ROY confined in hydrogen-bonded frameworks: coercing conformation of a chromophore†
Sishuang
Tang
a,
Anna
Yusov
 a,
Yuantao
Li
a,
Melissa
Tan
a,
Yunhui
Hao
a,
Zongzhe
Li
a,
Yuantao
Li
a,
Melissa
Tan
a,
Yunhui
Hao
a,
Zongzhe
Li
 a,
Yu-Sheng
Chen
b,
Chunhua T.
Hu
a,
Yu-Sheng
Chen
b,
Chunhua T.
Hu
 a,
Bart
Kahr
a,
Bart
Kahr
 a and
Michael D.
Ward
a and
Michael D.
Ward
 *a
*a
aDepartment of Chemistry and the Molecular Design Institute, New York University, 100 Washington Square East, New York, NY 10003, USA
bChemMatCARS, Center for Advanced Radiation Sources, The University of Chicago, Lemont, Illinois 60439, USA. E-mail: mdw3@nyu.edu
First published on 23rd June 2020
Abstract
5-Methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile is a crystalline compound rich in conformational polymorphs largely owing to the flexible torsion angle that leads to distinct colors, earning it the moniker ROY (Red-Orange-Yellow). Guanidinium organosulfonate hydrogen-bonded frameworks form six crystalline inclusion compounds with ROY, described here, in which the framework limits conformational twisting out of plane. Three of the six inclusion compounds enforce greater planarity and π-conjugation than any of nine ROY polymorphs that have been characterized by single crystal X-ray diffraction.
Introduction
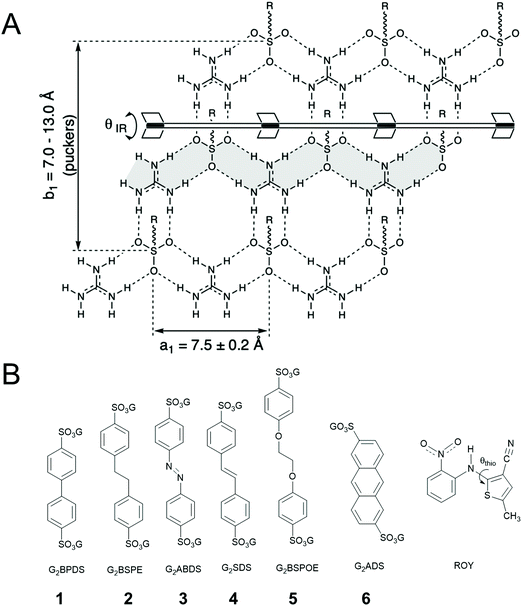
Hydrogen bonding guides the assembly of molecular components into organized supramolecular structures, from natural systems such as DNA to the design and synthesis of new materials. This themed issue is dedicated to Jean-Marie Lehn, who demonstrated in the early 1990s how hydrogen bonding can generate assemblies by molecular design, including cocrystals with molecular “strands” and “ribbons”1,2 and polymeric liquid crystals.3 The concepts emerged in parallel with other reports that illustrated the utility of hydrogen bonds in materials design, from hydrogen-bonded supramolecular polymers4,5 to hydrogen-bonded assemblies and frameworks,6–10 the subject of this contribution. Nearly contemporaneous with the honoree, Etter and Ward reported a series of compounds based on a 2D network assembled through complementary hydrogen-bonding between guanidinium (G) and sulfonate (S) groups of organosulfonates.11 Since that time, our laboratory has created a large family of GS frameworks built from this 2D network with cavities having sizes and shapes that can be regulated by the choice of organosulfonate and the framework architecture, enabling the encapsulation of a large variety of guest molecules,12,13 including laser dyes and other luminophores.14,15 Supramolecular hosts have been reported to exert influence on the conformation of their flexible guest molecules, suggesting an opportunity to regulate crystal properties through manipulation of guest conformation using the diverse library of GS frameworks.16,17 Herein, we describe the inclusion of 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile – also known as ROY for its Red, Orange and Yellow crystal polymorphs – in various GS frameworks (Fig. 1). ROY has become the most vivid example of conformational polymorphism, a term coined by Bernstein and Hagler in 1978,18 wherein crystal packing forces and molecular conformation are strongly interdependent, sometimes resulting in differently colored crystal forms.19–22 ROY molecules sequestered in GS host frameworks focuses conformational analysis on the interactions of the chromophores with the framework, as opposed to interactions with one another.Results and discussion
ROY is associated with eleven polymorphs, including nine with associated crystal structures.23–29 ROY owes its rich polymorphic behavior to the conformational flexibility of the S–C–N–C linkage, characterized by the torsion angle θthio (Fig. 1B). The ROY polymorphs are distinguished by their lattice energy, crystal entropy and conformational energy.24 The θthio values influence the conjugation in ROY, leading to the range of colors among the polymorphs, For example, θthio = 21.7° for the red form, 34.0° < θthio < 52.6° for the orange forms, and 104.1° < θthio < 112.8° for the yellow forms.GS frameworks have proven highly versatile for guest inclusion because of the unusual persistence of two-dimensional (2D) guanidinium sulfonate (GS) network, which usually adopts a quasi-hexagonal symmetry owing to complementary 3-fold symmetry and hydrogen bond donors and acceptors. The pendant organic substituents attached to the sulfonate moiety project from the GS network, serving as pillars (for disulfonates with –SO3− groups on opposite ends) that support lamellar stacking as well as inclusion cavities between the sheets. The resilience of the GS network to a wide range of pillars and guests can be attributed to the strength of the charge-assisted hydrogen bonds and a unique structural compliance through puckering (defined by θIR, Fig. 1) of the GS sheet about a hydrogen-bonded hinge connecting adjacent GS ribbons, which provides a “shrink-wrapping” pathway for achieving close packing with retention of the hydrogen bond connectivity in the GS network. Moreover, the 2D character of the GS network permits an indefinite number of “projection topologies” defined by the pattern of “up-down” orientations of the organosulfonate groups from opposite sides of each GS sheet. This enables the lamellar architectures to form inclusion cavities with various sizes and shapes – as a consequence of templating by the guest molecules during crystal assembly – thereby accommodating a wide range of guests. The frameworks alone typically are colorless (guanidinium azobenzenedisulfonate, G2ABDS, is an exception here), suggesting that the GS frameworks can sequester ROY molecules and enable determination of its conformation and associated color in a sequestered environment.
Crystallizations of GS⊃(ROY) inclusion compounds were performed at the microscale by slow evaporation of solvent from solutions containing the guanidinium organosulfonate apohost and ROY. ROY crystals often formed concomitant with the inclusion compound; therefore crystallization was performed with an excess of the apohost to favor inclusion compound formation (see ESI†). The concomitant formation of ROY crystals prevented confirmation of inclusion compound stoichiometry by NMR spectroscopy and complicated determination of inclusion compound phase purity by powder X-ray diffraction (PXRD). Although the possibility of polymorphism can never be excluded, polymorphism has never been observed in guanidinium organosulfonate compounds, and there was no evidence of polymorphism by visual inspection and Raman spectroscopy among the single crystals of each inclusion compound.
For example, crystallization of ROY with guanidinium 4,4′-biphenyldisulfonate (G2BPDS) by slow evaporation of methanol:acetonitrile solutions containing the dissolved G2BPDS apohost afforded single crystals of G2BPDS⊃(ROY)2/3 (1) as orangish-red {010} plates (Fig. S1, Table 1 and Table S2, ESI†). Single crystal X-ray diffraction confirmed the bilayer architecture (Fig. 2 and Fig. S2, ESI†), in which the long axes of the ROY molecules are aligned along one-dimensional channels flanked by the BPDS pillars (Fig. 3). Despite the non-integral stoichiometry, the ROY molecules are commensurate with the channel axis, with two ROY molecules commensurate with three pillars along the GS ribbons. The torsion angle θthio of the ROY molecules is 10.5°, smaller than that of the red form R of ROY (21.7°), signaling more π conjugation of the phenyl and thiophene rings. The single crystal structure suggests that the near-planar conformation of ROY is a consequence of enforcement due to confinement in the narrow 1D channel (ca. 6.5 Å).
| G2BPDS (1) | G2BSPE (2) | G2ABDS (3) | G2SDS (4) | G2BSPOE (5) | G2ADS (6) | |
|---|---|---|---|---|---|---|
| Color architecture | Red bilayer | Red bilayer | Red bilayer | Red bilayer | Red double-brick | Yellow zigzag |
| θ thio (degrees) | 10.53 | 14.32/14.74 | 16.15/17.33 | 25.08 | 33.86 | 56.38 |
| ν CN (cm−1) | 2208 | 2215 | 2205 | 2215 | 2224 | 2228 |
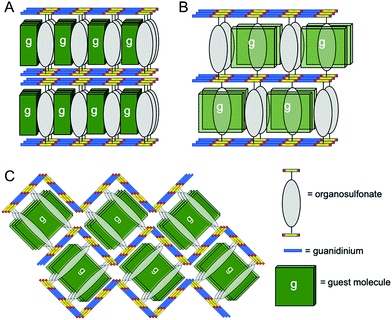 | ||
| Fig. 2 Schematic representation of (A) bilayer, (B) zigzag and (C) double brick architectures in GS frameworks reported here. | ||
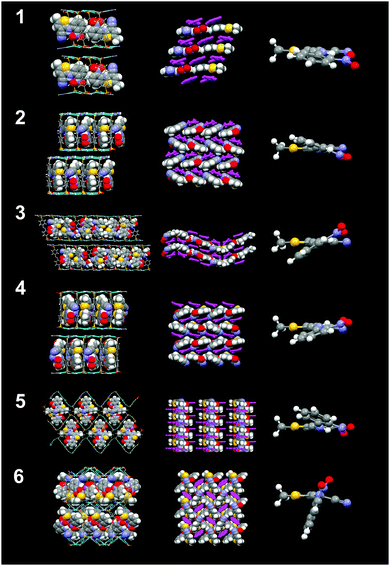 | ||
| Fig. 3 Crystal structures of inclusion compounds 1–6 (top to bottom) and their respective views from the top (second column), their respective ROY conformations (third column). | ||
The bilayer architecture also was observed for the G2BSPE, G2ABDS and G2SDS inclusion compounds with ROY. In these cases, however, the longer pillars result in the long axis of ROY aligned nearly parallel to the long axis of the pillars, reminiscent of enforced alignment of oligothiophene guests in GS frameworks.30 Crystallization of ROY with guanidinium 1,2-bis(4-sulfonatophenyl)ethane (G2BSPE) afforded single crystals of G2BSPE⊃ROY (2) as red {001} plates (Fig. S1, ESI†), along with a mixture of several ROY polymorphs. Single crystal X-ray diffraction revealed the bilayer architecture (Fig. S3, ESI†), with torsion angles for the two ROY molecules in each asymmetric unit of θthio = 14.3° and 14.7°. In this case, the long axes of the ROY guests are nearly parallel to the long axes of the pillars.
Crystallization with guanidinium azobenzenedisulfonate (G2ABDS) produced single crystal slabs of G2ABDS⊃(ROY)3/4(methanol)1/4 (3) as (101) plates (Fig. S1, ESI†). Although these crystals were red, azobenzene alone is red masking the true color of ROY. Nonetheless, ROY in 3 adopted two conformations with torsion angles of 16.2° and 17.3°, which would be consistent with the “red” form of ROY. Although this compound adopts the bilayer architecture (Fig. 3 and Fig. S4, ESI†), the unit cell (a = 35.6995(9) Å; b = 7.3345(2) Å; c = 48.5783(12) Å) is unusually large and the ROY guest arrangement is unusual for GS inclusion compounds. The large lattice constant along the 〈101〉 direction is a consequence of unusual ordering of the guest molecules, wherein each channel contains alternating ROY tetrads and ROY pairs. This is a consequence of an unusual hydrogen-bonding pattern in the GS sheets consisting of a repeating pattern of four GS ribbons with the customary quasi-hexagonal motif connected by hydrogen bonds through the so-called “shifted ribbon” motif, which is occasionally observed in GS compounds (Fig. 3 and Fig. S5, ESI†). The nitro groups of ROY molecules in the tetrad are oriented opposite to the nitro groups of the ROY pairs. Crystallization with guanidinium 4,4′-stilbenedisulfonate (G2SDS) afforded single crystal G2SDS⊃ROY (4) as 〈100〉 needles (Fig. S1, ESI†). Although 4 crystallizes in a bilayer architecture (Fig. 3 and Fig. S6, ESI†), the torsion angle θthio of 25.1° is considerably larger than observed in compounds 1–3. The long axes of ROY, like in BSPE frameworks, are parallel with the long axes of pillars.
Crystallization with guanidinium 1,2-bis(4-sulfonatophenoxy)ethane (G2BSPOE) afforded single crystals of G2BSPOE⊃ROY (5) as red 〈100〉 needles (Fig. S1, ESI†), but with the double-brick architecture (Fig. 2, 3 and Fig. S7, ESI†), in which the projection of pillars on pairs of adjacent GS ribbons alternate across each GS sheet. The formation of the double-brick framework may be attributed to the large volume of the BSPOE pillar, which can frustrate inclusion of ROY in the smaller inclusion cavities of the bilayer architecture. The flexibility of BSPOE appears important, however, as the GS sheet puckers significantly such that the framework conforms to the ROY guests, which are nestled in pockets surrounding by two pairs of BSPOE pillars. The torsion angle θthio = 33.9°, is significantly higher than those in bilayer architectures. Unlike the aforementioned compounds, the ROY thiophene ring is substantially offset from the phenyl ring of the BSPOE pillar, suggested negligible π–π interactions and structure-directing influence.
Crystallization with guanidinium 2,6-anthracene disulfonate (G2ADS) afforded single crystals of G2ADS⊃(ROY)2 (6) as yellow {001} plates (Fig. S1, ESI†), with the orthorhombic zigzag brick architecture (Fig. 2, 3 and Fig. S8, ESI†), in which the projections of the ADS pillars alternates in a zigzag manner across each GS sheet. This architecture, which typically is associated with larger inclusion cavities compared with the bilayer architecture, is likely a manifestation of the larger volume and greater rigidity of the ADS pillar compared with BPDS (185 Å3versus 166 Å3). Pairs of ROY molecules were encapsulated in the pockets flanked by ADS pillars. The torsion angle is θthio = 56.4°. The ROY thiophene ring is nearly perpendicular to its neighboring ADS pillar, indicating negligible π–π interaction and structure-directing influence.
The θthio values for the included ROY molecules are in the range 10.5° ≤ θthio ≤ 56.4°, similar to the θthio values for the red and orange ROY polymorphs. The Raman spectra for 1–6 revealed νCN stretching mode frequencies that decreased with decreasing torsion angle θthio, aligned with the trend reported for the red and orange ROY polymorphs (Fig. 4) and attributed to increased π-conjugation as θthio approaches zero.28 The confinement of the inclusion cavities in the GS frameworks exerts packing forces that result in θthio values far below those for the yellow forms or the recently reported “pumpkin orange” form.29θthio is smaller in inclusion compounds 1–3 than in the red ROY polymorph, which has the lowest value of θthio among the polymorphs. This molecular flattening of ROY can be attributed to the ability of GS inclusion compounds to “shrink wrap” around the guests and achieve close packing through variable pillar conformations, puckering of the GS sheet, and adopting different framework architectures, all on display in compounds 1–6. This feature is evident from the nearly uniform packing fractions of the inclusion compounds, which average 0.70 ± 0.02. (Table S2, ESI†). Moreover, close inspection of the crystal structures of compounds 1–4, which have the smallest values of θthio, reveals near-parallelism of (pillar)phenyl-ROY(phenyl) and (pillar)phenyl-ROY(thiophene) planes, with substantial ring–ring (π–π) overlap, and interplanar distances comparable or somewhat less than the sum of the van der Waals radii for these rings (Fig. S9, ESI†). In contrast, only the (pillar)phenyl-ROY(phenyl) rings are parallel in 5 and 6, enabling larger values of θthio, decreased π-conjugation, and larger νCN stretching frequencies. Vibrational Stark spectroscopy has demonstrated that the vibrational frequency of nitriles, especially aromatic nitriles, can be sensitive to the surrounding electronic field.31 The π–π interactions between the pillar phenyl rings and the ROY thiophene ring in compounds 1–4, as well as the coerced planarity from confinement, likely contribute to the trend in νCN stretching frequencies.
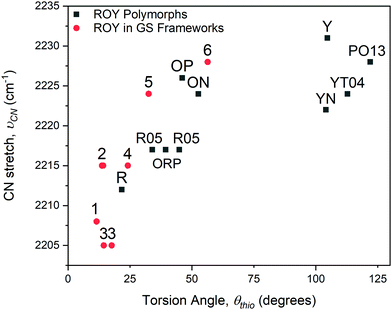 | ||
| Fig. 4 Dependence of the νCN stretching frequency on the ROY torsion angle, θthio, for inclusion compounds 1–6 and for the ROY polymorphs (denoted by their reported labels). | ||
Conclusions
Molecular building blocks with hydrogen bonding substituents have now produced a tremendous number of supramolecular assemblies and molecular frameworks. The guanidinium organosulfonate frameworks continue to surprise with their versatility, encapsulating a wide range of guest molecules, here confining ROY in channels and pockets that prevent severe conformational twisting out of plane. Among the nine solved crystal structures of ROY, the torsion angle θthio ranges from 21.7° to 112.7°. The GS frameworks here, however, constrain the twisting of ROY, with an upper limit of θthio = 56.4° in one of the inclusion compounds and three others with a value of θthio less than the lowest value among the polymorphs. These observations suggest that the properties of chromophores can be regulated by design of suitable molecular frameworks, particularly those that are amenable to adjustments in framework metrics with retention of generic architecture.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support of the Materials Research Science and Engineering Center (MRSEC) Program of the National Science Foundation under Award Number DMR-1420073, the National Science Foundation through DMR-1308677, and the NSF Chemistry Research Instrumentation and Facilities Program (CHE-0840277). Synchrotron X-ray data were collected at the ChemMatCARS Sector 15 at the Advanced Photon Source (APS). NSF's ChemMatCARS Sector 15 is principally supported by the Divisions of Chemistry (CHE) and Materials Research (DMR), National Science Foundation, under grant number NSF/CHE-1834750. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. MT is grateful for a Herman and Margaret Sokol graduate fellowship.Notes and references
- J.-M. Lehn, M. Mascal, A. DeCian and J. Fischer, Molecular recognition directed self-assembly of ordered supramolecular strands by cocrystallization of complementary molecular components, J. Chem. Soc., Chem. Commun., 1990, 479 RSC.
- J.-M. Lehn, M. Mascal, A. DeCian and J. Fischer, Molecular ribbons from molecular recognition directed self-assembly of self-complementary molecular components, J. Chem. Soc., Perkin Trans. 2, 1992, 461 RSC.
- C. Fouquey, J.-M. Lehn and A.-M. Levelut, Molecular recognition directed self-assembly of supramolecular liquid crystalline polymers from complementary chiral components, Adv. Mater., 1990, 2, 254 CrossRef CAS.
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Supramolecular polymers, Chem. Rev., 2001, 101, 4071 CrossRef CAS PubMed.
- R. P. Sijbesma, F. H. Beijer, L. Brunsveld, B. J. B. Folmer, J. H. K. Ky Hirschberg, R. F. M. Lange, J. K. L. Lowe and E. W. Meijer, Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding, Science, 1977, 278, 1601 CrossRef PubMed.
- P. Brunet, M. Simard and J. D. Wuest, Molecular tectonics. Porous hydrogen-bonded networks with unprecedented structural integrity, J. Am. Chem. Soc., 1997, 119, 2737 CrossRef CAS.
- M. Simard, D. Su and J. D. Wuest, Use of hydrogen bonds to control molecular aggregation. Self-assembly of three-dimensional networks with large chambers, J. Am. Chem. Soc., 1991, 113, 4696 CrossRef CAS.
- M. W. Hosseini, Molecular tectonics:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) From simple tectons to complex molecular networks, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS PubMed.
From simple tectons to complex molecular networks, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS PubMed. - F. Garcia-Tellado, S. J. Geib, S. Goswami and A. D. Hamilton, Molecular recognition in the solid state: Controlled assembly of hydrogen-bonded molecular sheets, J. Am. Chem. Soc., 1991, 113, 9265 CrossRef CAS.
- O. Felix, M. W. Hosseini and A. De Cian, Design of 2-D hydrogen bonded molecular networks using pyromellitate dianion, cyclic bisamidinium dication as complementary tectons, Solid State Sci., 2001, 3, 789 CrossRef CAS.
- V. A. Russell, M. C. Etter and M. D. Ward, Layered materials by molecular design: Structural enforcement by hydrogen bonding in guanidinium alkane- and arenesulfonates, J. Am. Chem. Soc., 1994, 116, 1941 CrossRef CAS.
- K. T. Holman, A. M. Pivovar, J. A. Swift and M. D. Ward, Metric engineering of soft molecular host frameworks, Acc. Chem. Res., 2001, 34, 107 CrossRef CAS PubMed.
- T. Adachi and M. D. Ward, Versatile and hydrogen-bonded host frameworks, Acc. Chem. Res., 2016, 49, 2669 CrossRef CAS PubMed.
- M. Handke, T. Adachi, C. Hu and M. D. Ward, Encapsulation of isolated luminophores within supramolecular cages, Angew. Chem., Int. Ed., 2017, 56, 13901 CrossRef CAS.
- A. C. Soegiarto and M. D. Ward, Directed organization of dye aggregates in hydrogen-bonded host frameworks, Cryst. Growth Des., 2009, 9, 3803 CrossRef CAS.
- B.-Q. Ma, Y. Zhang and P. Coppens, Multiple conformations of benzil in resorcinarene-based supramolecular host matrixes, J. Org. Chem., 2003, 68, 9467 CrossRef CAS PubMed.
- B.-Q. Ma and P. Coppens, Variable conformation of benzophenone in a series of resorcinarene-based supramolecular frameworks, Cryst. Growth Des., 2004, 4, 1377 CrossRef CAS.
- J. Bernstein and A. T. Hagler, Conformational polymorphism. The influence of crystal structure on molecular conformation, J. Am. Chem. Soc., 1978, 100, 673 CrossRef CAS.
- P. H. Toma, M. P. Kelley, T. B. Borchardt, S. R. Byrn and B. Kahr, Chromoisomers and polymorphs of 9-phenylacridinium hydrogen sulfate, Chem. Mater., 1994, 6, 1317 CrossRef CAS.
- B. A. Nogueira, C. Castiglioni and R. Fausto, Color polymorphism in organic crystals, Chem. Commun., 2020, 3, 34 CrossRef.
- S. R. Byrn, D. Curtin and I. Paul, X-ray crystal structures of the yellow and white forms of dimethyl 3,6-dichloro-2,5-dihydroxyterephthalate and a study of the conversion of the yellow form to the white form in the solid state, J. Am. Chem. Soc., 1972, 94, 890 CrossRef CAS.
- M. Tristani-Kendra, C. J. Eckhardt, J. Bernstein and E. Goldstein, Strong coupling in the optical spectra of polymorphs of a squarylium dye, Chem. Phys. Lett., 1983, 98, 57 CrossRef CAS.
- G. Stephenson, T. Borchardt, S. Byrn, J. Boyer, S. Snorek, C. Bunnell and L. Yu, Conformational and color polymorphism of 5-methyl-2-[(2-nitrophenyl) amino]-3-thiophenecarbonitrile, J. Pharm. Sci., 1995, 84, 1385 CrossRef CAS PubMed.
- L. Yu, A. G. Stephenson, C. A. Mitchell, C. A. Bunnell, S. V. Snorek, J. J. Bowyer, T. B. Borchardt, J. G. Stowell and S. R. Byrn, Thermochemistry and conformational polymorphism of a hexamorphic crystal system, J. Am. Chem. Soc., 2000, 122, 585 CrossRef CAS.
- S. Chen, H. Xi and L. Yu, Cross-nucleation between ROY polymorphs, J. Am. Chem. Soc., 2005, 127, 17439 CrossRef CAS PubMed.
- S. Chen, I. Guzei and L. Yu, New polymorphs of ROY and new record of coexisting polymorphs of solved structures, J. Am. Chem. Soc., 2005, 127, 9881 CrossRef CAS PubMed.
- M. Tan, A. G. Shtukenberg, S. Zhu, W. Xu, E. Dooryhee, S. M. Nichols, M. D. Ward, B. Kahr and Q. Zhu, ROY revisited, again: the eighth solved structure, Faraday Discuss., 2018, 211, 477 RSC.
- L. Yu, Polymorphism in molecular solids: an extraordinary system of red, orange, and yellow crystals, Acc. Chem. Res., 2010, 43, 1257 CrossRef CAS PubMed.
- K. S. Gunshurst, J. Nyman and S. X. M. Boerrigter, The PO13 crystal structure of ROY, CrystEngComm, 2019, 21, 1363 RSC.
- A. C. Soegiarto, A. Comotti and M. D. Ward, Controlled orientation of polyconjugated guest molecules in tunable host cavities, J. Am. Chem. Soc., 2010, 132, 14603 CrossRef CAS PubMed.
- S. S. Andrews and S. G. Boxer, Vibrational Stark effects of nitriles II. Physical origins of Stark effects from experiment and perturbation models, J. Phys. Chem. A, 2002, 106, 469 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and characterization details and additional figures. CCDC 1992984–1992989. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0qm00200c |
| This journal is © the Partner Organisations 2020 |