDF), and the European Union Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 832488. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.Notes and references
- H. Weihe and H. U. Güdel, J. Am. Chem. Soc., 1998, 120, 2870–2879 CrossRef CAS.
- R. D. Cannon and R. P. White, Prog. Inorg. Chem., 1998, 36, 195–298 Search PubMed.
- J. K. McCusker, J. B. Vincent, E. A. Schmitt, M. L. Mino, K. Shin, D. K. Coggin, P. M. Hagen, J. C. Huffman, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1991, 113, 3012–3302 CrossRef CAS.
- C. Cañada-Vilalta, T. A. O'Brien, E. K. Brechin, M. Pink, E. R. Davidson and G. Christou, Inorg. Chem., 2004, 43, 5505–5521 CrossRef PubMed.
- D. Gatteschi, R. Sessoli and A. Cornia, Chem. Commun., 2000, 725–732 RSC.
- V. O. Garlea, S. E. Nagler, J. L. Zarestky, C. Stassis, D. Vaknin, P. Kögerler, D. F. McMorrow, C. Niedermayer, D. A. Tennant, B. Lake, Y. Qiu, M. Exler, J. Schnack and M. Luban, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 024414 CrossRef.
- (a) D. M. Low, L. F. Jones, A. Bell, E. K. Brechin, T. Mallah, E. Rivière, S. J. Teat and E. J. L. McInnes, Angew. Chem., Int. Ed., 2013, 42, 3781–3784 CrossRef PubMed; (b) R. Shaw, R. H. Laye, L. F. Jones, D. M. Low, C. Talbot-Eeckelaers, Q. Wei, C. J. Milios, S. Teat, M. Helliwell, J. Raftery, M. Evangelisti, M. Affronte, D. Collison, E. K. Brechin and E. J. L. McInnes, Inorg. Chem., 2007, 46, 4968–4978 CrossRef CAS PubMed; (c) E. Garlatti, S. Carretta, J. Schnack, G. Amoretti and P. Santini, Appl. Phys. Lett., 2013, 103, 202410 CrossRef.
- G. W. Powell, H. N. Lancashire, E. K. Brechin, D. Collison, S. L. Heath, T. Mallah and W. Wernsdorfer, Angew. Chem., Int. Ed., 2004, 43, 5772–5775 CrossRef CAS PubMed.
- H. Docherty, J. Peng, A. P. Dominey and S. P. Thomas, Nat. Chem., 2017, 9, 595–600 CrossRef PubMed.
- C. Xie, Y. Duan, W. Xu, H. Zhang and X. Li, Angew. Chem., Int. Ed., 2017, 56, 14953–14957 CrossRef CAS PubMed.
- R. A. Revia and M. Zhang, Mater. Today, 2016, 19, 157–168 CrossRef CAS PubMed.
- (a) A. Bino, M. Ardon, D. Lee, B. Spingler and S. J. Lippard, J. Am. Chem. Soc., 2002, 124, 4578–4579 CrossRef CAS PubMed; (b) J. van Slageren, P. Rosa, A. Caneschi, R. Sessoli, H. Casellas, Y. V. Rakitin, L. Cianchi, F. Del Giallo, G. Spina, A. Bino, A.-L. Barra, T. Guidi, S. Carretta and R. Caciuffo, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 014422 CrossRef; (c) O. Sadeghi, L. N. Zakharov and M. Nyman, Science, 2015, 347, 1359–1362 CrossRef CAS PubMed; (d) O. Sadeghi, C. Falaise, P. I. Molina, R. Hufschmid, C. F. Campana, B. C. Noll, N. D. Browning and M. Nyman, Inorg. Chem., 2016, 55, 11078–11088 CrossRef CAS PubMed; (e) N. A. G. Bandeira, O. Sadeghi, T. J. Woods, Y.-Z. Zhang, J. Schnack, K. R. Dunbar, M. Nyman and C. Bo, J. Phys. Chem. A, 2017, 121, 1310–1318 CrossRef CAS PubMed.
- (a) C. Vecchini, D. H. Ryan, L. M. D. Cranswick, M. Evangelisti, W. Kockelmann, P. G. Radaelli, A. Candini, M. Affronte, I. A. Gass, E. K. Brechin and O. Moze, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 224403 CrossRef; (b) M. Evangelisti, A. Candini, A. Ghirri, M. Affronte, G. W. Powell, I. A. Gass, P. A. Wood, S. Parsons, E. K. Brechin, D. Collison and S. L. Heath, Phys. Rev. Lett., 2006, 97, 167202 CrossRef CAS PubMed; (c) I. A. Gass, C. J. Milios, M. Evangelisti, S. L. Heath, D. Collison, S. Parsons and E. K. Brechin, Polyhedron, 2007, 26, 1835–1837 CrossRef CAS; (d) I. A. Gass, E. K. Brechin and M. Evangelisti, Polyhedron, 2013, 52, 1177–1180 CrossRef CAS.
- A. E. Dearle, D. J. Cutler, M. Coletta, E. Lee, S. Dey, S. Sanz, H. W. L. Fraser, G. S. Nichol, G. Rajaraman, J. Schnack, L. Cronin and E. K. Brechin, Chem. Commun., 2022, 58, 52–22 RSC.
- A. E. Dearle, D. J. Cutler, H. W. L. Fraser, S. Sanz, E. Lee, S. Dey, I. F. Diaz-Ortega, G. S. Nichol, H. Nojiri, M. Evangelisti, G. Rajaraman, J. Schnack, L. Cronin and E. K. Brechin, Angew. Chem., Int. Ed., 2019, 58, 16903–16906 CrossRef CAS PubMed.
- (a) M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef; (b) H. N. Miras, J. Yan, D.-L. Liang and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403–7430 RSC.
- X.-Y. Zheng, H. Zhang, Z. Wang, P. Liu, M.-H. Du, Y.-Z. Han, R.-J. Wei, Z.-W. Ouyang, X.-J. Kong, G. i.-L. Zhuang, L.-S. Long and L.-S. Zheng, Angew. Chem., Int. Ed., 2017, 56, 11475–11479 CrossRef CAS PubMed.
- (a) L. F. Jones, P. Jensen, B. Moubaraki, K. J. Berry, J. F. Boas, J. R. Pilbrow and K. S. Murray, J. Mater. Chem., 2006, 16, 260–2697 RSC; (b) S. Mukherjee, R. Bagai, K. A. Abboud and G. Christou, Inorg. Chem., 2011, 50, 3489–3851 Search PubMed; (c) K. C. Mondal, V. Mereacre, G. E. Kostakis, Y. Lan, C. E. Anson, I. Prisecaru, O. Waldmann and A. K. Powell, Chem. – Eur. J., 2015, 21, 10835–10842 CrossRef CAS PubMed.
- (a) K. Bärwinkel, H.-J. Schmidt and J. Schnack, J. Magn. Magn. Mater., 2000, 212, 240–250 CrossRef; (b) R. Schnalle and J. Schnack, Int. Rev. Phys. Chem., 2010, 29, 403–452 Search PubMed; (c) T. Heitmann and J. Schnack, Phys. Rev. B, 2019, 99, 134405 CrossRef CAS.
- (a) M. K. Singh and G. Rajaraman, Inorg. Chem., 2019, 58, 3175–3188 CrossRef CAS PubMed; (b) D. J. Cutler, M. K. Singh, G. S. Nichol, M. Evangelisti, J. Schnack, L. Cronin and E. K. Brechin, Chem. Commun., 2021, 57, 8925–8928 RSC.
- (a) M. Coletta, T. G. Tziotzi, M. Gray, G. S. Nichol, M. K. Singh, C. J. Milios and E. K. Brechin, Chem. Commun., 2021, 57, 4122–4125 RSC; (b) M. K. Singh, Dalton Trans., 2020, 49, 4539–4548 RSC; (c) M. Coletta, S. Sanz, D. J. Cutler, S. J. Teat, K. J. Gagnon, M. K. Singh, E. K. Brechin and S. J. Dalgarno, Dalton Trans., 2020, 49, 14790–14797 RSC; (d) M. K. Singh, T. Rajeshkumar, R. Kumar, S. K. Singh and G. Rajaraman, Inorg. Chem., 2018, 57, 1846–1858 CrossRef CAS PubMed; (e) M. K. Singh, N. Yadav and G. Rajaraman, Chem. Commun., 2015, 51, 17732–17735 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of methods and materials, physical characterisation data, computational details. CCDC 2163355. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt01477g |
| This journal is © The Royal Society of Chemistry 2022 |