Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymeric carbon nitrides and related metal-free materials for energy and environmental applications
Jesús
Barrio
 ,
Michael
Volokh
,
Michael
Volokh
 and
Menny
Shalom
and
Menny
Shalom
 *
*
Department of Chemistry and Ilse Katz Institute for Nanoscale Science and Nanotechnology, Ben-Gurion University of the Negev, Beer-Sheva 8410501, Israel. E-mail: mennysh@bgu.ac.il
First published on 28th May 2020
Abstract
Carbon nitride polymers have emerged as a new class of materials for a wide range of applications such as photo- and electro-catalysis, sensors, bioimaging and more due to their chemical, photophysical and catalytic properties as well as their low-price, facile synthesis and high stability under harsh chemical conditions. In this review we begin with a broad overview of carbon-based materials, arriving at the focus of this review, polymeric carbon nitrides (CNs). After a brief overview of applications, we delve into their various synthetic methods, with an emphasis on achieving control on the nanoscale features of this intriguing polymeric semiconductor. The main synthetic pathways include co-polymerization at various stages, templating, an ionothermal pathway and harnessing of supramolecular pre-organization, which are discussed in detail along with CN growth and deposition on substrates. Finally, we give our perspectives on the evolution of this field, the current limitations, and elaboration of achievable control over the chemical composition, the electronic structure and the morphology of this family of materials.
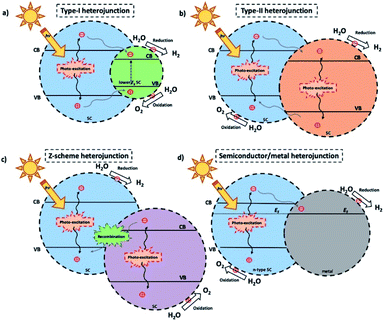
1. Carbon materials: synthesis, applications and challenges
Carbon materials ranging from graphene,1 carbon nanotubes (CNTs)2 or fullerenes3 to activated carbon and aerogels4 are widely used for environmental and energy-related purposes5–8 such as batteries,9,10 supercapacitors11 and electrocatalysis12,13 due to their conductivity, nanosized-derived effects (e.g., dirac points in graphene, metallic or semiconductor CNTs and electron accepting properties of fullerenes) and the possibility of inducing defects by chemical design.14 There are numerous synthetic pathways to synthetize this class of materials, such as chemical vapor deposition (CVD),15–17 pyrolysis of C-rich molecules,18–20 hydrothermal means, etc.21–25 Nevertheless, in most catalysis and energy-related scenarios, these materials serve as a support or as a platform for an additional active component or molecular complex to perform a given reaction.26 The morphology and properties of the final carbon-based material strongly depend on the synthetic pathway, as well as on the precursor.27 Additionally, utilizing foreign elements like templating agents or doping species28–30 can precisely tune the porous structure and electronic configuration, towards their implementation in a wider range of applications.31,322. Inserting heteroatoms into carbon matrices
To modify the electronic structure of C-materials and enable semiconductor-like properties, such as UV/visible light absorption, the formation of defects through the insertion of heteroatoms has emerged as a promising approach. Chemical elements that differ in parameters like the atomic radius or electronegativity can induce effects on the materials that are beneficial for their application in energy-related fields, i.e., the creation of a band gap or selectivity towards a given reaction. Furthermore, combining several heteroatoms in a C-matrix can produce synergistic effects such as the formation of reactive centers and a change in its intrinsic mechanical, chemical, physical and electronic properties.29 The insertion of nitrogen into a carbonaceous matrix is among the most frequently reported methods, given the straightforward synthetic pathways for such an insertion; for example, a thermal treatment of precursors containing C–N or a hydrothermal synthesis. Nevertheless, other heteroatoms, such as B, P, S, or metals, display a better affinity towards reactant molecules for various chemical reactions and therefore their inclusion within C-materials has been a common subject of research.33Non-metallic elements have been typically included by a co-polymerization route from a C-source with heteroatom-containing molecules at high temperatures or by direct thermal calcination of (bio)molecules, such as amino acids and ionic liquids.34–38 Wohlgemuth et al. obtained carbon microspheres and aerogels co-doped with nitrogen and sulfur by the hydrothermal treatment of glucose along with amino acids containing sulfur, and they studied their electrochemical performance for the oxygen reduction reaction (ORR).39,40 Furthermore, S-doped C-materials were recently explored for the nitrogen reduction reaction (NRR); Xia et al. prepared S-doped carbon nanospheres by a hydrothermal reaction comprising glucose and benzyl disulfide. The resulting materials showed excellent selectivity and a faradaic efficiency of 7.47% at −0.7 V versus (vs.) RHE, much higher than the undoped material (1.45%).41 The presence of heteroatoms within carbon materials strongly affects their reactivity in fields such as electrocatalysis, batteries or supercapacitors. B and P, for example, display a lower electronegativity than carbon, which results in a charge redistribution and modifies the adsorption modes of reactant molecules.42,43 The energy redistribution imprinted by electron-deficient heteroatoms in carbon materials has been widely reported by various groups; boron-doped graphene, for example, showed enhanced NRR performance (10.8% faradaic efficiency at −0.5 V) due to the lower energy barrier for N2 electroreduction within BC3 structures.44 Furthermore, the presence of BC3 trimers within the hexagonal lattice of C materials induces a p-type semiconductor behavior and the resulting material displays unique sensing capabilities (e.g., to NO2 and NH3 toxic gases).45 Phosphorous incorporation by pyrolysis or hydrothermal treatment also results in materials with catalytic sites, which are widely utilized as supercapacitors and electrocatalysts.46–51 From the synthetic point of view, achieving homogeneity in the distribution of dopant atoms within the lattice is a standing challenge; our group developed an approach to produce homogeneous heteroatom-doped carbons by utilizing polycyclic aromatic hydrocarbons (PAHs) and heteroatom-containing species which melt during a thermal reaction. During the polycondensation of PAHs with heteroatom-containing molecules (or metal salts), their sublimation is quenched, and the structural carbon is completely preserved thanks to the formation of new covalent or coordination bonds. Using this method, boron, nitrogen, oxygen, or different metals have been successfully included within carbon materials and their performance has been tested in Li-ion batteries or an electrochemical oxygen evolution reaction (OER, see Fig. 1).52,53
 | ||
| Fig. 1 Synthetic procedure schemes for (a) C–N–B–O and (b) metal-incorporated crystalline carbons using a molten-state synthesis. Reproduced with permission from ref. 52 and 53, copyright (2018) Wiley-VCH. | ||
2.1. N-Doped carbons, C2N, C3N4 and other C–N containing polymers
Given nitrogen's electron-accepting ability and different atomic radius, its incorporation into a C-matrix alters the electronic structure and modifies the charge distribution (uneven across a C–N bond) and the neighboring carbon atoms display a relatively positive charge that makes them more reactive in various catalytic scenarios.54,55 Modulating the amount of nitrogen in this class of materials, ranging from N-doped carbons, C–N based organic polymers, and CxNyHz derivatives, dramatically alters their electronic structure.Carbon and nitrogen-based organic polymers allow a relatively easy design, functionality and processing, which make them suitable for a wide range of applications ranging from photo- and electro-catalysis to gas storage and more.56–62 Their porosity and the tunability of their chemical composition promoted their utilization as precursors for the synthesis of N-doped carbon materials.63–65 Additionally, metal–organic frameworks (MOFs) and other highly ordered pre-organized molecular compounds have also been utilized for the high-temperature synthesis of heteroatom-modified carbon materials.66–70
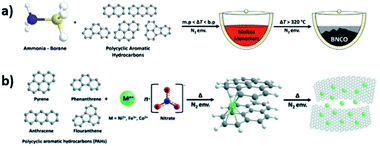
Increasing the nitrogen amount in carbon materials can be done by using N-rich precursors; the thermal condensation of such molecules, e.g., barbituric acid, urea or triaminopyrimidine, amongst others, can imprint graphitic or pyridinic nitrogen sites into the structure and yield C3N and C2N structures with varying electronic and porous structures.71–73 Already in 1986, Kouvetakis et al. have shown the preparation of BC3 and C5N planar structures by thermal condensation of a monomer containing B and N at high temperatures (800–1400 °C).74 Mahmood et al. reported for the first time the synthesis of a C2N material suitable for field-effect transistors by a wet-chemical reaction between hexaaminobenzene trihydrochloride and hexaketocyclohexane octahydrate in N-methyl-2-pyrrolidone (NMP) as shown in Fig. 2.75
 | ||
| Fig. 2 Schematic representation of the synthesis of a C2N material (a), and its corresponding digital images as prepared (b), solution cast over SiO2 after condensation at high temperatures (c) and in a film over a PET substrate (d). Reproduced with permission from ref. 75, copyright (2015) Nature publishing group. | ||
The same group later reported the synthesis of two-dimensional (2D) polyaniline (C3N) frameworks by the direct pyrolysis of hexaaminobenzene trihydrochloride single crystals.76 Since these reports, several groups have thoroughly exploited these graphitic materials for various applications, such as photocatalysis or as anodes in Li-ion batteries.77–79 Nitrogen-rich frameworks (C3N3, C3N4 or C3N5) are more commonly exploited in photocatalytic scenarios given their typical semiconducting behavior that allows the harvesting of visible light with the concomitant generation of an electron–hole (e−–h+) pair, which can reduce (electron) or oxidize (hole) molecules.80,81 C3N3 materials are the less explored of this kind, and theoretical predictions have shown that a C3N3 monolayer has a band gap (Eg) of 1.6 eV and could potentially be used in devices for H2 separation from impurity gases.82 Experimentally, this stoichiometry has been reported by our group and others by either high pressure-high temperature treatments, which results in the inclusion of P atoms (C3N3P) or by the thermal condensation of a supramolecular assembly containing triaminopyrimidine and cyanuric acid at low temperatures.83,84 C3N4 materials, commonly referred to as “carbon nitrides” or “melon” (first discovered by Liebig85,86 and typically labelled “CN”), have been the most widely studied and reported. The first C3N4 structures reported were obtained through solvothermal means,87 along with chemical and physical vapor deposition variants, which were summarized by Kroke et al. in a comprehensive review in 2004.88 Despite the fact that C3N4 is commonly referred to as a 2D analogue of graphene, the C3N4 stoichiometry, composed of tri-s-triazine units with remaining H atoms at the boundaries (due to a non-ideal condensation), is structurally a diamond-like CN allotrope, a hard material reported to be harder than diamond.89–91
Increasing the N-to-C ratio in carbon nitrides can result in novel properties, such as increased basicity; nevertheless, the synthesis of such materials involves the insertion of unstable N–N bonds. Kumar et al. showed the synthesis of a novel C3N5 material by the thermal deammoniation of melem hydrazine. The prepared framework exhibited a narrow Eg of 1.76 eV and was evaluated in several applications ranging from photo-electrochemistry to dye adsorption.92 Additionally, Kim et al. recently prepared thermodynamically stable, mesoporous C3N7 and C3N6 by the thermal treatment of a tetrazole derivative at temperatures lower than 300 °C. DFT calculations confirm the formation of N–N bonds in the form of tetrazines and triazoles and the catalytic activity of the nitrogen sites was assessed in the ORR, which was enhanced using C3N7.93 Furthermore, the inclusion of B atoms has allowed the formation of semiconducting C9N4 monolayers for an electrocatalytic NRR, as shown by Zhang et al.94
3. Graphitic carbon nitrides: introductory aspects and applications
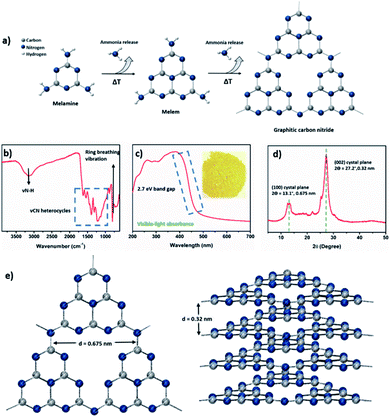
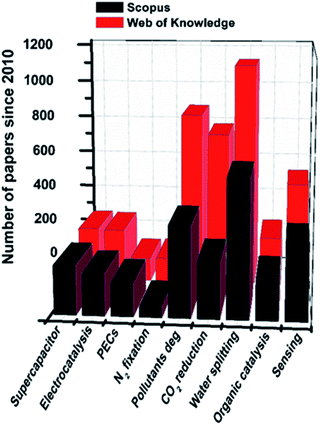
Graphitic carbon nitride (a melon-based C3N4, to which we refer here as CN) is a 2D semiconductor based on C and N and residual hydrogen atoms, with remarkable chemical and electronic properties.95 It has been widely used in energy-related scenarios since the 2009 pioneering report that showed its ability to split water under visible light.96 The synthesis of this material entails the thermal treatment of C–N monomers at high temperatures, which undergo a solid-state condensation reaction that releases ammonia and results in the formation of in-plane aromatic heptazine units with terminal amine groups, which interact with each other by van der Waals forces (Fig. 3).97–99Many organic monomers containing carbon, nitrogen, and often heteroatoms like oxygen or sulfur have been used for the synthesis of CN such as: dicyanamide,100 melamine,101–105 urea,106–109 thiourea,110–112 triazoles,113 and others,114–120 at different time intervals121 and temperatures ranging from 400 to 650 °C.122–125 The utilization of different precursors yields CN materials with a wide range of properties and possible applications.126,127 To this end, we refer the reader to excellent previously published reviews for a wider picture on the synthesis and characterization of CNs using a single precursor.128–134 To date, CN has been applied mainly in photo-(electro)catalytic reactions,135–139 with H2 production through water splitting,140–150 CO2 reduction151–153 and degradation of organic pollutants154–156 being the most comprehensive and commonly reported. Nevertheless, the fine control over the electronic structure or morphology of CNs has allowed their exploitation in a wide scope of additional applications157–159 as illustrated in Fig. 4, including water oxidation,160–162 organic transformations,163–170 supercapacitors,171 biosensing,172–178 nitrogen fixation,179–186 emulsion stabilizers,187–189 H2O2 production,190 and even as micromotors.191
The solid-state reaction that solid monomers undergo during the CN synthesis, along with the required high temperatures and the poor solubility of the final product in common solvents, impedes a full control over their chemical and electronic properties, as well as their functionalization or implementation in devices. Therefore, achieving high performance in a given application with a realistic perspective of industrial application is not common. In the next two sections, we review the state of the art of the synthetic pathways to control the nanostructure and/or the chemical composition of CN, via either a direct synthetic pathway or coupled to a post-synthetic treatment.
4. Synthetic pathways for the control of CN properties
During the last decade, different synthetic approaches have emerged in order to modify CN's properties towards an enhanced performance in photocatalytic scenarios. Improving optical absorption by tailoring energy band positions, achieving a high number of active sites through morphology and porosity control, and prolonging the lifetime of the photogenerated charge carriers have been the aims of the various demonstrated synthetic pathways. However, controlling these parameters is very challenging in a solid-state reaction and despite the recent progress, a complete and rational design of efficient photocatalytic CN systems from the reactant level is still a standing challenge.4.1. Copolymerization
Copolymerization is one of the most common synthetic approaches to the preparation of CN materials with extended light absorption due to its simplicity. In this technique, a mixture of two or more small organic monomers, obtained by grinding, ball-milling or dissolution in common solvents, reacts at high temperature typically through Schiff-base reactions192 or nucleophilic attacks. Zhang et al. reported in 2010 the first CN material prepared by copolymerization, where 2-cyanoguanidine (dicyandiamide, DCDA) and various amounts of barbituric acid (BA) were dissolved in water before a thermal treatment at 550 °C (Fig. 5a).193 In this work the C/N ratio increased up to 0.96 due to the replacement of nitrogen atoms in the melon ring by carbon, which entailed the narrowing of the CN's band gap to 1.58 eV (from the ‘pristine’ Eg of CN, ca. 2.7 eV) and a substantial enhancement of H2 production through water splitting in a water–triethanolamine (TEOA) solution with Pt serving as a co-catalyst. Since this pioneering work, CN prepared by copolymerization of DCDA and BA has been widely used in different scenarios such as glutathione detection through photoactivated oxidase mimetics,194 degradation of aniline195 or detection of Cu2+.196 The modification of the π-conjugated system of CN was studied by Zhang et al., who copolymerized DCDA with a wide library of different organic monomers containing an aromatic ring.197 The highest photocatalytic performance for the hydrogen evolution reaction (HER) was obtained with a 5% loading of 2-aminobenzonitrile (147 μmol h−1); the presence of aromatic carbon species in the CN structure was confirmed by 13C-solid-state nuclear magnetic resonance (NMR), which showed new peaks at 115 and 126 ppm. | ||
| Fig. 5 (a) Copolymerization reaction between dicyanamide and barbituric acid. Reproduced with permission from ref. 193, copyright (2010) Wiley-VCH. (b) Formation of fluorescent CN quantum dots by a copolymerization reaction of urea and citric acid. Reproduced with permission from ref. 202, copyright (2013) Royal Society of Chemistry. | ||
Generally, a wide variety of monomers have been utilized for copolymerization with DCDA, which is the most common C–N building block in this type of synthesis.198 Thiourea, for example, can decompose into gas bubbles during calcination, thereby inducing nanoporosity when copolymerized with DCDA and resulting in a 3.4 fold enhancement of the specific surface area (SA), with an average pore size of 3.7 nm, and an enhanced photocatalytic activity in the photodegradation of methylene blue (MB) dye.199 A decrease in the Eg of CN from 2.75 to 2.58 and 2.54 eV was achieved by copolymerizing DCDA with uracil and diaminopyrimidine, respectively. These molecules replace some of the nitrogen with carbon and allow additional fine-tuning of the electronic structure and morphology towards enhanced e−–h+ separation and photocatalytic performance in different model reactions.200,201 Despite the wider use of DCDA as the main C–N building block for copolymerization, other precursors are also common. The high solubility and reactivity of urea allow the formation of fluorescent CN quantum dots (CNQDs) by copolymerization under hydrothermal conditions in the presence of sodium citrate (molar ratio 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, see Fig. 5b). The size of the obtained CNQDs ranges from 2.6 to 5.5 nm, with thicknesses of 1.5–2.5 nm and the final material contains a high oxygen content, a consequence of the hydrothermal treatment and of the incorporation of oxygen from sodium citrate. Furthermore, the fluorescence quantum yield reached 42%, resulting in a remarkable performance for HEK 293T cell imaging.202
1, see Fig. 5b). The size of the obtained CNQDs ranges from 2.6 to 5.5 nm, with thicknesses of 1.5–2.5 nm and the final material contains a high oxygen content, a consequence of the hydrothermal treatment and of the incorporation of oxygen from sodium citrate. Furthermore, the fluorescence quantum yield reached 42%, resulting in a remarkable performance for HEK 293T cell imaging.202
A similar photoluminescent behavior was shown by introducing phenyl groups into CN through the copolymerization of urea with a small amount of trimesic acid. Song et al. observed that this treatment causes a downshift of the conduction band (CB) level, which results in a shift of the emission from blue (with two peaks at 443 and 457 nm) to green (522 nm) upon excitation at 340 nm. The fluorescence quantum yield of 14% allowed, for the first time, the application of CN for the imaging of latent fingerprints.203 Highly photoactive CN materials were also achieved by copolymerization of different precursor molecules with urea;204 aromatic moieties were induced by phenylurea,205 azoles,206,207 benzoic acid and others.208 These materials exhibited a narrower Eg and improved charge transfer and separation of photogenerated excitons towards improved performance in H2 production through water splitting.
Additionally, as mentioned previously, the copolymerization of urea with BA and other C-rich molecules can improve the optical absorption and charge separation by the creation of surface molecular heterojunctions that boost the HER and the CO2 photoreduction performance.209,210 A nanotube morphology with enhanced carrier mobility can also be obtained from mixtures of urea and oxamide; they display a similar structure and reactivity and therefore interact during thermal condensation to form a cross-linked complex, which upon further calcination yields a CN polymer with 1.3% AQY in the HER under green light illumination,211 as a result of an enhancement in light harvesting and a red shift of the absorption edge up to 650 nm.212 Melamine is also widely applied in copolymerization due to its compatibility with a wide range of commercially available triazine-based molecules. In 2010, the formation of N-rich (N/C ratio of 1.64) CN hollow vessels through the copolymerization of melamine and cyanuric chloride at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was reported.213 In this case, the reaction took place in a sealed quartz tube at 800 °C; the released gases during the condensation increase the inner pressure, which in turn contributes to the modification of the morphology and crystallinity of the final CN and leads to strong photoluminescence (PL) in the green–blue region.
1 was reported.213 In this case, the reaction took place in a sealed quartz tube at 800 °C; the released gases during the condensation increase the inner pressure, which in turn contributes to the modification of the morphology and crystallinity of the final CN and leads to strong photoluminescence (PL) in the green–blue region.
Cyanuric chloride can be transformed into 2,4,6-trihydrazino-1,3,5-triazine (THDT) by a reaction with hydrazine hydrate at 130 °C under reflux; the resulting monomer shows a high N/C ratio and its copolymerization with melamine alters the morphology and chemical composition of the final CN. The high nitrogen content, up to 77.2% as determined by X-ray photoelectron spectroscopy (XPS), disturbs the band structure of CN, resulting in an Eg = 1.98 eV and a 2.3-fold enhancement of the HER for a sample with a 0.15 mass ratio of THDT.214
Triaminopyrimidine, which contains one more C atom, successfully substitutes one nitrogen in the triazine ring of CN upon thermal condensation with melamine. This allows the narrowing of the Eg to 2.4 eV by a negative shift in the valence band (VB), resulting in enhanced performance in NO removal.215 As shown, the choice of the precursor monomers to include in a copolymerization determines the microstructure, band structure and charge separation properties, thus affecting the overall performance in a given reaction.
Several groups have reported a higher photocatalytic activity for a carbon nitride derived from urea vs. a CN derived from other C–N monomers, such as melamine, cyanamide or thiourea.126,216 Similarly, Zhang et al. studied the copolymerization of common C–N monomers (melamine, DCDA, thiourea and urea) with the amino acid glycine. Of all the copolymers formed, the one with urea showed the highest hydrogen evolution performance.217 Recently, the copolymerization of more than two C–N monomers has been described by Vidyasagar et al.218 In this work, phenylurea was copolymerized along with a melamine/urea mixture for a successful incorporation of phenyl groups. The assembly of phenyl units within the CN was confirmed by solid-state NMR, and the narrowing of the band gap contributes to a superior performance in CO2 reduction to formic acid and degradation of rhodamine B (RhB) dye.218
4.2. Doping
The first evidence of the formation of a hybrid material through copolymerization was provided by Wang et al. in 2009, who reported the synthesis of a transition metal-modified CN by the copolymerization of DCDA with FeCl3.221 The iron ions were included in the so-called “nitrogen pots” emulating the chemical structures of systems present in nature (e.g., chlorophyll, heme groups and coordination modes of phthalocyanines). The crystal structure of pristine CN is modified, with a significant shift in the in-plane period to 0.681 nm (vs. 0.713 nm), and the coordination of iron to the nitrogen atoms in CN is confirmed by the absence of diffraction peaks corresponding to iron species. The prepared materials show enhanced optical absorption, confirming the modification of the electronic structure, which resulted in the enhancement of the photocatalytic activity towards RhB dye degradation. Since then different metal-modified CN materials have been shown. For example, Ding et al. extended this study to other transition metal cations, such as Cu2+, Mn2+, Ni2+ and Co3+. In this work, XPS characterization shows a systematic shift to lower binding energy of the N 1s species in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C units upon coordination with the metal cation, as well as the presence of all the metallic elements.
N–C units upon coordination with the metal cation, as well as the presence of all the metallic elements.
Furthermore, the improved charge separation properties, proven by PL measurements, resulted in an enhanced photocatalytic performance in the oxidation of benzene to phenol for all hybrid materials.222 Iron doping was evaluated with different C–N building blocks as well, such as DCDA, glucose formamide and citric acid, to achieve iron and carbon doping.223,224 In both cases, the improved electron transfer resulted in an enhanced photocatalytic performance, for RhB degradation and the HER, respectively. Additionally, Hu et al. demonstrated how Fe-doped CN, synthetized from the pyrolysis of melamine and Fe(NO3)3·9H2O, is capable of performing N2 photofixation thanks to the activation of N2 molecules by Fe–N bonds.225 These examples show that doping with transition metals gives access to a wide array of materials with improved photocatalytic activity.226 Copper, nickel, manganese or cobalt doping can be easily achieved by polymerization of a C–N building block, namely DCDA, urea and others,227 with the corresponding transition metal chlorides228–236 or nitrates.237 The use of vanadate salts has also been reported.238 Furthermore, the doping amount used in the synthesis can promote the coexistence of metal cations with different oxidation states, as shown recently by Dai et al., who prepared CN materials synergistically doped with Cu(I) and Cu(II).239
Precious metals typically allow very high selectivity in organic reactions and, despite their high cost, they have also been incorporated as a dopant into CN lattices, thereby achieving improved photocatalytic performance. Eid et al. prepared homogeneous Pd-doped CN by the thermal polymerization of melamine with K2PdCl4 in the presence of nitric acid. The resulting CN nanowires show remarkable performance in the CO oxidation reaction thanks to the physicochemical properties of the nanowires and the catalytic activity of Pd atoms, achieving complete CO to CO2 conversion at 283 °C. The authors also demonstrated a synergistic effect between Pd and Cu atoms, which reduced the temperature needed for this oxidation down to 149 °C.240 Furthermore, Pd/Cu-doped CN showed enhanced electrocatalytic performance for CO2 reduction due to the quick charge transfer in the material, confirmed by impedance spectroscopy.241 Similarly, Au–Pd co-doped CN nanofibers were synthesized through copolymerization of melamine with HAuCl4 and K2PdCl4,242,243 and Pt–Pd with Na2PtCl4 and the same palladium salt.244 In all cases, the oxidation of CO was substantially enhanced compared to non-doped CN fibers.
Choi et al. carried out the complexation of Al3+ ions with N and O ligands by a rationally designed in situ keto–enol cyclization of urea and aluminum acetylacetonate (Al(acac)3). The inclusion of Al3+ ions, proven by XPS, significantly enhances light absorption; an Eg = 2.71 eV was observed and both the CB and VB shifted to more positive values. The overall AQY for the HER reached 6.2% under 420 nm light illumination.245 Molecular silver species have also been incorporated into CN polymers by the copolymerization of silver tricyanomethanide with cyanamide, resulting in an improvement of the optical absorption and an enhanced performance in the selective hydrogenation of alkynes.246
Rare-earth metals such as yttrium and lanthanides (e.g., Eu and Ce) have also been included in CN materials through copolymerization, generally achieving enhanced optical absorption, improved charge separation properties and an overall high photocatalytic activity.247–250
Alkali metals are another class of widely utilized metals along with a broad variety of semiconductor materials or molecular catalysts for enhancing their photocatalytic performance.251–256 The first reports on the doping of CN with Na+ and K+, through the copolymerization of DCDA with NaOH and KOH respectively, showed the feasibility of effectively tuning the band gap with increasing alkali metal doping levels (Fig. 6).257,258 Including these cations within CN lattices typically increases the interplanar d spacing, which influences the SA and electronic band structure (effectively narrowing the band gaps to 2.50–2.55 eV, depending on the doping amount). The coordination of either Na+ or K+ to the pyridinic nitrogens of the triazine lattice results in hybridization between the dopant orbitals and molecular orbitals of CN, which strongly modifies the potential of the VB and CB, as confirmed by the VB analysis through XPS. This strong alteration of the reduction and oxidation potential of CN materials allows an enhancement in their performance in the photocatalytic degradation of organic pollutants. Since the pioneer studies on alkali-metal doping and the complementary theoretical reports confirming a strong electron transfer from the alkali metal to the CN moiety, which narrows the highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) gap,259 several studies have been reported on this topic, with different precursor salts260–262 and C–N building blocks,263 with applications in solar fuel production.264
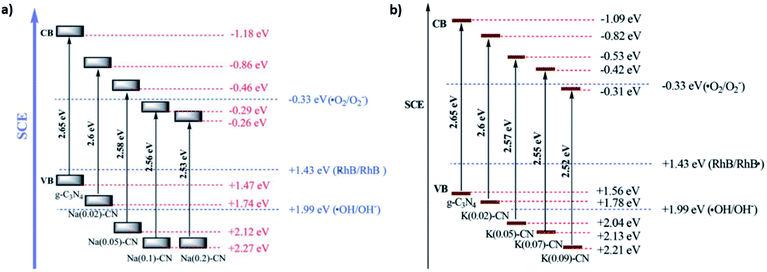 | ||
| Fig. 6 Variation of the electronic structure of CN doped with (a) potassium and (b) sodium. Reproduced with permission from ref. 257 and 258, copyright (2014, 2015 respectively) Royal Society of Chemistry. | ||
Additionally, the mediation of CN synthesis by K ions can induce useful chemical modifications, such as the creation of cyano groups, probably as a result of the formation and preservation of stable potassium cyanide intermediates.265 The presence of cyano groups at the surface of a CN photocatalyst can substantially improve the photocatalytic activity (e.g., the degradation of organic pollutants,266 HER,267 or photocatalytic NRR).268 Besides the more widespread utilization of Na+ and K+, theoretical and experimental studies show that other cations, namely Li+ or Mg+, also significantly alter CN's electronic structure, which has beneficial effects in photocatalytic scenarios.269,270
Also in 2010, the fluorination of CN solids was carried out by Wang et al., who copolymerized DCDA and NH4F. Based on the strong electronegativity of fluoride ions, the authors hypothesized that C–F bonds are formed, inducing a partial C-sp3 character within the material, thus lowering the in-plane order of the material. Furthermore, the enlarged optical absorption corresponded to Eg = 2.63 eV, and the photochemical properties were strongly modified; namely, the hydrogen production through water splitting was 2.7 times higher than that of bulk CN, and a substantial improvement in benzene oxidation to phenol was observed.272 The same authors also reported co-doping of CN with boron and fluorine as a result of the calcination of DCDA with the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate.273 These CNBF materials show excellent performance in the photooxidation reaction of cyclohexane due to their higher SA (444 m2 g−1, determined by nitrogen sorption measurements) coming from an increased interlayer distance (d), lower Eg, and enhanced charge separation properties derived from charge localization on the surface terminal sites.
Since these studies, doping of CN frameworks through copolymerization has become a bustling research field; the rational selection of heteroatom-containing organic monomers and different kinds of building blocks can result in the inclusion of non-metals such as B, P, S, O and halogens, which modify the selectivity in a given photocatalytic reaction and act as collection centers for e− or h+, enhancing the charge carriers' lifetime.
Boron is one of the most commonly utilized heteroatoms for incorporation into CN frameworks, due to its similar size to carbon and nitrogen, lower electronegativity, strong binding to nitrogen and influence on the final material's optical properties. These materials range from B-doped CN to BCN to C-doped boron nitride (BN).274 Wang et al. and others achieved the preparation of B-doped CN through the polymerization of DCDA and a borane ammonia complex;275 in this work, the B atoms were included mainly at the bay-carbon site, as proven by solid-state NMR and the enhanced catalytic activity of the material, which was tested for the oxidation of aliphatic C–H bonds of several substrates.276 Sodium tetraphenylboron ((C6H5)4BNa) has been utilized along with urea to achieve a fine control of the surface of the CN material.277 The phenyl leaving groups induce high SA in the final CN (up to 144 m2 g−1), which promotes its facile delamination, reaching thin films of approximately 2–5 nm thickness. Furthermore, XPS proves the formation of N–B–N bonds, which strongly affects the band gap and photophysical properties of the materials.278 The prepared materials show an enhancement in the photocatalytic HER through water splitting with TEOA as a hole scavenger and Pt as a co-catalyst. Boric acid (H3BO3) along with melamine, DCDA or thiourea has also been reported to achieve homogeneous B-doping in CN frameworks and the altered electronic properties and morphology result in enhanced photocatalytic performances in the photodegradation of organic pollutants,279 photoreduction of uranium species (UO22+)280 and cycloaddition of CO2 to epoxide.281
Sulfur doping is complicated to achieve, due to the tendency of sulfur-containing moieties to leave (usually in the form of sulfates) upon calcination. Nevertheless, the use of monomers which contain sulfur for the synthesis of CN has been proven to successfully achieve its inclusion within the lattice. For this, trithiocyanuric acid and thiourea have typically been the monomers of choice since the first report describing their pyrolysis and transformation into S-doped CN for water oxidation and reduction, respectively.117,282 Indeed, various reports described the preparation of SCN materials with proven photocatalytic performance in water splitting,283 degradation of dyes and organic pollutants,284–286 oxygen reduction287 and CO2 reduction.288 SCN has also been applied in bioimaging applications; namely, its confinement through top-down techniques such as ultrasonication yields highly fluorescent S-doped CN QDs with redshifted emission, good biocompatibility and low toxicity, as shown in the imaging of PC-3 cells.289 Furthermore, the enhanced chemiluminescence of SCN vs. bulk-CN was shown by Zhu et al. The strong electrochemiluminescence signals of SCN can be quenched in the presence of Cu2+, which allows the quantification of L-cysteine.290 Selenium has also been incorporated into CN materials and they have been utilized in different scenarios. Qiao et al. showed the synthesis of Se–CN by copolymerization of DCDA, cyanuric acid (CA) and SeO2, which resulted in a Se loading of 2.1 atomic %. This material could oxidize tetramethylbenzidine (TMB) in the presence of H2O2.291 Kumar et al. followed the same synthetic route and utilized the prepared Se–CN for the efficient photocatalytic production of formaldehyde from CO2.292
Phosphorus atoms within CN frameworks can strongly alter electrical or thermal conductivity, permitting their use in different applications, mainly relying on the enhanced charge mobility in the P-containing CN.293 One of the first reports on the synthesis of PCN through copolymerization involved the thermal treatment of DCDA with the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate.294 Ionic liquids have been previously used for the introduction of dopants such as B or F, and in this case, EDS analysis determined that the presence of the phosphate salt in the initial reactant resulted in a weight percentage (wt%) of 0.5% P in the final material; the inclusion of P in the CN matrix was confirmed by XPS. The enhancement in photocatalytic performance induced by the extended optical absorption and the improvement in the charge separation properties was confirmed by visible-light degradation of RhB and MO.295 Since then, the most commonly utilized species for the synthesis of PCN through copolymerization have been phosphate salts277,296–298 or phosphonic acids, associated with common monomers such as DCDA or melamine.299,300 In these scenarios, the photocatalytic activity towards the degradation of model organic dyes or a water-splitting HER was substantially enhanced due to the more beneficial electronic structure after P inclusion in CN frameworks.
Phosphonitrilic chloride trimer monomer (PCT) has also been utilized for the formation of co-polymers containing C–N–P elements, taking advantage of its high phosphorous content and a melting point (112–115 °C) that allows the formation of a homogeneous medium upon thermal treatment. In 2014, Zhou et al. carried out the copolymerization of guanidinium hydrochloride as a CN precursor with PCT; the resulting material possessed P atoms, which replaced carbon in the CN lattice, as shown by XPS and 31P solid-state NMR. As a result, the photocatalytic performance in hydrogen production and RhB degradation was substantially enhanced.301 The same monomer was copolymerized with thiourea and the resulting material was tested for photocatalytic NO removal.302 Our group reported the thermal copolymerization of PCT with 2,4-diamino-6-phenyl-1,3,5-triazine (DPT) and the formation of C–N–P materials with remarkable fire-resistance performance.303 In this case, both monomers create a liquid-state intermediate that allows an enhanced reactivity at higher temperature and the formation of CNP materials through ring opening of PCT and the nucleophilic attack of the amine group at the chlorine, before the final condensation takes place (Fig. 7). The inclusion of P in the CN framework reaches up to 32 wt%, and the prepared materials show an impressive resistance to oxidation.
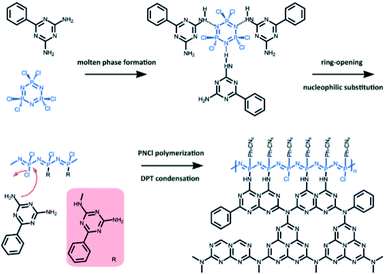 | ||
| Fig. 7 Synthetic pathway for carbon-nitrogen-phosphorous (CNP) materials by copolymerization of 2,4-diamino-6-phenyl-1,3,5-triazine (DPT) and phosphonitrilic chloride trimer (PCT). Reproduced with permission from ref. 303, copyright (2018) Wiley-VCH. | ||
Another pnictogen (group 15), antimony (Sb), was included within CN frameworks by copolymerization of urea and sodium antimonate (NaSbO3). The resulting N–Sb(III) bonds were shown to perform as charge-capture centers, leading to a strong enhancement of the photocatalytic HER, with a 6.4% AQY at 420 nm.304 Doping CN semiconductors with oxygen was predicted to improve visible-light harvesting through the improvement of charge carrier mobility.305 Qiu et al. achieved the extension of the optical absorption of CN up to 700 nm by inserting oxygen in its lattice, partially replacing nitrogen atoms. In this work, the doping with oxygen was achieved by copolymerization of urea with oxalic acid, and the resulting material showed an increased photocatalytic performance in the degradation of bisphenol A (BPA), with hydroxyl radicals playing the major role in the photocatalytic reaction.306 Another oxygen-rich monomer, citric acid, was copolymerized with thiourea in order to achieve simultaneous oxygen- and sulfur-doping in the final CN material. In this case, the oxygen doping was obtained by the partial retention of carboxylic acid groups after the hydrothermal reaction at 200 °C; this strongly altered the PL properties, resulting in blue fluorescence with a 14.5% AQY and allowing a remarkable performance in the detection of mercury ions and cell imaging.307
Obtaining the inclusion of several heteroatoms that synergistically contribute to the modification of the electronic structure and improvement of the overall photocatalytic performance can be achieved by rationally selecting heteroatom-containing organic monomers and will take place at the elevated temperatures of the synthesis. Thiourea copolymerized with PCT incorporates both sulfur and phosphorous atoms in the CN lattice, which improves light harvesting and SA compared to pristine or single-element-doped CN.308 Similarly, thiourea along with ionic liquids containing PF6 groups or phosphate salts, successfully yields S–P co-doped CN frameworks.309 Furthermore, DFT calculations performed by Liu et al. show that doping of P atoms preferentially occurs by replacing C atoms or occupying the interstitial sites of CN, while S or O doping can replace N or be included in an interstitial site.310
The modification of CN layers with halogens has been predicted to occur more feasibly in the interstitial position; their introduction has been proven to lead to new density states and redistribution of the HOMO and LUMO energy levels. Fluoride perturbs the VB and HOMO due to its high electronegativity; meanwhile, chloride, bromide and iodide are involved in the conduction band and LUMO. Overall, the calculations performed by Zhu et al. illustrated that halogen-doped CN possesses a narrower Eg, increased light absorption and reduced work function.311 Experimentally, after the first report by Wang et al.272 describing the fluorination of CN polymers, Zhang et al. extended this study by carrying out the copolymerization of DCDA with various ammonium halide salts (NH4X, X = F, Cl, Br and I) and testing the resulting halogen-modified CN materials as photocatalysts for the HER; CN–I showed the highest performance, reaching an AQY of 2.4% at 420 nm.312 The extension of the aromatic CN system thanks to halogen insertion results in an enhanced optical absorption; the band gap narrowing was proven by VB analysis through XPS (supported by DFT calculations). This finding can be explained by the delocalization of electrons that interact with the π-electron system of CN. Further evidence of the photocatalytic enhancement of CN by iodine doping was provided by Guo et al., who achieved simultaneous K and I inclusion into CN frameworks by copolymerization of DCDA and KI.313 Additionally, Lan et al. studied the effect of the C–N precursor in the synthesis of Br-modified CN materials. Urea, DCDA, NH4SCN and thiourea were chosen for the copolymerization along with NH4Br.314 As predicted by others,126,216 Br–CN prepared with urea resulted in higher H2 evolution rates and photocatalytic OER with Pt and cobalt oxide as co-catalysts, respectively.
In addition to the popular method of CN doping with heteroatoms through direct copolymerization, it is also possible to include foreign elements within CN matrices in a post-synthetic manner, which can successfully alter the electronic structure and enhance the photocatalytic performance of CN-derived materials.219,220,315
Lui et al. showed the incorporation of sulfur atoms by the thermal treatment of pre-formed CN under a H2S atmosphere and observed an enhancement of photocatalytic activity in several model reactions including the HER, phenol degradation and the photogeneration of hydroxyl radicals. The sulfur atoms are incorporated by replacing lattice nitrogen atoms. Since new C–S bonds are formed, the band gap is enlarged by the widening of the modified CN's VB stemming from the interaction of S 3p states with N 2p states.316 Another a chalcogen, i.e., Se, was introduced by the thermal treatment of a mixture consisting of CN and diphenyl selenide in ethanol, which resulted in the exfoliation of the product into thin nanosheets with thicknesses in the range of 0.7–2 nm. The Se atoms replace heptazine nitrogens and strongly modify the electronic structure towards enhanced photocatalytic performance in the CO2 reduction and HER.317 Other non-metals have been inserted within CN using similar approaches, for example, boron in the form of borate. The treatment of CN with NaBH4 in water results in the formation of Na3BO3, H3BO3 and hydrogen, which is a strong reducing agent that attacks N atoms and creates nitrogen defects, allowing the insertion of BO3− species. The reductive treatment results in an SA enhancement and in a narrowed band gap down from 2.71 to 2.66 eV, which contributes synergistically to the enhanced photocatalytic activity tested for NO removal.318 Phosphorous doping was achieved by wet grinding a urea-derived CN along with NaH2PO2, followed by thermal treatment at 400 °C. The introduced P atoms replace corner-site C atoms, which results in Eg = 2.52 eV with a negative shift of both the CB and VB. The enhanced SA and hydrophilicity contribute as well to an improved HER performance (AQY of 6.52% at 420 nm).319
The introduction of metals by a simple treatment of CN with metallic species has also been demonstrated. Typically, N atoms from CN, which serve as Lewis bases, coordinate to the utilized metal (cation) Lewis acid. Doping with alkaline metals, such as potassium or cesium, by the thermal treatment of a mixture containing prepared CN with either KCl or CsCl was recently reported.320,321 Cu2+ has also been widely inserted into CN matrices in a post-synthetic manner; Ju et al. showed that Cu-modified CN, prepared by a reaction between urea-derived CN and CuCl2, exhibits a better separation of the HOMO and LUMO distributions, which in turn leads to an enhanced production of reactive oxygen species (ROS) upon visible-light illumination.322 Furthermore, Vázquez-González et al. followed the same synthetic approach and demonstrated that Cu–CN can act as a heterogeneous catalyst mimicking the function of horseradish peroxidase.323 Other cations (e.g., Ru3+ and Zn2+) were incorporated following similar procedures and their photocatalytic performances were tested for organic catalysis and the HER.324,325 Lanthanum was also loaded by hydrothermally treating CN nanosheets with La(NO3)3; the resulting material showed strong fluorescence and outstanding performance in the detection of Fe3+ ions.326 Doping with halogens, such as iodine, was described by Han et al., who ball-milled melamine-derived CN with iodine in different ratios and observed, using XPS, that the formed C–I bonds could narrow the Eg down to 2.37 eV and enhance the photocatalytic HER, reaching an AQY of 3% at 420 nm.327
4.3. Templating
Already in 2005, Groenewolt et al. carried out the synthesis of CN nanoparticles (NPs) in different solid silica matrices with pore sizes in the 5–70 nm range.330 The porous templates were filled with cyanamide (CY) and the nanosized CN material was obtained via a thermal treatment at 550 °C. The first evidence of an enhancement in the catalytic performance by templated CN was given later in 2006 by Goettmann et al., who utilized SiO2 NPs of 12 nm for the synthesis of mesoporous CN, using CY as a molecular precursor. Electron microscopy along with the analysis of nitrogen sorption using the Brunauer–Emmett–Teller (BET) model confirmed weak microporosity and enhancement of SA up to 439 m2 g−1, which resulted in improved activity for the Friedel–Crafts acylation of benzene with hexanoyl chloride, reaching 90% conversion and a TOF of 6200 h−1.331 Vinu et al. used two different precursors, namely ethylenediamine (EDA) and CCl4 along with SBA-15 (a family of mesoporous silica templates with different pore diameters),332 for the synthesis of well-ordered materials with a pore diameter range of 4.2–6.4 nm.333 The catalytic activity was tested in Friedel–Crafts acylation of benzene using hexanoyl chloride and the prepared materials show a 100% conversion to caprophenone thanks to their high SA, reaching 830 m2 g−1.333 EDA and CCl4 were further utilized for the synthesis of CN nanotubes templated by porous anodic aluminum oxide (AAO) and as well for preparing mesoporous nanoparticles (>150 nm size) templated with ultra-small silica nanoparticles. After the formation of the nanotubes at high temperature and the removal of the AAO template by treatment with NaOH, the prepared materials were utilized as a nanotubular support for the deposition of Pt nanoparticles; this hybrid nanostructure was used for the hydrogenation of cyclohexene to cyclohexane.334 Furthermore, the high nitrogen content in the prepared CN nanoparticles gives rise to numerous basic sites in the form of amine or imine groups that promote the trans-esterification of β-keto esters, in synergy with the enhanced SA (645 m2 g−1).335 Confined nanorods of a 260 nm diameter with directional charge transport properties were synthesized utilizing an AAO template and CY as a C–N precursor; the confinement inside the nanochannels of the template enhances the orientation and crystallinity of the resulting CN with a lower HOMO given the lack of both free NH2 groups and defects within the structure (Fig. 9a). The photoactivity of the nanorods was substantially enhanced compared to bulk CN in the H2 and O2 evolution reactions,336 as was also confirmed by others.337 Further insight into HER performance enhancement using templating was provided by Sun et al. who used hollow silica nanoparticles along with CY for the formation of CN nanospheres, which after the template removal and light-induced deposition of Pt as a co-catalyst, showed a high HER AQY of 7.5% at 420.5 nm. This fact is attributed mainly to the maximized light harvesting due to inner reflections and photonic effects within the nanostructure of the CN vesicle (Fig. 9b).338 Si and co-workers reported a similar strategy, using silica spheres as nanoreactors to achieve the confined thermal polymerization of cyanamide precursors under pressurized conditions. The resulting nanosized CN showed a nanoporous structure as well as improved crystallinity and yielded a 44- and 30-fold HER enhancement under visible-light irradiation compared to bulk CN and a material prepared under atmospheric pressure, respectively.339
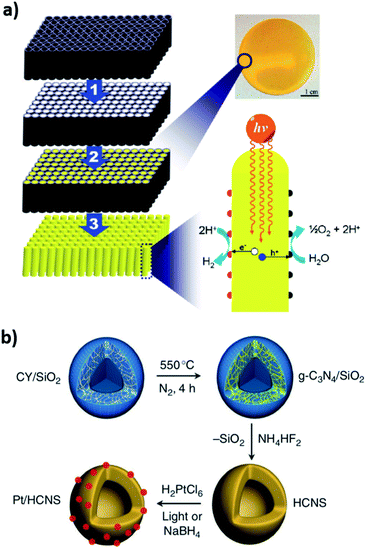 | ||
| Fig. 9 (a) Synthesis of CN nanorods confined in an AAO template. Reproduced from ref. 336. Copyright (2011) American Chemical Society. (b) Synthesis of CN nanospheres utilizing silica nanoparticles. Reproduced with permission from ref. 338, copyright (2012) Nature publishing group. | ||
SiO2 nanoparticles have also been utilized to alter the electronic structure of CN forming nanojunctions and improve the photocatalytic performance in the degradation of RhB. Shalom et al. demonstrated that the polymerization of a DCDA/SiO2 mixture yields a CN/SiO2 hybrid composite, which exhibits improved charge separation efficiency upon illumination besides enhanced SA.340 In the same way, aluminum oxide (Al2O3) loaded with nickel was utilized to generate CN through the polymerization of EDA and CCl4 resulting in Ni NPs covered with 3–4 layers (calculated) of CN. The CN/Ni/Al2O3 composites were used for the hydrogenation of p-nitrobenzoic acid at different pH values, where they showed remarkable conversion for more than 20 cycles. This performance stems from the donation of electrons from Ni to CN, which enhances its capability to activate hydrogen for its reaction with nitro compounds.341 Despite the variety in the utilized templates in this synthetic approach,342–345 in general, SBA-15 has been the most commonly used hard material to tailor the nanostructure of CN polymers. Carrying out the polymerization at different temperatures,346 modifying SBA-15 by cross-linking or protonation347,348 and testing the photocatalytic performance in different model reactions349 have been some of the recent developments in this research line. Furthermore, SBA-15 as well as common silica NPs allow the combination of this synthetic pathway with other modifications such as doping or copolymerization, by simple manipulation of the initial reactants (cast onto a hard template).
Utilizing thiourea to achieve S-doping282,350 or common C–N monomers along with metal salts (Fe2+,351 Cu2+,352,353 and others354,355) together with templating agents permitted combining the electronic modification of CN towards an improved charge transfer and/or separation with a precise engineering of the morphology and porosity, with the aim of improving the photocatalytic activity in several model reactions. Additionally, Chen et al. carried out the copolymerization of DCDA with thiophene within a silica NP template to create mesopores in a CN framework that included conjugated functional groups, thus achieving synergistic molecular and textural engineering of the prepared material towards an efficient photocatalyst for the selective oxidation of alcohols.356
Parameters such as the crystal structure and elemental composition are strongly altered by the type of template and its relative amount; for example, when using Pluronic P123, the molar C/N ratio varies from 0.82 to 2.06 due to the insertion of carbons from Pluronic P123 into the structure of the final CN. Ionic surfactants as cetyltrimethylammonium chloride and cetyltrimethylammonium bromide (CTA) lead to SA = 80 m2 g−1, while Triton X-100 provides high organization of the pore walls, with pore sizes ranging from 3.8 to 15 nm and SA = 76 m2 g−1. Furthermore, utilizing ionic liquids as templates proved to induce nanoporosity, as observed by transmission electron microscopy (TEM) with pore sizes of 5.6 nm and SA = 80 m2 g−1 in the case of 1-butyl-3-methylimidazolium dicyanamide.357 Yan et al. used melamine along with P123 for the synthesis of worm-like CN with a narrow pore size distribution; the utilization of Pluronic P123 also resulted in a certain carbon enrichment that shifted the absorbance edge up to 800 nm. Consequently, the prepared material showed good photocatalytic HER activity with Pt as a cocatalyst and produced 148.2 μmol h−1 H2 under visible light irradiation. Additionally, when the incident wavelength was longer than 700 nm, the material still produced 3.6 μmol h−1.358 A smaller templating agent (e.g., sucrose) was also used with melamine to produce mesoporous CN. Upon thermal condensation, sucrose firstly melts and then decomposes, releasing carbon-based gases that induce porosity in the final CN. BET analysis confirmed the SA enhancement up to 121 m2 g−1, which contributed to the enhancement of the photocatalytic HER activity.359 The utilization of biopolymers or biofibers as soft templating agents has been shown as another effective path to modify textural and photophysical properties. Zhang et al. used DCDA along with alginate or gelatin to modify the microstructure and electronic properties of CN and observed 5- and 6-fold SA enhancement when using alginate and gelatin, respectively. An additional consequence of these conditions is the insertion of carbon atoms, which in turn results in unpaired electrons within the CN lattice, measurable using electron paramagnetic resonance (EPR). Furthermore, the prepared materials were tested as photocathodes in a photoelectrochemical cell, showing a 2.6-fold enhancement of the photocurrent which remained stable during repeated on/off cycles.360 Additionally, mesoporous tubular CN with carbon doping was obtained using kapok fiber as a templating agent along with urea. This novel approach modifies the band structure toward an enhanced optical absorption and fast charge carrier separation, resulting in enhanced HER photocatalytic performance.361 Recently, urea was also condensed with a melamine formaldehyde resin; the resulting CN shows a nanotube morphology with enhanced SA, hydrophilicity and light harvesting properties, which result in a remarkable HER performance, reaching a 19.2% AQY.362
4.4. Ionothermal synthesis
The crystallinity of CN materials is a key factor for an efficient charge transfer and for the separation of photoexcited states and therefore for the achievement of a high photocatalytic activity. Merschjann et al. and others have shown that the electronic transport in CNs occurs perpendicularly to the 2D sheets' plane, in contrast to the situation in graphene-related materials.363,364 Therefore, achieving the closest possible, defect-free, highly ordered packing of the CN layers can substantially improve the electron and hole mobilities, one of the main bottlenecks of CN photocatalysts. Unlike the copolymerization of an organic monomer with a single alkaline salt, which typically results merely in a CN doped with the alkali metal or the anionic counterpart,365–370 using eutectic salt mixtures for the preparation of CNs induces both crystallinity and a higher degree of condensation in the resulting CN materials, and therefore has emerged as an effective approach to obtain high photocatalytic performance.371,372A eutectic mixture is composed of two or more compounds that melt simultaneously at temperatures ranging from 100 up to 1000 °C, allowing a miscible multicomponent liquid phase for the synthesis of inorganic crystalline materials and carbon–nitrogen based materials.373,374 Kuhn et al. described in 2008 the synthesis of triazine-based porous polymers in molten ZnCl2 that promoted the crystallinity of the framework. In this work the aromatic nitriles undergo dynamic trimerization within the ionic melt in a quartz ampule at 400 °C for 40 h, reaching an almost complete conversion. Fourier-transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) confirmed the formation of a crystalline polymer with a hexagonal pore packing and a specific surface area and pore volume of 791 m2 g−1 and 0.4 cm3 g−1, respectively.375 The utilization of a salt-melt mixture for the synthesis of CN was shown in a report by Bojdys et al. in 2008, where the condensation of DCDA was carried out in a LiCl/KCl mixture as the solvent. The CN obtained via this synthetic route shows an optimal degree of condensation, as proven by the absence of detectable amine groups in FTIR and corroborated by its high crystallinity reflected in the XRD patterns, where the (100) and (002) diffractions were observed at 12.1° and 26.5° respectively, corresponding to d = 7.3 and 3.36 Å and further supported by TEM.376 Later in 2011, Winhier et al. confirmed that the previously reported proposition of heptazine-based structures consists of a triazine-based framework, poly(triazine imide) (PTI).377 Li+ and Cl− intercalated PTI was prepared through the thermal treatment of DCDA in a eutectic mixture of LiCl and KCl, and the resulting material consisted of 2D networks composed of planar layers based on imide-bridged triazine units with Li+ and Cl− ions as confirmed by electron energy loss spectroscopy (EELS). 15N-NMR confirmed the triazine-based structure and the absence of heptazine motifs. Poly(triazine imides) have been studied in depth ever since; in 2013, the use of LiBr and KBr for their synthesis expanded the d spacing from 3.38 (observed in PTI prepared in LiCl/KCl) to 3.52 Å and led to a theoretical band gap between 1.6 and 2.0 eV.378 Chong et al. have shown the replacement of Br− by F− ions by washing with concentrated ammonium fluoride, obtaining PTI with intercalated fluoride ions and a smaller interlayer distance of 3.32 Å.379 The high photocatalytic performance of PTI materials was described later for both water half-splitting reactions with high H2 and O2 production rates.380–383 Further modification of the texture and electronic properties of the resulting material can be obtained by using organic monomers in order to enhance their optical absorption384 and by combining metallic salts with different loadings385 (e.g., NaCl, CuCl2, ZnCl2 and others).386–389 In 2018, Heymann et al. showed the electrochemical synthesis of PTI by coupling melamine radicals in a three-electrode setup; this novel approach allows the synthesis of PTI materials without intercalated ionic species. Additionally, despite the well-known high dispersibility of PTI,390 in this work, the high solubility of the obtained product allowed its analysis using liquid-state NMR.391
By selecting a novel monomer with a condensation temperature lower than that of the standard C–N based reactants, Dontsova et al. prepared well-defined potassium poly(heptazine imides) (PHTIK). Here, substituted triazoles were used along with a KCl/LiCl mixture. Given the temperature of the first reaction step (300 °C) and the melting point of the eutectic mixture (352 °C), the structure of the final material was directed towards a crystalline, heptazine-based structure113 with the capability to reduce water and exchange ions within the solid state.392 Since this first report on the ionothermal synthesis of PHTIK from triazole derivatives, several reports have been published on the modification of the properties of the final CN material produced from different reactant molecules393–395 or metallic salts.396 Their remarkable performances in photooxidation processes and catalyzing organic syntheses have also been described.397–399 Besides using raw C–N monomers with a condensation temperature lower than the melting point of the eutectic mixture, thermally treating common organic molecules to obtain melon units before the ionothermal synthetic step has emerged as an approach for the synthesis of highly photoactive materials. Lin et al. preheated melamine at 500 °C to prepare tri-s-triazine units (or s-heptazines400) and further heated the product in a KCl/LiCl mixture in order to achieve highly crystalline CN. Theoretical and experimental results confirmed the improved photogenerated charge carrier mobility of the tri-s-triazine-based CN vs. PTI, or melon type (Fig. 10), which resulted in a remarkable 50.7% AQY in the HER under 405 nm light illumination in a natural photosynthetic environment.401 Zhang et al. used a copolymerized CN material derived from urea and oxamide for the synthesis of a highly crystalline network based on heptazine units in a LiCl/KCl mixture. The narrowed interlayer distance and enhanced light absorption result in significant hydrogen production with a 57% and 10% AQY at 420 and 525 nm, respectively.402
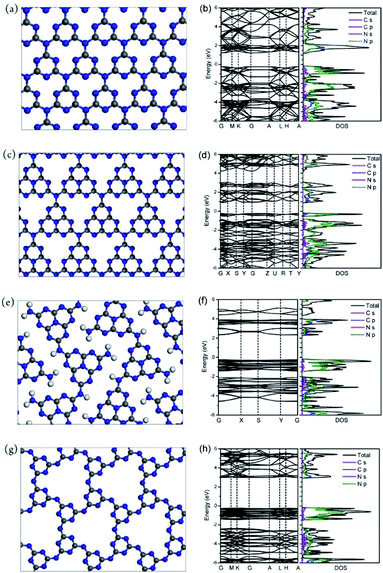 | ||
| Fig. 10 Models and electronic structure of different CN structures. (a and b) Triazine-based, (c and d) tri-s-triazine-based, (e and f) melon and (g and h) PTI. Reproduced with permission from ref. 401, copyright (2016) American Chemical Society. | ||
The variety of CN structures that can be obtained via ionothermal synthesis and the optimization of synthetic parameters (e.g., monomer-to-salt ratio or condensation path) has allowed, amongst others, the formation of isotype triazine–heptazine heterojunctions. They typically enhance the lifetime of photogenerated charge carriers thanks to the band alignment between different electronic structures, thereby promoting the photocatalytic activity;403,404 see also Section 5.4.
In 2017, Jin et al. polymerized urea in various amounts of the eutectic mixture LiCl/KCl at 450–550 °C. Temperatures above 500 °C resulted in PTI structures given their high thermal stability compared to heptazine structures. Nevertheless, at a fixed synthesis temperature of 500 °C, the salt-to-urea ratio determines the structural transformation from PTI into heptazines: a high molar ratio of molten salt to urea promotes the formation of a triazine-based structure, while a moderate ratio favors the heptazine form. The PTI/heptazine structure was confirmed by XRD, TEM and solid-state NMR and its photocatalytic performance (for the HER in this case) was substantially enhanced by the improved charge separation at the isotype heterojunction (Fig. 11).405 The presence of cations in the CN material resulting from an ionothermal synthetic procedure resulted in varying photocatalytic HER performance when salts were added to the reaction mixture. Li et al. polymerized melamine in the presence of a KCl–LiCl eutectic mixture and observed that the addition of certain cations, such as Rb+ or K+, promotes the assembly of the CNK material into nanorods and furthermore boosts the photocatalytic activity due to a dielectric screening effect.406
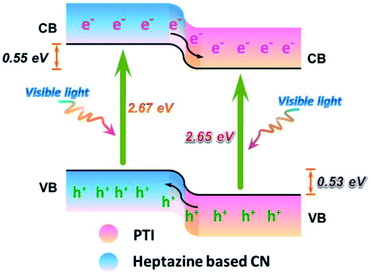 | ||
| Fig. 11 Charge separation at a PTI/heptazine CN heterojunction. Reproduced with permission from ref. 405, copyright (2017) American Chemical Society. | ||
4.5. Supramolecular design
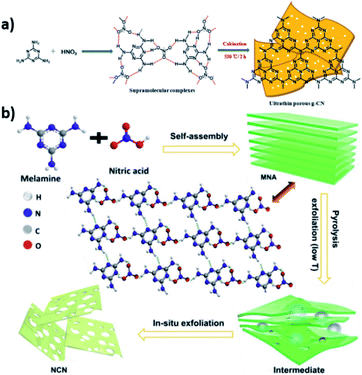
The use of supramolecular structures as a tool for the rational design of CNs has attracted widespread attention during the last 7 years, due to the possibility of imprinting novel morphologies and electronic structures from the molecular level all the way to the final product after calcination. In this approach, C–N-based monomers interact by non-covalent interactions, forming versatile self-assembled supramolecular frameworks of different dimensionalities in a given solvent. Their features strongly depend on the intermolecular forces at play (hydrogen and halogen bonding, π–π stacking, electrostatic interactions, etc.).407,408 The thermal condensation of the described supramolecular structures at high temperatures (300–650 °C) can induce the morphology and the monomer sequence onto the final CN material, thereby allowing the rational design of properties for a given application while surpassing the drawbacks of solid-state reactions.409The formation of a supramolecular assembly between sulfuric acid and melamine was further explored by others, confirming the quenching of sublimation during thermal treatment, the enhanced porosity, and the presence of trace amounts of sulfur, thereby modifying the chemical composition in the final bulk material, which promotes the photoactivity in various model reactions.413,414 Hydrochloric acid is the most commonly used strong acid for the synthesis of C–N-based supramolecular assemblies that serve as reactants for a high temperature solid-state reaction, and a large amount of work has been carried out since the first reports, showing the possibility of tuning the morphology and photoactivity of the final CN by using melamine hydrochloride as the starting reactant.415–418
Our group and others studied the effect of chloride ions on the features of the initial supramolecular assembly with melamine.419 Upon protonation, melamine is arranged in a 3D fashion with a repeating unit in plane,420 and this structure has also been observed in other supramolecular structures, where treatment with HCl induces directionality.421 The morphology imprinted by the supramolecular assembly strongly alters the physical features of the CN material, promoting the formation of ultrathin nanosheets or enhanced porosity.422 Furthermore, Zhang et al. confirmed the strong alteration of the band structure, which results in a positive shift of the CB and a negative shift of the VB.423 Bromide can also direct supramolecular assemblies based on melamine; our group showed that the sequential treatment of melamine with HCl and HBr results in the selective coordination of each halogen acid at a different binding site, namely an amine group or pyridinic nitrogen. Additionally, molecular dynamics simulations showed a stronger interaction of both amine and pyridinic N with Br rather than with Cl; therefore the modifications imprinted by HBr in the melamine-HCl supramolecular assembly strongly alter its chemical nature and dimensionality. The latter CN material showed a novel dot-like morphology with remarkable performance for the HER, reaching an AQY of 3.2% and 2.1% upon illumination at 405 and 455 nm, respectively.424 Treating melamine with nitric acid (HNO3) is one of the most effective approaches to direct the growth of the prepared CN towards one direction. Melamine and nitric acid interact by hydrogen bonding, forming supramolecular complexes with a rod-like shape and different sizes depending on the acid concentration or reaction time (Fig. 12).425 The rod-like morphology of the supramolecular assembly (melamine–HNO3) can effectively imprint a 1D morphology on the final CN material after condensation, as shown for the first time by Tahir et al. in 2014.426 Other groups have shown the application of 1D carbon nitrides, prepared using this method, in different catalytic scenarios such as dye photodegradation, an electrocatalytic ORR or a photocatalytic HER.427–430 Furthermore, several groups have studied the self-assembly of melamine with different strong acids and their combinations; CN obtained from self-assembled melamine–HNO3 proved to have the highest photocatalytic performance.431,432 Additionally, the use of supramolecular assemblies comprising melamine and nitric acid has allowed the tailoring of the chemical composition and electronic structure by further inserting heteroatoms,433 promoting the exfoliation of CN into ultrathin films (Fig. 12b),434 and generating homojunctions that improve the charge separation efficiency.435 Furthermore, while the protonation of standard C–N monomers before the pyrolysis does not typically modify the bulk chemical composition of the CN polymers, the use of acids containing heteroatoms can successfully alter it by inserting a given dopant element. Weng et al. and others described the synthesis of BN and BCN nanosheets from a hydrogen-bonded framework precursor containing melamine and boric acid after pyrolysis at 1050 °C,436,437 where hydrogen bonds are established between the hydroxyl groups of boric acid and both the amine and pyridinic nitrogens of melamine.438
The possibility of fine-tuning the CM lattice by using different solvents was confirmed, and thus the porosity and electronic properties of the resulting CNs were optimized towards enhanced photocatalytic activity. Nevertheless, due to the chemical composition of melamine and cyanuric acid and the release of both amine groups and O atoms, the chemical composition of the final CN polymer remains unaltered.445 Since the first reports on the utilization of CM lattices for the preparation of nanostructured CN, the modification of CM assemblies for the preparation of various CN materials with novel electronic structures and morphologies along with their versatile applications have been widely explored, which resulted in the accumulation of a vast body of knowledge.446–449
Inserting suitable C–N monomers modifies the dimensionality of the CM supramolecular assembly and the electronic properties of the final CN material by increasing the C-to-N ratio, which effectively narrows the optical band gap; small organic molecules, such as barbituric acid,450–452 aromatic moieties,453–456 urea,457 caffeine,458 nucleobases459 and others,460–463 have been shown to undergo supramolecular assembly with melamine and cyanuric acid, thus enhancing the photocatalytic activity of the final CN material (Fig. 13a). Furthermore, modulating the CM assembly with heteroatom-containing molecules allows the successful inclusion of the desired heteroatom within the framework of the final CN material, thereby modifying its bulk composition; boric acid or phosphoric acid can be integrated within the CM by hydrothermal treatment and preserve a certain amount of the doping agent, which promotes the charge transfer and separation of photoexcited states (Fig. 13b).464–466 Additionally, the utilization of sodium persulfate (Na2S2O8) along with the in situ generated CM lattice results in an enhanced porosity of the final CN, thanks to the release of sulfur-containing species, which improves the photo-electrochemical performance.467
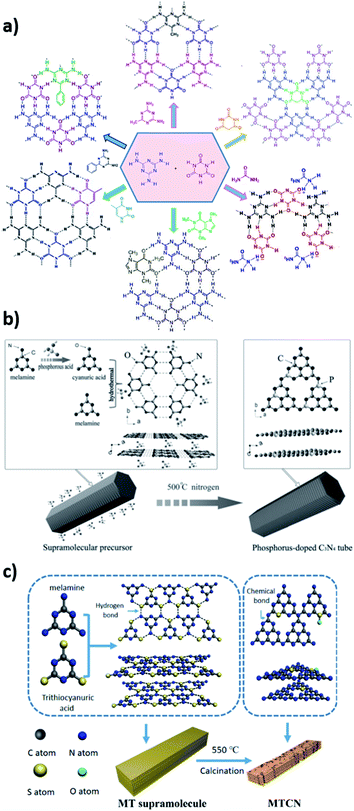 | ||
| Fig. 13 (a) Supramolecular structures based on the cyanuric acid–melamine lattice. Reproduced from ref. 409, copyright (2018) Wiley-VCH. (b) Synthesis of phosphorus-doped CN nanotubes. Reproduced from ref. 464, copyright (2016) Wiley-VCH. (c) Synthesis of a supramolecular assembly between melamine and trithiocyanuric acid and its conversion to sulfur-doped CN. Reproduced from ref. 473, copyright (2018) Elsevier publishing group. | ||
The replacement of melamine with a similar C-rich triazine-like molecule in the CM lattice modifies the chemical and electronic properties of CN. Xu et al. showed that when substituting a phenyl-modified triazine-based molecule such as benzoguanamine for melamine, a molten state was induced during the thermal condensation, which allowed the formation of homogeneous films of C-rich CN polymers over conductive substrates, opening the door to its application in solar cells.468 Additionally, the resulting phenyl-modified CN material, upon exfoliation, yields CN QDs with a remarkable PL behavior.469 Other C-rich molecules such as triaminopyrimidine (very similar to melamine but with one carbon replacing a nitrogen in the triazine ring), when self-assembled with cyanuric acid (CA), lead to the formation of C3N3O materials after thermal condensation at low temperatures, with photocatalytic activity for dye degradation, the HER and CO2 reduction.84
In addition to the widespread utilization of the CM lattice as a versatile platform for the design of C–N based supramolecular structures, melamine and other C–N monomers can also self-assemble with a wide variety of suitable monomers; this results in a diversity of supramolecular structures that modify the final CN properties towards an enhanced photoactivity. Trithiocyanuric acid (TTC), for example, has been widely used along with melamine for the synthesis of sulfur-doped CN, as shown by Feng et al.470 After this report, several groups have reported the formation of a M-TTC supramolecular assembly and its conversion into S-doped CN, with sulfur atoms typically replacing carbon in the CN framework; they used the latter for different photocatalytic scenarios including dye degradation,471 reduction of Cr(VI),472 the HER473 and formation of liquid crystals (Fig. 13c).474 Additionally, a large number of small organic molecules have been utilized and self-assembled with melamine; urea,475 glucose,476 oxalic acid,477 chitosan478 and others479–481 can interact by hydrogen bonding with melamine and form supramolecular aggregates, which after thermal treatment preserve certain C-doping. The clever selection of the building blocks taking part in the supramolecular self-assembly permits the obtention of semiconducting domains with a non-negligible photoactivity at low temperatures (250 °C); benzoquinone derivatives or chloranilic acid are some of the species that, after assembly with melamine and condensation at mild temperatures, have resulted in semiconducting polymers with photocatalytic activity in dye degradation and water oxidation.482,483
Additionally, several reports have performed the self-assembly of melamine with inorganic salts and explored the features of the final CN. Chen et al. preorganized melamine with [Al(NO3)3·9H2O], which interacts via hydrogen bonding between the amine groups of melamine and the water molecules of the hydrated complex. The thermal condensation of the supramolecular arrangement results in aluminum oxide species that can be removed using an acidic wash, resulting in enhanced SA and photocatalytic activity.484 Following this report, ionic complexes such as ammonium oxalate or hydroxylammonium chloride have been proven to form a supramolecular precursor with melamine by hydrogen bonding between amino groups and carbonyl or hydroxyl groups. The thermal condensation of these supramolecular precursors directs the morphology of the final CN towards porous nanotubes and enhanced photocatalytic activity in the HER or CO2 reduction.485,486
In the case of CM lattices, the solvent determines the aggregation mode of the supramolecular assembly and therefore the properties of the CN. In 2013, Shalom et al. prepared a CM lattice in different solvents, namely water, chloroform and ethanol. They observed that given the different surface energies of the hydrogen-bonded structures formed the aggregate displayed different morphologies, namely rods, needles and pancake-like structures, which introduced different structural motifs and photophysical properties into the final CN.445 Later this phenomenon was further studied in CM–barbituric acid (CMB) networks. A supramolecular CMB assembly in water yields a rod-like morphology, while in ethanol, spheres were formed. Upon thermal condensation, macroscopic (up to 4–5 cm long) CN wires grow spontaneously from the supramolecular spheres, whose properties strongly differ from the substrate, resulting in two distinct CN populations that can be used for water purification or H2 production through water splitting.496 Furthermore, recently Wang et al. studied the variation of the hydrogen-bond lengths in a CM framework and its effect on the resulting CN material. The self-assembled precursors were freeze-dried in order to decrease the length and increase the strength of the hydrogen bonds.497,498 Upon calcination, the distorted supramolecular structure results in a nanocage-like CN with enhanced SA, which can photocatalyze CO2 reduction.499 For heteroatom-containing supramolecular assemblies, our group has shown that the solubility of the initial monomers in a given solvent can dramatically alter their inclusion within the hydrogen-bonded framework. We have shown that preorganizing melamine with 1,3,4-thiadiazole-2,5-dithiol in DMSO results in a higher integration of the S-containing moiety in the starting assembly, which implies a more defined structural rearrangement compared to the assemblies prepared in water or common organic solvents. The unique chemical composition and structure achieved when using DMSO as the solvent are induced in the final CN material, resulting in a trace amount of sulfur doping, a beneficial electronic structure for photocatalysis and an enhanced SA.500
The manipulation of CM lattices by sequential solvent treatment also allows the formation of thermodynamically driven gradients of energy levels; Sun et al. prepared CM supramolecular assemblies in various solvents and confirmed the stronger aggregation of CM lattices in acetone and ethanol. Additionally, the prepared assemblies were subsequently immersed in a second solvent and this solvent's capability to disrupt the hydrogen bonding between the monomers was explored. As expected, polar solvents could penetrate within the structure, causing a complete rearrangement, while non-polar solvents modify only the aggregate's surface, which in turn leads to the formation of two interconnected CNs with an energy band gradient after thermal treatment, enhancing the lifetime of the photogenerated electron–hole pairs and the overall photocatalytic activity.501 Nevertheless, the introduction of a second solvent for the synthesis of supramolecular structures that serve as CN reactants is still in its infancy, and nowadays, most assemblies are still prepared in only one solvent. This fact limits the library of available monomers to those dispersible in a common solvent. However, in polymer chemistry and other fields, the advantages of using two non-miscible solvents have been demonstrated for the interfacial polymerization of supramolecular structures, allowing the interaction of several different units dispersed in non-miscible solvents (Fig. 14a).502 This concept was recently utilized in our group for the synthesis of CM frameworks. Specifically, we have shown the formation of CM frameworks in water–chloroform interfacial systems. Molecular dynamics simulations showed that both monomers tend to interact at the interface rather than in the bulk of either solvent. This thermodynamic tendency induces a complete rearrangement of the morphology. The thermal condensation of this system results in hollow CN tubes with altered electronic properties.503 Additionally, this approach allows a straightforward modification of CM units, either in situ by drop-casting a dopant monomer (such as picric acid from the aqueous phase, which inserts C–C bonds within the final CN polymer) or by preorganizing melamine before the mixing of the immiscible phases (water and chloroform, Fig. 14b). Particularly, Dolai et al. used a monomer with both hydrogen-bond donor and acceptor sites (i.e., the amino acid creatine) to selectively bind melamine and CA at the interface, thereby directing the modified CM units towards one direction. The latter CN material shows an altered morphology and electronic properties, which promote its photocatalytic activity towards several model reactions.504
 | ||
| Fig. 14 (a) Schematic representation of the interfacial supramolecular polymerization. Reproduced from ref. 502, copyright (2017) Wiley-VCH. (b) Synthesis of cyanuric acid–melamine (CM) frameworks in a water–chloroform interfacial (immiscible) system. Reproduced from ref. 503, copyright (2019) Royal Society of Chemistry. | ||
In 2018 this concept was established as a tool for the rational design of CN properties; our group showed the synthesis of melaminium chloride hemihydrate single crystals, where protonated melaminium residues are connected by chloride ions and water molecules. The crystals exhibited a 1D needle shaped morphology, which after thermal treatment was induced in the final CN material, resulting in macroscopic centimeter-long CN needles suitable for optoelectronic applications. Additionally, the chemical and photophysical properties of the final material could be fine-tuned by the reaction temperature (up to 650 °C) as well as by the chemical composition of the starting crystal through the insertion of a C-rich monomer such as benzoguanamine.510 Several groups have exploited novel single-crystal structures based on triazine molecules for the synthesis of modified CN materials. Zhu et al. used crystalline copper chloride templated by protonated melamine with the form [H2mela]2[CuCl5]Cl, consisting of [CuCl5]n3n− chains with Cl− anions and layers of doubly protonated melaminium cations ([H2mela]), bridged by N–H⋯Cl hydrogen bonds.511 The pyrolysis of this precursor allowed the release of HCl(g), which favors the formation of Cu–N bonds and results in the successful inclusion of up to 25.9 wt% of Cu within the final CN material. Furthermore, they studied the crystal preorganization effect by comparing the resulting material to the one obtained by the simple copolymerization of melamine with a metallic salt, concluding that the formation of highly ordered crystals promotes the inclusion of metallic Cu2+, whereas in other scenarios CuO NPs were formed on the CN surface. The photocatalytic activity was substantially enhanced as a result of the capability of Cu–N bonds to produce hydroxyl radicals which efficiently degrade standard organic pollutants such as RhB, MB or MO.512 Jiang et al. further used a wide variety of metal chlorides (Fe3+, Ni2+, Co2+ and Mn2+) for the synthesis of metal–organic crystal precursors; the latter were obtained by slowly cooling a saturated solution containing melamine and the metal chloride. The use of the highly crystalline precursors for the synthesis of CN resulted in the formation of nanotubes with enhanced photocatalytic activity for RhB degradation and the HER.513 Besides metal–organic crystals, using co-crystals formed by non-covalent interactions between different organic monomers can lead to a novel layered morphology, as well as a certain degree of carbon doping in the CN. Fang et al. showed the formation of co-crystals comprising terephthalic acid (TPA) and melamine, which interact by hydrogen bonding between the carboxylic groups of TPA and the amines of melamine, and their utilization as precursors for high temperature reactions. The hydrogen-bonded framework is organized as 2D molecular sheets. Additionally, the aromatic character of TPA allows the preparation of carbon-rich frameworks with a long range order, thereby promoting the charge separation of photogenerated charge carriers in the final CN material. The resulting C-rich material showed simultaneous H2 and O2 production, with an AQY of 5.3% for the HER.514 Additionally, Zhang et al. utilized a 3D hydrogen bonded crystal obtained by the recrystallization of urea from DMF. Upon thermal condensation, the DMF within the crystal structure is released promoting the formation of nanosheets with an enhanced surface area and photocatalytic performance.515
5. Post-synthetic modifications
Despite the relatively easy manipulation of CN parameters from the synthetic point of view, where electronic properties, texture and surface chemistry can be tailored, the post-synthetic modification of these materials is more complicated given the stability and low dispersibility of CN polymers in most solvents. The polarity of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond within the lattice, or the remaining –NH2 groups, a result of non-ideal condensation, permits the tailoring of the electronic properties and structure in a post-synthetic manner. Since covalent transformations within the CN structure are complex, several means have emerged in order to induce doping or defects, which improve the charge transfer and the photocatalytic performance, including consecutive thermal treatments,516–521 hydrothermal and solvothermal means,522–529 protonation530–532 and others.533 In this section, the focus is on the recent developments in post-synthetic modifications of CN, as well as the formation of hybrid composites with other layered materials, NPs, molecular complexes and so forth.
N– bond within the lattice, or the remaining –NH2 groups, a result of non-ideal condensation, permits the tailoring of the electronic properties and structure in a post-synthetic manner. Since covalent transformations within the CN structure are complex, several means have emerged in order to induce doping or defects, which improve the charge transfer and the photocatalytic performance, including consecutive thermal treatments,516–521 hydrothermal and solvothermal means,522–529 protonation530–532 and others.533 In this section, the focus is on the recent developments in post-synthetic modifications of CN, as well as the formation of hybrid composites with other layered materials, NPs, molecular complexes and so forth.
5.1. Covalent functionalization
Covalent chemistry offers a wide range of possible organic motifs to include within CN frameworks; the electronic properties and other parameters, such as colloidal stability or surface chemistry, are strongly affected by the attached functional group. Furthermore, this approach allows the formation of nanostructures and features, which is not possible to achieve by other conventional means.534 The first studies involving covalent functionalization of CN frameworks were performed by taking advantage of the electron lone pairs of the –NH2 terminal groups; in 2010, Guo et al. described the amidation of 1,2,4,5-benzene tetracarboxylic dianhydride (PMDA) and the CN amine groups for the extension of the polymer conjugation towards improved visible-light harvesting properties. First principles calculations confirmed that the PMDA moiety is perpendicular to the CN plane, promoting the repulsion between 2D layers, and 13C-NMR proved the presence of carbonyl carbons in the imide group. The improved conjugation leads to extended light absorption and promotes photocatalytic activity as demonstrated for MO photodegradation.535 In a similar manner, the conjugation was further extended by the introduction of perylene imidies into the CN framework, which promoted the photocatalytic removal of nitric oxide.536,537Lotsch and co-workers screened a wide variety of heptazine units functionalized in the 2, 5 and 8 positions with different moieties (e.g., alkyl chains, p-tolyl, p-benzoic acid, melonates and triphtalimide). The melonate form, obtained by salt-melt synthesis, improves the coordination to a platinum co-catalyst by cyanamide defects and therefore enhances the charge-transfer efficiency, resulting in a HER with 9.3% AQY.538 The introduction of cyano terminal groups through different synthetic pathways was also investigated by several groups showing a general enhancement of the photocatalytic activity thanks to the modification of the electronic structure. In these studies, the materials were prepared by mild treatment with a strong reducing agent such as NaBH4 or photoinduced in the presence of thiocyanates.539,540 Further treatment of the melonates with HCl yields urea-modified CNs through the hydrolyzation of the cyanamide defect group. This library of materials was shown to produce hydrogen through the water splitting reaction even in the absence of a Pt co-catalyst. When Pt was loaded, an AQY of 18% was obtained with methanol as a sacrificial electron donor, one of the highest photocatalytic activities for polymeric photocatalysts.541
Schiff-base chemistry involving terminal –NH2 groups has been widely used to prepare CNs with extended conjugation and enhanced photocatalytic performances.542 The thermal treatment at mild temperatures of CN with various aromatic carboxylic acids has been demonstrated to successfully graft the organic moiety to the solid polymer by Tian et al., who synthesized aromatic heterocycle-grafted CNs and observed that the electronic properties and photocatalytic performance strongly depended on the structure of the formed copolymer.543 The conjugation of CN frameworks through terminal amino groups can also modify their semiconductor behavior. Vidyasagar et al. successfully grafted CN with 2,5-thiophenedicarboxylic acid. The introduction of the organic moiety, proven by XPS and solid-state NMR, strongly modifies the band structure of CN, yielding both n- and p-type semiconducting domains, probed by Mott–Schottky analyses. The minimization of e−–h+ recombination enhanced both photocatalytic activity and charge-capacitance behavior.544 Besides small conjugated molecules or functional groups, the –NH2 groups in the edge planes of CN also allow covalent bonding to larger molecular materials or graphitic nanostructures; Pan et al. covalently embedded heptazine units within a covalent-organic framework (COF) by reacting them with 1,3,5-triformyl phloroglucinol and 2,4,6-tris (4-aminophenyl)-1,3,5-triazine in DMSO (Fig. 15a).545 Lu et al. immobilized Zn-phthalocyanine on CN through amide bonding in order to improve the light absorption and overall photocatalytic performance in organic dye degradation.546 The same effect has been observed with covalently bonded porphirins to CN, which promote light harvesting and lead to a remarkable HER and CO2 reduction performance.547,548 The amidation reaction has also been utilized for the covalent bonding of CN to carbon nanotubes (CNTs), which promoted the electron transfer in the ORR and the photocatalytic activity for H2O2 production (Fig. 15b).549
 | ||
| Fig. 15 Covalent functionalization schemes. (a) Synthesis of heptazine-functionalized covalent organic frameworks (COFs). Reproduced from ref. 545, copyright (2018) Wiley-VCH. (b) Synthesis of covalently bonded CN to a carbon nanotube. Reproduced from ref. 549, copyright (2018) Elsevier publishing group. (c) 1,3-Dipolar cycloaddition reactions for the covalent functionalization of CN. Reproduced from ref. 550, copyright (2014) Royal Society of Chemistry. | ||
Besides the covalent functionalizations taking advantage of the lone pair of terminal amine groups, cycloadditions, alkylations and other reactions have been carried out on CN by making use of the polar –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond within the framework. In 2014, Zhang et al. carried out the 1,3-dipolar cycloaddition reaction of azomethine ylides, known as Prato's reaction, for the functionalization of CN frameworks with a wide library of organic groups, including aryls, ionic liquids, ferrocene and more (Fig. 15c). In this work, the reaction takes place in toluene at 120 °C for 21 h, where azomethine ylide is first generated, in situ, by condensation of an α-amino acid and an aldehyde bearing the functional group to be inserted. The electronic structure of functionalized CN was tailored according to the functional group and a general light-absorption enhancement was observed. The photocatalytic activity was tested for the C–H oxidation reaction of β-isophorone to keto-isophorone, where the CN functionalized with ferrocene achieved a 96% conversion with 82% selectivity.550 Graphitic nanostructures like fullerenes can also be covalently bonded to CN by a solid-state mechanochemical route; Chen et al. utilized ball-milling to functionalize CN with a C60 fullerene in the presence of lithium hydroxide (LiOH) through the four-membered ring of azetidine.
N– bond within the framework. In 2014, Zhang et al. carried out the 1,3-dipolar cycloaddition reaction of azomethine ylides, known as Prato's reaction, for the functionalization of CN frameworks with a wide library of organic groups, including aryls, ionic liquids, ferrocene and more (Fig. 15c). In this work, the reaction takes place in toluene at 120 °C for 21 h, where azomethine ylide is first generated, in situ, by condensation of an α-amino acid and an aldehyde bearing the functional group to be inserted. The electronic structure of functionalized CN was tailored according to the functional group and a general light-absorption enhancement was observed. The photocatalytic activity was tested for the C–H oxidation reaction of β-isophorone to keto-isophorone, where the CN functionalized with ferrocene achieved a 96% conversion with 82% selectivity.550 Graphitic nanostructures like fullerenes can also be covalently bonded to CN by a solid-state mechanochemical route; Chen et al. utilized ball-milling to functionalize CN with a C60 fullerene in the presence of lithium hydroxide (LiOH) through the four-membered ring of azetidine.
The CN/C60 hybrid was used as a photocatalyst for the hydrogen evolution reaction under visible light with eosin Y as a sensitizer, without a noble-metal cocatalyst, showing a 4-fold increase in activity over pristine CN due to electron transfer from the sensitizer to the hybrid system and an improved charge separation efficiency.551 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond reactivity within CN also allows the covalent bonding of alkyl chains, forming a new C–C bond by using alkyl halides (the C
N bond reactivity within CN also allows the covalent bonding of alkyl chains, forming a new C–C bond by using alkyl halides (the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond serves as an excellent radical acceptor in this alkyl halide radical mechanism). The inclusion of such chains promotes the exfoliation of CN layers, by improving their solubility in common solvents such as tetrahydrofuran (THF).552 Related mechanisms can use dihalides and allow further functionalization with bio-compatible molecules as quinoxalines for organocatalysis.553
N bond serves as an excellent radical acceptor in this alkyl halide radical mechanism). The inclusion of such chains promotes the exfoliation of CN layers, by improving their solubility in common solvents such as tetrahydrofuran (THF).552 Related mechanisms can use dihalides and allow further functionalization with bio-compatible molecules as quinoxalines for organocatalysis.553
The chemical oxidation of CN nanosheets through acidic hydrothermal treatment has also been studied as a feasible way to improve the solubility of the material and its reactivity toward further functionalization.554 Along these lines, Teng et al. synthetized edge-functionalized CN via Hummers' method.555 Mulliken charge distribution confirmed that the surface acid groups improve the charge separation efficiency, thereby facilitating the formation of hydrogen peroxide and displaying a remarkable performance in the photocatalytic disinfection of water. A more complex covalent functionalization has been carried out utilizing halogenated organic monomers for the synthesis of CN frameworks modified with functional groups. Our group demonstrated that the calcination at 350 °C of a triazine-based molecule modified with halogenated aromatics, prepared by a reaction between DCDA and halogenated benzonitriles, yields CN frameworks with photophysical properties strongly dependent on the functional group.173 The added halogenated group serves as a highly active chemically reactive center, which allows its substitution by phenyl and tert-butyl propionate groups via Suzuki and reductive-Heck cross-coupling reactions, respectively. Other complex modifications of CN nanosheets promote their exfoliation and dispersibility in common solvents and furthermore enhance their photocatalytic activity towards the oxidation of benzyl alcohol as a model reaction.556
The capability of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in the heterocyclic ring to act as a radical acceptor in intermolecular alkyl radical additions allows the formation of CN-based hydrogels (CN–H) through the photoinduced generation of radicals and introduction of cross-linkers, functional groups, etc. The photoinduced grafting of molecules over CN polymers has been proven to permit the introduction of moieties such as fluoride or sulfonic groups as well as polymeric chains that modify the electronic structure, texture and colloidal properties of CN, toward its application in multiple fields.557–562 Furthermore, the formation of CN-based hydrogels with a defined 3D shape which maintain the semiconducting features of the matrix while surpassing its intrinsic drawbacks (grain boundaries or fast recombination) is an approach that has lately gathered attention. The tunability of the morphology, mechanical strength and photophysical properties of CN-based hydrogels through the selection of functional groups and cross-linkers may permit their application on an industrial scale in a wide range of fields.563–565
N bond in the heterocyclic ring to act as a radical acceptor in intermolecular alkyl radical additions allows the formation of CN-based hydrogels (CN–H) through the photoinduced generation of radicals and introduction of cross-linkers, functional groups, etc. The photoinduced grafting of molecules over CN polymers has been proven to permit the introduction of moieties such as fluoride or sulfonic groups as well as polymeric chains that modify the electronic structure, texture and colloidal properties of CN, toward its application in multiple fields.557–562 Furthermore, the formation of CN-based hydrogels with a defined 3D shape which maintain the semiconducting features of the matrix while surpassing its intrinsic drawbacks (grain boundaries or fast recombination) is an approach that has lately gathered attention. The tunability of the morphology, mechanical strength and photophysical properties of CN-based hydrogels through the selection of functional groups and cross-linkers may permit their application on an industrial scale in a wide range of fields.563–565
Thus far, the reports on CN–H were mostly based on non-covalent interactions with external agents, such as peptides,566 ionic liquids,567 graphitic materials568–571 and others.572–574 Typically, they involve the self-assembly of CN nanosheets by π–π or hydrogen-bonding interactions to form the desired 3D networks. Photopolymerization in the presence of cross-linkers has been used to covalently functionalize the surface of CN networks and form 3D structures; it yields robust CN–H with remarkable mechanical and photocatalytic properties. In 2017, Sun et al. have shown that CN generates surface radicals that induce in situ polymerization of acrylamide derivatives that yield photoactive CN–H. The resulting materials show strong mechanical strength and deformation restorability; these hydrogels were applied for the adsorption and degradation of organic dyes and the photocatalytic HER (Fig. 16).575 Liu et al. used poly(N-isopropylacrylamide) (NIPA) and CN for the formation of CN–H; the gelation occurred by photocatalytic generation of hydroxyl radicals, which initiate the polymerization. Furthermore, the temperature-responsive character of NIPA allowed the formation of smart coatings with tailorable turbidity by temperature control.576 The colloidal properties of the CN used for the formation of CN–H has been proven to be crucial for the modulation of the final mechanical properties. For example, Kumru et al. showed that CN–H derived from urea-CN with a zeta potential of −40.9 mV displays a storage modulus at 0.1% strain of 8320 Pa.577 Sulfonic acid was also grafted on CN and served both as the reinforcer and the initiator, resulting in CN–H with enhanced compressibility, tissue adhesive properties and moderate flexibility.578 The same authors also reported the effect of the amount of CN used in the preparation of CN–H in an ethylene glycol (EG) dispersant. Thus, they could achieve dispersions with up to 4 wt% of CN, yielding hydrogels with a remarkably high storage modulus of 720 kPa.579 Ye et al. showed a wide range of novel applications for CN–H. Specifically, they used CN–H obtained through photopolymerization of CN with acrylamide. The prepared hydrogels showed good mechanical properties as well as self-healing capabilities, and their performance as a UV irradiation protective film was tested for the first time and showed 95% filtered UV radiation (315–400 nm).580
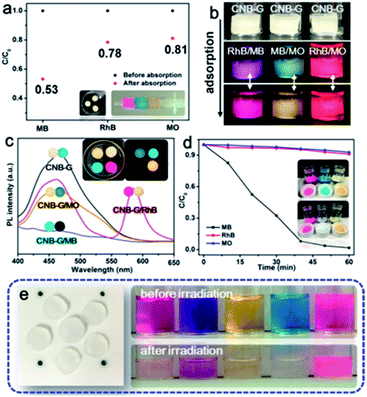 | ||
| Fig. 16 Application of CN-based hydrogels (CN–H) for dye adsorption and degradation. (a) Adsorption capacity with different organic dyes, (b) digital images of the CN–H adsorption capacity with mixed dyes, (c) photoluminescence spectra before and after dye adsorption, (d) photodegradation of CN–H of MB, RhB and MO, and (e) digital images of the photodegradation of various dyes with CN–H. Reproduced from ref. 575, copyright (2017) American Chemical Society. | ||
5.2. Exfoliation
The exfoliation of layered materials is an established approach for the obtention of isolated flakes or 2D nanosheets. The exfoliation of a bulk layered material into thin sheets increases the number of active sites and therefore its reactivity. Furthermore, the choice of the exfoliation technique, namely mechanical, thermal or chemical (in a liquid phase), defines the yield and the morphology of the nanosheets, thus determining their performance in a given application.581 In particular, liquid-phase exfoliation has been one of the most widespread techniques due to the possibility of weakening the van der Waals (vdW) interactions between the 2D sheets in layered materials through the insertion of surfactants and ions, and the possibility of modulating the exfoliation degree by controlling parameters such as shear force, temperature or ultrasonic frequency.582Various 2D layered materials (graphene,583 BN,584 transition metal dichalcogenides,585 and others586–588) have been successfully exfoliated using such techniques.
The exfoliation of CN is of great interest given the potential synthetic prospects and applications of nanosheet-based devices. CN layers interact by weak vdW forces, which can be easily disturbed by mechanical means. CN in the form of nanosheets is typically endowed with a significantly higher SA and more abundant surface active sites, a larger Eg thanks to quantum confinement effects, and improved electron transport along the in-plane direction, which allows the enhancement of intrinsic properties, such as photoresponse.589,590
Zhang et al. showed the exfoliation of CN in solvents with various polarities and observed that the relatively higher polarity of water benefited the exfoliation; ultrathin sheets were obtained at a concentration of 0.15 mg mL−1 and with a zeta potential of −30.3 mV. Furthermore, the CN layers in solution had diameters of 70–160 nm and a thickness of 2.5 nm. This suspension remained stable for months even at pH values ranging from 3 to 11, with the electronic structure strongly altered compared to the bulk counterpart. DFT calculations confirmed that single-layered CN has an increased density of states at the CB edge compared to the bulk—an indication of a higher charge-carrier concentration—which supports the measurement of an increased photoresponse.591 Yang et al. further studied the liquid-phase exfoliation of CN in solvents (including 2-propanol (IPA), NMP, water, ethanol and acetone) and the influence of sonication time, as well as solvent–material interactions. They found that NMP and IPA were the most appropriate solvents, given their surface energies (40 mJ cm−2vs. 70 mJ cm−2 for vdW-bonded surfaces). After 10 h of sonication, the CN dispersion consisted of 2 nm-thick nanosheets that remained stable up to 4 months. The XRD pattern of the thin-layered material slightly differs from that of the bulk counterpart; the relative intensity of the (002) diffraction peak, corresponding to the interplanar stacking, significantly decreases. Additionally, the CN nanosheets showed a band gap of 2.65 eV and a 3.75% AQY in the HER owing to the high SA and the resulting larger number of exposed catalytic sites.592
Since the pioneering reports of liquid-phase exfoliation of CN, different groups have established relationships between the utilization of different solvents, including alcohols,593 sulfonic group-containing solvents,594,595 hydrogen peroxide,596 and others597,598 with the nanoscale features of the prepared sheets.599 The polymerization degree of the CN used in the exfoliation strongly determines the photophysical behavior of the prepared nanosheets, as shown by Zhou et al., who developed electrochemiluminescent sensors for the detection of multiple metal ions based on CN nanosheets with different condensation degrees that display different responses to each metal ion.600 The thermal exfoliation of CN can also yield 2 nm-thick nanosheets with SA ∼300 m2 g−1. This procedure can be carried out by the thermal condensation of pre-formed CN at high temperatures and in air, which typically results in the oxidation of exposed nitrogen atoms.601,602 Niu et al. confirmed that the electronic properties of such nanosheets are also strongly altered as shown by a 0.2 eV increase of Eg (2.97 vs. 2.77), which correlated with a blue shift of the fluorescence emission maximum of 20 nm. These quantum confinement-derived effects are also reflected in the good electron conductivity along the in-plane direction of the nanosheet compared to its bulk counterpart and the enhanced photocatalytic activity for the generation of hydroxyl radicals.603 Additionally, Xia et al. established that the atmosphere used during the thermal exfoliation can induce chemical modifications in the nanosheets, for example, amine functionalization by thermal exfoliation of bulk CN under a continuous NH3 flow.604 In order to reduce the thickness of the discussed 2 nm-thick CN nanosheets and obtain single atomic monolayers, a chemical exfoliation of bulk CN can be performed, as reported in 2013 by Xu et al.; bulk CN was stirred in the presence of H2SO4 (98 wt%) for 8 h, followed by a liquid-phase exfoliation in water and reflux in methanol at 65 °C for 6 h. The intercalation of H2SO4 within the CN layers resulted in a shift of the (002) diffraction peak down to 25.0° indicating the expansion of the inter layer distance to 0.356 nm; the latter exfoliation yielded 0.4 nm-thick layers with tens of nanometers lateral size. The modification of the dimensionality strongly affected the optical properties, as the Eg increases from 2.64 to 2.92 eV given the decrease in conjugation and the quantum confinement effect (also confirmed by a fluorescence emission peak blue shift from 464 in the bulk to 438 nm). The modification of the nanosheet morphology and electronic properties resulted in an enhanced photocatalytic HER and photodegradation of phenol.605
The utilization of strong acids has been further studied by several groups, which reported the chemical cleavage of the CN framework, thus enhancing its solubility, which even allowed recording liquid-state NMR spectra.606,607 The exfoliation of layered materials by mechanical means, e.g., scotch tape, which has been widely utilized,608–611 was also applied for CN. For example, Bojdys et al. showed the mechanical and chemical exfoliation of poly(triazine imide) with intercalated potassium metal that resulted in thin layers, scrolls and bundles with macroscopic lateral dimensions. Furthermore, the reported liquid-phase exfoliation resulted in atomically thin PTI sheets with Eg = 2.6 eV.612 The liquid-phase exfoliation of crystalline PTI materials was later studied by Schwinghammer et al., who showed that 1–2 nm-thick nanosheets of PTI/Li+Cl− could be obtained by simple sequential ultrasonication in water (Fig. 17). The well-dispersed PTI nanosheets remained stable in both acidic and alkaline environments and showed improved photocatalytic HER performance.613 Further work on the exfoliation of PTI materials resulted in the complete dissolution of PTI·Li+Br− in the presence of polar aprotic organic solvents such as NMP, DMF or DMSO, with mass concentrations ca. 0.8 ± 0.05 mg mL−1. The spontaneous dissolution occurred as a result of a free energy gain upon solvent coordination, where the energies of the interlayer interactions remain similar to that of the solvent–nanosheet interface.614
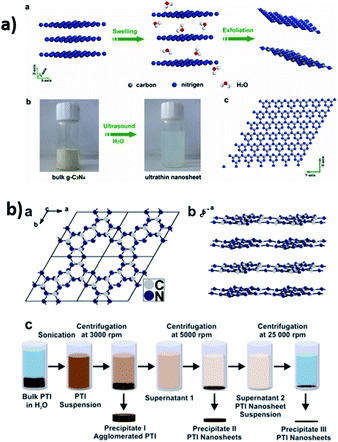 | ||
| Fig. 17 (a) Scheme of the synthesis of dispersed CN nanosheets by liquid-phase exfoliation (a swelling step, followed by exfoliation using ultrasonication as an external mechanical force). Reproduced from ref. 591, copyright (2012) American Chemical Society. (b) Scheme of the preparation of ultrathin PTI materials by sequential exfoliation. Reproduced from ref. 613, copyright (2014) American Chemical Society. | ||
Utilizing intercalating agents to promote the separation of CN layers is a very effective approach to enhancing the yield of the nanosheets prepared via liquid-phase exfoliation. Xu et al. carried out the liquid-phase exfoliation of CN in the presence of ammonium chloride (NH4Cl); the thermal treatment of CN/NH4Cl resulted in the decomposition of ammonium ions, which distorted the vdW interactions between layers, resulting in 2–3 nm sheets with enhanced SA and performance in the photoelectrochemical detection of copper(II) ions.615 Melamine has been utilized as an intercalating agent as well; Ma et al. treated CN with DMF and subsequently added melamine to achieve an optimal enlargement of the interlayer distance of CN. The resulting material possessed an enhanced SA (116.76 m2 g−1), a larger bandgap (by 0.13 eV) and higher electron–hole mobility, which promoted the photocatalytic activity in a visible light-driven HER.616 Ji et al. made the most of a planar conjugated molecule, such as 1-pyrene-butyrate, to exfoliate and modify the CN's surface via π–π interactions. The latter was anchored to a DNA probe and used as an electrochemiluminescent DNA biosensor.617 Reversible chemical exfoliation was achieved by the intercalation of alkaline metal hydroxides and the re-assembly of CN layers. Li et al. utilized OH-modified CN618 for the in situ generation of water molecules upon acidic treatment, which promoted the exfoliation into thin nanosheets of 1–2 nm thickness and a yield of 48% at pH = 1.3. They were able to re-assemble the thin layers using a dilute alkali treatment; they exhibited a superior photocatalytic performance for IPA degradation and the HER thanks to their improved visible light absorption and hydrophilicity induced by the hydroxyl groups at the surface.619
5.3. Hybrid composites based on CN
A hybrid composite (HC) combines the functionalities of its individual constituent domains. In our context, the novel properties result from the coupling between CN and the other domain(s). A metal domain may exhibit plasmonic effects and catalytic activities, while a semiconductor absorbs light, produces charge carriers and, depending on the band alignment at the SC–SC interface, can facilitate charge separation (in photovoltaics and photocatalysis) or recombination (in enhanced photoluminescence or light-emitting diodes). Such interfaces can exist on the macroscale, e.g., in CN films deposited on highly doped metal oxides or in the case of CN films deposited on various substrates with nanostructured features (vertically aligned ZnO nanorods,620 for example), or on the nanoscale as a post-synthetic modification of CN, as discussed in this subsection (for example we refer the reader to a recent review from Liao et al., which summarizes many HCs used for the HER).146In type-I heterojunctions, the CB and VB of one SC engulf the bands of the other (Fig. 18a, ‘straddling gap’). Though this band alignment favors radiative recombination in the narrow-Eg SC, redox reactions can still take place, e.g., CN/antimonene,622 CN/ZnV2O5 (ref. 623) and CN/ZnIn2S4.624
In type-II heterojunctions, e− and h+ spatial separation is favored across the formed interface, which is effective in enhancing the lifetime of photogenerated charges (see photocatalytic examples in a recent review by Shen and co-workers).625 As CN is an n-type SC, the construction of a heterojunction with a p-type SC results in a built-in internal electric field, which helps drive charge separation,626 as was demonstrated using BiOCl/CN627 and Cu2O/CN.628
Despite their efficiency in promoting charge separation, after the migration across the interface, the redox potential difference for a reaction at the surface is weakened in a type-II alignment (Fig. 18b, CB of the orange particle and VB of the blue SC). In order to maintain optimal reduction and oxidation potentials, while at the same time achieving the spatial charge separation of the photogenerated excitons, a Z-scheme charge transfer pathway between two semiconductors has emerged as an efficient strategy.629 In this case (Fig. 18c),630 photoexcited electrons from the ‘purple’ CB recombine with photogenerated holes of the ‘blue’ SC. In this manner, the remaining, spatially separated charges display optimal reduction and oxidation potentials as shown for various systems of CN with metal oxides,631–633 sulfides,634etc. of different morphologies, where a continuous interface is present. The described Z-scheme can also be supplemented by a third material at the interface, an electron mediator such as metal NPs or reduced graphene oxide (rGO).630,635 An interesting combination of materials was achieved by Li and co-workers, who formed a Z-scheme catalyst where CN is the light absorber, meso-tetra(4-carboxyphenyl) porphyrin (TCPP) both acts as an antenna and provides excited electrons to perform the HER, and the third component of this hybrid is a Cu NP, which acts as the mediator.636 In this work, the intimate connection between CN and porphyrin, which is usually very weak owing to π–π stacking and H bonding, was more robust thanks to the Cu linker.
Besides SC/SC junctions, interfacing CN with metal NPs, also known as Mott–Schottky junctions, has a catalytic effect in part due to a charge transfer between the SC and the metal. The metal NP, in turn, acts as a reactive binding site for a reduction or oxidation process. We recommend a thorough review by Li and Antonietti on this subject, where they discuss the different possible N-doped carbon/metal and CN/metal Mott–Schottky heterojunctions.637 Since CN is n-type, we limit our discussion to CN-to-metal electron transfer, which serves as an electron sink (Fig. 18d). This is relevant also for Z-scheme interfaces, where a Pt NP, for example, can catalyze the HER process using electrons from a CN, thus enhancing the interfacial recombination at the direct Z-scheme interface (2D CN sheets/3D TiO2 microflowers), leaving behind holes in TiO2 that can oxidize water and/or hole scavengers as suggested by Wang et al.638
Graphene, carbon nanotubes or fullerenes can act as electron acceptors, promoting charge separation upon photoexcitation; they also successfully transfer the excited electrons to the reactant molecules. An intimate contact between the two counterparts can result in specific chemical bonds, such as covalent C–O–C moieties in the case of reduced graphene oxide (rGO) and CN, which results in a narrowing of the band gap and an improvement in light-harvesting properties.641 Simple ultrasonication in toluene of CN powder and C60 results in a CN/C60 HC tested for photocatalytic oxidation of (aromatic) sulfides to sulfoxides.642
Other composites, which incorporate CN, are constantly explored including different metal-free non-carbon-based 2D materials such as phosphorene643 and antimonene,622 due to their strong vdW interactions.621 Many other hybrids between CN and quasi-2D and other functional materials such as MOFs are reported; see for example, a review by Wang et al.644
Metal-containing compounds can exhibit semiconductor or catalytic properties (or both). Here, we briefly survey some SC/SC heterojunctions, while the catalysts are in the next subsection. The use of metal oxide materials is driven by their generally superior stability. Some recent examples include: the hydrothermal decoration of CN on rutile and anatase TiO2 nanotubes by Kumar et al.;645 CN–WO3 heterojunctions;635 Jiang and co-workers’ CN with a nanotube morphology decorated with CoOx NPs (CoO and Co3O4) for the HER, in which case they confirmed, using Kelvin-probe spectroscopy, that smaller CoO NPs (ca. 8 nm) form a type-II heterojunction, while larger Co3O4 NPs (∼10–50 nm) form a type-I heterojunction with CN;646 a bimetallic oxide (NiCo2O4) on exfoliated CN sheets with enhanced HER performance owing to a formed junction between the p-type oxide and n-type CN showing superior performance relative to NiO/CN and Co3O4/CN;647 2D/2D CN/Co3O4 or CN/V2O5 for the HER;631 and 2D/2D InSe/CN for the OER.648
Type II interfaces based on CN and metal chalcogenides are also common. A recent example of a complex morphology was demonstrated in the form of an octahedral CdS/CN hybrid for the HER and oxidation of toluene and other aromatic alkanes. In this case, first, a hydrothermal synthesis exploiting a coordination polymer with Cd–S bonds was used as the precursor for the formation of μm-sized porous CdS octahedra, which was then modified with melamine in an alkaline hydrothermal environment, finally forming a porous core–shell CdS structure with an external octahedral porous CdS shell/CN HC.649
Molecular complexes can be combined with CN in the liquid phase; for example, the boron-containing dye 4,4-difluoro-8-phenyl-4-bora-3a,4adiaza-s-indacene (BODIPY) effectively extracts holes from CN in an OER scenario.531 Metal-containing catalysts such as Ni(II) bis(diphosphine) can act as H2-evolution catalysts in a photocatalytic system, where they are coupled to a CN (specifically, with a cyanamide surface functionalization), which performs selective oxidation of benzylic alcohols to aldehydes in water.654 CO2 reduction and the HER using molecular catalysts such as Co(bpy)32+ connected to CN using a molecular bridge were also reported.655
The extreme case of minimization would be the use of single atoms on the CN's surface.656–660 In this architecture, the CN acts as the support for the metal. It is extremely hard to prove the dispersion of single atoms and their actual oxidation state. For example, the previously mentioned silver incorporation through copolymerization by Chen et al. has shown incorporation of an Ag+-complex into the CN matrix, but XPS measurements indicate Ag(0) to Ag+ atomic ratios from 61% to 39%.246 To successfully incorporate metal atoms, the CN matrix may be altered, as exemplified by Pérez-Ramírez and co-workers, who hosted Pd in CN by forming phosphorous doping (P was better than other heteroatoms due to increased electron density).657 Recent theoretical calculations for different transition metals on the surface of CN for the electrocatalytic nitrogen reduction reaction (NRR) were performed by Zhang et al.; they predicted that Mo and V would be the best non-precious metal atomic dopant based on the nitrogen adsorption geometry (standing-on and lying-on, respectively).661 Chen et al. have also performed a calculational screening, reaching the conclusion that a W atom supported over a single CN layer would be a better catalyst than a stepped Ru(0001) surface, which has the best activity towards the NRR amongst bulk single-metal catalysts.662
Already, in the first reports of the photocatalytic activity of CN published a decade ago, two distinct approaches were demonstrated by Wang and co-workers: in the first, Pt NPs were photodeposited on CN to improve the HER.96 In this synthetic mechanism, photoexcited electrons reduce a Pt source in the surrounding solution, forming a CN/Pt NP HC; in the second, a photocatalytic activity enhancement is achieved through metal doping, an approach that was discussed in Section 4.2.1. In this approach (for example, Fe(III) species within a CN matrix663), metal-nitrogen bonds at the surface are responsible for the catalytic activity. The latter approach can be extended from metal-ion-containing motifs to metal-based materials. Zhang et al. have reported using a core@shell Ni@NiO/CN hybrid for the HER.664 Liao et al. have used a simple in situ thermal decomposition in air of a Ni(II) precursor physically adsorbed from a solution on CN to achieve a uniform distribution of NiO NPs throughout CN sheets. This hybrid material exhibits high OER activity due to the formation of a conductive interface and the existence of Ni–N bonds at the interface, which are responsible for charge accumulation on the Ni atom, lowering the adsorption free energy of the OER intermediates.665 This is an example of how a doping-based catalytic mechanism can exist in CN/NP HCs.
The photodeposition of metals is very common, as mentioned for the case of Pt: when a solution with a metal complex is illuminated in the presence of CN, the photogenerated electrons reduce the metal on its surface, typically forming metal NPs on the CN's surface (as Cu636). Recently, Wang et al. have shown that gold can not only be photodeposited, but can also be deposited via piezo-catalytic electron excitation.666 This mechanism allows the excitation of electrons following an external ultrasonic stimulus; the excited electrons can be used for H2O2 generation, and this effect is stronger when the CN has fewer vacancies.
Other ways of forming metal/CN HCs include reduction of the metal by combination of a reducing agent and high temperature (e.g., refluxing CN with RuCl3 and ascorbic acid).667 or thermal reduction with H2(g) after a wet impregnation with a metal salt, as in the case of the Ni0 NP/CN HC, which was reported for successful photocatalytic CO2 methanation (Sabatier reaction),668 in part due to better charge transfer in the hybrid.
The HC combinations are not limited to single junctions, and the formation of multicomponent structures shows great promise, which augment the HC with high SA, charge separation, catalytically active sites and so forth. For example, ternary CN/NiCoP2 MOF/porous carbon HCs were demonstrated for the HER; they take advantage of an improved charge separation at the metal-phosphide/CN interface, the low overpotential of the NiCoP2 cocatalyst, the support functionality of the porous MOF and the improved conductivity of the carbon mediator.669 Other examples include combination of a metal/SC junction in addition to a SC/SC junction (e.g., CuInS2/Au/CN,670 which is useful amongst other applications for the previously discussed Z-scheme photocatalysts) or to a SC/enzyme.671
6. Deposition on substrates
One of the main obstacles for the widespread application of CN is the difficulty in depositing CN materials on electrodes while at the same time maintaining their advantageous properties developed in powder. A successful CN or CN-based hybrid composite on a substrate would continue fulfilling its function under operating conditions (in acidic/alkaline environments, under illumination, under applied voltage bias, etc.). Because of the high importance of this challenging mission, much research has been dedicated towards such an achievement. Our group and others (e.g., Mat Teridi, Zhang, Xue and Pang, Yang, Bian and their co-workers) have recently published review papers discussing deposition techniques and properties of CN films for photoelectrochemical (PEC) and related applications, such as electrocatalysts, photovoltaics, and energy storage.135,136,672–675 For this reason, we will focus only on the most recent progress and refer the reader to the above-mentioned reviews for further information regarding performance, mechanisms, and application-specific prospects.When discussing deposition techniques, the main distinction is whether the CN is formed on the substrate itself or first synthesized ex situ and then deposited using a given casting technique. Generally, the latter results in an inferior electrical connection and poor mechanical stability due to uncontrolled adhesion of exfoliated CN onto substrates. A good demonstration of this fact is the comparison Ruan et al. performed for CN on fluorine-doped tin oxide (FTO), where a bulk CN film (obtained by ultrasonication in water and IPA, followed by drop-casting a CN suspension in Nafion resin) was compared to a compact and dense film prepared by other methods (discussed later) and was shown to display inferior crystallinity and performance.676 For this reason, high-performance CN films are usually not prepared using this method. Nevertheless, advances in the field of functionalization and heterojunction formation can still be successfully measured (recent examples are the studies of Zhang and co-workers who formed a CN/Cu-containing complex hybrid via ball milling and tested it for IR biosensing;677 another is the solar aqueous battery678 demonstrated by Lotsch and co-workers). Furthermore, the deposition of ex situ synthesized material by casting can be used for the facile electrochemical test of changes of CN properties (e.g., the influence of exposure to γ-radiation).679 Exceptions include physical deposition techniques, where a simple precursor dispersion is needed (spray coating composites680 and electrospinning681 are representative examples).
Some recent progress of note was achieved by the (photo)functionalization of CN sheets, improving their dispersibility in organic solvents for further spray coating682 (opening the exciting practical path of inkjet printing) and casting. The latter was used to fabricate thermoset CN-in-polyester films558 and also to incorporate a thin CN layer as an electron transporting layer in an inverted perovskite solar cell by spin-casting.683 Related reports of CN incorporation described treatment with a functionalizing acid684 and iodine doping,685 but in these cases the CN was cast as an additive and not as a standalone film. Similarly, CN was blended with mesoporous TiO2 in a polymeric matrix (polyvinylidene fluoride and N-phenethyl-4-piperidone), which was then doctor-bladed.686 For further insight, we refer the reader to a recent review by Schmidt and co-workers on the combination of CN with polymers.687 Our group has also recently demonstrated that covalent modification of CN precursors with polycyclic aromatic hydrocarbons (PAH, such as anthracene and pyrene) amongst other advantages also improves the casting (i.e., drop-casting or spin-coating) of the resulting CNs (after calcination) on both rigid and flexible substrates, thereby allowing the use of such materials in applications such as electropolymerization and photoanodes in PECs.688
The ‘direct’ growth methods of CN on electrodes include variations of gas-phase and liquid-phase syntheses:135 the thermal vapor of various subliming/evaporating CN precursors can condense on the surface of the substrate or nucleate on pre-formed ‘seeds’; liquid-phase methods rely on the immersion of the substrate into liquid media which include molten CN precursors, other molten phases (e.g., sulfur689) and solvothermal solutions.690,691 An intermediate situation occurs when a CN-precursor layer is in intimate contact with the substrate and is being thermally condensed, with (strong) CN–substrate interactions present, as is the case of soaking precursors692 and for the method reported by our group, where a paste of supramolecular precursors is doctor-bladed on a substrate prior to its thermal condensation at elevated temperatures in a closed (not sealed) tube.494,693,694
The facility and scalability of the doctor-blade method along with the benefits of using supramolecular aggregates (e.g., precise control over composition) as precursors are its most appealing features. Furthermore, it allows the incorporation of conductive elements, such as rGO, to improve the electron diffusion length within the film (up to ca. 36 μm).693 We have found that to improve the packing and order of the CN layer (motivated by Durrant and co-workers’ report on electron trap states at the surface695 and the electron transport properties of crystalline CN. See discussion in Section 4.4) it is better to use a molecular precursor rather than a supramolecular precursor. To this end, we have dipped the substrate into a hot supersaturated melamine solution to deposit a melamine film, which after calcination formed a crystalline CN layer.696 Melamine alone cannot be doctor-bladed, but upon addition of GO, a viscous powder is formed, which allows the deposition.694
Recent advances achieved using vapor-based methods include multi-step deposition resulting in pinhole-free layers,697 heteroatom-incorporation698 and heterojunctions of CN containing different heteroatoms,699 metal-ion incorporation700 and control over crystallinity in ∼0.2 μm-thick formaldehyde-doped CN layers.701
It is important to note that the discussion in this section focused on the deposition of thin CN layers over substrates with relatively low roughness (as is the case for the standard transparent conductive oxides (TCOs), which are coated on rigid glass or flexible polymer-based substrates in photoelectronic devices thanks to their combination of electronic conductance and optical transparency). However, the same principles can be applied to interface CN with different (porous) materials, such as carbon-based materials, membranes (serving as a hard template),702,703 inorganic nanostructures,620,692,704etc. All the above-mentioned approaches can be used for the formation of semiconductor heterojunctions704,705 between CN and other materials (to improve photoelectrode performance, for example, by improving light harvesting, charge separation and charge carrier migration paths). An elegant demonstration of a complex HC for PECs was shown by Qin et al., who thermally polymerized CN over a substrate with vertically aligned TiO2 nanorods, followed by metal ion soaking and gas-phase phosphidation, which both dopes the CN with P and forms metal (M = Fe, Cu) phosphide oxidation catalysts. This synthesis yields FTO/TiO2/(P-doped)CN/MxP HC photoanodes.706
For some applications, such as photocatalysis, free-standing films are of value. To this end, on top of common synthetic pathways such as filtration or freeze-drying,707 the deposition on substrates can be used as a first step before peeling-off700,708 or before the transformation of the substrate itself into an electrode, as our group has demonstrated by the calcination of a filter paper coated with CN precursors, transforming it into a free-standing C/CN hybrid.709
Significant progress is underway in the application of CN as a photoelectrode in the emerging organic PEC devices710 or in hybrid scenarios where CN complements the better-established inorganic layers,711 with possible metal-based cocatalysts.712 In addition, the ability to deposit large-scale porous films713 of CN paves the way also for its application as a particulate photocatalyst,714i.e., a film of photoactive material (composite) that can perform energy conversion reactions, such as hydrogen production, without connection to an external electric bias, as demonstrated by the Domen group for other materials.715–717
7. Prospects and concluding remarks
In this review, we summarized the recent developments in the synthesis, growth methods and applications of carbon nitride-based materials, ranging from carbons, N-doped carbons, C2N, C3N4 to other C–N containing polymers. We have mainly focused on melon carbon nitrides (commonly referred to in the literature as C3N4) owing to their attractive properties, e.g., tunable band gap, low-price, stability under harsh conditions and suitable energy band positions, enabling various reduction and oxidation reactions along with a wide range of applications.The exploration and development of synthetic routes, characterization techniques and fundamental understanding of structural features play an essential role in opening the path towards the utilization of these materials in practical applications. We discussed the synthetic methods in detail, including templating and doping techniques, as well as emerging approaches, such as the use of supramolecular chemistry and covalent functionalization, with a special emphasis on the rational design of a modified-CN with controlled chemical, photophysical and catalytic properties. The methods to affect and study their electronic and optical properties and their photo-, electro- and photoelectro-catalytic performance for various energy-related applications were elaborated upon. The manipulation of the electronic and catalytic properties by interfacing with other materials, by single atom doping and by molecular modification was shown. A special emphasis was placed on the correlation between their structural properties and their activity in a given reaction. In the last part, the recent progress in the growth and deposition of carbon nitride materials on surfaces and their utilization as photoelectrodes were briefly introduced, with a focus on the deposition techniques and the subsequent use of CN films as photoelectrodes for water splitting. Based on the current status and challenges in the synthesis and incorporation of (modified) CN materials into practical devices, in our opinion, future research should pay attention to the following:
1. The synthetic paths should be further improved, to allow a better design of the final properties, such as the specific surface area, morphology, optical band gap, crystallinity and reaction yield. Moreover, the development of alternative synthetic methods (low temperature reaction, solvothermal, etc.) may lead to the reduction of the costs inherent to the required high-temperature synthesis.
2. A thorough evaluation of the chemical processes occurring during thermal condensation by in situ techniques and operando methods would improve both mechanistic understanding and reproducibility. Specifically, spectroscopic means coupled to thermogravimetric analysis or electrochemical means, in situ heating electronic microscopy and other techniques could bring about a deeper understanding of the chemical, electronic and morphological transformations occurring during the thermal condensation and polymerization, which form CxNy(Hz) polymers.
3. The understanding of the photophysical properties, i.e., charge separation and transfer properties as well as energy band structure is crucial for the further integration of CN materials as practical absorbers and catalysts.
4. For photodriven reactions, the effective optical band gap should be reduced to about 2 eV to permit an effective utilization of the solar spectrum and achieve higher overall efficiencies.
5. We envision the emergence of CN materials in related research fields, such as solar cells, photoelectrochemical cells, imaging, biomedical and others, especially in conjugation with other organic and inorganic materials as hybrid nanostructures and composites, where control over the interface morphology and defects has room for further improvement without increasing complexity.
6. For practical applications, the scalability of the preparation protocols and the long-term stability under operational conditions will define the real-world feasibility of the material utilization in a given application.
All these efforts together with the substantial ongoing progress in the field will make CN materials competitive candidate semiconductors for many energy-related applications and other fields spanning photo- and electro-catalysis, organic transformation, sensors and more in the future.
Conflicts of interest
The authors declare no conflicts of interest.Abbreviations
| 2D | Two-dimensional |
| AQY | Average quantum yield |
| BET | Brunauer–Emmett–Teller model |
| CB | Conduction band |
| DFT | Density functional theory |
| e− | Electron (negative charge carrier) |
| E F | Fermi level (Fermi energy) |
| E g | Band gap (electronic) |
| EDS | Energy-dispersive X-ray spectroscopy |
| EELS | Electron energy loss spectroscopy |
| EPR | Electron paramagnetic resonance |
| FTIR | Fourier-transform infrared spectroscopy |
| h+ | Hole (positive charge carrier) |
| HC | Hybrid composite |
| HER | Hydrogen evolution reaction |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| MOF | Metal–organic framework |
| NMR | Nuclear magnetic resonance spectroscopy |
| NP | Nanoparticle |
| NRR | Nitrogen reduction reaction |
| OER | Oxygen evolution reaction |
| QD | Quantum dot |
| ROS | Reactive oxygen species |
| S A | Specific surface area |
| SC | Semiconductor |
| TEM | Transmission electron microscopy |
| TCO | Transparent conductive oxide (e.g., ITO, FTO, and AZO) |
| TOF | Turnover frequency |
| vs. | Versus |
| VB | Valance band |
| vdW | van der Waals |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
Chemicals, materials and solvents
| AAO | Anodic aluminum oxide |
| acac | Acetylacetonate |
| BA | Barbituric acid |
| BN | Boron nitride |
| BCN | Boron-doped carbon nitride |
| BPA | Bisphenol A |
| CY | Cyanamide |
| CA | Cyanuric acid |
| CN | Carbon nitride polymers |
| CN–H | Carbon nitride-based hydrogel |
| CNQDs | Carbon nitride quantum dots |
| CM | Cyanuric acid–melamine |
| CMB | Cyanuric acid–melamine–barbituric acid |
| COF | Covalent-organic framework |
| DCDA | Dicyandiamide |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| DPT | 2,4-Diamino-6-phenyl-1,3,5-triazine |
| EDA | Ethylenediamine |
| FTO | Fluorine-doped tin oxide |
| HRP | Horseradish peroxidase |
| IPA | Isopropyl alcohol (2-propanol) |
| MB | Methylene blue dye |
| MO | Methyl orange |
| NIPA | Poly(N-isopropylacrylamide) |
| NMP | N-Methylpyrrolidone |
| PAH | Polycyclic aromatic hydrocarbon |
| PCN | Phosphorous-doped carbon nitride |
| PCT | Phosphonitrilic chloride trimer |
| PHTIK | Potassium poly(heptazine imide) |
| PMDA | 1,2,4,5-Benzene tetracarboxylic dianhydride |
| PTI | Poly(triazine imide) |
| rGO | Reduced graphene oxide |
| RhB | Rhodamine B dye |
| SBA-15 | Mesoporous silica nanoparticles |
| SCN | Sulfur-doped carbon nitride |
| TEOA | Triethanolamine |
| THDT | 2,4,6-Trihydrazino-1,3,5-triazine |
| TMB | Tetramethylbenzidine |
Acknowledgements
We acknowledge the financial support of the Israel Science Foundation (ISF), grant no. 1161/17 and the Minerva Center No. 117873. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. [849068]).References
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- T. W. Ebbesen and P. M. Ajayan, Nature, 1992, 358, 220–222 CrossRef CAS.
- R. Taylor and D. R. M. Walton, Nature, 1993, 363, 685–693 CrossRef CAS.
- M. Antonietti, N. Fechler and T.-P. Fellinger, Chem. Mater., 2013, 26, 196–210 CrossRef.
- M. Oschatz and M. Antonietti, Energy Environ. Sci., 2018, 11, 57–70 RSC.
- C. Hu, Y. Lin, J. W. Connell, H.-M. Cheng, Y. Gogotsi, M.-M. Titirici and L. Dai, Adv. Mater., 2019, 31, 1806128 CrossRef PubMed.
- M.-M. Titirici, R. J. White, N. Brun, V. L. Budarin, D. S. Su, F. del Monte, J. H. Clark and M. J. MacLachlan, Chem. Soc. Rev., 2015, 44, 250–290 RSC.
- M. Antonietti, N. Lopez-Salas and A. Primo, Adv. Mater., 2019, 31, 1805719 CrossRef PubMed.
- Z. Xu, J. Park, G. Yoon, H. Kim and K. Kang, Small Methods, 2019, 3, 1800227 CrossRef CAS.
- K. Tang, L. Fu, R. J. White, L. Yu, M.-M. Titirici, M. Antonietti and J. Maier, Adv. Energy Mater., 2012, 2, 873–877 CrossRef CAS.
- G. A. Tiruye, D. Muñoz-Torrero, T. Berthold, J. Palma, M. Antonietti, N. Fechler and R. Marcilla, J. Mater. Chem. A, 2017, 5, 16263–16272 RSC.
- R. Daiyan, X. Tan, R. Chen, W. H. Saputera, H. A. Tahini, E. Lovell, Y. H. Ng, S. C. Smith, L. Dai, X. Lu and R. Amal, ACS Energy Lett., 2018, 3, 2292–2298 CrossRef CAS.
- A. B. Jorge, R. Jervis, A. P. Periasamy, M. Qiao, J. Feng, L. N. Tran and M.-M. Titirici, Adv. Energy Mater., 2020, 10, 1902494 CrossRef CAS.
- C. Tang, H.-F. Wang, X. Chen, B.-Q. Li, T.-Z. Hou, B. Zhang, Q. Zhang, M.-M. Titirici and F. Wei, Adv. Mater., 2016, 28, 6845–6851 CrossRef CAS PubMed.
- J. Kong, A. M. Cassell and H. Dai, Chem. Phys. Lett., 1998, 292, 567–574 CrossRef CAS.
- A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Nano Lett., 2008, 9, 30–35 CrossRef PubMed.
- L. Cai and G. Yu, Small Methods, 2019, 1900071 CrossRef.
- C. Lavorato, A. Primo, R. Molinari and H. García, ACS Catal., 2014, 4, 497–504 CrossRef CAS.
- J. Xu, J. Zhu, X. Yang, S. Cao, J. Yu, M. Shalom and M. Antonietti, Adv. Mater., 2016, 28, 6727–6733 CrossRef CAS PubMed.
- L. Zhao, N. Baccile, S. Gross, Y. Zhang, W. Wei, Y. Sun, M. Antonietti and M.-M. Titirici, Carbon, 2010, 48, 3778–3787 CrossRef CAS.
- B. Hu, K. Wang, L. Wu, S.-H. Yu, M. Antonietti and M.-M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS PubMed.
- M.-M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103–116 RSC.
- L. K. Gil-Herrera, Á. Blanco, B. H. Juárez and C. López, Small, 2016, 12, 4357–4362 CrossRef CAS PubMed.
- M. Sevilla and A. B. Fuertes, Carbon, 2009, 47, 2281–2289 CrossRef CAS.
- M. Sevilla and A. B. Fuertes, Chem.–Eur. J., 2009, 15, 4195–4203 CrossRef CAS PubMed.
- Y. Chen, X. Zhang and Z. Zhou, Small Methods, 2019, 3, 1900050 CrossRef.
- N. Fechler and M. Antonietti, Nano Today, 2015, 10, 593–614 CrossRef CAS.
- R. Yan, T. Heil, V. Presser, R. Walczak, M. Antonietti and M. Oschatz, Adv. Sustainable Syst., 2018, 2, 1700128 CrossRef.
- J. P. Paraknowitsch and A. Thomas, Energy Environ. Sci., 2013, 6, 2839–2855 RSC.
- M. Antonietti and M. Oschatz, Adv. Mater., 2018, 30, 1706836 CrossRef PubMed.
- L. K. Gil-Herrera, J. A. Pariente, F. Gallego-Gómez, F. Gándara, B. H. Juárez, Á. Blanco and C. López, Adv. Funct. Mater., 2018, 28, 1703885 CrossRef.
- H.-L. Jiang, B. Liu, Y.-Q. Lan, K. Kuratani, T. Akita, H. Shioyama, F. Zong and Q. Xu, J. Am. Chem. Soc., 2011, 133, 11854–11857 CrossRef CAS PubMed.
- W. Chen, M. Wan, Q. Liu, X. Xiong, F. Yu and Y. Huang, Small Methods, 2019, 3, 1800323 CrossRef CAS.
- H. Zhu, J. Yin, X. Wang, H. Wang and X. Yang, Adv. Funct. Mater., 2013, 23, 1305–1312 CrossRef CAS.
- L.-F. Chen, Z.-H. Huang, H.-W. Liang, H.-L. Gao and S.-H. Yu, Adv. Funct. Mater., 2014, 24, 5104–5111 CrossRef CAS.
- N. Fechler, T.-P. Fellinger and M. Antonietti, J. Mater. Chem. A, 2013, 1, 14097–14102 RSC.
- D. Kim, O. L. Li and N. Saito, Phys. Chem. Chem. Phys., 2015, 17, 407–413 RSC.
- Y.-Q. Zhao, M. Lu, P.-Y. Tao, Y.-J. Zhang, X.-T. Gong, Z. Yang, G.-Q. Zhang and H.-L. Li, J. Power Sources, 2016, 307, 391–400 CrossRef CAS.
- S.-A. Wohlgemuth, F. Vilela, M.-M. Titirici and M. Antonietti, Green Chem., 2012, 14, 741–749 RSC.
- S.-A. Wohlgemuth, R. J. White, M.-G. Willinger, M.-M. Titirici and M. Antonietti, Green Chem., 2012, 14, 1515–1523 RSC.
- L. Xia, X. Wu, Y. Wang, Z. Niu, Q. Liu, T. Li, X. Shi, A. M. Asiri and X. Sun, Small Methods, 2019, 3, 1800251 CrossRef.
- J. Zhang and L. Dai, ACS Catal., 2015, 5, 7244–7253 CrossRef CAS.
- L. Zhang, Y. Wang, Z. Niu and J. Chen, Small Methods, 2019, 1800443 CrossRef.
- X. Yu, P. Han, Z. Wei, L. Huang, Z. Gu, S. Peng, J. Ma and G. Zheng, Joule, 2018, 2, 1610–1622 CrossRef CAS.
- R. Lv, G. Chen, Q. Li, A. McCreary, A. Botello-Méndez, S. V. Morozov, L. Liang, X. Declerck, N. Perea-López, D. A. Cullen, S. Feng, A. L. Elías, R. Cruz-Silva, K. Fujisawa, M. Endo, F. Kang, J.-C. Charlier, V. Meunier, M. Pan, A. R. Harutyunyan, K. S. Novoselov and M. Terrones, Proc. Natl. Acad. Sci., 2015, 112, 14527–14532 CrossRef CAS PubMed.
- J. Yi, Y. Qing, C. Wu, Y. Zeng, Y. Wu, X. Lu and Y. Tong, J. Power Sources, 2017, 351, 130–137 CrossRef CAS.
- J. Wu, C. Jin, Z. Yang, J. Tian and R. Yang, Carbon, 2015, 82, 562–571 CrossRef CAS.
- M.-S. Balogun, W. Qiu, Y. Huang, H. Yang, R. Xu, W. Zhao, G.-R. Li, H. Ji and Y. Tong, Adv. Mater., 2017, 29, 1702095 CrossRef PubMed.
- X. Gong, S. Liu, C. Ouyang, P. Strasser and R. Yang, ACS Catal., 2015, 5, 920–927 CrossRef CAS.
- J. Patiño, N. López-Salas, M. C. Gutiérrez, D. Carriazo, M. L. Ferrer and F. del Monte, J. Mater. Chem. A, 2016, 4, 1251–1263 RSC.
- Y. Wen, B. Wang, C. Huang, L. Wang and D. Hulicova-Jurcakova, Chem.–Eur. J., 2015, 21, 80–85 CrossRef CAS PubMed.
- J. Tzadikov, M. Auinat, J. Barrio, M. Volokh, G. Peng, C. Gervais, Y. Ein-Eli and M. Shalom, ChemSusChem, 2018, 11, 2912–2920 CrossRef CAS PubMed.
- J. Tzadikov, M. Amsellem, H. Amlani, J. Barrio, A. Azoulay, M. Volokh, S. Kozuch and M. Shalom, Angew. Chem., Int. Ed., 2019, 58, 14964–14968 CrossRef CAS PubMed.
- A. Zahoor, M. Christy, Y. J. Hwang, Y. R. Lim, P. Kim and K. S. Nahm, Appl. Catal., B, 2014, 147, 633–641 CrossRef CAS.
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361–365 CrossRef CAS PubMed.
- K. Sakaushi and M. Antonietti, Acc. Chem. Res., 2015, 48, 1591–1600 CrossRef CAS PubMed.
- W. Wang and A. D. Schlüter, Macromol. Rapid Commun., 2019, 40, 1800719 CrossRef PubMed.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed.
- L. Merí-Bofí, S. Royuela, F. Zamora, M. L. Ruiz-González, J. L. Segura, R. Muñoz-Olivas and M. J. Mancheño, J. Mater. Chem. A, 2017, 5, 17973–17981 RSC.
- D. Rodríguez-San-Miguel and F. Zamora, Chem. Soc. Rev., 2019, 48, 4375–4386 RSC.
- Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed.
- V. S. Vyas, F. Haase, L. Stegbauer, G. Savasci, F. Podjaski, C. Ochsenfeld and B. V Lotsch, Nat. Commun., 2015, 6, 8508 CrossRef CAS PubMed.
- J. Romero, D. Rodriguez-San-Miguel, A. Ribera, R. Mas-Ballesté, T. F. Otero, I. Manet, F. Licio, G. Abellán, F. Zamora and E. Coronado, J. Mater. Chem. A, 2017, 5, 4343–4351 RSC.
- X. Liu, L. Zhou, Y. Zhao, L. Bian, X. Feng and Q. Pu, ACS Appl. Mater. Interfaces, 2013, 5, 10280–10287 CrossRef CAS PubMed.
- Z. Xiang, D. Cao, L. Huang, J. Shui, M. Wang and L. Dai, Adv. Mater., 2014, 26, 3315–3320 CrossRef CAS PubMed.
- K. Shen, X. Chen, J. Chen and Y. Li, ACS Catal., 2016, 6, 5887–5903 CrossRef CAS.
- R. Walczak, B. Kurpil, A. Savateev, T. Heil, J. Schmidt, Q. Qin, M. Antonietti and M. Oschatz, Angew. Chem., Int. Ed., 2018, 57, 10765–10770 CrossRef CAS PubMed.
- C. Van Nguyen, S. Lee, Y. G. Chung, W.-H. Chiang and K. C.-W. Wu, Appl. Catal., B, 2019, 257, 117888 CrossRef CAS.
- A. Aijaz, N. Fujiwara and Q. Xu, J. Am. Chem. Soc., 2014, 136, 6790–6793 CrossRef CAS PubMed.
- J. Hwang, R. Walczak, M. Oschatz, N. V Tarakina and B. V. K. J. Schmidt, Small, 2019, 15, 1901986 CrossRef PubMed.
- T. Jordan, M. Shalom, M. Antonietti and N. Fechler, Asia-Pacific J. Chem. Eng., 2016, 11, 866–873 CrossRef CAS.
- Z. Zhou, F. He, Y. Shen, X. Chen, Y. Yang, S. Liu, T. Mori and Y. Zhang, Chem. Commun., 2017, 53, 2044–2047 RSC.
- X. Feng, L. Bian, J. Ma, L. Zhou, X. Wang, G. Guo and Q. Pu, Chem. Commun., 2019, 55, 3963–3966 RSC.
- J. Kouvetakis, R. B. Kaner, M. L. Sattler and N. Bartlett, J. Chem. Soc., Chem. Commun., 1986, 1758–1759 RSC.
- J. Mahmood, E. K. Lee, M. Jung, D. Shin, I.-Y. Jeon, S.-M. Jung, H.-J. Choi, J.-M. Seo, S.-Y. Bae, S.-D. Sohn, N. Park, J. H. Oh, H.-J. Shin and J.-B. Baek, Nat. Commun., 2015, 6, 6486 CrossRef CAS PubMed.
- J. Mahmood, E. K. Lee, M. Jung, D. Shin, H.-J. Choi, J.-M. Seo, S.-M. Jung, D. Kim, F. Li, M. S. Lah, N. Park, H.-J. Shin, J. H. Oh and J.-B. Baek, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7414–7419 CrossRef CAS PubMed.
- M. R. A. Kishore and P. Ravindran, ChemPhysChem, 2017, 18, 1526–1532 CrossRef CAS PubMed.
- S. Yang, W. Li, C. Ye, G. Wang, H. Tian, C. Zhu, P. He, G. Ding, X. Xie, Y. Liu, Y. Lifshitz, S.-T. Lee, Z. Kang and M. Jiang, Adv. Mater., 2017, 29, 1605625 CrossRef PubMed.
- J. Xu, J. Mahmood, Y. Dou, S. Dou, F. Li, L. Dai and J.-B. Baek, Adv. Mater., 2017, 29, 1702007 CrossRef PubMed.
- F. Opoku, K. K. Govender, C. G. C. E. van Sittert and P. P. Govender, Adv. Sustainable Syst., 2017, 1, 1700006 CrossRef.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- Z. Ma, X. Zhao, Q. Tang and Z. Zhou, Int. J. Hydrogen Energy, 2014, 39, 5037–5042 CrossRef CAS.
- Q. Wang, H. Gou, L. Zhu, H.-T. Huang, A. Biswas, B. L. Chaloux, A. Epshteyn, J. P. Yesinowski, Z. Liu, G. Cody, M. Ma, Z. Zhao, Y. Fei, C. Prescher, E. Greenberg, V. B. Prakapenka and T. A. Strobel, ACS Mater. Lett., 2019, 1, 14–19 CrossRef CAS.
- J. Barrio, N. Karjule, J. Qin and M. Shalom, ChemCatChem, 2019, 11, 6295–6300 CrossRef CAS.
- J. Liebig, Justus Liebigs Ann. Chem., 1844, 50, 337–363 CrossRef.
- J. Liebig, Justus Liebigs Ann. Chem., 1855, 95, 257–282 CrossRef.
- H. Montigaud, B. Tanguy, G. Demazeau, I. Alves and S. Courjault, J. Mater. Sci., 2000, 35, 2547–2552 CrossRef CAS.
- E. Kroke and M. Schwarz, Coord. Chem. Rev., 2004, 248, 493–532 CrossRef CAS.
- A. Y. Liu and M. L. Cohen, Phys. Rev. B: Solid State, 1990, 41, 10727–10734 CrossRef CAS PubMed.
- A. Y. Liu and M. L. Cohen, Science, 1989, 245, 841–842 CrossRef CAS PubMed.
- C. Niu, Y. Z. Lu and C. M. Lieber, Science, 1993, 261, 334–337 CrossRef CAS PubMed.
- P. Kumar, E. Vahidzadeh, U. K. Thakur, P. Kar, K. M. Alam, A. Goswami, N. Mahdi, K. Cui, G. M. Bernard, V. K. Michaelis and K. Shankar, J. Am. Chem. Soc., 2019, 141, 5415–5436 CrossRef CAS PubMed.
- I. Y. Kim, S. Kim, S. Premkumar, J.-H. Yang, S. Umapathy and A. Vinu, Small, 2019, n/a, 1903572 Search PubMed.
- J. Zhang, Y. Zhao, Z. Wang, G. Yang, J. Tian, D. Ma and Y. Wang, New J. Chem., 2020, 44, 422–427 RSC.
- T. S. Miller, A. B. Jorge, T. M. Suter, A. Sella, F. Corà and P. F. McMillan, Phys. Chem. Chem. Phys., 2017, 19, 15613–15638 RSC.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- D. Ni, Y. Zhang, Y. Shen, S. Liu and Y. Zhang, Chin. Chem. Lett., 2020, 31, 115–118 CrossRef CAS.
- R. Schlo, A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J. Mu and J. M. Carlsson, J. Mater. Chem., 2008, 4893–4908 Search PubMed.
- B. Zhu, B. Cheng, L. Zhang and J. Yu, Carbon Energy, 2019, 1, 32–56 CrossRef.
- W. Chao, F. Huiqing, R. Xiaohu, M. Jiangwei, F. Jiawen and W. Weijia, ChemSusChem, 2018, 11, 700–708 CrossRef PubMed.
- X. Li, J. Zhang, L. Shen, Y. Ma, W. Lei, Q. Cui and G. Zou, Appl. Phys. A, 2009, 94, 387–392 CrossRef CAS.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2009, 25, 10397–10401 CrossRef CAS PubMed.
- W. Tu, Y. Xu, J. Wang, B. Zhang, T. Zhou, S. Yin, S. Wu, C. Li, Y. Huang, Y. Zhou, Z. Zou, J. Robertson, M. Kraft and R. Xu, ACS Sustainable Chem. Eng., 2017, 5, 7260–7268 CrossRef CAS.
- J. Yang, X. Wu, X. Li, Y. Liu, M. Gao, X. Liu, L. Kong and S. Yang, Appl. Phys. A, 2011, 105, 161 CrossRef CAS.
- H.-S. Zhai, L. Cao and X.-H. Xia, Chin. Chem. Lett., 2013, 24, 103–106 CrossRef CAS.
- J. Xu, Y. Li, S. Peng, G. Lu and S. Li, Phys. Chem. Chem. Phys., 2013, 15, 7657–7665 RSC.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC.
- J. Liu, T. Zhang, Z. Wang, G. Dawson and W. Chen, J. Mater. Chem., 2011, 21, 14398–14401 RSC.
- J. Bai, Q. Han, Z. Cheng and L. Qu, Chem.–Asian J., 2018, 13, 3160–3164 CrossRef CAS PubMed.
- G. Zhang, J. Zhang, M. Zhang and X. Wang, J. Mater. Chem., 2012, 22, 8083–8091 RSC.
- F. Dong, Y. Sun, L. Wu, M. Fu and Z. Wu, Catal. Sci. Technol., 2012, 2, 1332–1335 RSC.
- Y. Hong, E. Liu, J. Shi, X. Lin, L. Sheng, M. Zhang, L. Wang and J. Chen, Int. J. Hydrogen Energy, 2019, 44, 7194–7204 CrossRef CAS.
- D. Dontsova, S. Pronkin, M. Wehle, Z. Chen, C. Fettkenhauer, G. Clavel and M. Antonietti, Chem. Mater., 2015, 27, 5170–5179 CrossRef CAS.
- Y. Cui, G. Zhang, Z. Lin and X. Wang, Appl. Catal., B, 2016, 181, 413–419 CrossRef CAS.
- A. T. Montoya and E. G. Gillan, ACS Appl. Nano Mater., 2018, 1, 5944–5956 CrossRef CAS.
- A. Savateev, S. Pronkin, J. D. Epping, M. G. Willinger, M. Antonietti and D. Dontsova, J. Mater. Chem. A, 2017, 5, 8394–8401 RSC.
- J. Zhang, J. Sun, K. Maeda, K. Domen, P. Liu, M. Antonietti, X. Fu and X. Wang, Energy Environ. Sci., 2011, 4, 675 RSC.
- R. C. Dante, P. Martín-Ramos, F. M. Sánchez-Arévalo, L. Huerta, M. Bizarro, L. M. Navas-Gracia and J. Martín-Gil, J. Solid State Chem., 2013, 201, 153–163 CrossRef CAS.
- B. Long, J. Lin and X. Wang, J. Mater. Chem. A, 2014, 2, 2942–2951 RSC.
- Y. Cui, J. Zhang, G. Zhang, J. Huang, P. Liu, M. Antonietti and X. Wang, J. Mater. Chem., 2011, 21, 13032–13039 RSC.
- Z. Zhang, Y. Zhang, L. Lu, Y. Si, S. Zhang, Y. Chen, K. Dai, P. Duan, L. Duan and J. Liu, Appl. Surf. Sci., 2017, 391, 369–375 CrossRef CAS.
- H.-J. Li, D.-J. Qian and M. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 25162–25170 CrossRef CAS PubMed.
- Y. Zhang, Q. Pan, G. Chai, M. Liang, G. Dong, Q. Zhang and J. Qiu, Sci. Rep., 2013, 3, 1943 CrossRef PubMed.
- H. Zhang and A. Yu, J. Phys. Chem. C, 2014, 118, 11628–11635 CrossRef CAS.
- P. Wu, J. Wang, J. Zhao, L. Guo and F. E. Osterloh, J. Mater. Chem. A, 2014, 2, 20338–20344 RSC.
- H. Lan, L. Li, X. An, F. Liu, C. Chen, H. Liu and J. Qu, Appl. Catal., B, 2017, 204, 49–57 CrossRef CAS.
- G. Dong, Y. Zhang, Q. Pan and J. Qiu, J. Photochem. Photobiol., C, 2014, 20, 33–50 CrossRef CAS.
- Y. Zheng, J. Liu, J. J. Liang, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2012, 5, 6717–6731 RSC.
- J. Zhu, P. Xiao, H. Li and A. C. Carabineiro, ACS Appl. Mater. Interfaces, 2014, 6, 16449–16465 CrossRef CAS PubMed.
- C. Shaowen, L. Jingxiang, Y. Jiaguo and J. Mietek, Adv. Mater., 2015, 27, 2150–2176 CrossRef PubMed.
- J. Wen, J. Xie, X. Chen and X. Li, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS.
- K. S. Lakhi, D.-H. Park, K. Al-Bahily, W. Cha, B. Viswanathan, J.-H. Choy and A. Vinu, Chem. Soc. Rev., 2017, 46, 72–101 RSC.
- W. J. Ong, L. L. Tan, Y. H. Ng, S. T. Yong and S. P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS PubMed.
- S. Sun and S. Liang, Nanoscale, 2017, 9, 10544–10578 RSC.
- M. Volokh, G. Peng, J. Barrio and M. Shalom, Angew. Chem., Int. Ed., 2019, 58, 6138–6151 CrossRef CAS PubMed.
- J. Safaei, N. A. Mohamed, M. F. Mohamad Noh, M. F. Soh, N. A. Ludin, M. A. Ibrahim, W. N. Roslam Wan Isahak and M. A. Mat Teridi, J. Mater. Chem. A, 2018, 6, 22346–22380 RSC.
- S. Kumar, S. Karthikeyan and A. Lee, Catalysts, 2018, 8, 74 CrossRef.
- L. Wang, Y. Tong, J. Feng, J. Hou, J. Li, X. Hou and J. Liang, Sustainable Mater. Technol., 2019, 19, e00089 CrossRef CAS.
- F. K. Kessler, Y. Zheng, D. Schwarz, C. Merschjann, W. Schnick, X. Wang and M. J. Bojdys, Nat. Rev. Mater., 2017, 2, 17030 CrossRef CAS.
- Y. Yang, S. Wang, Y. Li, J. Wang and L. Wang, Chem.–Asian J., 2017, 12, 1421–1434 CrossRef CAS PubMed.
- A. Naseri, M. Samadi, A. Pourjavadi, A. Z. Moshfegh and S. Ramakrishna, J. Mater. Chem. A, 2017, 5, 23406–23433 RSC.
- J. Yi, W. El-Alami, Y. Song, H. Li, P. M. Ajayan and H. Xu, Chem. Eng. J., 2020, 382, 122812 CrossRef CAS.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed.
- C. Butchosa, P. Guiglion and M. A. Zwijnenburg, J. Phys. Chem. C, 2014, 118, 24833–24842 CrossRef CAS.
- Y. Zhu, D. Zhang, L. Gong, L. Zhang and Z. Xia, Front. Mater., 2019, 6, 1–10 CrossRef.
- G. Liao, Y. Gong, L. Zhang, H. Gao, G.-J. Yang and B. Fang, Energy Environ. Sci., 2019, 12, 2080–2147 RSC.
- S. Patnaik, S. Martha and K. M. Parida, RSC Adv., 2016, 6, 46929–46951 RSC.
- M. Z. Rahman, K. Davey and C. B. Mullins, Adv. Sci., 2018, 5, 1800820 CrossRef PubMed.
- M. S. Nasir, G. Yang, I. Ayub, S. Wang, L. Wang, X. Wang, W. Yan, S. Peng and S. Ramakarishna, Appl. Catal., B, 2019, 257, 117855 CrossRef CAS.
- Y. Wang, A. Vogel, M. Sachs, R. S. Sprick, L. Wilbraham, S. J. A. Moniz, R. Godin, M. A. Zwijnenburg, J. R. Durrant, A. I. Cooper and J. Tang, Nat. Energy, 2019, 4, 746–760 CrossRef CAS.
- J. Lin, Z. Pan and X. Wang, ACS Sustainable Chem. Eng., 2014, 2, 353–358 CrossRef CAS.
- Y. Fang and X. Wang, Chem. Commun., 2018, 54, 5674–5687 RSC.
- S. N. Talapaneni, G. Singh, I. Y. Kim, K. AlBahily, A. H. Al-Muhtaseb, A. S. Karakoti, E. Tavakkoli and A. Vinu, Adv. Mater., 2019, 0, 1904635 Search PubMed.
- V. Hasija, P. Raizada, A. Sudhaik, K. Sharma, A. Kumar, P. Singh, S. B. Jonnalagadda and V. K. Thakur, Appl. Mater. Today, 2019, 15, 494–524 CrossRef.
- R. Hu, X. Wang, S. Dai, D. Shao, T. Hayat and A. Alsaedi, Chem. Eng. J., 2015, 260, 469–477 CrossRef CAS.
- A. L. Stroyuk, A. E. Raevskaya and S. Y. Kuchmy, Theor. Exp. Chem., 2019, 55, 147–172 CrossRef CAS.
- J. Liu, H. Wang and M. Antonietti, Chem. Soc. Rev., 2016, 45, 2308–2326 RSC.
- Z. Zhou, Y. Zhang, Y. Shen, S. Liu and Y. Zhang, Chem. Soc. Rev., 2018, 47, 2298–2321 RSC.
- J. Xu, M. Antonietti and M. Shalom, Chem.–Asian J., 2016, 11, 2499–2512 CrossRef CAS PubMed.
- A. B. Jorge, D. J. Martin, M. T. S. Dhanoa, A. S. Rahman, N. Makwana, J. Tang, A. Sella, F. Corà, S. Firth, J. A. Darr and P. F. McMillan, J. Phys. Chem. C, 2013, 117, 7178–7185 CrossRef CAS.
- K. Maeda, X. Wang, Y. Nishihara, D. Lu, M. Antonietti and K. Domen, J. Phys. Chem. C, 2009, 113, 4940–4947 CrossRef CAS.
- G. Zhang, Z.-A. Lan and X. Wang, Chem. Sci., 2017, 8, 5261–5274 RSC.
- A. Savateev, I. Ghosh, B. König and M. Antonietti, Angew. Chem., Int. Ed., 2018, 57, 15936–15947 CrossRef CAS PubMed.
- A. Savateev and M. Antonietti, ACS Catal., 2018, 8, 9790–9808 CrossRef CAS.
- Y. Guo, Y. Wang, S. Zhao, Z. Liu, H. Chang and X. Zhao, Chem. Eng. J., 2019, 369, 553–562 CrossRef CAS.
- B. Kurpil, Y. Markushyna and A. Savateev, ACS Catal., 2019, 9, 1531–1538 CrossRef CAS.
- B. Kurpil, K. Otte, A. Mishchenko, P. Lamagni, W. Lipiński, N. Lock, M. Antonietti and A. Savateev, Nat. Commun., 2019, 10, 945 CrossRef PubMed.
- H. Liu, H. Li, J. Lu, S. Zeng, M. Wang, N. Luo, S. Xu and F. Wang, ACS Catal., 2018, 8, 4761–4771 CrossRef CAS.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- C. Zhou, Z. Zeng, G. Zeng, D. Huang, R. Xiao, M. Cheng, C. Zhang, W. Xiong, C. Lai, Y. Yang, W. Wang, H. Yi and B. Li, J. Hazard. Mater., 2019, 120815 CrossRef CAS PubMed.
- M. Ghaemmaghami and R. Mohammadi, Sustainable Energy Fuels, 2019, 3, 2176–2204 RSC.
- L. Chen and J. Song, Adv. Funct. Mater., 2017, 27, 1702695 CrossRef.
- J. Sun, R. Malishev, A. Azoulay, J. Tzadikov, M. Volokh, R. Jelinek and M. Shalom, Small, 2018, 14, 1800516 CrossRef PubMed.
- J. Xu and M. Shalom, ChemPhotoChem, 2019, 3, 170–179 CrossRef CAS.
- A.-J. Wang, H. Li, H. Huang, Z.-S. Qian and J.-J. Feng, J. Mater. Chem. C, 2016, 4, 8146–8160 RSC.
- Y. Dong, Q. Wang, H. Wu, Y. Chen, C.-H. Lu, Y. Chi and H.-H. Yang, Small, 2016, 12, 5376–5393 CrossRef CAS PubMed.
- M. M. Xavier, P. R. Nair and S. Mathew, Analyst, 2019, 144, 1475–1491 RSC.
- Y. Wang, R. Zhang, Z. Zhang, J. Cao and T. Ma, Adv. Mater. Interfaces, 2019, 1–32 Search PubMed.
- H. Wang, Y. Bu, G. Wu and X. Zou, Dalton Trans., 2019, 48, 11724–11731 RSC.
- Y. Shiraishi, S. Shiota, Y. Kofuji, M. Hashimoto, K. Chishiro, H. Hirakawa, S. Tanaka, S. Ichikawa and T. Hirai, ACS Appl. Energy Mater., 2018, 1, 4169–4177 CrossRef CAS.
- X. Chen, X. Zhao, Z. Kong, W.-J. Ong and N. Li, J. Mater. Chem. A, 2018, 6, 21941–21948 RSC.
- S. Cao, H. Chen, F. Jiang and X. Wang, Appl. Catal., B, 2018, 224, 222–229 CrossRef CAS.
- X. Chen, N. Li, Z. Kong, W.-J. Ong and X. Zhao, Mater. Horiz., 2018, 5, 9–27 RSC.
- Y. Ren, D. Zeng and W.-J. Ong, Chin. J. Catal., 2019, 40, 289–319 CrossRef CAS.
- G. Dong, W. Ho and C. Wang, J. Mater. Chem. A, 2015, 3, 23435–23441 RSC.
- Z. Wang, X. Hu, Z. Liu, G. Zou, G. Wang and K. Zhang, ACS Catal., 2019, 9, 10260–10278 CrossRef CAS.
- Q. Cao, Q. Cui, Y. Yang, J. Xu, C. Han and L. Li, Chem.–Eur. J., 2018, 24, 2286–2291 CrossRef CAS PubMed.
- C. Han, P. Meng, E. R. Waclawik, C. Zhang, X.-H. Li, H. Yang, M. Antonietti and J. Xu, Angew. Chem., Int. Ed., 2018, 57, 14857–14861 CrossRef CAS PubMed.
- C. Han, Q. Cui, P. Meng, E. R. Waclawik, H. Yang and J. Xu, Langmuir, 2018, 34, 10135–10143 CrossRef CAS PubMed.
- G. Moon, M. Fujitsuka, S. Kim, T. Majima, X. Wang and W. Choi, ACS Catal., 2017, 7, 2886–2895 CrossRef CAS.
- K. Villa, C. L. Manzanares Palenzuela, Z. Sofer, S. Matějková and M. Pumera, ACS Nano, 2018, 12, 12482–12491 CrossRef CAS PubMed.
- J. L. Segura, M. J. Mancheño and F. Zamora, Chem. Soc. Rev., 2016, 45, 5635–5671 RSC.
- J. Zhang, X. Chen, K. Takanabe, K. Maeda, K. Domen, J. D. Epping, X. Fu, M. Antonietti and X. Wang, Angew. Chem., Int. Ed., 2010, 49, 441–444 CrossRef CAS PubMed.
- G. Li, Q. Wang, Y. Wu, Y. Li and L. Guo, Spectrochim. Acta, Part A, 2019, 215, 307–312 CrossRef CAS PubMed.
- L. Li, Q. Meng, H. Lv, L. Shui, Y. Zhang, Z. Zhang, Z. Chen, M. Yuan, R. Nötzel, X. Wang, J.-M. Liu and G. Zhou, Appl. Surf. Sci., 2018, 428, 739–747 CrossRef CAS.
- L. Chen, Z. Song, X. Liu, L. Guo, M. Li and F. Fu, Analyst, 2018, 143, 1609–1614 RSC.
- Z. Jinshui, Z. Guigang, C. Xiufang, L. Sen, M. Lennart, D. Grzegorz, L. Grzegorz, A. Markus, B. Siegfried and W. Xinchen, Angew. Chem., 2012, 124, 3237–3241 CrossRef.
- J. Zhang, M. Zhang, S. Lin, X. Fu and X. Wang, J. Catal., 2014, 310, 24–30 CrossRef CAS.
- J. Xu, Y. Wang and Y. Zhu, Langmuir, 2013, 29, 10566–10572 CrossRef CAS PubMed.
- Y. Huang, D. Li, Z. Fang, R. Chen, B. Luo and W. Shi, Appl. Catal., B, 2019, 254, 128–134 CrossRef CAS.
- Z. Chen, P. Sun, B. Fan, Q. Liu, Z. Zhang and X. Fang, Appl. Catal., B, 2015, 170–171, 10–16 CrossRef CAS.
- J. Zhou, Y. Yang and C. Zhang, Chem. Commun., 2013, 49, 8605–8607 RSC.
- Z. Song, Z. Li, L. Lin, Y. Zhang, T. Lin, L. Chen, Z. Cai, S. Lin, L. Guo, F. Fu and X. Wang, Nanoscale, 2017, 9, 17737–17742 RSC.
- X. L. Wang and H. G. Yang, Appl. Catal., B, 2017, 205, 624–630 CrossRef CAS.
- G. Zhang and X. Wang, J. Catal., 2013, 307, 246–253 CrossRef CAS.
- K. Li and W.-D. Zhang, Small, 2018, 14, 1703599 CrossRef PubMed.
- A. Hayat, F. Raziq, M. Khan, J. Khan, S. K. B. Mane, A. Ahmad, M. U. Rahman and W. U. Khan, J. Colloid Interface Sci., 2019, 554, 627–639 CrossRef CAS PubMed.
- K. Li, M. Sun and W.-D. Zhang, Carbon, 2018, 134, 134–144 CrossRef CAS.
- J. Qin, S. Wang, H. Ren, Y. Hou and X. Wang, Appl. Catal., B, 2015, 179, 1–8 CrossRef CAS.
- W. Yan, Y. Yu, H. Zou, X. Wang, P. Li, W. Gao, J. Wang, S. Wu and K. Ding, Sol. RRL, 2018, 2, 1800058 CrossRef.
- G. Zhang, A. Savateev, Y. Zhao, L. Li and M. Antonietti, J. Mater. Chem. A, 2017, 5, 12723–12728 RSC.
- H. Tang, R. Wang, C. Zhao, Z. Chen, X. Yang, D. Bukhvalov, Z. Lin and Q. Liu, Chem. Eng. J., 2019, 374, 1064–1075 CrossRef CAS.
- Y. Li, J. Zhang, Q. Wang, Y. Jin, D. Huang, Q. Cui and G. Zou, J. Phys. Chem. B, 2010, 114, 9429–9434 CrossRef CAS PubMed.
- L. Luo, M. Zhang, P. Wang, Y. Wang and F. Wang, New J. Chem., 2018, 42, 1087–1091 RSC.
- W. Ho, Z. Zhang, W. Lin, S. Huang, X. Zhang, X. Wang and Y. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 5497–5505 CrossRef CAS PubMed.
- M. Ismael, Y. Wu, D. H. Taffa, P. Bottke and M. Wark, New J. Chem., 2019, 43, 6909–6920 RSC.
- G. Li, J. Shi, G. Zhang, Y. Fang, M. Anpo and X. Wang, Res. Chem. Intermed., 2017, 43, 5137–5152 CrossRef CAS.
- D. Vidyasagar, N. Manwar, A. Gupta, S. G. Ghugal, S. S. Umare and R. Boukherroub, Catal. Sci. Technol., 2019, 9, 822–832 RSC.
- L. Zhou, H. Zhang, H. Sun, S. Liu, M. O. Tade, S. Wang and W. Jin, Catal. Sci. Technol., 2016, 6, 7002–7023 RSC.
- L. Jiang, X. Yuan, Y. Pan, J. Liang, G. Zeng, Z. Wu and H. Wang, Appl. Catal., B, 2017, 217, 388–406 CrossRef CAS.
- B. X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Adv. Mater., 2009, 1609–1612 CrossRef.
- Z. Ding, X. Chen, M. Antonietti and X. Wang, ChemSusChem, 2011, 4, 274–281 CAS.
- Z. Shouwei, L. Jiaxing, Z. Meiyi, L. Jie, X. Jinzhang and W. Xiangke, Chem.–Eur. J., 2014, 20, 9805–9812 CrossRef PubMed.
- L.-F. Gao, T. Wen, J.-Y. Xu, X.-P. Zhai, M. Zhao, G.-W. Hu, P. Chen, Q. Wang and H.-L. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 617–624 CrossRef CAS PubMed.
- S. Hu, X. Chen, Q. Li, F. Li, Z. Fan, H. Wang, Y. Wang, B. Zheng and G. Wu, Appl. Catal., B, 2017, 201, 58–69 CrossRef CAS.
- Y. Yang, M. Guo, G. Zhang and W. Li, Carbon, 2017, 117, 120–125 CrossRef CAS.
- W. Wu, Z. Ruan, J. Li, Y. Li, Y. Jiang, X. Xu, D. Li, Y. Yuan and K. Lin, Nano-Micro Lett., 2019, 11, 10 CrossRef CAS.
- D. Das, D. Banerjee, M. Mondal, A. Shett, B. Das, N. S. Das, U. K. Ghorai and K. K. Chattopadhyay, Mater. Res. Bull., 2018, 101, 291–304 CrossRef CAS.
- G. Zhang, C. Huang and X. Wang, Small, 2015, 11, 1215–1221 CrossRef CAS PubMed.
- J. Mu, J. Li, X. Zhao, E.-C. Yang and X.-J. Zhao, RSC Adv., 2016, 6, 35568–35576 RSC.
- P.-W. Chen, K. Li, Y.-X. Yu and W.-D. Zhang, Appl. Surf. Sci., 2017, 392, 608–615 CrossRef CAS.
- Y. Fu, W. Zhan, Y. Guo, Y. Guo, Y. Wang and G. Lu, Green Energy Environ., 2017, 2, 142–150 CrossRef.
- W. Gong, J. Zou, S. Zhang, X. Zhou and J. Jiang, Electroanalysis, 2016, 28, 227–234 CrossRef CAS.
- X. Zou, R. Silva, A. Goswami and T. Asefa, Appl. Surf. Sci., 2015, 357, 221–228 CrossRef CAS.
- J.-C. Wang, C.-X. Cui, Y. Li, L. Liu, Y.-P. Zhang and W. Shi, J. Hazard. Mater., 2017, 339, 43–53 CrossRef CAS PubMed.
- Z. Li, C. Kong and G. Lu, J. Phys. Chem. C, 2015, 120, 56–63 CrossRef.
- J.-C. Wang, C.-X. Cui, Q.-Q. Kong, C.-Y. Ren, Z. Li, L. Qu, Y. Zhang and K. Jiang, ACS Sustainable Chem. Eng., 2018, 6, 8754–8761 CrossRef CAS.
- G. Ding, W. Wang, T. Jiang, B. Han, H. Fan and G. Yang, ChemCatChem, 2013, 5, 192–200 CrossRef CAS.
- Y. Dai, Y. Gu and Y. Bu, Appl. Surf. Sci., 2020, 500, 144036 CrossRef CAS.
- K. Eid, Y. Ahmad, A. Mohamed, A. Elsafy and S. Al-Qaradawi, Catalysts, 2018, 8, 411 CrossRef.
- K. Eid, M. H. Sliem, K. Jlassi, A. S. Eldesoky, G. G. Abdo, S. Y. Al-Qaradawi, M. A. Sharaf, A. M. Abdullah and A. A. Elzatahry, Inorg. Chem. Commun., 2019, 107460 CrossRef CAS.
- K. Eid, M. H. Sliem, A. S. Eldesoky, H. Al-Kandari and A. M. Abdullah, Int. J. Hydrogen Energy, 2019, 44, 17943–17953 CrossRef CAS.
- K. Eid, M. H. Sliem, H. Al-Kandari, M. A. Sharaf and A. M. Abdullah, Langmuir, 2019, 35, 3421–3431 CrossRef CAS PubMed.
- K. Eid, M. H. Sliem and A. M. Abdullah, Nanoscale, 2019, 11, 11755–11764 RSC.
- C. Hun Choi, L. Lin, S. Gim, S. Lee, H. Kim, X. Wang and W. Choi, ACS Catal., 2018, 8, 4241–4256 CrossRef.
- Z. Chen, S. Pronkin, T.-P. Fellinger, K. Kailasam, G. Vilé, D. Albani, F. Krumeich, R. Leary, J. Barnard, J. M. Thomas, J. Pérez-Ramírez, M. Antonietti and D. Dontsova, ACS Nano, 2016, 10, 3166–3175 CrossRef CAS PubMed.
- D. Xu, X. Li, J. Liu and L. Huang, J. Rare Earths, 2013, 31, 1085–1091 CrossRef CAS.
- Y. Wang, Y. Li, X. Bai, Q. Cai, C. Liu, Y. Zuo, S. Kang and L. Cui, Catal. Commun., 2016, 84, 179–182 CrossRef CAS.
- R. Jin, S. Hu, J. Gui and D. Liu, Bull. Korean Chem. Soc., 2015, 36, 17–23 CrossRef CAS.
- J. Xu, T. J. K. Brenner, Z. Chen, D. Neher, M. Antonietti and M. Shalom, ACS Appl. Mater. Interfaces, 2014, 6, 16481–16486 CrossRef CAS PubMed.
- D. Salinas, P. Araya and S. Guerrero, Appl. Catal., B, 2012, 117–118, 260–267 CrossRef CAS.
- H. Chu, L. Yang, Q. Zhang and Y. Wang, J. Catal., 2006, 241, 225–228 CrossRef CAS.
- G. Pekridis, N. Kaklidis, M. Konsolakis, C. Athanasiou, I. V. Yentekakis and G. E. Marnellos, Solid State Ionics, 2011, 192, 653–658 CrossRef CAS.
- Y. Chen, J. He, H. Tian, D. Wang and Q. Yang, J. Colloid Interface Sci., 2014, 428, 1–7 CrossRef CAS PubMed.
- J. Jiang, G. Lu, C. Miao, X. Wu, W. Wu and Q. Sun, Microporous Mesoporous Mater., 2013, 167, 213–220 CrossRef CAS.
- Y. Markushyna, C. A. Smith and A. Savateev, Eur. J. Org. Chem., 2020, 2020, 1294–1309 CrossRef CAS.
- J. Zhang, S. Hu and Y. Wang, RSC Adv., 2014, 4, 62912–62919 RSC.
- S. Hu, F. Li, Z. Fan, F. Wang, Y. Zhao and Z. Lv, Dalton Trans., 2015, 44, 1084–1092 RSC.
- N. Hou, W.-M. Sun, F.-Y. Du and H.-S. Wu, Optik, 2019, 183, 455–462 CrossRef CAS.
- Y. Zhang, S. Zong, C. Cheng, J. Shi, X. Guan, Y. Lu and L. Guo, Int. J. Hydrogen Energy, 2018, 43, 13953–13961 CrossRef CAS.
- Y. Shang, Y. Ma, X. Chen, X. Xiong and J. Pan, Mol. Catal., 2017, 433, 128–135 CrossRef CAS.
- M. Zhang, X. Bai, D. Liu, J. Wang and Y. Zhu, Appl. Catal., B, 2015, 164, 77–81 CrossRef CAS.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Mater., 2017, 29, 1605148 CrossRef PubMed.
- L. Zhang, N. Ding, M. Hashimoto, K. Iwasaki, N. Chikamori, K. Nakata, Y. Xu, J. Shi, H. Wu, Y. Luo, D. Li, A. Fujishima and Q. Meng, Nano Res., 2018, 11, 2295–2309 CrossRef CAS.
- N. Zhou, P. Qiu, H. Chen and F. Jiang, J. Taiwan Inst. Chem. Eng., 2018, 83, 99–106 CrossRef CAS.
- H. Tan, X. Gu, P. Kong, Z. Lian, B. Li and Z. Zheng, Appl. Catal., B, 2019, 242, 67–75 CrossRef CAS.
- H. Tan, P. Kong, M. Liu, X. Gu and Z. Zheng, Chem. Commun., 2019, 55, 12503–12506 RSC.
- W. Wang, H. Zhang, S. Zhang, Y. Liu, G. Wang, C. Sun and H. Zhao, Angew. Chem., Int. Ed., 2019, 58, 16644–16650 CrossRef CAS PubMed.
- L. Ruan, G. Xu, L. Gu, C. Li, Y. Zhu and Y. Lu, Mater. Res. Bull., 2015, 66, 156–162 CrossRef CAS.
- Q. Deng and Q. Li, J. Mater. Sci., 2018, 53, 506–515 CrossRef CAS.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- Y. Wang, Y. Di, M. Antonietti, H. Li, X. Chen and X. Wang, Chem. Mater., 2010, 22, 5119–5121 CrossRef CAS.
- Y. Wang, J. Zhang, X. Wang, M. Antonietti and H. Li, Angew. Chem., Int. Ed., 2010, 49, 3356–3359 CrossRef CAS PubMed.
- M. Jalaly, F. José Gotor, M. Semnan and M. Jesús Sayagués, Sci. Rep., 2017, 7, 3453 CrossRef PubMed.
- S. Thaweesak, S. Wang, M. Lyu, M. Xiao, P. Peerakiatkhajohn and L. Wang, Dalton Trans., 2017, 46, 10714–10720 RSC.
- Y. Wang, H. Li, J. Yao, X. Wang and M. Antonietti, Chem. Sci., 2011, 2, 446–450 RSC.
- Y. Luo, J. Wang, S. Yu, Y. Cao, K. Ma, Y. Pu, W. Zou, C. Tang, F. Gao and L. Dong, J. Mater. Res., 2018, 33, 1268–1278 CrossRef CAS.
- Z. Lin and X. Wang, Angew. Chem., Int. Ed., 2013, 52, 1735–1738 CrossRef CAS PubMed.
- J. Zou, Y. Yu, W. Yan, J. Meng, S. Zhang and J. Wang, J. Mater. Sci., 2019, 54, 6867–6881 CrossRef CAS.
- C. Lu, R. Chen, X. Wu, M. Fan, Y. Liu, Z. Le, S. Jiang and S. Song, Appl. Surf. Sci., 2016, 360, 1016–1022 CrossRef CAS.
- J. Zhu, T. Diao, W. Wang, X. Xu, X. Sun, S. A. C. Carabineiro and Z. Zhao, Appl. Catal., B, 2017, 219, 92–100 CrossRef CAS.
- J. Hong, X. Xia, Y. Wang and R. Xu, J. Mater. Chem., 2012, 22, 15006–15012 RSC.
- J. Chen, Z. Hong, Y. Chen, B. Lin and B. Gao, Mater. Lett., 2015, 145, 129–132 CrossRef CAS.
- K.-Y. A. Lin, Z.-Y. Zhang and T. Wi-Afedzi, J. Water Process Eng., 2018, 24, 83–89 CrossRef.
- C. Rajkumar, P. Veerakumar, S.-M. Chen, B. Thirumalraj and K.-C. Lin, ACS Sustainable Chem. Eng., 2018, 6, 16021–16031 CrossRef CAS.
- K.-Y. A. Lin and Z.-Y. Zhang, Chem. Eng. J., 2017, 313, 1320–1327 CrossRef CAS.
- J. Jin, H. Wu, S. Wang, Y. Ding and S. Yin, Int. J. Hydrogen Energy, 2017, 42, 20579–20588 CrossRef CAS.
- K. Wang, Q. Li, B. Liu, B. Cheng, W. Ho and J. Yu, Appl. Catal., B, 2015, 176–177, 44–52 CAS.
- X. Dai, Z. Han, H. Fan and S. Ai, J. Nanopart. Res., 2018, 20, 315 CrossRef.
- R. Zhu, Y. Zhang, X. Fang, X. Cui, J. Wang, C. Yue, W. Fang, H. Zhao and Z. Li, J. Mater. Chem. B, 2019, 7, 2320–2329 RSC.
- F. Qiao, J. Wang, S. Ai and L. Li, Sens. Actuators, B, 2015, 216, 418–427 CrossRef CAS.
- A. Kumar, R. K. Yadav, N.-J. Park and J.-O. Baeg, ACS Appl. Nano Mater., 2017, 1, 47–54 CrossRef.
- X. Ma, Y. Lv, J. Xu, Y. Liu, R. Zhang and Y. Zhu, J. Phys. Chem. C, 2012, 116, 23485–23493 CrossRef CAS.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- L. Zhang, X. Chen, J. Guan, Y. Jiang, T. Hou and X. Mu, Mater. Res. Bull., 2013, 48, 3485–3491 CrossRef CAS.
- S. Hu, L. Ma, J. You, F. Li, Z. Fan, F. Wang, D. Liu and J. Gui, RSC Adv., 2014, 4, 21657–21663 RSC.
- S. Hu, L. Ma, J. You, F. Li, Z. Fan, G. Lu, D. Liu and J. Gui, Appl. Surf. Sci., 2014, 311, 164–171 CrossRef CAS.
- B. Chai, J. Yan, C. Wang, Z. Ren and Y. Zhu, Appl. Surf. Sci., 2017, 391, 376–383 CrossRef CAS.
- J. Ran, T. Y. Ma, G. Gao, X.-W. Du and S. Z. Qiao, Energy Environ. Sci., 2015, 8, 3708–3717 RSC.
- Y.-P. Zhu, T.-Z. Ren and Z.-Y. Yuan, ACS Appl. Mater. Interfaces, 2015, 7, 16850–16856 CrossRef CAS PubMed.
- Y. Zhou, L. Zhang, J. Liu, X. Fan, B. Wang, M. Wang, W. Ren, J. Wang, M. Li and J. Shi, J. Mater. Chem. A, 2015, 3, 3862–3867 RSC.
- W. Zhang, J. Zhang, F. Dong and Y. Zhang, RSC Adv., 2016, 6, 88085–88089 RSC.
- W. Zhang, J. Barrio, C. Gervais, A. Kocjan, A. Yu, X. Wang and M. Shalom, Angew. Chem., Int. Ed., 2018, 57, 9764–9769 CrossRef CAS PubMed.
- Z. Liang, Y. Xia, G. Ba, H. Li, Q. Deng and W. Hou, Catal. Sci. Technol., 2019, 9, 6627–6637 RSC.
- J. Cui, S. Liang, X. Wang and J. Zhang, Mater. Chem. Phys., 2015, 161, 194–200 CrossRef CAS.
- P. Qiu, C. Xu, H. Chen, F. Jiang, X. Wang, R. Lu and X. Zhang, Appl. Catal., B, 2017, 206, 319–327 CrossRef CAS.
- Y.-C. Lu, J. Chen, A.-J. Wang, N. Bao, J.-J. Feng, W. Wang and L. Shao, J. Mater. Chem. C, 2015, 3, 73–78 RSC.
- L. Jiang, X. Yuan, G. Zeng, X. Chen, Z. Wu, J. Liang, J. Zhang, H. Wang and H. Wang, ACS Sustainable Chem. Eng., 2017, 5, 5831–5841 CrossRef CAS.
- X. Han, A. Yuan, C. Yao, F. Xi, J. Liu and X. Dong, J. Mater. Sci., 2019, 54, 1593–1605 CrossRef CAS.
- Q. Liu, J. Shen, X. Yu, X. Yang, W. Liu, J. Yang, H. Tang, H. Xu, H. Li, Y. Li and J. Xu, Appl. Catal., B, 2019, 248, 84–94 CrossRef CAS.
- B. Zhu, J. Zhang, C. Jiang, B. Cheng and J. Yu, Appl. Catal., B, 2017, 207, 27–34 CrossRef CAS.
- Z. Guigang, Z. Mingwen, Y. Xinxin, Q. Xiaoqing, L. Sen and W. Xinchen, Adv. Mater., 2013, 26, 805–809 Search PubMed.
- Y. Guo, T. Chen, Q. Liu, Z. Zhang and X. Fang, J. Phys. Chem. C, 2016, 120, 25328–25337 CrossRef CAS.
- Z. A. Lan, G. Zhang and X. Wang, Appl. Catal., B, 2016, 192, 116–125 CrossRef CAS.
- Y. Zhu, L. Gong, D. Zhang, X. Wang, J. Zhang, L. Zhang, L. Dai and Z. Xia, Nano Energy, 2019, 63, 103819 CrossRef CAS.
- G. Liu, P. Niu, C. Sun, S. C. Smith, Z. Chen, G. Qing (Max) Lu and H.-M. Cheng, J. Am. Chem. Soc., 2010, 132, 11642–11648 CrossRef CAS PubMed.
- H. Ou, C. Tang, Y. Zhang, A. M. Asiri, M.-M. Titirici and X. Wang, J. Catal., 2019, 375, 104–112 CrossRef CAS.
- J. Cao, J. Zhang, X. Dong, H. Fu, X. Zhang, X. Lv, Y. Li and G. Jiang, Appl. Catal., B, 2019, 249, 266–274 CrossRef CAS.
- B. Wang, H. Cai, D. Zhao, M. Song, P. Guo, S. Shen, D. Li and S. Yang, Appl. Catal., B, 2019, 244, 486–493 CrossRef CAS.
- A. Tripathi and S. Narayanan, Appl. Surf. Sci., 2019, 479, 1–11 CrossRef CAS.
- W. Xu, X. An, Q. Zhang, Z. Li, Q. Zhang, Z. Yao, X. Wang, S. Wang, J. Zheng, J. Zhang, W. Wu and M. Wu, ACS Sustainable Chem. Eng., 2019, 7, 12351–12357 CAS.
- E. Ju, K. Dong, Z. Chen, Z. Liu, C. Liu, Y. Huang, Z. Wang, F. Pu, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2016, 55, 11467–11471 CrossRef CAS PubMed.
- M. Vázquez-González, W.-C. Liao, R. Cazelles, S. Wang, X. Yu, V. Gutkin and I. Willner, ACS Nano, 2017, 11, 3247–3253 CrossRef PubMed.
- P. Sharma and Y. Sasson, Green Chem., 2017, 19, 844–852 RSC.
- Y. Wang, X. Zhou, W. Xu, Y. Sun, T. Wang, Y. Zhang, J. Dong, W. Hou, N. Wu, L. Wu, B. Zhou, Y. Wu, Y. Du and W. Zhong, Appl. Catal., A, 2019, 582, 117118 CrossRef CAS.
- H. Zhang, Y. Huang, X. Lin, F. Lu, Z. Zhang and Z. Hu, Sens. Actuators, B, 2018, 255, 2218–2222 CrossRef CAS.
- Q. Han, C. Hu, F. Zhao, Z. Zhang, N. Chen and L. Qu, J. Mater. Chem. A, 2015, 3, 4612–4619 RSC.
- Z. Yang, Y. Zhang and Z. Schnepp, J. Mater. Chem. A, 2015, 3, 14081–14092 RSC.
- A. Thomas, F. Goettmann and M. Antonietti, Chem. Mater., 2008, 20, 738–755 CrossRef CAS.
- M. Groenewolt and M. Antonietti, Adv. Mater., 2005, 17, 1789–1792 CrossRef CAS.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467–4471 CrossRef CAS PubMed.
- M. Kruk, M. Jaroniec, C. Hyun Ko and R. Ryoo, Chem. Mater., 2000, 12, 1961–1968 CrossRef CAS.
- A. Vinu, Adv. Funct. Mater., 2008, 18, 816–827 CrossRef CAS.
- S.-W. Bian, Z. Ma and W.-G. Song, J. Phys. Chem. C, 2009, 113, 8668–8672 CrossRef CAS.
- X. Jin, V. V. Balasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. Q. Lu and A. Vinu, Angew. Chem., Int. Ed., 2009, 48, 7884–7887 CrossRef CAS PubMed.
- X.-H. Li, J. Zhang, X. Chen, A. Fischer, A. Thomas, M. Antonietti and X. Wang, Chem. Mater., 2011, 23, 4344–4348 CrossRef CAS.
- J. Liu, J. Huang, H. Zhou and M. Antonietti, ACS Appl. Mater. Interfaces, 2014, 6, 8434–8440 CrossRef CAS PubMed.
- J. Sun, J. Zhang, M. Zhang, M. Antonietti, X. Fu and X. Wang, Nat. Commun., 2012, 3, 1139 CrossRef.
- Y. Si, Z. Sun, L. Huang, M. Chen and L. Wu, J. Mater. Chem. A, 2019, 7, 8952–8959 RSC.
- M. Shalom, S. Inal, D. Neher and M. Antonietti, Catal. Today, 2014, 225, 185–190 CrossRef CAS.
- T. Fu, M. Wang, W. Cai, Y. Cui, F. Gao, L. Peng, W. Chen and W. Ding, ACS Catal., 2014, 4, 2536–2543 CrossRef CAS.
- D. Chen, J. Yang and H. Ding, Appl. Surf. Sci., 2017, 391, 384–391 CrossRef CAS.
- L. Shi, L. Liang, F. Wang, M. Liu, K. Chen, K. Sun, N. Zhang and J. Sun, ACS Sustainable Chem. Eng., 2015, 3, 3412–3419 CrossRef CAS.
- J. Zhang, M. Zhang, C. Yang and X. Wang, Adv. Mater., 2014, 26, 4121–4126 CrossRef CAS PubMed.
- M. Hu, J. Reboul, S. Furukawa, L. Radhakrishnan, Y. Zhang, P. Srinivasu, H. Iwai, H. Wang, Y. Nemoto, N. Suzuki, S. Kitagawa and Y. Yamauchi, Chem. Commun., 2011, 47, 8124–8126 RSC.
- J. Liu, J. Yan, H. Ji, Y. Xu, L. Huang, Y. Li, Y. Song, Q. Zhang, H. Xu and H. Li, Mater. Sci. Semicond. Process., 2016, 46, 59–68 CrossRef CAS.
- H.-M. Zhao, C.-M. Di, L. Wang, Y. Chun and Q.-H. Xu, Microporous Mesoporous Mater., 2015, 208, 98–104 CrossRef CAS.
- J. Zhang, F. Guo and X. Wang, Adv. Funct. Mater., 2013, 23, 3008–3014 CrossRef CAS.
- Y. Ling, G. Liao, W. Feng, Y. Liu and L. Li, J. Photochem. Photobiol., A, 2017, 349, 108–114 CrossRef CAS.
- M. Jourshabani, Z. Shariatinia and A. Badiei, Langmuir, 2017, 33, 7062–7078 CrossRef CAS PubMed.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- S. Hu, X. Qu, P. Li, F. Wang, Q. Li, L. Song, Y. Zhao and X. Kang, Chem. Eng. J., 2018, 334, 410–418 CrossRef CAS.
- S. Le, T. Jiang, Q. Zhao, X. Liu, Y. Li, B. Fang and M. Gong, RSC Adv., 2016, 6, 38811–38819 RSC.
- Y. Guo, Q. Liu, Z. Li, Z. Zhang and X. Fang, Appl. Catal., B, 2018, 221, 362–370 CrossRef CAS.
- K. Leng, W. Mai, X. Zhang, R. Liu, X. Lin, J. Huang, H. Lou, Y. Xie, R. Fu and D. Wu, Chem. Commun., 2018, 54, 7159–7162 RSC.
- Y. Chen, J. Zhang, M. Zhang and X. Wang, Chem. Sci., 2013, 4, 3244 RSC.
- W. Yong, W. Xinchen, A. Markus and Z. Yuanjian, ChemSusChem, 2010, 3, 435–439 CrossRef PubMed.
- H. Yan, Chem. Commun., 2012, 48, 3430 RSC.
- F. He, G. Chen, Y. Zhou, Y. Yu, Y. Zheng and S. Hao, Chem. Commun., 2015, 51, 16244–16246 RSC.
- Y. Zhang, Z. Schnepp, J. Cao, S. Ouyang, Y. Li, J. Ye and S. Liu, Sci. Rep., 2013, 3, 2163 CrossRef PubMed.
- M. A. Mohamed, M. F. M. Zain, L. J. Minggu, M. B. Kassim, J. Jaafar, N. A. Saidina Amin, Z. A. Mohd Hir and M. S. Rosmi, Int. J. Hydrogen Energy, 2019, 44, 13098–13105 CrossRef CAS.
- X. Zhao, Y. Zhang, X. Zhao, X. Wang, Y. Zhao, H.-Q. Tan, H. Zhu, W. Ho, H. Sun and Y.-G. Li, ACS Appl. Mater. Interfaces, 2019, 11, 27934–27943 CrossRef CAS PubMed.
- C. Merschjann, S. Tschierlei, T. Tyborski, K. Kailasam, S. Orthmann, D. Hollmann, T. Schedel-Niedrig, A. Thomas and S. Lochbrunner, Adv. Mater., 2015, 27, 7993–7999 CrossRef CAS PubMed.
- C. Merschjann, T. Tyborski, S. Orthmann, F. Yang, K. Schwarzburg, M. Lublow, M.-C. Lux-Steiner and T. Schedel-Niedrig, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 205204 CrossRef.
- M. Wu, J.-M. Yan, X. Tang, M. Zhao and Q. Jiang, ChemSusChem, 2014, 7, 2654–2658 CrossRef CAS PubMed.
- L. Shi, L. Yao and W. Si, J. Mater. Sci.: Mater. Electron., 2019, 30, 1714–1723 CrossRef CAS.
- A. Savateev, Z. P. Chen and D. Dontsova, RSC Adv., 2016, 6, 2910–2913 RSC.
- C. Liu, Y. Zhang, F. Dong, A. H. Reshak, L. Ye, N. Pinna, C. Zeng, T. Zhang and H. Huang, Appl. Catal., B, 2017, 203, 465–474 CrossRef CAS.
- Z. Jun-Jun, G. Jie-Min, W. Hong-Hui, W. Xiao, L. Xin-Hao and C. Jie-Sheng, ChemCatChem, 2016, 8, 3441–3445 CrossRef.
- X. Qian, X. Meng, J. Sun, L. Jiang, Y. Wang, J. Zhang, X. Hu, M. Shalom and J. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 27226–27232 CrossRef CAS PubMed.
- L. Lin, Z. Yu and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 6164–6175 CrossRef CAS PubMed.
- A. Savateev and M. Antonietti, ChemCatChem, 2019, 11, 6166–6176 CrossRef CAS.
- X. Liu, N. Fechler and M. Antonietti, Chem. Soc. Rev., 2013, 42, 8237–8265 RSC.
- B. P. Jelle and S. E. Kalnæs, Cost-Effective Energy Effic. Build. Retrofit., 2017, pp. 57–118 Search PubMed.
- P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450–3453 CrossRef CAS PubMed.
- M. J. Bojdys, J.-O. Müller, M. Antonietti and A. Thomas, Chem.–Eur. J., 2008, 14, 8177–8182 CrossRef CAS PubMed.
- E. Wirnhier, M. Döblinger, D. Gunzelmann, J. Senker, B. V Lotsch and W. Schnick, Chem.–Eur. J., 2011, 17, 3213–3221 CrossRef CAS PubMed.
- G. Algara-Siller, N. Severin, S. Y. Chong, T. Björkman, R. G. Palgrave, A. Laybourn, M. Antonietti, Y. Z. Khimyak, A. V Krasheninnikov, J. P. Rabe, U. Kaiser, A. I. Cooper, A. Thomas and M. J. Bojdys, Angew. Chem., Int. Ed., 2014, 53, 7450–7455 CrossRef CAS PubMed.
- S. Y. Chong, J. T. A. Jones, Y. Z. Khimyak, A. I. Cooper, A. Thomas, M. Antonietti and M. J. Bojdys, J. Mater. Chem. A, 2013, 1, 1102–1107 RSC.
- L. Lin, C. Wang, W. Ren, H. Ou, Y. Zhang and X. Wang, Chem. Sci., 2017, 8, 5506–5511 RSC.
- Y. Ham, K. Maeda, D. Cha, K. Takanabe and K. Domen, Chem.–Asian J., 2013, 8, 218–224 CrossRef CAS PubMed.
- A. Belen Jorge, D. James Martin, M. T. S. Dhanoa, A. S. Rahman, N. Makwana, J. Tang, A. Sella, F. Corà, S. Firth, J. A. Darr and P. F. McMillan, J. Phys. Chem. C, 2013, 117, 7178–7185 CrossRef.
- K. Schwinghammer, M. B. Mesch, V. Duppel, C. Ziegler, J. Senker and B. V Lotsch, J. Am. Chem. Soc., 2014, 136, 1730–1733 CrossRef CAS PubMed.
- M. K. Bhunia, K. Yamauchi and K. Takanabe, Angew. Chem., Int. Ed., 2014, 53, 11001–11005 CrossRef CAS PubMed.
- E. J. McDermott, E. Wirnhier, W. Schnick, K. Singh Virdi, C. Scheu, Y. Kauffmann, W. D. Kaplan, E. Z. Kurmaev and A. Moewes, J. Phys. Chem. C, 2013, 117, 8806–8812 CrossRef CAS.
- J. Zhao, L. Ma, H. Wang, Y. Zhao, J. Zhang and S. Hu, Appl. Surf. Sci., 2015, 332, 625–630 CrossRef CAS.
- C. Fettkenhauer, J. Weber, M. Antonietti and D. Dontsova, RSC Adv., 2014, 4, 40803–40811 RSC.
- X. Yan, Z. Jia, H. Che, S. Chen, P. Hu, J. Wang and L. Wang, Appl. Catal., B, 2018, 234, 19–25 CrossRef CAS.
- C. Fettkenhauer, G. Clavel, K. Kailasam, M. Antonietti and D. Dontsova, Green Chem., 2015, 17, 3350–3361 RSC.
- Y. Wang, Y. Li, W. Ju, J. Wang, H. Yao, L. Zhang, J. Wang and Z. Li, Carbon, 2016, 102, 477–486 CrossRef CAS.
- L. Heymann, B. Schiller, H. Noei, A. Stierle and C. Klinke, ACS Omega, 2018, 3, 3892–3900 CrossRef CAS PubMed.
- A. Savateev, S. Pronkin, M. G. Willinger, M. Antonietti and D. Dontsova, Chem.–Asian J., 2017, 12, 1517–1522 CrossRef CAS PubMed.
- B. Kurpil, A. Savateev, V. Papaefthimiou, S. Zafeiratos, T. Heil, S. Özenler, D. Dontsova and M. Antonietti, Appl. Catal., B, 2017, 217, 622–628 CrossRef CAS.
- A. Savateev, S. Pronkin, J. D. Epping, M. G. Willinger, C. Wolff, D. Neher, M. Antonietti and D. Dontsova, ChemCatChem, 2017, 9, 167–174 CrossRef CAS.
- G. Zhang, G. Li, T. Heil, S. Zafeiratos, F. Lai, A. Savateev, M. Antonietti and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 3433–3437 CrossRef CAS PubMed.
- Z. Chen, A. Savateev, S. Pronkin, V. Papaefthimiou, C. Wolff, M. G. Willinger, E. Willinger, D. Neher, M. Antonietti and D. Dontsova, Adv. Mater., 2017, 29, 1700555 CrossRef PubMed.
- A. Savateev, D. Dontsova, B. Kurpil and M. Antonietti, J. Catal., 2017, 350, 203–211 CrossRef CAS.
- Y. Markushyna, C. Teutloff, B. Kurpil, D. Cruz, I. Lauermann, Y. Zhao, M. Antonietti and A. Savateev, Appl. Catal., B, 2019, 248, 211–217 CrossRef CAS.
- I. Ghosh, J. Khamrai, A. Savateev, N. Shlapakov, M. Antonietti and B. König, Science, 2019, 365, 360 CrossRef CAS PubMed.
- A. Schwarzer, T. Saplinova and E. Kroke, Coord. Chem. Rev., 2013, 257, 2032–2062 CrossRef CAS.
- L. Lin, H. Ou, Y. Zhang and X. Wang, ACS Catal., 2016, 6, 3921–3931 CrossRef CAS.
- G. Zhang, G. Li, Z.-A. Lan, L. Lin, A. Savateev, T. Heil, S. Zafeiratos, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2017, 56, 13445–13449 CrossRef CAS PubMed.
- L. Jiang, X. Yuan, G. Zeng, J. Liang, Z. Wu, H. Wang, J. Zhang, T. Xiong and H. Li, Environ. Sci.: Nano, 2018, 5, 2604–2617 RSC.
- J. Zhang, M. Zhang, R.-Q. Sun and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 10145–10149 CrossRef CAS PubMed.
- A. Jin, Y. Jia, C. Chen, X. Liu, J. Jiang, X. Chen and F. Zhang, J. Phys. Chem. C, 2017, 121, 21497–21509 CrossRef CAS.
- X. Li, S. A. Bartlett, J. M. Hook, I. Sergeyev, E. B. Clatworthy, A. F. Masters and T. Maschmeyer, J. Mater. Chem. A, 2019, 7, 18987–18995 RSC.
- J. Lehn, Angew. Chem., Int. Ed., 1988, 27, 89–112 CrossRef.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- J. Barrio and M. Shalom, ChemCatChem, 2018, 10, 5573–5586 CrossRef CAS.
- J. Xu, G. Wu, Z. Wang and X. Zhang, Chem. Sci., 2012, 3, 3227 RSC.
- L. Xie, Y. Ding, X. Wang and W. Xu, Phys. Chem. Chem. Phys., 2019, 21, 9357–9361 RSC.
- H. Yan, Y. Chen and S. Xu, Int. J. Hydrogen Energy, 2012, 37, 125–133 CrossRef CAS.
- J. Shen, H. Yang, Q. Shen and Y. Feng, Eur. J. Inorg. Chem., 2015, 2611–2618 CrossRef CAS.
- N. D. Shcherban, S. M. Filonenko, M. L. Ovcharov, A. M. Mishura, M. A. Skoryk, A. Aho and D. Y. Murzin, ChemistrySelect, 2016, 1, 4987–4993 CrossRef.
- G. Dong and L. Zhang, J. Mater. Chem., 2012, 22, 1160–1166 RSC.
- X. Wu, C. Liu, X. Li, X. Zhang, C. Wang and Y. Liu, Mater. Sci. Semicond. Process., 2015, 32, 76–81 CrossRef CAS.
- X. Jiang, J. Li, J. Fang, L. Gao, W. Cai, X. Li, A. Xu and X. Ruan, J. Photochem. Photobiol., A, 2017, 336, 54–62 CrossRef CAS.
- M. Wu, Y. Gong, T. Nie, J. Zhang, R. Wang, H. Wang and B. He, J. Mater. Chem. A, 2019, 7, 5324–5332 RSC.
- S. Sun, E. Fan, H. Xu, W. Cao, G. Shao, B. Fan, H. Wang and R. Zhang, Nanotechnology, 2019, 30, 315601 CrossRef CAS PubMed.
- J. Barrio and M. Shalom, Mater. Sci. Semicond. Process., 2018, 73, 78–82 CrossRef CAS.
- J. Barrio, L. Lin, X. Wang and M. Shalom, ACS Sustainable Chem. Eng., 2018, 6, 519–530 CrossRef CAS.
- Y. Hong, C. Li, D. Li, Z. Fang, B. Luo, X. Yan, H. Shen, B. Mao and W. Shi, Nanoscale, 2017, 9, 14103–14110 RSC.
- L. Zhang, N. Ding, J. Wu, K. Iwasaki, L. Lin, Y. Yamaguchi, Y. Shibayama, J. Shi, H. Wu, Y. Luo, K. Nakata, D. Li, X. Wang, A. Fujishima and Q. Meng, Catal. Sci. Technol., 2018, 8, 3846–3852 RSC.
- J. Barrio, A. Grafmüller, J. Tzadikov and M. Shalom, Appl. Catal., B, 2018, 237, 681–688 CrossRef CAS.
- B. Zhu, G. Xu, X. Li, Z. Wang, J. Lv, X. Shu, J. Huang, Z. Zheng and Y. Wu, J. Mater. Res., 2019, 1–12 Search PubMed.
- M. Tahir, C. Cao, F. K. Butt, S. Butt, F. Idrees, Z. Ali, I. Aslam, M. Tanveer, A. Mahmood and N. Mahmood, CrystEngComm, 2014, 16, 1825 RSC.
- S. Wan, M. Ou, Y. Wang, Y. Zeng, Y. Xiong, F. Song, J. Ding, W. Cai, S. Zhang and Q. Zhong, Appl. Catal., B, 2019, 258, 118011 CrossRef CAS.
- M. Tahir, C. Cao, N. Mahmood, F. K. Butt, A. Mahmood, F. Idrees, S. Hussain, M. Tanveer, Z. Ali and I. Aslam, ACS Appl. Mater. Interfaces, 2014, 6, 1258–1265 CrossRef CAS PubMed.
- M. Tahir, N. Mahmood, J. Zhu, A. Mahmood, F. K. Butt, S. Rizwan, I. Aslam, M. Tanveer, F. Idrees, I. Shakir, C. Cao and Y. Hou, Sci. Rep., 2015, 5, 12389 CrossRef PubMed.
- J. Huang, Y. Cao, H. Wang, H. Yu, F. Peng, H. Zou and Z. Liu, Chem. Eng. J., 2019, 373, 687–699 CrossRef CAS.
- L. Shi, F. Wang, L. Liang, K. Chen, M. Liu, R. Zhu and J. Sun, Catal. Commun., 2017, 89, 129–132 CrossRef CAS.
- G. Ge and Z. Zhao, Catal. Sci. Technol., 2019, 9, 266–270 RSC.
- Q. Chen, H. Dou, S. Zheng, X. Rao and Y. Zhang, J. Photochem. Photobiol., A, 2019, 382, 111931 CrossRef CAS.
- X. Gao, J. Feng, D. Su, Y. Ma, G. Wang, H. Ma and J. Zhang, Nano Energy, 2019, 59, 598–609 CrossRef CAS.
- H. Li, H. Tian, X. Wang, M. Pi, S. Wei, H. Zhu, D. Zhang and S. Chen, ACS Appl. Energy Mater., 2019, 2, 4692–4699 CrossRef CAS.
- Q. Weng, X. Wang, C. Zhi, Y. Bando and D. Golberg, ACS Nano, 2013, 7, 1558–1565 CrossRef CAS PubMed.
- L. Chen, M. Zhou, Z. Luo, M. Wakeel, A. M. Asiri and X. Wang, Appl. Catal., B, 2019, 241, 246–255 CrossRef CAS.
- A. Roy, A. Choudhury and C. N. R. Rao, J. Mol. Struct., 2002, 613, 61–66 CrossRef CAS.
- Y.-S. Jun, E. Z. Lee, X. Wang, W. H. Hong, G. D. Stucky and A. Thomas, Adv. Funct. Mater., 2013, 23, 3661–3667 CrossRef CAS.
- Y.-S. Jun, J. Park, S. U. Lee, A. Thomas, W. H. Hong and G. D. Stucky, Angew. Chem., Int. Ed., 2013, 23, 3661–3667 CAS.
- C. T. Seto and G. M. Whitesides, J. Am. Chem. Soc., 1990, 112, 6409–6411 CrossRef CAS.
- J. a. Zerkowski, C. T. Seto and G. M. Whitesides, J. Am. Chem. Soc., 1992, 114, 5473–5475 CrossRef CAS.
- B. Roy, P. Bairi and A. K. Nandi, RSC Adv., 2014, 4, 1708–1734 RSC.
- C. Li, B. J. Cafferty, S. C. Karunakaran, G. B. Schuster and N. V. Hud, Phys. Chem. Chem. Phys., 2016, 18, 20091–20096 RSC.
- M. Shalom, S. Inal, C. Fettkenhauer, D. Neher and M. Antonietti, J. Am. Chem. Soc., 2013, 135, 7118–7121 CrossRef CAS PubMed.
- Z. Sun, W. Wang, Q. Chen, Y. Pu, H. He, W. Zhuang, J. He and L. Huang, J. Mater. Chem. A, 2020, 8, 3160–3167 RSC.
- B. Shen, Z. Hong, Y. Chen, B. Lin and B. Gao, Mater. Lett., 2014, 118, 208–211 CrossRef CAS.
- J. Hu, J. Tian and C. Li, ACS Appl. Mater. Interfaces, 2017, 9, 11615–11625 CrossRef CAS PubMed.
- N.-N. Vu, C.-C. Nguyen, S. Kaliaguine and T.-O. Do, ChemSusChem, 2019, 12, 291–302 CrossRef CAS PubMed.
- M. Shalom, M. Guttentag, C. Fettkenhauer, S. Inal, D. Neher, A. Llobet and M. Antonietti, Chem. Mater., 2014, 26, 5812–5818 CrossRef CAS.
- Q. Zheng, D. P. Durkin, J. E. Elenewski, Y. Sun, N. A. Banek, L. Hua, H. Chen, M. J. Wagner, W. Zhang and D. Shuai, Environ. Sci. Technol., 2016, 50, 12938–12948 CrossRef CAS PubMed.
- C. Zhou, C. Lai, D. Huang, G. Zeng, C. Zhang, M. Cheng, L. Hu, J. Wan, W. Xiong, M. Wen, X. Wen and L. Qin, Appl. Catal., B, 2018, 220, 202–210 CrossRef CAS.
- Y. Ishida, L. Chabanne, M. Antonietti and M. Shalom, Langmuir, 2014, 30, 447–451 CrossRef CAS PubMed.
- D. Tang, Y. Chen, M. Yin, Q. Yang, Y. Zhou and L. Zhou, Mater. Sci. Semicond. Process., 2020, 105, 104735 CrossRef CAS.
- C. Zhao, Z. Chen, J. Xu, Q. Liu, H. Xu, H. Tang, G. Li, Y. Jiang, F. Qu, Z. Lin and X. Yang, Appl. Catal., B, 2019, 256, 117867 CrossRef CAS.
- N. Karjule, J. Barrio, J. Tzadikov and M. Shalom, Chem.–Eur. J., 2020 DOI:10.1002/chem.201905875.
- Y. Liao, S. Zhu, J. Ma, Z. Sun, C. Yin, C. Zhu, X. Lou and D. Zhang, ChemCatChem, 2014, 6, 3419–3425 CrossRef CAS.
- T. Jordan, N. Fechler, J. Xu, T. J. K. Brenner, M. Antonietti and M. Shalom, ChemCatChem, 2015, 7, 2826–2830 CrossRef CAS.
- S. Wan, Q. Zhong, M. Ou and S. Zhang, RSC Adv., 2016, 6, 101208–101215 RSC.
- S. Wan, M. Ou, Q. Zhong, S. Zhang and W. Cai, Adv. Opt. Mater., 2017, 5, 1700536 CrossRef.
- X. Song, D. Tang, Y. Chen, M. Yin, Q. Yang, Z. Chen and L. Zhou, ACS Omega, 2019, 4, 6114–6125 CrossRef CAS PubMed.
- Y. Chen, F. Ding, A. Khaing, D. Yang and Z. Jiang, Appl. Surf. Sci., 2019, 479, 757–764 CrossRef CAS.
- W. Che, W. Cheng, T. Yao, F. Tang, W. Liu, H. Su, Y. Huang, Q. Liu, J. Liu, F. Hu, Z. Pan, Z. Sun and S. Wei, J. Am. Chem. Soc., 2017, 139, 3021–3026 CrossRef CAS PubMed.
- S. Guo, Z. Deng, M. Li, B. Jiang, C. Tian, Q. Pan and H. Fu, Angew. Chem., Int. Ed., 2016, 55, 1830–1834 CrossRef CAS PubMed.
- Z. Wang, M. Chen, Y. Huang, X. Shi, Y. Zhang, T. Huang, J. Cao, W. Ho and S. C. Lee, Appl. Catal., B, 2018, 239, 352–361 CrossRef CAS.
- S. Wang, F. He, X. Zhao, J. Zhang, Z. Ao, H. Wu, Y. Yin, L. Shi, X. Xu, C. Zhao, S. Wang and H. Sun, Appl. Catal., B, 2019, 257, 117931 CrossRef CAS.
- L. Yin, S. Wang, C. Yang, S. Lyu and X. Wang, ChemSusChem, 2019, 12, 3320–3325 CrossRef CAS PubMed.
- J. Xu, T. J. K. Brenner, L. Chabanne, D. Neher, M. Antonietti and M. Shalom, J. Am. Chem. Soc., 2014, 136, 13486–13489 CrossRef CAS PubMed.
- Q. Cui, J. Xu, X. Wang, L. Li, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2016, 55, 3672–3676 CrossRef CAS PubMed.
- L.-L. Feng, Y. Zou, C. Li, S. Gao, L.-J. Zhou, Q. Sun, M. Fan, H. Wang, D. Wang, G.-D. Li and X. Zou, Int. J. Hydrogen Energy, 2014, 39, 15373–15379 CrossRef CAS.
- Q. Fan, J. Liu, Y. Yu, S. Zuo and B. Li, Appl. Surf. Sci., 2017, 391, 360–368 CrossRef CAS.
- Y. Zheng, Z. Yu, F. Lin, F. Guo, A. K. Alamry, A. L. Taib, M. A. Asiri and X. Wang, Molecules, 2017, 22, 572 CrossRef PubMed.
- H. Wang, Y. Bian, J. Hu and L. Dai, Appl. Catal., B, 2018, 238, 592–598 CrossRef CAS.
- J.-Y. Song, H.-J. Kang, J. C. Won, Y. H. Kim, Y.-S. Jun and H. S. Jeong, Mater. Horiz., 2019, 6, 1726–1732 RSC.
- X. Wang, C. Zhou, R. Shi, Q. Liu, G. I. N. Waterhouse, L. Wu, C. H. Tung and T. Zhang, Nano Res., 2019, 12, 2385–2389 CrossRef CAS.
- P. Zhang, X. Li, C. Shao and Y. Liu, J. Mater. Chem. A, 2015, 3, 3281–3284 RSC.
- X. Han, L. Tian, H. Jiang, L. Kong, J. Lv, J. Shan, J. Wang and X. Fan, RSC Adv., 2017, 7, 14372–14381 RSC.
- H. Li, F. Li, Z. Wang, Y. Jiao, Y. Liu, P. Wang, X. Zhang, X. Qin, Y. Dai and B. Huang, Appl. Catal., B, 2018, 229, 114–120 CrossRef CAS.
- Y. Qin, Y. Ding and H. Tang, J. Environ. Chem. Eng., 2016, 4, 4374–4384 CrossRef CAS.
- Z. Chen, T.-T. Fan, X. Yu, Q.-L. Wu, Q.-H. Zhu, L.-Z. Zhang, J.-H. Li, W.-P. Fang and X.-D. Yi, J. Mater. Chem. A, 2018, 6, 15310–15319 RSC.
- N. Tian, K. Xiao, Y. Zhang, X. Lu, L. Ye, P. Gao, T. Ma and H. Huang, Appl. Catal., B, 2019, 253, 196–205 CrossRef CAS.
- L. Li, Y. Zhao, M. Antonietti and M. Shalom, Small, 2016, 12, 6090–6097 CrossRef CAS PubMed.
- L. Li, M. Shalom, Y. Zhao, J. Barrio and M. Antonietti, J. Mater. Chem. A, 2017, 5, 18502–18508 RSC.
- Z. Chen, M. Antonietti and D. Dontsova, Chem.–Eur. J., 2015, 21, 10805–10811 CrossRef CAS PubMed.
- L. Kui, X. Xin and Z. Wei-De, ChemCatChem, 2016, 8, 2128–2135 CrossRef.
- Z. Mo, X. Zhu, Z. Jiang, Y. Song, D. Liu, H. Li, X. Yang, Y. She, Y. Lei, S. Yuan, H. Li, L. Song, Q. Yan and H. Xu, Appl. Catal., B, 2019, 256, 117854 CrossRef CAS.
- L. Kampschulte, M. Lackinger, A.-K. Maier, R. S. K. Kishore, S. Griessl, M. Schmittel and W. M. Heckl, J. Phys. Chem. B, 2006, 110, 10829–10836 CrossRef CAS PubMed.
- C. C. Robertson, J. S. Wright, E. J. Carrington, R. N. Perutz, C. A. Hunter and L. Brammer, Chem. Sci., 2017, 8, 5392–5398 RSC.
- P. Li, H. D. Arman, H. Wang, L. Weng, K. Alfooty, R. F. Angawi and B. Chen, Cryst. Growth Des., 2015, 15, 1871–1875 CrossRef CAS.
- J. L. Cook, C. A. Hunter, C. M. R. Low, A. Perez-Velasco and J. G. Vinter, Angew. Chem., Int. Ed., 2007, 46, 3706–3709 CrossRef CAS PubMed.
- Z.-F. Huang, J. Song, L. Pan, Z. Wang, X. Zhang, J.-J. Zou, W. Mi, X. Zhang and L. Wang, Nano Energy, 2015, 12, 646–656 CrossRef CAS.
- J.-W. Zhang, S. Gong, N. Mahmood, L. Pan, X. Zhang and J.-J. Zou, Appl. Catal., B, 2018, 221, 9–16 CrossRef CAS.
- X. Weinan, C. Gang, L. Chunmei, S. Jingxue, H. Zhonghui, Z. Yansong, H. Yidong and M. Qingqiang, ChemCatChem, 2016, 8, 2838–2845 CrossRef.
- G. Peng, L. Xing, J. Barrio, M. Volokh and M. Shalom, Angew. Chem., Int. Ed., 2018, 57, 1186–1192 CrossRef CAS PubMed.
- L. Jiang, X. Yuan, G. Zeng, J. Liang, Z. Wu, H. Yu, D. Mo, H. Wang, Z. Xiao and C. Zhou, J. Colloid Interface Sci., 2019, 536, 17–29 CrossRef CAS PubMed.
- J. Barrio and M. Shalom, ACS Appl. Mater. Interfaces, 2018, 10, 39688–39694 CrossRef CAS PubMed.
- X. Hu, M. Vatankhah-Varnoosfaderani, J. Zhou, Q. Li and S. S. Sheiko, Adv. Mater., 2015, 27, 6899–6905 CrossRef CAS PubMed.
- L.-S. Teo, C.-Y. Chen and J.-F. Kuo, Macromolecules, 1997, 30, 1793–1799 CrossRef CAS.
- J. Wang, S. Cao and J. Yu, Sol. RRL, 2019, 1900435 Search PubMed.
- N. Karjule, J. Barrio, A. Tashakory and M. Shalom, Sol. RRL, 2020, 2000017 CrossRef.
- J. Sun, J. Xu, A. Grafmueller, X. Huang, C. Liedel, G. Algara-Siller, M. Willinger, C. Yang, Y. Fu, X. Wang and M. Shalom, Appl. Catal., B, 2017, 205, 1–10 CrossRef CAS.
- B. Qin, S. Zhang, Q. Song, Z. Huang, J.-F. Xu and X. Zhang, Angew. Chem., Int. Ed., 2017, 56, 7639–7643 CrossRef CAS PubMed.
- S. Dolai, J. Barrio, G. Peng, A. Grafmueller and M. Shalom, Nanoscale, 2019, 11, 5564–5570 RSC.
- S. Dolai, N. Karjule, A. Azoulay and J. Barrio, RSC Adv., 2019, 9, 26091–26096 RSC.
- R. S. Feigelson, in Handbook of Crystal Growth, Elsevier, 2015, pp. 1–83 Search PubMed.
- B. Spingler, S. Schnidrig, T. Todorova and F. Wild, CrystEngComm, 2012, 14, 751–757 RSC.
- A. Azoulay, J. Barrio and M. Shalom, Isr. J. Chem., 2019 DOI:10.1002/ijch.201900056.
- J. Gao, J. Wang, X. Qian, Y. Dong, H. Xu, R. Song, C. Yan, H. Zhu, Q. Zhong, G. Qian and J. Yao, J. Solid State Chem., 2015, 228, 60–64 CrossRef CAS.
- J. Wang, H. Xu, X. Qian, Y. Dong, J. Gao, G. Qian and J. Yao, Chem.–Asian J., 2015, 10, 1276–1280 CrossRef CAS PubMed.
- J. Barrio, L. Lin, P. Amo-Ochoa, J. Tzadikov, G. Peng, J. Sun, F. Zamora, X. Wang and M. Shalom, Small, 2018, 14, 1800633 CrossRef PubMed.
- D.-F. Weng, B.-W. Wang, Z.-M. Wang and S. Gao, CrystEngComm, 2011, 13, 4683–4688 RSC.
- J.-N. Zhu, X.-Q. Zhu, F.-F. Cheng, P. Li, F. Wang, Y.-W. Xiao and W.-W. Xiong, Appl. Catal., B, 2019, 256, 117830 CrossRef CAS.
- Z. Jiang, X. Zhang, H. Chen, X. Hu and P. Yang, ChemCatChem, 2019, 11, 4558–4567 CrossRef CAS.
- X. Fang, R. Gao, Y. Yang and D. Yan, iScience, 2019, 16, 22–30 CrossRef CAS PubMed.
- J.-H. Zhang, M.-J. Wei, Z.-W. Wei, M. Pan and C.-Y. Su, ACS Appl. Nano Mater., 2020, 3, 1010–1018 CrossRef CAS.
- Z. Liu, G. Wang, H.-S. Chen and P. Yang, Chem. Commun., 2018, 54, 4720–4723 RSC.
- Z. Jiang, X. Zhang, J. Wang, L. Chen, H.-S. Chen and P. Yang, Chem. Commun., 2018, 54, 13519–13522 RSC.
- J. Liu, Z. Wei, W. Fang, Z. Jiang and W. Shangguan, ChemCatChem, 2019, 11, 6275–6281 CrossRef CAS.
- J. Fu, B. Zhu, C. Jiang, B. Cheng, W. You and J. Yu, Small, 2017, 13, 1–9 Search PubMed.
- W. Ren, J. Cheng, H. Ou, C. Huang, M. Titirici and X. Wang, ChemSusChem, 2019, 12, 3257–3262 CrossRef CAS PubMed.
- M. Gao, J. Feng, Z. Zhang, M. Gu, J. Wang, W. Zeng, Y. Lv, Y. Ren, T. Wei and Z. Fan, ACS Appl. Nano Mater., 2018, 1, 6733–6741 CrossRef CAS.
- T. Sano, S. Tsutsui, K. Koike, T. Hirakawa, Y. Teramoto, N. Negishi and K. Takeuchi, J. Mater. Chem. A, 2013, 1, 6489–6496 RSC.
- G. Dong, Z. Ai and L. Zhang, RSC Adv., 2014, 4, 5553–5560 RSC.
- S. Liu, D. Li, H. Sun, H. M. Ang, M. O. Tadé and S. Wang, J. Colloid Interface Sci., 2016, 468, 176–182 CrossRef CAS PubMed.
- L. Xu, J. Xia, L. Wang, H. Ji, J. Qian, H. Xu, K. Wang and H. Li, Eur. J. Inorg. Chem., 2014, 2014, 3665–3673 CrossRef CAS.
- Q. Yang, Z. Li, C. Chen, Z. Zhang and X. Fang, RSC Adv., 2019, 9, 4404–4414 RSC.
- W. Jiang, Q. Ruan, J. Xie, X. Chen, Y. Zhu and J. Tang, Appl. Catal., B, 2018, 236, 428–435 CrossRef CAS.
- Q. Han, B. Wang, J. Gao, Z. Cheng, Y. Zhao, Z. Zhang and L. Qu, ACS Nano, 2016, 10, 2745–2751 CrossRef CAS PubMed.
- Y. Xu, Q. Guo, L. Huang, H. Feng, C. Zhang, H. Xu and M. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 31934–31942 CrossRef CAS PubMed.
- Y. Zhang, A. Thomas, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2008, 131, 50–51 CrossRef PubMed.
- C. Ye, X.-Z. Wang, J.-X. Li, Z.-J. Li, X.-B. Li, L.-P. Zhang, B. Chen, C.-H. Tung and L.-Z. Wu, ACS Catal., 2016, 6, 8336–8341 CrossRef CAS.
- T. Y. Ma, Y. Tang, S. Dai and S. Z. Qiao, Small, 2014, 10, 2382–2389 CrossRef CAS PubMed.
- X. Qu, S. Hu, J. Bai, P. Li, G. Lu and X. Kang, New J. Chem., 2018, 42, 4998–5004 RSC.
- D. K. Chauhan, S. Jain, V. R. Battula and K. Kailasam, Carbon, 2019, 152, 40–58 CrossRef CAS.
- Y. Guo, S. Chu, S. Yan, Y. Wang and Z. Zou, Chem. Commun., 2010, 46, 7325–7327 RSC.
- G. Dong, L. Yang, F. Wang, L. Zang and C. Wang, ACS Catal., 2016, 6, 6511–6519 CrossRef CAS.
- J. Hu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Small, 2018, 14, 1800416 CrossRef PubMed.
- V. W. Lau, I. Moudrakovski, T. Botari, S. Weinberger, M. B. Mesch, V. Duppel, J. Senker, V. Blum and B. V Lotsch, Nat. Commun., 2016, 7, 12165 CrossRef CAS PubMed.
- G. Liu, G. Zhao, W. Zhou, Y. Liu, H. Pang, H. Zhang, D. Hao, X. Meng, P. Li, T. Kako and J. Ye, Adv. Funct. Mater., 2016, 26, 6822–6829 CrossRef CAS.
- L. Li, D. Cruz, A. Savateev, G. Zhang, M. Antonietti and Y. Zhao, Appl. Catal., B, 2018, 229, 249–253 CrossRef CAS.
- V. W. Lau, V. W. Yu, F. Ehrat, T. Botari, I. Moudrakovski, T. Simon, V. Duppel, E. Medina, J. K. Stolarczyk, J. Feldmann, V. Blum and B. V Lotsch, Adv. Energy Mater., 2017, 7, 1602251 CrossRef.
- X. Fan, L. Zhang, M. Wang, W. Huang, Y. Zhou, M. Li, R. Cheng and J. Shi, Appl. Catal., B, 2016, 182, 68–73 CrossRef CAS.
- J. Tian, L. Zhang, X. Fan, Y. Zhou, M. Wang, R. Cheng, M. Li, X. Kan, X. Jin, Z. Liu, Y. Gao and J. Shi, J. Mater. Chem. A, 2016, 4, 13814–13821 RSC.
- D. Vidyasagar, S. G. Ghugal, S. S. Umare and M. Banavoth, Sci. Rep., 2019, 9, 7186 CrossRef PubMed.
- J. Pan, L. Guo, S. Zhang, N. Wang, S. Jin and B. Tan, Chem.–Asian J., 2018, 13, 1674–1677 CrossRef CAS PubMed.
- W. Lu, T. Xu, Y. Wang, H. Hu, N. Li, X. Jiang and W. Chen, Appl. Catal., B, 2016, 180, 20–28 CrossRef CAS.
- G. Zhao, H. Pang, G. Liu, P. Li, H. Liu, H. Zhang, L. Shi and J. Ye, Appl. Catal., B, 2017, 200, 141–149 CrossRef CAS.
- S. Tian, S. Chen, X. Ren, R. Cao, H. Hu and F. Bai, Nano Res., 2019, 12, 3109–3115 CrossRef CAS.
- S. Zhao, T. Guo, X. Li, T. Xu, B. Yang and X. Zhao, Appl. Catal., B, 2018, 224, 725–732 CrossRef CAS.
- P. Zhang, H. Li and Y. Wang, Chem. Commun., 2014, 50, 6312–6315 RSC.
- X. Chen, H. Chen, J. Guan, J. Zhen, Z. Sun, P. Du, Y. Lu and S. Yang, Nanoscale, 2017, 9, 5615–5623 RSC.
- X.-H. Song, L. Feng, S.-L. Deng, S.-Y. Xie and L.-S. Zheng, Adv. Mater. Interfaces, 2017, 4, 1700339 CrossRef.
- A. Rashidizadeh, H. Ghafuri, H. Reza Esmaili Zand and N. Goodarzi, ACS Omega, 2019, 4, 12544–12554 CrossRef CAS PubMed.
- Y.-L. Thi Ngo, J. S. Chung and S. H. Hur, Dyes Pigm., 2019, 168, 180–188 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 2002, 80, 1339 CrossRef.
- J. Sun, R. Phatake, A. Azoulay, G. Peng, C. Han, J. Barrio, J. Xu, X. Wang and M. Shalom, Chem.–Eur. J., 2018, 24, 14921–14927 CrossRef CAS PubMed.
- B. Kumru, M. Antonietti and B. V. K. J. Schmidt, Langmuir, 2017, 33, 9897–9906 CrossRef CAS PubMed.
- B. Kumru, J. Barrio, J. Zhang, M. Antonietti, M. Shalom and B. V. K. J. Schmidt, ACS Appl. Mater. Interfaces, 2019, 11, 9462–9469 CrossRef CAS PubMed.
- M. A. Douzandegi Fard, H. Ghafuri and A. Rashidizadeh, Microporous Mesoporous Mater., 2019, 274, 83–93 CrossRef CAS.
- B. Kumru, V. Molinari, M. Hilgart, F. Rummel, M. Schäffler and B. V. K. J. Schmidt, Polym. Chem., 2019, 10, 3647–3656 RSC.
- Q. Cao, B. Kumru, M. Antonietti and B. V. K. J. Schmidt, Macromolecules, 2019, 52, 4989–4996 CrossRef CAS PubMed.
- Q. Cao, B. Kumru, M. Antonietti and B. V. K. J. Schmidt, Mater. Horiz., 2020, 7, 762–786 RSC.
- C. Hu, Y.-R. Lin and H.-C. Yang, ChemSusChem, 2019, 12, 1794–1806 CrossRef CAS PubMed.
- M. Li, H. Liao, Q. Deng, Y. Wu, F. Xiao, X. Wei and D. Tu, J. Macromol. Sci., Part A: Pure Appl.Chem., 2018, 55, 408–413 CrossRef CAS.
- M. Zarei, H. Ahmadzadeh, E. K. Goharshadi and A. Farzaneh, Anal. Chim. Acta, 2015, 887, 245–252 CrossRef CAS PubMed.
- J. Wan Ko, W. Seok Choi, J. Kim, S. Keun Kuk, S. Ha Lee and C. Beum Park, Biomacromolecules, 2017, 18, 3551–3556 CrossRef PubMed.
- J. Yan, M.-T. F. Rodrigues, Z. Song, H. Li, H. Xu, H. Liu, J. Wu, Y. Xu, Y. Song, Y. Liu, P. Yu, W. Yang, R. Vajtai, H. Li, S. Yuan and P. M. Ajayan, Adv. Funct. Mater., 2017, 27, 1700653 CrossRef.
- X. Wang, Y. Liang, W. An, J. Hu, Y. Zhu and W. Cui, Appl. Catal., B, 2017, 219, 53–62 CrossRef CAS.
- L. Tang, C. Jia, Y. Xue, L. Li, A. Wang, G. Xu, N. Liu and M. Wu, Appl. Catal., B, 2017, 219, 241–248 CrossRef CAS.
- Q. Han, Z. Cheng, J. Gao, Y. Zhao, Z. Zhang, L. Dai and L. Qu, Adv. Funct. Mater., 2017, 27, 1606352 CrossRef.
- Z. Tong, D. Yang, J. Shi, Y. Nan, Y. Sun and Z. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 25693–25701 CrossRef CAS PubMed.
- M. Zhang, W. Jiang, D. Liu, J. Wang, Y. Liu, Y. Zhu and Y. Zhu, Appl. Catal., B, 2016, 183, 263–268 CrossRef CAS.
- Y. Zhang, Z. Zhou, Y. Shen, Q. Zhou, J. Wang, A. Liu, S. Liu and Y. Zhang, ACS Nano, 2016, 10, 9036–9043 CrossRef CAS PubMed.
- W. Jiang, W. Luo, R. Zong, W. Yao, Z. Li and Y. Zhu, Small, 2016, 12, 4370–4378 CrossRef CAS PubMed.
- J. Sun, B. V. K. J. Schmidt, X. Wang and M. Shalom, ACS Appl. Mater. Interfaces, 2017, 9, 2029–2034 CrossRef CAS PubMed.
- J. Liu, T. An, Z. Chen, Z. Wang, H. Zhou, T. Fan, D. Zhang and M. Antonietti, J. Mater. Chem. A, 2017, 5, 8933–8938 RSC.
- B. Kumru, M. Shalom, M. Antonietti and B. V. K. J. Schmidt, Macromolecules, 2017, 50, 1862–1869 CrossRef CAS.
- B. Kumru, V. Molinari, R. Dünnebacke, K. G. Blank and B. V. K. J. Schmidt, Macromol. Rapid Commun., 2019, 40, 1800712 Search PubMed.
- B. Kumru, V. Molinari, M. Shalom, M. Antonietti and B. V. K. J. Schmidt, Soft Matter, 2018, 14, 2655–2664 RSC.
- B. Ye, C. Yao, M. Yan, H. Zhang, F. Xi, J. Liu, B. Li and X. Dong, Macromol. Mater. Eng., 2019, 304, 1800500 CrossRef.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1226419 CrossRef.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun’Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- Y. Lin, T. V. Williams and J. W. Connell, J. Phys. Chem. Lett., 2009, 1, 277–283 CrossRef.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- M. Pumera and Z. Sofer, Adv. Mater., 2017, 29, 1605299 CrossRef PubMed.
- Y. Niu, J. Villalva, R. Frisenda, G. Sanchez-Santolino, L. Ruiz-González, E. M. Pérez, M. García-Hernández, E. Burzurí and A. Castellanos-Gomez, 2D Mater., 2019, 6, 35023 CrossRef CAS.
- C. Gibaja, D. Rodriguez-San-Miguel, P. Ares, J. Gómez-Herrero, M. Varela, R. Gillen, J. Maultzsch, F. Hauke, A. Hirsch, G. Abellán and F. Zamora, Angew. Chem., Int. Ed., 2016, 55, 14345–14349 CrossRef CAS PubMed.
- J. Zhang, Y. Chen and X. Wang, Energy Environ. Sci., 2015, 8, 3092–3108 RSC.
- Z. Qin, Z. Huang, M. Wang, D. Liu, Y. Chen and L. Guo, Appl. Catal., B, 2020, 261, 118211 CrossRef CAS.
- X. Zhang, X. Xie, H. Wang, J. Zhang, B. Pan and Y. Xie, J. Am. Chem. Soc., 2012, 135, 18–21 CrossRef PubMed.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- X. She, H. Xu, Y. Xu, J. Yan, J. Xia, L. Xu, Y. Song, Y. Jiang, Q. Zhang and H. Li, J. Mater. Chem. A, 2014, 2, 2563–2570 RSC.
- C. Huang, J. Wen, Y. Shen, F. He, L. Mi, Z. Gan, J. Ma, S. Liu, H. Ma and Y. Zhang, Chem. Sci., 2018, 9, 7912–7915 RSC.
- Z. Gan, C. Huang, Y. Shen, Q. Zhou, D. Han, J. Ma, S. Liu and Y. Zhang, Chin. Chem. Lett., 2020, 31, 513–516 CrossRef CAS.
- X. Bai, S. Yan, J. Wang, L. Wang, W. Jiang, S. Wu, C. Sun and Y. Zhu, J. Mater. Chem. A, 2014, 2, 17521–17529 RSC.
- Q. Lin, L. Li, S. Liang, M. Liu, J. Bi and L. Wu, Appl. Catal., B, 2015, 163, 135–142 CrossRef CAS.
- S. P. Pattnaik, A. Behera, S. Martha, R. Acharya and K. Parida, J. Mater. Sci., 2019, 54, 5726–5742 CrossRef CAS.
- H. Zhang, L.-H. Guo, L. Zhao, B. Wan and Y. Yang, J. Phys. Chem. Lett., 2015, 6, 958–963 CrossRef CAS PubMed.
- Z. Zhou, Q. Shang, Y. Shen, L. Zhang, Y. Zhang, Y. Lv, Y. Li, S. Liu and Y. Zhang, Anal. Chem., 2016, 88, 6004–6010 CrossRef CAS PubMed.
- P. Qiu, H. Chen, C. Xu, N. Zhou, F. Jiang, X. Wang and Y. Fu, J. Mater. Chem. A, 2015, 3, 24237–24244 RSC.
- S. Guo, Y. Zhu, Y. Yan, Y. Min, J. Fan and Q. Xu, Appl. Catal., B, 2016, 185, 315–321 CrossRef CAS.
- P. Niu, L. Zhang, G. Liu and H.-M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- P. Xia, B. Zhu, J. Yu, S. Cao and M. Jaroniec, J. Mater. Chem. A, 2017, 5, 3230–3238 RSC.
- J. Xu, L. Zhang, R. Shi and Y. Zhu, J. Mater. Chem. A, 2013, 1, 14766 RSC.
- Z. Zhou, Y. Shen, Y. Li, A. Liu, S. Liu and Y. Zhang, ACS Nano, 2015, 9, 12480–12487 CrossRef CAS PubMed.
- Z. Zhou, J. Wang, J. Yu, Y. Shen, Y. Li, A. Liu, S. Liu and Y. Zhang, J. Am. Chem. Soc., 2015, 137, 2179–2182 CrossRef CAS PubMed.
- M. Yi and Z. Shen, J. Mater. Chem. A, 2015, 3, 11700–11715 RSC.
- H. Li, G. Lu, Y. Wang, Z. Yin, C. Cong, Q. He, L. Wang, F. Ding, T. Yu and H. Zhang, Small, 2013, 9, 1974–1981 CrossRef CAS PubMed.
- A. Castellanos-Gomez, L. Vicarelli, E. Prada, J. O. Island, K. L. Narasimha-Acharya, S. I. Blanter, D. J. Groenendijk, M. Buscema, G. A. Steele, J. V. Alvarez, H. W. Zandbergen, J. J. Palacios and H. S. J. van der Zant, 2D Mater., 2014, 1, 025001 CrossRef.
- P. Ares, F. Aguilar-Galindo, D. Rodríguez-San-Miguel, D. A. Aldave, S. Díaz-Tendero, M. Alcamí, F. Martín, J. Gómez-Herrero and F. Zamora, Adv. Mater., 2016, 28, 6332–6336 CrossRef CAS PubMed.
- M. J. Bojdys, N. Severin, J. P. Rabe, A. I. Cooper, A. Thomas and M. Antonietti, Macromol. Rapid Commun., 2013, 34, 850–854 CrossRef CAS PubMed.
- K. Schwinghammer, M. B. Mesch, V. Duppel, C. Ziegler, J. Senker and B. V. Lotsch, J. Am. Chem. Soc., 2014, 136, 1730–1733 CrossRef CAS PubMed.
- T. S. Miller, T. M. Suter, A. M. Telford, L. Picco, O. D. Payton, F. Russell-Pavier, P. L. Cullen, A. Sella, M. S. P. Shaffer, J. Nelson, V. Tileli, P. F. McMillan and C. A. Howard, Nano Lett., 2017, 17, 5891–5896 CrossRef CAS PubMed.
- H. Xu, J. Yan, X. She, L. Xu, J. Xia, Y. Xu, Y. Song, L. Huang and H. Li, Nanoscale, 2014, 6, 1406–1415 RSC.
- L. Ma, H. Fan, M. Li, H. Tian, J. Fang and G. Dong, J. Mater. Chem. A, 2015, 3, 22404–22412 RSC.
- J. Ji, J. Wen, Y. Shen, Y. Lv, Y. Chen, S. Liu, H. Ma and Y. Zhang, J. Am. Chem. Soc., 2017, 139, 11698–11701 CrossRef CAS PubMed.
- Y. Li, S. Ouyang, H. Xu, X. Wang, Y. Bi, Y. Zhang and J. Ye, J. Am. Chem. Soc., 2016, 138, 13289–13297 CrossRef CAS PubMed.
- Y. Li, S. Ouyang, H. Xu, W. Hou, M. Zhao, H. Chen and J. Ye, Adv. Funct. Mater., 2019, 29, 1901024 CrossRef.
- Š. Hajduk, S. P. Berglund, M. Podlogar, G. Dražić, F. F. Abdi, Z. C. Orel and M. Shalom, Adv. Mater. Interfaces, 2017, 4, 1700924 CrossRef.
- W.-J. Ong, Front. Mater., 2017, 4, 1–10 Search PubMed.
- J. Barrio, C. Gibaja, J. Tzadikov, M. Shalom and F. Zamora, Adv. Sustainable Syst., 2019, 3, 1800138 CrossRef.
- A. Bafaqeer, M. Tahir and N. A. S. Amin, Appl. Catal., B, 2019, 242, 312–326 CrossRef CAS.
- Z. Gao, K. Chen, L. Wang, B. Bai, H. Liu and Q. Wang, Appl. Catal., B, 2019, 118462 Search PubMed.
- M. S. Khan, F. Zhang, M. Osada, S. S. Mao and S. Shen, Sol. RRL, 2019, 1900435 CrossRef.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef PubMed.
- S. Yin, J. Di, M. Li, Y. Sun, J. Xia, H. Xu, W. Fan and H. Li, J. Mater. Sci., 2016, 51, 4769–4777 CrossRef CAS.
- S. Han, X. Hu, W. Yang, Q. Qian, X. Fang and Y. Zhu, ACS Appl. Energy Mater., 2019, 2, 1803–1811 CrossRef CAS.
- W. Zhang, A. R. Mohamed and W.-J. Ong, Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.201914925.
- J. Fu, J. Yu, C. Jiang and B. Cheng, Adv. Energy Mater., 2018, 8, 1–31 CAS.
- H. Xu, X. She, T. Fei, Y. Song, D. Liu, H. Li, X. Yang, J. Yang, H. Li, L. Song, P. M. Ajayan and J. Wu, ACS Nano, 2019, 13, 11294–11302 CrossRef CAS PubMed.
- L. Sun, B. Li, X. Chu, N. Sun, Y. Qu, X. Zhang, I. Khan, L. Bai and L. Jing, ACS Sustainable Chem. Eng., 2019, 7, 9916–9927 CrossRef CAS.
- R. Bhosale, S. Jain, C. P. Vinod, S. Kumar and S. Ogale, ACS Appl. Mater. Interfaces, 2019, 11, 6174–6183 CrossRef CAS PubMed.
- T. Di, B. Zhu, B. Cheng, J. Yu and J. Xu, J. Catal., 2017, 352, 532–541 CrossRef CAS.
- Z. Pan, G. Zhang and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 7102–7106 CrossRef CAS PubMed.
- K. Zhu, M. Zhang, X. Feng, L. Qin, S.-Z. Kang and X. Li, Appl. Catal., B, 2019, 118434 Search PubMed.
- X.-H. Li and M. Antonietti, Chem. Soc. Rev., 2013, 42, 6593 RSC.
- J. Wang, G. Wang, X. Wang, Y. Wu, Y. Su and H. Tang, Carbon, 2019, 149, 618–626 CrossRef CAS.
- Q. Han, N. Chen, J. Zhang and L. Qu, Mater. Horiz., 2017, 4, 832–850 RSC.
- C. Wang, G. Liu, K. Song, X. Wang, H. Wang, N. Zhao and F. He, ChemCatChem, 2019, 11, 6364–6371 CrossRef CAS.
- Y. Li, H. Zhang, P. Liu, D. Wang, Y. Li and H. Zhao, Small, 2013, 9, 3336–3344 CAS.
- X. Chen, K. Deng, P. Zhou and Z. Zhang, ChemSusChem, 2018, 11, 2444–2452 CrossRef CAS PubMed.
- J. Ran, W. Guo, H. Wang, B. Zhu, J. Yu and S.-Z. Qiao, Adv. Mater., 2018, 30, 1800128 CrossRef PubMed.
- C.-C. Wang, X.-H. Yi and P. Wang, Appl. Catal., B, 2019, 247, 24–48 CrossRef CAS.
- P. Kumar, P. Kar, A. P. Manuel, S. Zeng, U. K. Thakur, K. M. Alam, Y. Zhang, R. Kisslinger, K. Cui, G. M. Bernard, V. K. Michaelis and K. Shankar, Adv. Opt. Mater., 2019, 8, 1901275 CrossRef.
- Y. Zhu, T. Wan, X. Wen, D. Chu and Y. Jiang, Appl. Catal., B, 2019, 244, 814–822 CrossRef CAS.
- W. Chang, W. Xue, E. Liu, J. Fan and B. Zhao, Chem. Eng. J., 2019, 362, 392–401 CrossRef CAS.
- C. He, J. H. Zhang, W. X. Zhang and T. T. Li, J. Phys. Chem. Lett., 2019, 10, 3122–3128 CrossRef CAS PubMed.
- P. Chen, F. Liu, H. Ding, S. Chen, L. Chen, Y.-J. Li, C.-T. Au and S.-F. Yin, Appl. Catal., B, 2019, 252, 33–40 CrossRef CAS.
- C. A. Caputo, L. Wang, R. Beranek and E. Reisner, Chem. Sci., 2015, 6, 5690–5694 RSC.
- C. A. Caputo, M. A. Gross, V. W. Lau, C. Cavazza, B. V. Lotsch and E. Reisner, Angew. Chem., Int. Ed., 2014, 53, 11538–11542 CrossRef CAS PubMed.
- C. Li, S. Yu, L. Gu, J. Han, H. Dong, Y. Wang and G. Chen, Adv. Mater. Interfaces, 2018, 5, 1801297 CrossRef.
- H. Zhang, J. Wu, J. Han, L. Wang, W. Zhang, H. Dong, C. Li and Y. Wang, Chem. Eng. J., 2020, 385, 123764 CrossRef.
- H. Kasap, C. A. Caputo, B. C. M. Martindale, R. Godin, V. W. Lau, B. V. Lotsch, J. R. Durrant and E. Reisner, J. Am. Chem. Soc., 2016, 138, 9183–9192 CrossRef CAS PubMed.
- Z. Pan, P. Niu, M. Liu, G. Zhang, Z. Zhu and X. Wang, ChemSusChem, 2020, 13, 888–892 CrossRef CAS PubMed.
- S. Ding, M. J. Hülsey, J. Pérez-Ramírez and N. Yan, Joule, 2019, 3, 2897–2929 CrossRef CAS.
- Z. Chen, S. Mitchell, F. Krumeich, R. Hauert, S. Yakunin, M. V. Kovalenko and J. Pérez-Ramírez, ACS Sustainable Chem. Eng., 2019, 7, 5223–5230 CrossRef CAS.
- P. Huang, J. Huang, S. A. Pantovich, A. D. Carl, T. G. Fenton, C. A. Caputo, R. L. Grimm, A. I. Frenkel and G. Li, J. Am. Chem. Soc., 2018, 140, 16042–16047 CrossRef CAS PubMed.
- G. Gao, Y. Jiao, E. R. Waclawik and A. Du, J. Am. Chem. Soc., 2016, 138, 6292–6297 CrossRef CAS PubMed.
- G. Vilé, D. Albani, M. Nachtegaal, Z. Chen, D. Dontsova, M. Antonietti, N. López and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2015, 54, 11265–11269 CrossRef PubMed.
- L. Zhang, W. Zhao, W. Zhang, J. Chen and Z. Hu, Nano Res., 2019, 12, 1181–1186 CrossRef CAS.
- Z. Chen, J. Zhao, C. R. Cabrera and Z. Chen, Small Methods, 2019, 3, 1800368 CrossRef.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- G. Zhang, G. Li and X. Wang, ChemCatChem, 2015, 7, 2864–2870 CrossRef CAS.
- C. Liao, B. Yang, N. Zhang, M. Liu, G. Chen, X. Jiang, G. Chen, J. Yang, X. Liu, T. S. Chan, Y. J. Lu, R. Ma and W. Zhou, Adv. Funct. Mater., 2019, 29, 1–11 Search PubMed.
- K. Wang, D. Shao, L. Zhang, Y. Zhou, H. Wang and W. Wang, J. Mater. Chem. A, 2019, 7, 20383–20389 RSC.
- Y. Peng, W. Pan, N. Wang, J.-E. Lu and S. Chen, ChemSusChem, 2018, 11, 130–136 CrossRef CAS PubMed.
- J. Barrio, D. Mateo, J. Albero, H. García and M. Shalom, Adv. Energy Mater., 2019, 9, 1902738 CrossRef CAS.
- K. Li, Y. Zhang, Y.-Z. Lin, K. Wang and F. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 28918–28927 CrossRef CAS PubMed.
- W. Ye, J. Hu, X. Hu, W. Zhang, X. Ma and H. Wang, ChemCatChem, 2019, 11, 6372–6383 CrossRef CAS.
- Y. Wang, N. Zhang, R. Hübner, D. Tan, M. Löffler, S. Facsko, E. Zhang, Y. Ge, Z. Qi and C. Wu, Adv. Mater. Interfaces, 2019, 6, 1801664 CrossRef.
- W. Xiong, F. Huang and R.-Q. Zhang, Sustainable Energy Fuels, 2020, 4, 485–503 RSC.
- Y. Luo, Y. Yan, S. Zheng, H. Xue and H. Pang, J. Mater. Chem. A, 2019, 7, 901–924 RSC.
- W. Niu and Y. Yang, ACS Energy Lett., 2018, 3, 2796–2815 CrossRef CAS.
- J. Bian, L. Xi, C. Huang, K. M. Lange, R. Q. Zhang and M. Shalom, Adv. Energy Mater., 2016, 6, 4–9 Search PubMed.
- Q. Ruan, M. K. Bayazit, V. Kiran, J. Xie, Y. Wang and J. Tang, Chem. Commun., 2019, 55, 7191–7194 RSC.
- L. Zhao, J. Ji, Y. Shen, K. Wu, T. Zhao, H. Yang, Y. Lv, S. Liu and Y. Zhang, Chem.–Eur. J., 2019, 25, 15680–15686 CrossRef CAS PubMed.
- F. Podjaski, J. Kröger and B. V. Lotsch, Adv. Mater., 2018, 30, 1705477 CrossRef PubMed.
- N. A. Mohamed, H. Ullah, J. Safaei, A. F. Ismail, M. F. Mohamad Noh, M. F. Soh, M. A. Ibrahim, N. A. Ludin and M. A. Mat Teridi, J. Phys. Chem. C, 2019, 123, 9013–9026 CrossRef CAS.
- M. Sima, E. Vasile, A. Sima, N. Preda and C. Logofatu, Int. J. Hydrogen Energy, 2019, 44, 24430–24440 CrossRef CAS.
- C. Ott, F. Reiter, M. Baumgartner, M. Pielmeier, A. Vogel, P. Walke, S. Burger, M. Ehrenreich, G. Kieslich, D. Daisenberger, J. Armstrong, U. K. Thakur, P. Kumar, S. Chen, D. Donadio, L. S. Walter, R. T. Weitz, K. Shankar and T. Nilges, Adv. Funct. Mater., 2019, 29, 1900233 CrossRef.
- B. Kumru, D. Cruz, T. Heil, B. V. K. J. Schmidt and M. Antonietti, J. Am. Chem. Soc., 2018, 140, 17532–17537 CrossRef CAS PubMed.
- D. Cruz, J. Garcia Cerrillo, B. Kumru, N. Li, J. Dario Perea, B. V. K. J. Schmidt, I. Lauermann, C. J. Brabec and M. Antonietti, J. Am. Chem. Soc., 2019, 141, 12322–12328 CrossRef CAS PubMed.
- Z. Li, S. Wu, J. Zhang, Y. Yuan, Z. Wang and Z. Zhu, Sol. RRL, 2019, 4, 1900413 CrossRef.
- W. Cao, K. Lin, J. Li, L. Qiu, Y. Dong, J. Wang, D. Xia, R. Fan and Y. Yang, J. Mater. Chem. C, 2019, 7, 12717–12724 RSC.
- C. Murugan, K. B. Bhojanaa, W.-J. Ong, K. Jothivenkatachalam and A. Pandikumar, Int. J. Hydrogen Energy, 2019, 44, 30885–30898 CrossRef CAS.
- Q. Cao, B. Kumru, M. Antonietti and B. V. K. J. Schmidt, Mater. Horiz., 2020, 7, 762–786 RSC.
- N. Karjule, R. Phatake, M. Volokh, I. Hod and M. Shalom, Small Methods, 2019, 3, 1900401 CrossRef CAS.
- J. Xu, S. Cao, T. Brenner, X. Yang, J. Yu, M. Antonietti and M. Shalom, Adv. Funct. Mater., 2015, 25, 6265–6271 CrossRef CAS.
- X. Fan, T. Wang, B. Gao, H. Gong, H. Xue, H. Guo, L. Song, W. Xia, X. Huang and J. He, Langmuir, 2016, 32, 13322–13332 CrossRef CAS PubMed.
- X. Xie, X. Fan, X. Huang, T. Wang and J. He, RSC Adv., 2016, 6, 9916–9922 RSC.
- Y. Xiao, M. Chen and M. Zhang, ChemPhotoChem, 2019, 3, 101–106 CrossRef CAS.
- G. Peng, M. Volokh, J. Tzadikov, J. Sun and M. Shalom, Adv. Energy Mater., 2018, 8, 1–7 Search PubMed.
- G. Peng, J. Qin, M. Volokh, C. Liu and M. Shalom, J. Mater. Chem. A, 2019, 7, 11718–11723 RSC.
- R. Godin, Y. Wang, M. A. Zwijnenburg, J. Tang and J. R. Durrant, J. Am. Chem. Soc., 2017, 139, 5216–5224 CrossRef CAS PubMed.
- G. Peng, J. Albero, H. Garcia and M. Shalom, Angew. Chem., Int. Ed., 2018, 57, 15807–15811 CrossRef CAS PubMed.
- X. Lv, M. Cao, W. Shi, M. Wang and Y. Shen, Carbon, 2017, 117, 343–350 CrossRef CAS.
- M. Huang, Y.-L. Zhao, W. Xiong, S. V. Kershaw, Y. Yu, W. Li, T. Dudka and R.-Q. Zhang, Appl. Catal., B, 2018, 237, 783–790 CrossRef CAS.
- P. Luan, Q. Meng, J. Wu, Q. Li, X. Zhang, Y. Zhang, L. A. O'Dell, S. R. Raga, J. Pringle, J. C. Griffith, C. Sun, U. Bach and J. Zhang, ChemSusChem, 2020, 13, 328–333 CrossRef CAS PubMed.
- H. Li, H. Huang, Z. Wang, Z. Zheng, P. Wang, Y. Liu, X. Zhang, X. Qin, Y. Dai, Y. Li, H. Zou and B. Huang, Catal. Today, 2020, 340, 92–96 CrossRef CAS.
- W. Xiong, S. Chen, M. Huang, Z. Wang, Z. Lu and R. Zhang, ChemSusChem, 2018, 11, 2497–2501 CrossRef CAS PubMed.
- K. Xiao, B. Tu, L. Chen, T. Heil, L. Wen, L. Jiang and M. Antonietti, Angew. Chem., Int. Ed., 2019, 58, 12574–12579 CrossRef CAS PubMed.
- K. Xiao, L. Chen, R. Chen, T. Heil, S. D. C. Lemus, F. Fan, L. Wen, L. Jiang and M. Antonietti, Nat. Commun., 2019, 10, 74 CrossRef CAS PubMed.
- J. Albero, E. M. Barea, J. Xu, I. Mora-Seró, H. Garcia and M. Shalom, Adv. Mater. Interfaces, 2017, 4, 1600265 CrossRef.
- Y. Li, G. Liu, D. Jia, C. Li, L. Wang, J. Zheng, X. Liu and Z. Jiao, Catal. Lett., 2019, 149, 19–24 CrossRef CAS.
- D.-D. Qin, J.-J. Quan, S.-F. Duan, J. San Martin, Y. Lin, X. Zhu, X.-Q. Yao, J.-Z. Su, I. Rodríguez-Gutiérrez, C.-L. Tao and Y. Yan, ChemSusChem, 2019, 12, 898–907 CrossRef CAS PubMed.
- Y. Yang, Q. Zhang, R. Zhang, T. Ran, W. Wan and Y. Zhou, Front. Chem., 2018, 6, 1–10 CrossRef CAS PubMed.
- F. Jia, Y. Zhang, W. Hu, M. Lv, C. Jia and J. Liu, Front. Mater., 2019, 6, 1–9 CrossRef.
- G. Peng, J. Qin, M. Volokh and M. Shalom, ACS Appl. Mater. Interfaces, 2019, 11, 29139–29146 CrossRef CAS PubMed.
- S. Otep, T. Michinobu and Q. Zhang, Sol. RRL, 2019, 1900395 CrossRef.
- K. Sivula and R. van de Krol, Nat. Rev. Mater., 2016, 1, 15010 CrossRef CAS.
- I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem., 2017, 1, 0003 CrossRef CAS.
- K. S. Lakhi, D.-H. Park, K. Al-Bahily, W. Cha, B. Viswanathan, J.-H. Choy and A. Vinu, Chem. Soc. Rev., 2017, 46, 72–101 RSC.
- Q. Wang and K. Domen, Chem. Rev., 2020, 120, 919–985 CrossRef CAS PubMed.
- Y. Goto, T. Hisatomi, Q. Wang, T. Higashi, K. Ishikiriyama, T. Maeda, Y. Sakata, S. Okunaka, H. Tokudome, M. Katayama, S. Akiyama, H. Nishiyama, Y. Inoue, T. Takewaki, T. Setoyama, T. Minegishi, T. Takata, T. Yamada and K. Domen, Joule, 2018, 2, 509–520 CrossRef CAS.
- T. Takata and K. Domen, ACS Energy Lett., 2019, 542–549 CrossRef CAS.
- Q. Wang, T. Hisatomi, Q. Jia, H. Tokudome, M. Zhong, C. Wang, Z. Pan, T. Takata, M. Nakabayashi, N. Shibata, Y. Li, I. D. Sharp, A. Kudo, T. Yamada and K. Domen, Nat. Mater., 2016, 15, 611–615 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |