Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A pillar[5]arene-based covalent organic framework with pre-encoded selective host–guest recognition†
Lu
Liu
ac,
Yiming
Hu
 c,
Shaofeng
Huang
c,
Shaofeng
Huang
 c,
Yinghua
Jin
c,
Yinghua
Jin
 c,
Jingnan
Cui
c,
Jingnan
Cui
 a,
Weitao
Gong
a,
Weitao
Gong
 *ab and
Wei
Zhang
*ab and
Wei
Zhang
 *c
*c
aState Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: wtgong@dlut.edu.cn
bEngineering Laboratory of Boric and Magnesic Functional Material Preparative and Applied Technology, Dalian, Liaoning Province 116024, P. R. China
cDepartment of Chemistry, University of Colorado Boulder, Boulder, Colorado 80309, USA. E-mail: wei.zhang@colorado.edu
First published on 15th September 2021
Abstract
It is highly desirable to maintain both permanent accessible pores and selective molecular recognition capability of macrocyclic cavitands in the solid state. Integration of well-defined discrete macrocyclic hosts into ordered porous polymeric frameworks (e.g., covalent organic frameworks, COFs) represents a promising strategy to transform many supramolecular chemistry concepts and principles well established in the solution phase into the solid state, which can enable a broad range of practical applications, such as high-efficiency molecular separation, heterogeneous catalysis, and pollution remediation. However, it is still a challenging task to construct macrocycle-embedded COFs. In this work, a novel pillar[5]arene-derived (P5) hetero-porous COF, denoted as P5-COF, was rationally designed and synthesized. Featuring the unique backbone structure, P5-COF exhibited selective adsorption of C2H2 over C2H4 and C2H6, as well as significantly enhanced host–guest binding interaction with paraquat, in comparison with the pillar[5]arene-free COF analog, Model-COF. The present work established a new strategy for developing COFs with customizable molecular recognition/separation properties through the bottom-up “pre-porous macrocycle to porous framework” design.
Introduction
Covalent organic frameworks (COFs) represent a class of crystalline porous materials constructed with diverse organic building blocks via covalent bonds.1–5 Featuring high surface area, large pore volume, high chemical/thermal stability, and tunable pore topologies, COFs have become a promising platform for gas adsorption and separation,6–10 comparable to conventional porous materials, such as zeolites and metal–organic frameworks (MOFs).11–13 However, specific supramolecular host–guest interactions, which have been commonly employed in the solution phase to realize selective molecular binding, have rarely been explored in the COF system.14,15 One possible way to address this issue is to incorporate macrocyclic cavitands into COFs. Such a “pre-porous macrocycle to porous framework” design would combine the merits of both macrocyclic hosts, with inherent porosity and selective molecular recognition properties, and COFs with stable backbones and a large pore distribution for mass transfer.16–20Pillar[n]arenes,21–23 a class of macrocyclic cavitands with intrinsic confined pores and selective guest binding capability, are attractive candidates for integration into COFs as host molecules.24–26 Although interesting molecular recognition properties of pillar[n]arenes in the solution phase have been demonstrated, they generally exhibit poor guest binding capability in the solid state due to their random packing.27–32 The opening channels are largely blocked, and the intrinsic cavities are buried inside the solid matrix and not accessible to the guest species.33,34 Meanwhile, the supramolecular structures assembled via noncovalent interactions are vulnerable and tend to collapse upon guest removal.35,36 Therefore, it is challenging to maintain the permanent porosity and selective guest binding properties of pillar[n]arenes in the solid state. It is envisioned that the integration of pillar[n]arene macrocycles into COFs can overcome the above-mentioned drawbacks. To date, although amorphous porous organic polymers with pillar[n]arenes incorporated have been conceived and triggered some interesting applications,37–44 to the best of our knowledge, the pillar[n]arene-based crystalline COFs have not been realized, likely due to the synthetic challenges.
Herein, we report the rational design and synthesis of a new COF, denoted as P5-COF, derived from pre-porous pillar[5]arene-based diamine (APP5) and 1,3,5-triformylbenzene (TFB) via one-pot imine condensation. To demonstrate the critical role of pillar[5]arene macrocycles for the observed selective molecular recognition in the resulting P5-COF, a model COF without pillar[5]arene moieties, named Model-COF was also prepared. The experiments clearly showed that P5-COF exhibits superior performance in selective C2H2 gas adsorption over C2H4 and C2H6, as well as significantly enhanced host–guest binding interaction with paraquat in comparison with Model-COF, likely owing to the presence of pillar[5]arene moieties.
Results and discussion
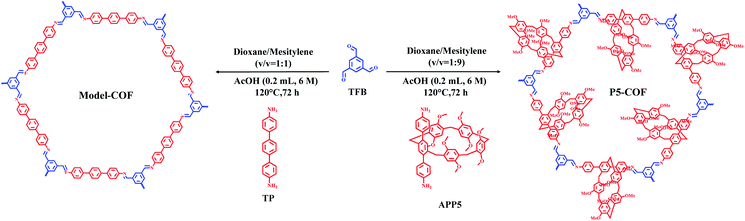
We synthesized P5-COF and Model-COF through imine condensation between 1,3,5-triformylbenzene (TFB) and APP5 or 4,4′-diamino-p-terphenyl (TP) in mixed solvents of dioxane, mesitylene and acetic acid aqueous solution at 120 °C for 3 days, respectively (Scheme 1). P5-COF and Model-COF were characterized by FT-IR spectroscopy, 13C CP-MAS NMR spectroscopy, TGA, SEM and PXRD analysis. The FT-IR spectra of both P5-COF and Model-COF show the disappearance of the aldehyde peak at 1693 cm−1 and amine peaks at 3449 and 3356 cm−1. Meanwhile, the appearance of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching peak at 1624 cm−1 supports the formation of imine bonds via the condensation of aldehyde and primary amine (Fig. S3 and S4†).
N stretching peak at 1624 cm−1 supports the formation of imine bonds via the condensation of aldehyde and primary amine (Fig. S3 and S4†).
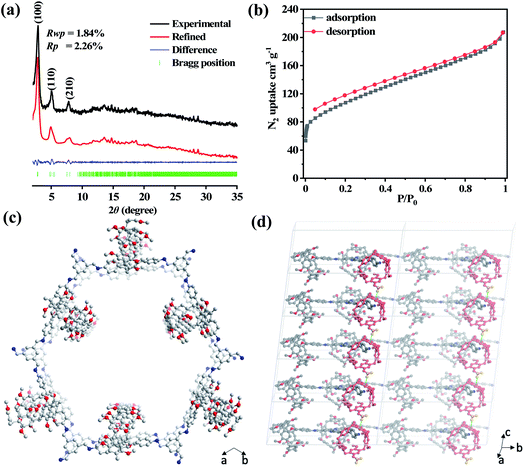
The cross-polarization magic-angle spinning (CP-MAS) NMR spectrum of P5-COF shows a peak around 157 ppm, corresponding to the carbon atom of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, and signals at 122–138 ppm, which can be assigned to the carbon atoms of the phenyl groups (Fig. S5 and S6†). The peaks with the chemical shifts around 55 and 37 ppm were assigned to the methoxy carbon and benzyl carbon in pillar[5]arenes, respectively (Fig. S5†). Thus, the solid-state NMR data support the structure of P5-COF. The thermogravimetric analysis (TGA) of the materials showed good thermal stability with a decomposition temperature of 420 °C for Model-COF and 400 °C for P5-COF (Fig. S7†). Furthermore, the morphologies of the two COFs were characterized by SEM and TEM (Fig. S8 and S9†). P5-COF shows irregular particles with rather smooth surfaces, whereas Model-COF shows a moss-like surface morphology. The good crystallinity of P5-COF was confirmed by powder X-ray diffraction (PXRD) (Fig. 1a). To determine the packing structure of P5-COF, the eclipsed and staggered stacking conformations of the pillar[5]arene structure were simulated. In order to construct the most energy stable packing pattern, we examined the single crystal packing mode of pillar[5]arene small molecules. We found that CH–π interactions between pillar[5]arene molecules largely stabilize the crystal structure.45 Thus, we introduced similar CH–π interactions when incorporating the pillar[5]arene moieties along the z axis to minimize the system energy. Accordingly, we built a P5-COF structure in the P3 space group with Pawley-refined unit cell parameters a = 37.2308 Å, b = 35.9837 Å, c = 13.3034 Å, α = 86.93°, β = 89.76°, and γ = 116.91° (Fig. 1c and d). The PXRD patterns of P5-COF exhibit three intense peaks at 2.88°, 5.04° and 7.77°, which correspond to the (100), (110) and (210) reflections, respectively. The Pawley-refined PXRD patterns agree well with the experimental results with Rp and Rwp values of 1.84% and 2.26%, respectively (Fig. 1a). The as-obtained Model-COF also exhibits good crystallinity, showing a strong and sharp peak at 2.42° in the PXRD spectrum, which could be assigned to the (100) Bragg degree reflection. The peaks at 4.69° and 5.36° were assigned to the (110) and (200) reflections, respectively (Fig. S10†). To explore the stability of these frameworks, P5-COF and Model-COF were immersed in different organic solvents and aqueous solutions (EtOH, THF, DMF, water, aqueous HCl (2 M), and NaOH (2 M)) for 24 hours. The PXRD data obtained before and after treatment barely showed any difference, which indicates high chemical stability of both COFs (Fig. S11†).
N bond, and signals at 122–138 ppm, which can be assigned to the carbon atoms of the phenyl groups (Fig. S5 and S6†). The peaks with the chemical shifts around 55 and 37 ppm were assigned to the methoxy carbon and benzyl carbon in pillar[5]arenes, respectively (Fig. S5†). Thus, the solid-state NMR data support the structure of P5-COF. The thermogravimetric analysis (TGA) of the materials showed good thermal stability with a decomposition temperature of 420 °C for Model-COF and 400 °C for P5-COF (Fig. S7†). Furthermore, the morphologies of the two COFs were characterized by SEM and TEM (Fig. S8 and S9†). P5-COF shows irregular particles with rather smooth surfaces, whereas Model-COF shows a moss-like surface morphology. The good crystallinity of P5-COF was confirmed by powder X-ray diffraction (PXRD) (Fig. 1a). To determine the packing structure of P5-COF, the eclipsed and staggered stacking conformations of the pillar[5]arene structure were simulated. In order to construct the most energy stable packing pattern, we examined the single crystal packing mode of pillar[5]arene small molecules. We found that CH–π interactions between pillar[5]arene molecules largely stabilize the crystal structure.45 Thus, we introduced similar CH–π interactions when incorporating the pillar[5]arene moieties along the z axis to minimize the system energy. Accordingly, we built a P5-COF structure in the P3 space group with Pawley-refined unit cell parameters a = 37.2308 Å, b = 35.9837 Å, c = 13.3034 Å, α = 86.93°, β = 89.76°, and γ = 116.91° (Fig. 1c and d). The PXRD patterns of P5-COF exhibit three intense peaks at 2.88°, 5.04° and 7.77°, which correspond to the (100), (110) and (210) reflections, respectively. The Pawley-refined PXRD patterns agree well with the experimental results with Rp and Rwp values of 1.84% and 2.26%, respectively (Fig. 1a). The as-obtained Model-COF also exhibits good crystallinity, showing a strong and sharp peak at 2.42° in the PXRD spectrum, which could be assigned to the (100) Bragg degree reflection. The peaks at 4.69° and 5.36° were assigned to the (110) and (200) reflections, respectively (Fig. S10†). To explore the stability of these frameworks, P5-COF and Model-COF were immersed in different organic solvents and aqueous solutions (EtOH, THF, DMF, water, aqueous HCl (2 M), and NaOH (2 M)) for 24 hours. The PXRD data obtained before and after treatment barely showed any difference, which indicates high chemical stability of both COFs (Fig. S11†).
Nitrogen sorption isotherms at 77 K were then measured to evaluate the porosity of the COFs. Both the isotherms of P5-COF and Model-COF present type-IV adsorption. The BET surface areas of P5-COF and Model-COF are calculated to be 381 m2 g−1 and 134 m2 g−1, respectively (Fig. 1b and S12†). The pore size distributions (PSDs) were evaluated with the quenched solid density functional theory (QSDFT) equilibrium model. Model-COF exhibits a pore size distribution in the range of 2.0–3.2 nm, which is in good agreement with the literature report (Fig. S14†).46P5-COF exhibits two major pores with the sizes of 0.78 nm and 1.43 nm, which likely result from the intrinsic pillarene macrocycles and internal aligned channels in the COF. It is worth noting that the pore diameter of 0.78 nm is in agreement with the cavity size of pillar[5]arenes, which supports the presence of the permanent pillar[5] cavity in the COF.47 The calculated total pore volumes of Model-COF and P5-COF are 0.53 and 0.32 cm3 g−1, respectively (Table S1†).
The unique pillar-shaped architectures and rigid π-rich cavities of pillar[n]arenes make them high affinity hosts for paraquat and other electron-deficient molecules. However, conventionally such guest-binding behavior has been mostly observed in the homogeneous solution.48–52 In the solid state, due to random molecular aggregation, many intrinsic pores are blocked, thus dramatically decreasing the guest-binding capacity.53 By integrating pillar[5]arenes into ordered COFs, we envisioned that such long-standing problems in their guest binding in a heterogeneous environment can be overcome.
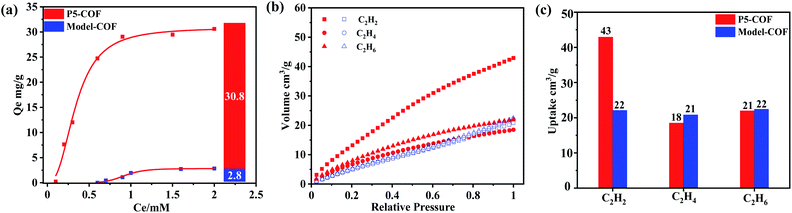
To explore the binding behavior of the P5-COF solid material toward paraquat in a heterogeneous environment, the absorption isotherm experiments with various concentrations of paraquat (0–0.2 mM) at room temperature were conducted. As shown in Fig. 2a, the Langmuir equilibrium isotherm model of paraquat matches very well with a good distribution coefficient. We found that P5-COF exhibits a good adsorption capacity for paraquat (qmax = 31 mg g−1). Furthermore, as shown in Fig. S16,† the adsorption efficiency remained >80% even after five cycles of the adsorption study, and P5-COF retained its chemical structure and crystallinity after five cycles based on PXRD and FT-IR spectra. By contrast, Model-COF did not show any obvious adsorption of paraquat, which is consistent with our assumption that the adsorbed paraquat by P5-COF was due to the host–guest interaction between the pillar[5]arene units of P5-COF and paraquat. These results support that when a well-defined molecular porous structure is introduced into an ordered porous polymer, the host–guest binding behavior (e.g., binding selectivity, affinity, etc.) could be transferred into the polymer matrix; hence, P5-COF bridged the gap between the organic porous polymers and the supramolecular macrocycles as pre-porous building blocks.
Given the encouraging results of the paraquat adsorption study, we next explored the gas adsorption properties of P5-COF. Hydrocarbons play a significant role in the petrochemical industry and have been considered as important energy sources due to the high value of these hydrocarbons as chemical feedstock and their wide applications. In this study, C2H2, C2H4, and C2H6 were chosen as the probe molecules to examine the gas adsorption properties. All measurements were conducted at 1 bar and 273 K. The uptakes of C2H6, C2H4, and C2H2 by Model-COF were 22, 21, and 22 cm3 g−1, respectively. At the same temperature and pressure, the uptakes of C2H6, C2H4, and C2H2 by P5-COF were 21, 18, and 43 cm3 g−1, respectively. It should be noted that the uptake of C2H2 by P5-COFwas increased by 110%, while the uptake of C2H6 and C2H4 was decreased slightly when compared to the amount adsorbed by Model-COF (Fig. 2c). This difference in acetylene uptake is presumably due to the more favorable interaction between pillar[5]arene moieties and acetylene molecules through hydrogen bonding and π–π, or C–H–O interactions,54–57 thus showing the potential of P5-COF in selective separation of acetylene. We also calculated the natural selectivity of C2H2 over C2H4, which is an important parameter in the industrial process of ethylene purification. P5-COF showed a natural selectivity of 2.4, which is comparable to that of many other organic frameworks.58–60 The selectivity calculated by the ideal adsorption solution theory (IAST) method is 3.2 with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 feeding mixture at a total pressure of 1 bar and 273 K. However, as shown in Table S2,† the IAST selectivity of C2H2/C2H4 is comparable to that of the well-known MOFs such as series ZJNU-14 (2.05), HUST-5 (1.8), and HUST-6 (1.42). In sharp contrast, the Model-COF showed nearly no selectivity for C2H2 over C2H4 at 273 K. Considering that these two COFs have a similar C2H4 uptake amount, the higher C2H2 uptake of P5-COF mainly results from the interaction between the adsorbent and adsorbate.
50 feeding mixture at a total pressure of 1 bar and 273 K. However, as shown in Table S2,† the IAST selectivity of C2H2/C2H4 is comparable to that of the well-known MOFs such as series ZJNU-14 (2.05), HUST-5 (1.8), and HUST-6 (1.42). In sharp contrast, the Model-COF showed nearly no selectivity for C2H2 over C2H4 at 273 K. Considering that these two COFs have a similar C2H4 uptake amount, the higher C2H2 uptake of P5-COF mainly results from the interaction between the adsorbent and adsorbate.
Conclusions
In summary, we have synthesized, for the first time, a pillar[5]arene-based COF (P5-COF), which showed heteroporosity and notable C2H2 and paraquat adsorption capabilities. More importantly, P5-COF exhibits superior performance in selective C2H2 gas adsorption over C2H4 and C2H6, as well as significantly enhanced adsorption of paraquat compared with that of pillar[5]arene-free Model-COF. We believe that the macrocyclic structural features of pillar[5]arenes, the connectivity of their intrinsic cavities, and pore size of the framework play a critical role in the performance characteristics of P5-COF. Our study opens new possibilities for developing novel COF materials with specific guest binding capability by integrating host moieties into porous frameworks and harnessing their supramolecular host–guest binding interactions in the solid state, which conventionally has been possible only in the solution phase.Data availability
All the data have been included in the ESI.†Author contributions
Conceptualization and supervision: W. Gong and W. Zhang; synthesis and characterization (XRD, PXRD, TGA, IR, adsorption): L. Liu, Y. Hu, and S. Huang; writing, reviewing, & editing: L. Liu, S. Huang, Y. Hu, Y. Jin, W. Gong and W. Zhang. The ideal adsorption solution theory (IAST) method calculations: S. Huang, all authors proof-read, provided comments, and approved the final version of this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the Natural Science Foundation of Liaoning Province [No. 2019-MS-046], the Open Funds of the State Key Laboratory of Rare Earth Resource Utilization (No. RERU2020004) and the Open Sharing Fund for the Large-scale Instruments and Equipments of Dalian University of Technology (No. DUTKFJJ2021086, DUTKFJJ2021122). W. Z. thanks the University of Colorado Boulder. L. L. acknowledges the support of the scholarship from the China Scholarship Council (CSC) under the Grant CSC No. 201906060129.Notes and references
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- K. Geng, T. He, R. Liu, S. Dalapati, K.-T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- S. Wang, Q. Sun, W. Chen, Y. Tang, B. Aguila, Y. Pan, A. Zheng, Z. Yang, L. Wojtas, S. Ma and F.-S. Xiao, Matter, 2020, 2, 416–427 CrossRef.
- Y. Jin, Y. Hu, M. Ortiz, S. Huang, Y. Ge and W. Zhang, Chem. Soc. Rev., 2020, 49, 4637–4666 RSC.
- Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed.
- T. Banerjee, K. Gottschling, G. Savasci, C. Ochsenfeld and B. V. Lotsch, ACS Energy Lett., 2018, 3, 400–409 CrossRef CAS PubMed.
- P. Wang, Q. Xu, Z. Li, W. Jiang, Q. Jiang and D. Jiang, Adv. Mater., 2018, 30, 1801991–1801998 CrossRef PubMed.
- X. Li and K. P. Loh, ACS Mater. Lett., 2019, 1, 327–335 CrossRef CAS.
- S. Huang, Y. Hu, L. L. Tan, S. Wan, S. Yazdi, Y. Jin and W. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 51517–51522 CrossRef CAS PubMed.
- P. Yang, W. Zhao, A. Shkurenko, Y. Belmabkhout, M. Eddaoudi, X. Dong, H. N. Alshareef and N. M. Khashab, J. Am. Chem. Soc., 2019, 141, 1847–1851 CrossRef CAS PubMed.
- Y. Zhang, J. Li, X. Yang, P. Zhang, J. Pang, B. Li and H. C. Zhou, Chem. Commun., 2019, 55, 2023–2026 RSC.
- L. Zhao, J. Gan, T. Xia, L. Jiang, J. Zhang, Y. Cui, G. Qian and Z. Yang, J. Mater. Chem. C, 2019, 7, 897–904 RSC.
- J. Chen, Y. Wang, C. Wang, R. Long, T. Chen and Y. Yao, Chem. Commun., 2019, 55, 6817–6826 RSC.
- T. Ogoshi, R. Sueto, M. Yagyu, R. Kojima, T. Kakuta, T. A. Yamagishi, K. Doitomi, A. K. Tummanapelli, H. Hirao, Y. Sakata, S. Akine and M. Mizuno, Nat. Commun., 2019, 10, 479 CrossRef PubMed.
- N. Huang, P. Wang, M. A. Addicoat, T. Heine and D. Jiang, Angew. Chem., Int. Ed., 2017, 56, 4982–4986 CrossRef CAS PubMed.
- Q. Xu, S. Tao, Q. Jiang and D. Jiang, J. Am. Chem. Soc., 2018, 140, 7429–7432 CrossRef CAS PubMed.
- X. Chen, Y. Li, L. Wang, Y. Xu, A. Nie, Q. Li, F. Wu, W. Sun, X. Zhang, R. Vajtai, P. M. Ajayan, L. Chen and Y. Wang, Adv. Mater., 2019, 31, 1901640–1901648 CrossRef PubMed.
- Y. Hu, N. Dunlap, S. Wan, S. Lu, S. Huang, I. Sellinger, M. Ortiz, Y. Jin, S. H. Lee and W. Zhang, J. Am. Chem. Soc., 2019, 141, 7518–7525 CrossRef CAS PubMed.
- L. Zhai, W. Wei, B. Ma, W. Ye, J. Wang, W. Chen, X. Yang, S. Cui, Z. Wu, C. Soutis, G. Zhu and L. Mi, ACS Mater. Lett., 2020, 2, 1691–1697 CrossRef CAS.
- T. Ogoshi, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- T. Ogoshi, T. A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- T. Ogoshi, T. Kakuta and T. A. Yamagishi, Angew. Chem., Int. Ed., 2019, 58, 2197–2206 CrossRef CAS PubMed.
- Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS PubMed.
- G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248–13251 CrossRef CAS PubMed.
- G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711–8717 CrossRef CAS PubMed.
- M. Pan and M. Xue, Chin. J. Chem., 2014, 32, 391–395 CrossRef CAS.
- L. L. Tan, H. Li, Y. Tao, S. X. Zhang, B. Wang and Y. W. Yang, Adv. Mater., 2014, 26, 7027–7031 CrossRef CAS PubMed.
- Y. Zhou, Y. Yao and F. Huang, Chin. J. Chem., 2015, 33, 356–360 CrossRef CAS.
- D. Dai, J. Yang, Y. C. Zou, J. R. Wu, L. L. Tan, Y. Wang, B. Li, T. Lu, B. Wang and Y. W. Yang, Angew. Chem., Int. Ed., 2021, 60, 8967–8975 CrossRef CAS PubMed.
- Z. Li, X. Li and Y. W. Yang, Small, 2019, 15, 1805509–1805519 CrossRef PubMed.
- Z. Li and Y.-W. Yang, Polym. Chem., 2021, 12, 4613–4620 RSC.
- T. Ogoshi, Y. Shimada, Y. Sakata, S. Akine and T. A. Yamagishi, J. Am. Chem. Soc., 2017, 139, 5664–5667 CrossRef CAS PubMed.
- D. Xia, L. Wang, X. Lv, J. Chao, X. Wei and P. Wang, Macromolecules, 2018, 51, 2716–2722 CrossRef CAS.
- T. Ogoshi, Y. Hamada, R. Sueto, R. Kojima, F. Sakakibara, Y. Nagata, Y. Sakata, S. Akine, T. Ono, T. Kakuta and T. Yamagishi, Cryst. Growth Des., 2020, 20, 7087–7092 CrossRef CAS.
- J. M. Seo, H. J. Noh, H. Y. Jeong and J. B. Baek, J. Am. Chem. Soc., 2019, 141, 11786–11790 CrossRef CAS PubMed.
- Y. Guan, M. Ni, X. Hu, T. Xiao, S. Xiong, C. Lin and L. Wang, Chem. Commun., 2012, 48, 8529–8531 RSC.
- X.-Y. Hu, P. Zhang, X. Wu, W. Xia, T. Xiao, J. Jiang, C. Lin and L. Wang, Polym. Chem., 2012, 3, 3060–3063 RSC.
- T. Ogoshi, T. Aoki, S. Ueda, Y. Tamura and T. A. Yamagishi, Chem. Commun., 2014, 50, 6607–6609 RSC.
- L. Wu, Y. Fang, Y. Jia, Y. Yang, J. Liao, N. Liu, X. Yang, W. Feng, J. Ming and L. Yuan, Dalton Trans., 2014, 43, 3835–3838 RSC.
- G. Yu, R. Zhao, D. Wu, F. Zhang, L. Shao, J. Zhou, J. Yang, G. Tang, X. Chen and F. Huang, Polym. Chem., 2016, 7, 6178–6188 RSC.
- X. S. Du, C. Y. Wang, Q. Jia, R. Deng, H. S. Tian, H. Y. Zhang, K. Meguellati and Y. W. Yang, Chem. Commun., 2017, 53, 5326–5329 RSC.
- R. Wang, Y. Sun, F. Zhang, M. Song, D. Tian and H. Li, Angew. Chem., Int. Ed., 2017, 56, 5294–5298 CrossRef CAS PubMed.
- Z. Zhang, L. Shao, Y. Zhou and J. Yang, Polym. Chem., 2017, 55, 3529–3533 CrossRef CAS.
- C. Li, K. Han, J. Li, H. Zhang, J. Ma, X. Shu, Z. Chen, L. Weng and X. Jia, Org. Lett., 2011, 14, 43–45 Search PubMed.
- S. Song, Y. Shi, N. Liu and F. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 10513–10523 CrossRef CAS PubMed.
- K. Jie, Y. Zhou, Q. Sun, B. Li, R. Zhao, D. E. Jiang, W. Guo, H. Chen, Z. Yang, F. Huang and S. Dai, Nat. Commun., 2020, 11, 1086 CrossRef CAS PubMed.
- Z. Li, J. Yang, G. Yu, J. He, Z. Abliz and F. Huang, Org. Lett., 2014, 16, 2066–2069 CrossRef CAS PubMed.
- X. Chi, X. Ji, L. Shao and F. Huang, Macromol. Rapid Commun., 2017, 38, 1600626–1600632 CrossRef PubMed.
- Y. Sun, W. Fu, C. Chen, J. Wang and Y. Yao, Chem. Commun., 2017, 53, 3725–3728 RSC.
- X. Mao, T. Liu, J. Bi, L. Luo, D. Tian and H. Li, Chem. Commun., 2016, 52, 4385–4388 RSC.
- L. Luo, G. Nie, D. Tian, H. Deng, L. Jiang and H. Li, Angew. Chem., Int. Ed., 2016, 55, 12713–12716 CrossRef CAS PubMed.
- A. Giri, A. Sahoo, T. K. Dutta and A. Patra, ACS Omega, 2020, 5, 28413–28424 CrossRef CAS PubMed.
- S. V. Athare and S. P. Gejji, J. Mol. Model., 2019, 26(3), 3–14 Search PubMed.
- S. V. Athare and S. P. Gejji, ChemistrySelect, 2019, 4, 9354–9359 CrossRef CAS.
- C. F. Matta, J. Hernandez-Trujillo, T. H. Tang and R. F. Bader, Chemistry, 2003, 9, 1940–1951 CrossRef CAS PubMed.
- L. L. Tan, Y. Zhu, H. Long, Y. Jin, W. Zhang and Y. W. Yang, Chem. Commun., 2017, 53, 6409–6412 RSC.
- Z. Zhang, S. B. Peh, Y. Wang, C. Kang, W. Fan and D. Zhao, Angew. Chem., Int. Ed., 2020, 59, 18927–18932 CrossRef CAS PubMed.
- F. Yu, B.-Q. Hu, X.-N. Wang, Y.-M. Zhao, J.-L. Li, B. Li and H.-C. Zhou, J. Mater. Chem. A, 2020, 8, 2083–2089 RSC.
- B. Zhu, J. W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest, M. J. Zaworotko and K. J. Chen, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR, 13C NMR, and IR spectra, solid-state NMR spectrum, SEM images, TGA curve, PXRD patterns and nitrogen adsorption isotherms. See DOI: 10.1039/d1sc03680g |
| This journal is © The Royal Society of Chemistry 2021 |