Open Access Article
Open Access ArticleAnti- and pro-oxidative mechanisms comparing the macular carotenoids zeaxanthin and lutein with other dietary carotenoids – a singlet oxygen, free-radical in vitro and ex vivo study
Fritz
Boehm
a,
Ruth
Edge
 b and
T. George
Truscott
*c
b and
T. George
Truscott
*c
aPhotobiology Research, Internationales Handelszentrum (IHZ), Friedrichstraße 95, 10117 Berlin, Germany
bDalton Cumbrian Facility, Westlakes Science Park, The University of Manchester, Cumbria, CA24 3HA, UK
cSchool of Chemical and Physical Sciences, Keele University, Staffordshire, ST5 5BG, UK. E-mail: t.g.truscott@keele.ac.uk
First published on 19th June 2020
Abstract
The interactions of dietary carotenoids, and particularly the xanthophylls in the macula, with singlet oxygen and three different oxy-radicals, (hydroxyl radical, nitrogen dioxide and the superoxide radical anion) are compared using pulsed laser and γ-techniques. The results give possible molecular mechanisms for the switch from anti-oxidant (protection) by carotenoids to pro-oxidant (damage) by carotenoids. The participation of oxygen in radical mechanisms in the presence of different carotenoids is compared for the different radicals. It is shown that the mechanistic role of oxygen differs very significantly for anti-/pro-oxidation by hydroxyl radicals when compared to nitrogen dioxide. Lutein was found to be an extremely good cell protector against hydroxyl radicals at all oxygen concentrations, including under physiological conditions.
Introduction
As well as being important anti-oxidants in the eye, carotenoids (CAR) are of significance in photosynthesis, the skin, and in several other aspects of human health. The anti-oxidant processes mainly concern quenching of singlet oxygen (1O2) and/or free radicals. The quenching of 1O2 by dietary carotenoids in ‘simple’ solvents and heterogeneous environments from micelles and liposomes to cells has already been extensively reported,1 and it is well established most quench 1O2 extremely efficiently, near the diffusion limit, (except lutein (LUT), with 10 conjugated double bonds, being typically 50% less efficient). However, some of this work deserves further comment, as follows: Ogilby and co-workers have thrown doubt on the relevance of such quenching – they were able to detect 1O2 in cells2 using individual HeLa mammalian cells and microscope-based time-resolved 1275 nm luminescence, but they could observe no quenching of the 1O2 by β-carotene (β-CAR). However, Telfer and co-workers show carotenoids quench some of the 1O2 in cells, but not all, due to the distance of the carotenoids from the source of the 1O2.3 This work, in photosynthetic systems, suggests that in a specific biological environment, quenching will only be efficient when the carotenoid is sufficiently close to the source of the 1O2. Virtually all studies have concerned the 1O2 quenching by carotenoids as non-aggregated, monomeric species. However, when aggregated, quenching by the xanthophylls (LUT and zeaxanthin (ZEA)) and probably all carotenoids is markedly reduced. The carotenoid concentration in the fovea of the eye approaches 1 mM, and the ratio of LUT to ZEA to meso-ZEA is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 So, the extension of singlet oxygen quenching to aggregated carotenoids should also be further considered. Clearly, there is more to learn about the interaction of β-CAR with 1O2 in the ex vivo and in vivo situation.
1.4 So, the extension of singlet oxygen quenching to aggregated carotenoids should also be further considered. Clearly, there is more to learn about the interaction of β-CAR with 1O2 in the ex vivo and in vivo situation.
While the overall process of 1O2 quenching simply converts the excess energy to heat via the carotenoids lowest triplet state, the reaction of carotenoids, with free radicals is much more complex, partly because individual radicals behave differently from each other and also because of a likely role of the oxygen concentration – this will also depend on the type of radical.
In this work, we review and give new results showing how three distinct radicals, the hydroxyl radical, nitrogen dioxide and the superoxide radical anion (OH˙, NO2˙ and O2˙− respectively) exhibit totally different chemistry in their interaction with carotenoids. Often this is ‘over simplified’ by just referring to carotenoid quenching of Reactive Oxygen Species (ROS). Here, we compare OH˙, NO2˙ and O2˙− reactivity with LUT, ZEA and other carotenoids, especially lycopene (LYC) – there are claims that LYC has a role in macular protection despite not accumulating in the macula.5,6
Experimental
Radiation processes
The radiation processes are well established.7 The NO2˙ radical is generated, in aqueous solutions, using 355 nm pulsed laser excitation of 1-nitronaphthalene (NN). This produces the NN triplet state (3NN) which can subsequently react with sodium nitrite, to yield the radical anion of nitronaphthalene (NN˙−) and the nitrogen dioxide radical (NO2˙).8–10The steady-state γ-radiolysis was performed in a Model 812 cobalt-60 irradiator (Foss Therapy Services Inc.) using a turntable system rotating at 12 rpm with an average absorbed dose rate of around 90–120 Gy min−1. All irradiations were carried out at room temperature and solutions were saturated, as appropriate, with research-grade nitrous oxide, argon, oxygen or mixtures. Dose rates were determined using a Radcal Corporation Accu-Dose+ base unit equipped with a 10 × 6−0.18 ion chamber (calibrated annually by PHE to traceable national standards) and were checked against standard Fricke dosimetry and found to be accurate to ±5%.
The radiolysis products of water7 can be simplified to the generation of main radical products OH˙ and the solvated electron, eaq−
| H2O → OH˙ + eaq− + H˙ + non radical species | (1) |
At all oxygen concentrations used the eaq− is totally converted to O2˙−
| eaq− + O2 → O2˙− | (2) |
The cell types used for the ex vivo studies have also been described previously.8–10
The individual carotenoids were gifts from Hoffmann-la-Roche (better than 99.9% via HPLC). For the ex vivo studies, dietary supplements were used, and the claimed amount checked via absorption spectroscopy. The duration of the diet (between 4 days and 2 weeks) was varied but maintaining a constant total amount of carotenoid, and this had no significant effect on our results.
The SOD (bovine) was supplied by Sigma-Aldrich as 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units and was used at 30
000 units and was used at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, units in a cell suspension volume maintained at 700 μl.
000, units in a cell suspension volume maintained at 700 μl.
The sources of all other chemicals have been described previously.8
While there may be a link between dietary intake and serum levels of specific carotenoids this is complex and varies from one individual to another. We simply used a high dose of each supplement to ensure maximum uptake for each carotenoid, as described previously for lycopene.10 No attempt was made to determine the amount of carotenoid nor the distribution in each cell. We examined the effect of changing the rate of dietary intake – i.e. shorter times at higher concentrations. This made no difference to the results obtained.
Results and discussion
Free radicals, of course, are characterized by an unpaired electron. When a free radical interacts with a carotenoid, several possible modes of reaction can occur, and these depend mainly on the nature of the free radical. The situation is, therefore, much more complex than the quenching of 1O2 by carotenoids. Many studies of the generation and reactions of carotenoid radical cations, and anions, have been reported in several reviews.11–13 One of the most well studied species is the carotenoid radical cation obtained via abstraction of an electron from the carotenoid by a strongly oxidising free radical.| CAR + R˙ → CAR˙+ + R− | (3) |
Typical examples of such oxidising free radicals that lead to electron transfer are chlorinated peroxyl radicals, such as CCl3O2˙, nitrogen dioxide (NO2˙), arylperoxyl radicals, dibromide radical anion (Br2˙−) and sulfonyl radicals (RSO2˙). However, as we will discuss below, the hydroxyl radical (OH˙), even though it is a strongly oxidising radical, mainly adds to the carotenoid rather than producing the carotenoid radical cation. Furthermore, radicals, such as CCl3O2˙, and possibly NO2˙, may give both the radical cation and also add to the carotenoid. Though, following extensive studies, only electron transfer has been previously observed for the reaction of β-CAR with NO2˙.14
Two significant results from pulse radiolysis and laser flash photolysis show (i) the carotenoid radicals are themselves strongly oxidising radicals which can cause damage by oxidising bio-substrates and (ii) ascorbic acid (and other reducing species) can remove (quench) such carotenoid radical cations and possibly ameliorate the potential deleterious problems associated with the carotenoid radical cation production. See, for example ref. 15 and 16.
It has been shown that, for heavy smokers, a high concentration of β-CAR can have a surprisingly damaging effect for lung cancer,17 and our speculation was that this may, in part, be due to smoke-based free radicals (e.g. NO2˙) reacting with β-CAR to generate the β-CAR radical cation in the lung, which can then damage biomolecules.10 Smokers have low levels of ascorbic acid,18 and so there is insufficient to efficiently quench any β-CAR radical cation generated via NO2˙.
Results from Skibsted and co-workers19,20 are consistent with the above, showing reactions of β-CAR radical cation with tyrosine and tryptophan, regenerating the parent β-CAR but oxidising the amino acids. Interestingly, Skibsted used pH conditions where the redox potentials (the standard reduction potentials) were the same for tyrosine and tryptophan (the reduction potential for β-CAR radical cation is independent of pH in the region studied). These workers found that tyrosine reacted an order of magnitude faster than tryptophan and speculate that this may account for tyrosine, rather than tryptophan, as the protein moiety reacting with β-CAR in the protective mechanism, which operates in the photosynthetic reaction center. As Skibsted points out, the driving force in these reactions depends on the “local” pH and in proteins the reverse reaction between a tyrosine radical and β-CAR may also be important.
Carotenoids and NO2˙
In early work we showed a significant protection of human cells against pulsed-laser generated NO2˙ by LYC.8 In this work we compared the percentage of dead cells (obtained via eosin staining techniques) with and without LYC supplementation under atmospheric conditions. The ratio was reported as a protection factor with values between 8.2 and 8.6. A somewhat lower protection was observed for β-CAR supplementation (3.0–3.5). However, these early studies involved the use of ‘water-solubilised’ carotenoids (rather than the carotenoid itself) and an in vitro procedure in which the ‘soluble’ carotenoids were simply added to the blood lymphocyte suspensions prior to the exposure to the NO2˙. That is, no dietary route to carotenoid supplementation was used. Nevertheless, these studies did show that LYC can be an efficient protective species against NO2˙, a major toxic component of polluted air.Subsequently,9 we reported a synergistic cell protection against NO2˙-induced cell kill when the actual diet was supplemented with β-CAR and with and without dietary vitamin C and E (typically 1000 mg day−1 and 800 mg day−1 respectively). The protection factor increasing from 2.0, 1.8 and 1.2 for β-CAR, vitamin E and vitamin C individually to 10 when all 3 were co-supplemented.
We now report similar measurements with LYC instead of β-CAR. Once again, control experiments with supplementation with only vitamin C and only vitamin E showed protection factors of 1.8 and 1.2 while with LYC (30 mg day−1 for 3 weeks) plus these two vitamins the protection factor was greatly increased to 21. Essentially, the same synergistic observations, in a non-biological system, have been reported by Liu et al.21 We have also previously reported similar synergism when the specific amounts of lycopene were replaced by either 2 weeks supplementation with tomato soup or with pre-boiled tomato juice.10
To understand the mechanism of such synergism it is noteworthy that the beneficial quenching of the damaging NO2˙ radical by a dietary carotenoid also generates the radical cation of the carotenoid, as described above, and this is a strongly oxidising species itself which can oxidise biological moieties such as tryptophan, tyrosine and cysteine19,20 leading to deleterious membrane protein damage and subsequent disease. However, we have previously shown in both methanol and micellar environments, that vitamin C quenches carotenoid radical cations15,16 and we suggest that such a process between vitamin C and the carotenoid radical cation could account for the observed synergistic effects. Furthermore, it could be related to the much discussed and surprising results showing β-CAR enhances lung cancer incidence in smokers.17 Of course, smokers are very low in vitamin C in the lungs!
The above does depend on the NO2˙ reacting with carotenoids substantially via electron transfer, as has been reported by Everett et al.14 However, no such information is available for LYC.
In new work we have studied the cell killing by NO2˙ as a function of the oxygen concentration. As discussed below in detail, we would expect no effect on carotenoid radical cation/vitamin C/E processes of oxygen concentration. If radical addition processes arise then addition of oxygen (to give, possibly, a damaging peroxyl radical) will be significant and the above mechanisms involving radical cations would not apply.
These new results show a cell kill of 53.7%, 54.2% and 57.8% at 0%, 20% and 100% oxygen, respectively, for the unprotected cells (i.e. no LYC, vitamin C or E supplementation).
For the protected cells (with the anti-oxidant supplement) the corresponding cell kill was 3.2%, 3.1% and 6.2%.
These lead to protection factors of 16.8 and 17.5 for 0% and 21% oxygen – that is no effect of the oxygen. For the 100% oxygen the protection factor is somewhat reduced to 9.3.
Our overall conclusion is that oxygen has played no role in normal physiological conditions and hence the value of LYC (and possibly β-CAR22) is worthwhile considering as a means of reduction damage (possibly lung damage) due the environmental NO2˙ in non-smokers. Of course, other processes involving other smoke-related radicals can also be related to the increased lung cancer in the presence of high β-CAR diets17 so such a beneficial value (for all dietary carotenoids) needs careful assessment.
Carotenoids and OH˙ – the role of oxygen
Here we compare the macular carotenoids, LUT and ZEA with previous data on LYC (with re-interpretation of some of those results), β-CAR and astaxanthin (ASTA). In this series of experiments, we used commercial carotenoid supplements. We avoided commercial supplement with carotenoid mixtures even though many people use such mixtures (e.g. LUT plus ZEA for AMD). We do not know the concentration of a carotenoid in each cell nor its position (near the aqueous interface or totally embedded in the membrane for example). Instead, our aim was to compare the different carotenoids where the cell loading is as high as can be achieved in normal use by the public – i.e. using amount of carotenoids well above that normally used (typically 70–100 mg day−1 for 2 weeks or variations on this as mentioned above – all combinations studied gave the same results) so that effectively all cells have the maximum carotenoid loading under normal conditions. The numerical protection factors we report may not be of significance. However, the comparisons in these factors, as a function of oxygen concentration, for the 5 carotenoids studied are of interest. Indeed, our comparative data may suggest one possible explanation as to why the macular chooses ZEA and LUT for best protection.The first carotenoid we choose to study the protection of human cells against damage by OH˙ (see eqn (1) and (2) above) generated via γ-radiation was LYC11 – chosen partly because it has been established it is the carotenoid which is the strongest reducing agent (i.e. the most easily oxidised)1 and also because it may be important with respect to protection against AMD despite not accumulating in the macular.5,6
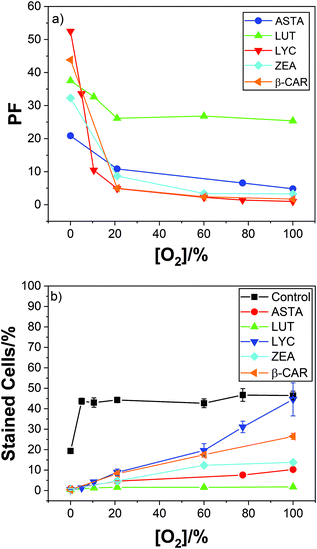
In air we reported a reduction in cell kill due to dietary LYC (typically about 100 mg day−1 over 2 weeks, but other time/dose combinations gave the same results and comparisons with added vitamin C and E also showed no effect on LYC protection against the cell kill) from near 44% in the absence of lycopene to near only 9% with the supplementation – this is a protection factor (PF) of near 5 ± 1.3. While this is a significant protection, we saw a most dramatic effect of high oxygen concentration on the PF as shown in Fig. 1.
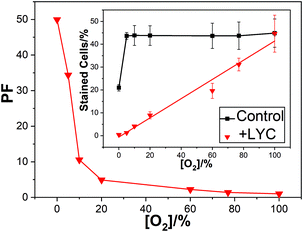 | ||
| Fig. 1 Mean PF against oxygen percentage for lymphocyte cells irradiated with 2000 Gy dose; inset shows the percentage of dead cells under these conditions. | ||
At 100% oxygen there was no protection at all while at 0% oxygen the PF was over 50. Thus, LYC gave near total protection against damage by OH˙ in the absence of oxygen.
Fig. 1 shows that in the presence of the LYC supplement, increasing the oxygen concentration reduces the protection of the cells. That is, despite the OH˙ concentration remaining constant between 0 and 100% oxygen, and the O2˙− concentration between 5 and 100%, there is a huge change in the PF. Clearly, a key finding is that in the presence of the LYC supplement the PF is affected, to a large extent, by the concentration of oxygen itself rather than by the free radical concentrations.
It is instructive to compare the present results using γ-radiation with those we have previously published8–10 for nitrogen dioxide (NO2˙), discussed above. Using similar lymphoid cell procedures, we obtained a PF of about 17 in the laser studies – that is, considerably more protection than seen in the γ-radiation work (PF near 5 in air-saturated conditions). While the laser study generated virtually only oxidising NO2˙ radicals the γ-radiation generates, under the conditions used in the presence of oxygen, both OH˙ radicals and the superoxide radical anion (discussed below). This comparison shows the LYC supplement protects against damage due to the oxidising NO2˙ radicals markedly better than against the OH˙ radical (the superoxide reactivity is discussed below).
In a totally non-biological environment (non-cellular and non-aqueous) key work by Burton and Ingold23 investigated the effect of oxygen concentration on processes involving lipid peroxidation leading to free radicals and oxygen radical addition reactions. In this study, adducts between the carotenoid and peroxyl radicals were also invoked to explain a switch from anti- to pro-oxidant behaviour of β-CAR as the oxygen concentration increased in their system.
One mechanism discussed1,23 to explain the effect of O2 concentration on the cell protection by the LYC supplement is related to the ability of O2 to add to neutral radicals to produce reactive peroxyl radicals. There is much evidence that the strongly oxidising OH˙ forms adducts with most substrates so that LYC, OH˙ neutral radical adduct (LYC–OH˙) is likely to be a significant product in our cellular studies.24–26 Another possible product is the neutral LYC radical (LYC˙) formed via hydrogen atom abstraction.27 In the presence of oxygen, both radicals may add molecular oxygen to form reactive peroxyl radicals leading to species such as LYC–OH–OO˙ as shown in eqn (4)–(6) below or LYC–OO˙ in the case of hydrogen abstraction followed by oxygen addition. We suggested, such peroxyl radicals are formed in increasing concentration as the oxygen concentration increases and proposed these killed the lymphoid cells. Our cell study showed a near linear relationship between oxygen concentration and cell kill (Fig. 1 inset) and the following postulated mechanism:
| LYC + OH˙ → LYC–OH˙ | (4) |
| LYC–OH˙ + O2 ⇄ LYC–OH–OO˙ | (5) |
| LYC–OH–OO˙ + cell → cell kill | (6) |
However, other possible mechanisms involving oxygen concentration are possible.28,29 Our new results concern the carotenoids which accumulate in the macula – ZEA and LUT and comparative results are now presented on the protection against OH˙ by these xanthophylls. In addition, we present results on another xanthophyll – ASTA and some results on the ‘parent’ carotenoid, β-CAR.
Typical results are given in Fig. 2 showing comparative plots of cell kill and protection factors as a function of oxygen percentage.
The effect of increasing oxygen concentration reducing the efficiency of cell protection by LYC, discussed above, is also observed with the 4 new carotenoids studied. But less so with the xanthophylls and especially so for LUT, which is still an effective cell protector even at 100% oxygen.
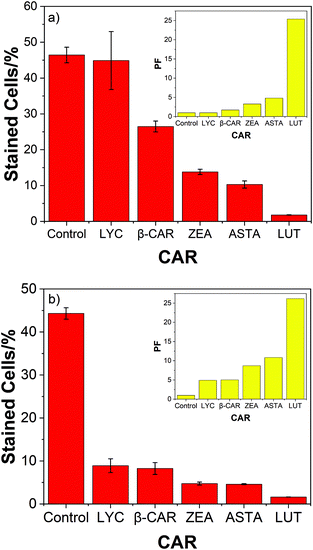
The high protective ability of lutein compared to the 4 other carotenoids studied is also evident from Fig. 3 – the corresponding bar charts for air and oxygen.
Two significant trends due to the presence of oxygen are clear. Now the hydrocarbons (LYC and β-CAR) show the lowest PF compared to the xanthophylls (ASTA, ZEA and LUT) and, LUT shows the highest PF, especially at high oxygen concentrations. In fact, the efficient cell protection by lutein shows, more-or-less, rather little change from 0–100% oxygen (37; 33; 26; 27; and 25 for oxygen percentages of 0; 10; 21; 60; and 100%).
So, overall, our work with OH˙ in the presence of oxygen suggests that while lutein is the poorest protector against one ROS – singlet oxygen, it is significantly the best against radicals which may arise in oxygenated conditions (as in the eye).
Carotenoids and O2˙− – the role of oxygen and SOD
The role and effectiveness of the first-line defense antioxidant, superoxide dismutase (SOD), is important and indispensable as an antioxidant, especially against O2˙−, which is perpetually generated in normal body metabolism.The product arising from carotenoids reacting with O2˙− is not a primary concern of this work and is not well understood. Generation of the carotenoid radical cation has been suggested:30
| CAR + O2˙− (+2H+) → CAR˙+ + H2O2 (at physiological pH) | (7) |
While others,31 using density function theory, show O2˙− acts as an electron donor species to generate the carotenoid radical anions:
| CAR + O2˙− → CAR˙− + O2 | (8) |
In our work on LYC protection9 we were able to calculate the theoretical concentration of OH˙, O2˙− and the solvated electron (e−aq) and this showed a significant cell kill by O2˙− and OH˙ but rather little kill by the e-aq. (5.6%, 8.75% and 2% respectively). As discussed above we report a significant and differing effect of oxygen concentration on the PFs of the 5 carotenoids studied and explained this in terms of the neutral radical adducts, CAR–OH˙, adding oxygen. Clearly, the effect of oxygen concentration on the cell kill by O2˙− could be important.
Furthermore, an aspect of our early work9 which was not previously presented was that at 100% oxygen the LYC gives no cell protection whatsoever – that is, no protection against OH˙ nor O2˙−. While at low oxygen levels (5%) there is only 1.3% cell kill in the presence of the LYC supplement. This implies LYC is protecting against OH˙ and O2˙− at this low oxygen concentration.
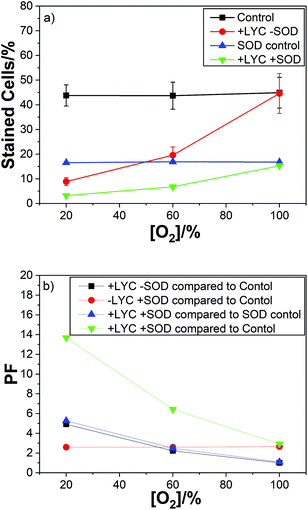
In order to gain more information on the effects of O2˙− we present results on the comparative effects, with and without superoxide dismutase (SOD) for LYC, ZEA and β-CAR. In particular, we report the results on protection factors with and without quenching of O2˙− by SOD.
For LYC we used air, 60% and 100% oxygen, with previously reported PFs of 5, 2 and 1(i.e. no protection at all at 100% oxygen). As can be easily seen from Fig. 4 there is no effect of oxygen concentration on the protection factor or cell kill count for just SOD, in the absence of LYC, (red plot).
As can be seen the PF observed for the SOD was constant at 2.6 for 21%, 60% and 100% oxygen – showing no effect of oxygen concentration on the protection of the cells by SOD (Fig. 4b). It is important to note the SOD was simply added to the cell suspension and so was predominantly localised outside the cells while the carotenoids (lycopene in this case) are present in the cells, i.e. in the membranes. So, a comparison between the individual protectors (SOD and lycopene here) is not likely to be useful. However, the same procedure was adopted for all the carotenoids studied so the comparison of the different carotenoids is worthwhile.
Of course, the combination of LYC and SOD gives the best protection – ranging from 14 to 3 for air to 100% oxygen. In good agreement with the protection for the individual antioxidants. The data also shows (compare the black and blue plots) no effect of the presence of SOD on the protection by LYC at any of the oxygen concentrations studied.
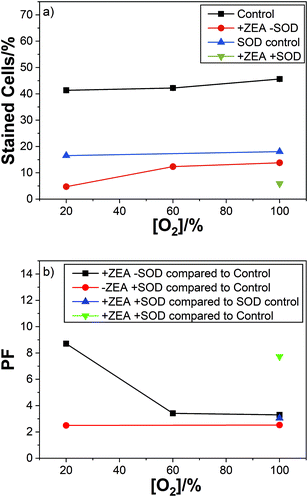
The corresponding results for ZEA are summarised in the Fig. 5.
We obtained similar results with the other carotenoids studied. Showing no effect of SOD on the carotenoid cell protection with respect to oxygen concentration.
We compare the PF for all carotenoids studied against O2˙− and OH˙ (combined) to that of SOD (Table 1 below).
| Carotenoid | % O2 | PF | % O2 | PF |
|---|---|---|---|---|
| LYC | 21 | 4.9 | 100 | 1.0 |
| β-CAR | 21 | 5.0 | 100 | 1.7 |
| ASTA | 21 | 10.8 | 100 | 4.8 |
| ZEA | 21 | 8.7 | 100 | 3.3 |
| LUT | 21 | 26.0 | 100 | 25.0 |
| SOD | 21 | 2.6 | 100 | 2.6 |
As can be seen under near physiological conditions LUT shows the highest PF by a significant amount, at all oxygen concentrations. As noted above, the important comparisons are between the individual carotenoids PFs against O2˙− + OH˙ themselves rather than each with SOD. Possibly this reflects an important property of LUT – to protect efficiently against a wide range of oxidising radicals including OH˙ and O2˙− at all oxygen concentrations, whereas SOD only protects efficiently against O2˙−. Possibly the outcome of the interaction of carotenoids, including LUT, with O2˙− leads to other potentially damaging products such as carotenoid radicals,30,31 with no such potential damage from SOD.
Conclusions
While the role and relative efficiencies of the dietary carotenoids to quench singlet oxygen in solvents and some heterogeneous environments are well established this process is less clear in cells. In a single mammalian HeLa cell, 1O2 was detected, but no quenching of the 1O2 by β-CAR was observed, although such quenching has been reported in photosynthetic systems. A further complication, especially for the macular pigment, ZEA, is the marked reduction in 1O2 quenching due to aggregation – much less effects of aggregation for LUT being reported.For ROS other than 1O2 we have shown the three radicals NO2˙, OH˙ and O2˙− behave quite differently in reactivity with carotenoids.
The concentration of oxygen is shown to be pivotal in understanding the protection of cells against OH˙ radicals. Particularly the effectiveness of the macular pigment LUT (compared to other dietary carotenoids including ZEA) to protect human cells against OH˙ damage at physiological (and higher) oxygen concentrations is shown.
Neither NO2˙ and O2˙− showed and any marked effects of oxygen concentration. However, the NO2˙ radical was shown to be quenched by dietary carotenoids but this quenching leads to the production of carotenoid radical cations and pointed to the value of reducing agents, especially ascorbic acid, to prevent a switch to damaging pro-oxidation. The possible relevance of this to the increased lung cancer risk of heavy smokers by β-carotene was invoked and extended to a possible benefit for LYC/vitamin C mixtures.
Of course, SOD is a key in vivo protector against the O2˙− radicals. However, our new results indicate that LUT protects against O2˙− (by an order of magnitude) more effectively than SOD. While our ex vivo results are just a model system, the efficient human cell protection provided by LUT at all oxygen concentrations, not provided by other carotenoids, may indicate one reason why it is LUT that accumulates in the macula.
Overall, there is still much to learn about a possible anti/pro-oxidation switch for dietary carotenoid interactions with individual ROS components and this requirement is often hidden by ‘simple’ statements concerning dietary carotenoids quenching ROS.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the support of Keele University and The University of Manchester's Dalton Cumbrian Facility (DCF), a partner in the National Nuclear User Facility, the EPSRC UK National Ion Beam Centre and the Henry Royce Institute.We would also like to acknowledge Dr Joan Roberts for the invitation to present this work at the World Congress on Light and Life (17th Congress of the International Union of Photobiology and 18th Congress of the European Society for Photobiology), 25-30 August 2019, Barcelona, Spain.
References
- R. Edge and T. G. Truscott, Antioxidants, 2018, 7, 1 CrossRef PubMed.
- G. N. Bosio, T. Breitenbach, J. Parisi, M. Reigosa, F. H. Blaikie, B. W. Pedersen, E. F. F. Silva, D. O. Martire and P. R. Ogilby, J. Am. Chem. Soc., 2013, 135, 272 CrossRef CAS PubMed.
- A. Telfer, Plant Cell Physiol., 2014, 55, 1216 CrossRef CAS PubMed.
- R. A. Bone, J. T. Landrum, L. M. Friedes, C. M. Gomez, M. D. Kilburn, E. Menendez, I. Vidal and W. Wang, Exp. Eye Res., 1997, 64, 211 CrossRef CAS PubMed.
- N. Cardinault, J.-H. Abalain, B. Sairafi, C. Coudray, P. Grolier, M. Rambeau and J.-C. Carré, Clin. Chim. Acta, 2005, 357, 34 CrossRef CAS PubMed.
- J. A. Mares-Perlman, W. Brady, R. Klein, B. Klein, P. Bowen and M. Stacewiczsapuntzakis, et al. , Arch. Ophthalmol., 1995, 113, 1518 CrossRef CAS PubMed.
- R. V. Bensasson, E. J. Land and T. G. Truscott, Excited states in biology and medicine, Oxford University Press, UK, 1993, ch. 1–4 Search PubMed.
- F. Boehm, J. Tinkler and T. G. Truscott, Nat. Med., 1995, 1, 98 CrossRef CAS PubMed.
- F. Boehm, R. Edge, D. J. McGarvey and T. G. Truscott, FEBS Lett., 1998, 436, 387 CrossRef.
- F. Böhm, R. Edge, M. Burke and T. G. Truscott, J. Photochem. Photobiol., B, 2001, 64, 176 CrossRef.
- F. Boehm, R. Edge, T. G. Truscott and C. Witt, FEBS Lett., 2016, 590, 1086 CrossRef CAS PubMed.
- F. Böhm, R. Edge and T. G. Truscott, Mol. Nutr. Food Res., 2012, 56, 205 CrossRef PubMed.
- R. Alvarez, B. Vaz, H. Gronemayer and A. R. de Lera, Chem. Rev., 2014, 114, 1 CrossRef CAS PubMed.
- S. A. Everett, M. F. Dennis, K. B. Patel, S. Maddix, S. C. Kundu and R. L. Willson, Am. Soc. Biochem. Mol. Biol., 1996, 271, 3988 CAS.
- F. Böhm, R. Edge, E. J. Land, D. J. McGarvey and T. G. Truscott, J. Am. Chem. Soc., 1997, 119, 621 CrossRef.
- M. Burke, R. Edge, E. J. Land and T. G. Truscott, J. Photochem. Photobiol., B, 2001, 60, 1 CrossRef CAS.
- The α-Tocopherol, β-carotene Cancer Prevention Study Group, N. Engl. J. Med., 1996, 330, 1029 Search PubMed.
- X. D. Xang and R. M. Russell, Nutr. Res., 1999, 57, 263 Search PubMed.
- H. Cheng, R.-M. Han, M.-K. Lyu, J.-P. Zhang and L. H. Skibsted, J. Phys. Chem. B, 2015, 119, 6603 CrossRef CAS PubMed.
- H.-T. Chang, H. Cheng, R.-M. Han, P. Wang, J.-P. Zhang and L. H. Skibsted, J. Agric. Food Chem., 2017, 65, 908 CrossRef CAS PubMed.
- D. Liu, J. Shi, A. C. Ibarra, Y. Kakuda and S. J. Xue, Food Sci. Technol., 2008, 41, 1344 CAS.
- R. Góralczyk, Nutr. Cancer, 2009, 61, 767 CrossRef PubMed.
- G. W. Burton and K. U. Ingold, Science, 1984, 224, 569 CrossRef CAS PubMed.
- S. Steenken, Chem. Rev., 1989, 89, 503–520 CrossRef CAS.
- C.-H. Chen, R.-H. Han, R. Liang, L.-M. Fu, P. Wang, A. Xi-Cheng, J.-P. Zhang and L. H. Skibsted, J. Phys. Chem. B, 2011, 115, 2082 CrossRef CAS PubMed.
- A. Mortensen, L. H. Skibsted and T. G. Truscott, Arch. Biochem. Biophys., 2001, 385, 13 CrossRef CAS PubMed.
- A. El-Agamey and D. J. McGarvey, Free Radical Res., 2007, 41, 295 CrossRef CAS PubMed.
- A. El-Agamey, A. Cantrell, E. J. Land, D. J. McGarvey and T. G. Truscott, Photochem. Photobiol. Sci., 2004, 3, 802 RSC.
- A. El-Agamey and D. J. McGarvey, Free Radical Biol. Med., 2016, 90, 75 CrossRef CAS PubMed.
- T. G. Truscott, J. Photochem. Photobiol., B, 1996, 35, 233 CrossRef CAS.
- A. Galano, R. Vargast and A. Martinez, Phys. Chem. Chem. Phys., 2010, 12, 193 RSC.
| This journal is © The Royal Society of Chemistry and Owner Societies 2020 |