Open Access Article
Open Access ArticleHigh aldehyde dehydrogenase activity does not protect colon cancer cells against TPCS2a-sensitized photokilling
Judith Jing Wen
Wong
 and
Pål Kristian
Selbo
and
Pål Kristian
Selbo
 *
*
Department of Radiation Biology, Institute for Cancer Research, The Norwegian Radium Hospital, Oslo University Hospital Montebello, 0379 Oslo, Norway. E-mail: Selbo@rr-research.no; Tel: (+47)22781469
First published on 28th February 2020
Abstract
Aldehyde dehydrogenases (ALDH) are detoxifying enzymes that are upregulated in cancer stem cells (CSCs) and may cause chemo- and ionizing radiation (IR) therapy resistance. By using the ALDEFLUOR assay, CD133 + human colon cancer cells HT-29, were FACSorted into three populations: ALDHbright, ALDHdim and unsorted (bulk) and treated with chemo-, radio- or photodynamic therapy (PDT) using the clinical relevant photosensitizer disulfonated tetraphenyl chlorin (TPCS2a/fimaporfin). Here we show that there is no difference in cytotoxic responses to TPCS2a-PDT in ALHDbright, ALDHdim or bulk cancer cells. Likewise, both 5-FU and oxaliplatin chemotherapy efficacy was not reduced in ALDHbright as compared to ALDHdim cancer cells. However, we found that ALHDbright HT-29 cells are significantly less sensitive to ionizing radiation compared to ALDHdim cells. This study demonstrates that the cytotoxic response to PDT (using TPCS2a as photosensitizer) is independent of ALDH activity in HT-29 cancer cells. Our results further strengthen the use of TPCS2a to target CSCs.
Aldehyde dehydrogenases (ALDHs) constitute a group of enzymes that have been associated with cancer progression and cancer therapy resistance.1 ALDHs have diverse cellular activity, including vital role in detoxification of aldehydes to carboxylic acids, thereby preventing generation of reactive oxygen species (ROS) and lipid peroxidation.1 In addition, ALDHs are involved in the synthesis of retinoic acid, which is important for cell survival, proliferation, embryogenesis and development of the immune system.1 Overexpression of ALDH1 is used as a marker for both normal stem and progenitor cells and cancer stem cells (CSCs).2,3 High ALDH1 activity provides a survival advantage of CSC as they are more equipped to resist accumulation of toxic aldehydes induced by increased metabolic activity, ionizing radiation or ROS-generating drugs.1,4 In this communication, we present results obtained in fluorescence-activated cell sorted (BD FACS Aria II cell sorter from Becton Dickinson (BD Biosciences, San Jose, USA)) human colon cancer cells with high (ALDHbright) and low (ALDHdim) ALDH activity. We compared these populations with regard to cytotoxic responses to chemotherapy, ionizing radiation or photodynamic therapy (PDT). For PDT, we selected the photosensitizer disulfonated tetraphenyl chlorin (TPCS2a/fimaporfin, PCI Biotech AS, Oslo, Norway) as TPCS2a is a clinical relevant photosensitizer used in the drug delivery technology photochemical internalization (PCI).5,6
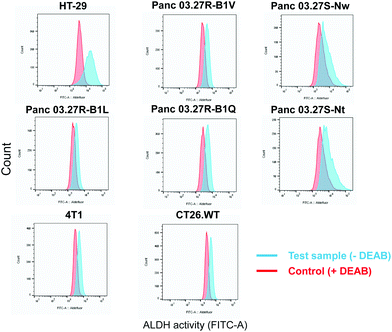
The ALDEFLUOR assay (STEMCELL Technologies, Vancouver, Canada) was performed to evaluate ALDH activity and cell sorting. The assay is based on the use of BODIPY-aminoacetaldehyde (BAAA) which is a substrate of ALDH which convert BAAA into BODIPY-aminoacetate (BAA−) that is highly fluorescent and retained in live cells due to its negative charge.7 Thus, cells with high and low ALDH activity can be distinguished and sorted using flow cytometry based on the fluorescent signal from BAA−. The ALDH inhibitor, N,N-diethylaminobenzaldehyde (DEAB), was included as a control providing adequate gating strategy for flow cytometry. By flow cytometry (BD LSR II, BD Biosciences), we screened a panel of eight cancer cell lines for ALDH activity which included; HT-29 (human colorectal adenocarcinoma, ATCC®HTB-38™), 5-FU-resistant and sensitive Panc 03.27-derived monoclonal cell lines (human pancreatic adenocarcinoma generated as previously described,8 provided by Dr. Stephan Krauss), CT26.WT (murine undifferentiated colon carcinoma, ATCC®CRL-2638™) and 4T1 (murine triple negative mammary carcinoma, ATCC®CRL-2539™) (Fig. 1).
A shift in fluorescence was observed in both murine cell lines, CT26.WT and 4T1, and in three of the 5-FU-resistant Panc 03.27-derived cell lines, Panc 03.27R-B1L, Panc 03.27R-B1Q and Panc 03.27R-B1LV, indicating homogenous ALDH activity. Interestingly, the 5-FU-sensitive Panc 03.27S-Nt and Pan03.27S-Nw cell lines, displayed heterogeneous ALDH activity compared to Panc 03.27R-B1L, -B1Q and B1V.
We have previously shown that the 5-FU-resistant Panc 03.27R-B1L, -B1Q and -B1V are hypersensitive to TPCS2a-PDT compared to the 5-FU sensitive clones.9 Based on this, and the lack of information regarding the PDT-effect on ALDHbrightversus ALDHdim cancer cells in the literature, we wanted to explore the cytotoxic effect of TPCS2a-PDT with regard to ALDH activity within the same cell line to exclude inter-cell line genetic/proteomic variations.
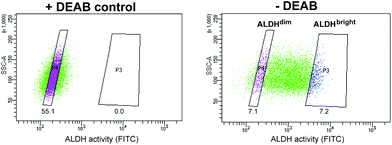
Of all cell line tested, the HT-29 cell line exhibited the highest heterogeneous mixture of ALDH activity, where the median fluorescence intensity in cells incubated with BAA− was more than 5-fold higher than the DEAB control (Fig. 1). Thus, HT-29 was selected for fluorescence activated cell sorting (FACS) and subsequent evaluation of responses to chemo-, radio-, and photodynamic therapy (PDT). By means of the ALDEFLUOR assay, HT-29 cells were FACSorted into three populations: (1) Cells that exhibited very high fluorescence intensity (near 10% of the cells gated with the highest BAA− signals), indicating high ALDH activity, were designated ALDHbright (Fig. 2). (2) Correspondingly, cells that displayed very low fluorescence intensity (near 10% of the cells gated with the lowest BAA− signals) were defined as ALDHdim. (3) Finally, unsorted cells were included to represent the bulk population. In all experiments, cells were sorted directly onto 96-well- or 6-well plates (Nunc, Thermo Fisher Scientific, Waltham, MA, USA) containing sterile filtered (0.22 μm) conditioned medium mixed with fresh McCoy's 5a medium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The culture medium was supplemented with 10% fetal bovine serum, 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin (Sigma-Aldrich). The sorted cells were allowed to attach overnight and subjected to treatment as indicated.
1). The culture medium was supplemented with 10% fetal bovine serum, 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin (Sigma-Aldrich). The sorted cells were allowed to attach overnight and subjected to treatment as indicated.
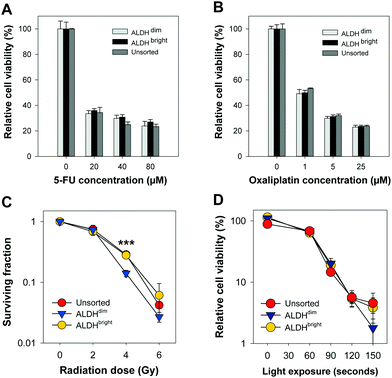
High ALDH activity has been associated with chemoresistance in different cancer types.10–13 We assessed the chemotherapy response of FACSorted HT-29 cells to increasing concentrations of 5-FU or oxaliplatin (both from Sigma-Aldrich) (Fig. 3A and B).
Chemotherapy-induced cytotoxic responses were measured using the MTT viability assay (0.25 mg ml−1, 4 hours incubation). Surprisingly, the cell viability was found to be similar in all FACSorted populations at all concentrations tested which indicate that ALDH activity does not significantly affect 5-FU and oxaliplatin sensitivity in the HT-29 cell line. Our results are in contrast with Kozovska et al. that reported inhibition of ALDH using DEAB in combination with 5-FU or cisplatin significantly reduced cell viability in HT-29 cells.14 On the other hand, Prasmickaite et al. demonstrated similar sensitivity of the anti-melanoma drug dacarbazine in ALDEFLUOR-sorted cells isolated from malignant melanoma patients which indicate that ALDH alone might not be sufficient to select for chemoresistant malignant melanoma cells.15 In 5-FU- and oxaliplatin-resistant HT-29 cells, a 16-to-30 fold enrichment of the cancer stem cell marker CD133 was observed which may indicate that CD133 alone or in combination with ALDEFLUOR may be more suitable to select for resistant HT-29 cells.16 Moreover, CD133 + cells were found to be highly resistant to 5-FU and oxaliplatin in human colon cancer cells derived from patients.17 Data from our lab18 indicate that HT-29 exhibit high CD133 expression. As we did not include CD133 expression as a parameter for gating in our FACS, we cannot exclude that sorting based on a combination between ALDH and CD133 would have resulted in isolation of a chemo-resistant population. Therefore, the HT-29 cytotoxicity data obtained after 5-FU or oxaliplatin chemotherapy and the ALDEFLUOR assay results showing reduced ALDH activity in the 5-FU-resistant Panc 03.27 cell lines (Fig. 1) suggests that resistance to 5-FU may not be directly linked to ALDHs.9 As this is in conflict with existing literature, we suggest that more experimental research on the role of ALDH in response to 5-FU treatment is important, e.g. including ALDH knock-out models and evaluations in other cancer cell lines with heterogeneous mixture of ALDH activity.
Clonogenic assay was used to determine cell survival/death after ionizing radiation treatment of ALDHbright, ALDHdim and unsorted HT-29 cells in 6-well culture plates (Nunc). The cells were treated with a single fraction irradiation up to 6 Gy (160 kV, 6.3 mA, X-ray generator, Faxitron CP160, Tuscon, AZ, USA). When sufficiently large colonies in control plates were formed (10–14 day post-treatment), colonies were ethanol fixed, methylene blue stained and counted manually. A colony was defined to consist of at least 50 cells.19 Interestingly, based on three independent biological replicates, a slightly higher plating efficiency of ALDHbright (53.3 ± 2.5%) was observed compared to ALDHdim (43.0 ± 5.3%, not significant, p = 0.152). The plating efficiency of ALDHdim cells was also slightly lower compared to unsorted cells (49.3 ± 5.3%, not significant, p = 0.404). ALDHbright and unsorted HT-29 cells tended towards a higher ionizing radiation resistance than ALDHdim cells but only showed a significant difference after irradiation with 4 Gy (Fig. 3C). The surviving fraction (SF) of ALDHdim cells was significantly lower (∼2-fold) at 4 Gy (SF: 14.1 ± 0.98%, p < 0.001) compared to ALDHbright (SF: 27.9 ± 1.2%) and unsorted cells (SF: 28.7 ± 4.1%, p < 0.001). This observation is in agreement with existing studies which reported radioresistance in cells with high ALDH activity as well as in CSCs selected using other markers.20,21
PDT is based on the use of a light sensitive drug (photosensitizer) that is nontoxic in the dark and which accumulates in tumour tissues. Light exposure of the tumour tissue results in excitation of the photosensitizer leading to energy transfer from the photosensitizer to molecular oxygen (O2) or to other cellular components, resulting in generation of cytotoxic concentration of reactive oxygen species (ROS), of which singlet oxygen (1O2) is the most abundant. PDT-induced ROS-generation results in peroxidation of vital cellular components and initiation of cell death mechanisms such as apoptosis, necrosis or autophagy.22
In this work, we used the clinical relevant PCI photosensitizer TPCS2a (fimaporfin23) to compare PDT efficacy in HT-29 colorectal adenocarcinoma cells with either very high or very low ALDH activity. Cells were incubated with 0.4 μg ml−1 TPCS2a (PCI Biotech AS) for 18 hours, washed twice with PBS and chased for 4 hours in drug-free medium to remove plasma membrane-bound TPCS2a to mimic a PCI protocol. The cells were subjected to broadband blue light irradiation (λmax = 435 nm) with an output of 9.6 mW cm−2 (LumiSource, PCI Biotech AS). Cell viability was evaluated 72 hours post-light exposure by using the MTT assay, which is widely accepted in the field of PDT and has been used for 30 years to assess cell viability.24 Furthermore, we have also shown that there is a good consistency between this assay and the clonogenic cell assay.25,26 Of high interest, no statistical significant differences (p > 0.1 at all light exposure times) in cell viability was found between ALDHdim and ALDHbright cells treated with TPCS2a-PDT (Fig. 3D). Of relevance, we previously demonstrated that TPCS2a is not a substrate for the CSC markers ABCG2 and ABCB1 (P-gp) transporter26–28 which may explain why no difference in TPCS2a sensitivity was observed in ALDH-sorted cells. As this study is on the importance of ALDH activity and its influence on TPCS2a-PDT efficacy in only the HT-29 cell line, this should be verified in other cell lines in future studies. In addition, further investigation to establish the role of ALDH activity in PDT using other photosensitizers is warranted. In conclusion, we show that ALDHdim cells are more sensitive to ionizing radiation at 4 Gy compared to bulk and ALDHbright populations, which is in line with the literature. However, we report that TPCS2a-PDT is equally efficient in both ALDHbright and ALDHdim HT-29 cancer cell populations. Our data further strengthen the use of TPCS2a-based PCI of CSC-targeting therapeutics.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The present work was financially supported by South-Eastern Norway Health Authority (funding number: 2017068 (P. K. S.) and 2016023 (J. J. W. W.)) and The Norwegian Radium Hospital Research Foundation (funding number: FU0803 (P. K. S.). We would like to thank Dr. Kaja Lund and Professor Stefan Krauss (University of Oslo/Oslo University Hospital) for kindly providing us the Panc 03.27 monoclonal cell lines. We also thank Idun Dale Rein and Heidi Ødegaard Notø at the Flow Cytometry Core Facility (Institute for Cancer Research, Oslo University Hospital) for help with cell sorting and technical expertise.References
- S. S. Dinavahi, C. G. Bazewicz, R. Gowda and G. P. Robertson, Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics, Trends Pharmacol. Sci., 2019, 40(10), 774–789 CrossRef CAS PubMed.
- C. Ginestier, M. H. Hur, E. Charafe-Jauffret, F. Monville, J. Dutcher and M. Brown, et al., ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome, Cell Stem Cell, 2007, 1(5), 555–567 CrossRef CAS PubMed.
- E. H. Huang, M. J. Hynes, T. Zhang, C. Ginestier, G. Dontu and H. Appelman, et al., Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis, Cancer Res., 2009, 69(8), 3382–3389 CrossRef CAS PubMed.
- E. Batlle and H. Clevers, Cancer stem cells revisited, Nat. Med., 2017, 23(10), 1124–1134 CrossRef CAS PubMed.
- A. A. Sultan, W. Jerjes, K. Berg, A. Hogset, C. A. Mosse and R. Hamoudi, et al., Disulfonated tetraphenyl chlorin (TPCS2a)-induced photochemical internalisation of bleomycin in patients with solid malignancies: a phase 1, dose-escalation, first-in-man trial, Lancet Oncol., 2016, 17(9), 1217–1229 CrossRef CAS PubMed.
- P. K. Selbo, A. Weyergang, A. Hogset, O. J. Norum, M. B. Berstad and M. Vikdal, et al., Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules, J. Controlled Release, 2010, 148(1), 2–12 CrossRef CAS PubMed.
- R. W. Storms, A. P. Trujillo, J. B. Springer, L. Shah, O. M. Colvin and S. M. Ludeman, et al., Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity, Proc. Natl. Acad. Sci. U. S. A., 1999, 96(16), 9118–9123 CrossRef CAS PubMed.
- K. Lund, J. L. Dembinski, N. Solberg, A. Urbanucci, I. G. Mills and S. Krauss, Slug-dependent upregulation of L1CAM is responsible for the increased invasion potential of pancreatic cancer cells following long-term 5-FU treatment, PLoS One, 2015, 10(4), e0123684 CrossRef PubMed.
- K. Lund, C. E. Olsen, J. J. W. Wong, P. A. Olsen, N. T. Solberg and A. Hogset, et al., 5-FU resistant EMT-like pancreatic cancer cells are hypersensitive to photochemical internalization of the novel endoglin-targeting immunotoxin CD105-saporin, J. Exp. Clin. Cancer Res., 2017, 36(1), 187 CrossRef PubMed.
- D. Kim, B. H. Choi, I. G. Ryoo and M. K. Kwak, High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling, Cell Death Dis., 2018, 9(9), 896 CrossRef PubMed.
- L. Cortes-Dericks, L. Froment, R. Boesch, R. A. Schmid and G. Karoubi, Cisplatin-resistant cells in malignant pleural mesothelioma cell lines show ALDH(high)CD44(+) phenotype and sphere-forming capacity, BMC Cancer, 2014, 14, 304 CrossRef PubMed.
- G. Vassalli, Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells, Stem Cells Int., 2019, 2019, 3904645 CrossRef PubMed.
- Y. Touil, W. Igoudjil, M. Corvaisier, A. F. Dessein, J. Vandomme and D. Monte, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis, Clin. Cancer Res., 2014, 20(4), 837–846 CrossRef CAS PubMed.
- Z. Kozovska, A. Patsalias, V. Bajzik, E. Durinikova, L. Demkova and S. Jargasova, et al., ALDH1A inhibition sensitizes colon cancer cells to chemotherapy, BMC Cancer, 2018, 18(1), 656 CrossRef CAS PubMed.
- L. Prasmickaite, B. O. Engesaeter, N. Skrbo, T. Hellenes, A. Kristian and N. K. Oliver, et al., Aldehyde dehydrogenase (ALDH) activity does not select for cells with enhanced aggressive properties in malignant melanoma, PLoS One, 2010, 5(5), e10731 CrossRef PubMed.
- N. A. Dallas, L. Xia, F. Fan, M. J. Gray, P. Gaur and G. van Buren 2nd, et al., Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition, Cancer Res., 2009, 69(5), 1951–1957 CrossRef CAS PubMed.
- M. Todaro, M. P. Alea, A. B. Di Stefano, P. Cammareri, L. Vermeulen and F. Iovino, et al., Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4, Cell Stem Cell, 2007, 1(4), 389–402 CrossRef CAS PubMed.
- C. E. Olsen, L. H. Cheung, A. Weyergang, K. Berg, D. A. Vallera and M. G. Rosenblum, et al., Design, Characterization, and Evaluation of scFvCD133/rGelonin: A CD133-Targeting Recombinant Immunotoxin for Use in Combination with Photochemical Internalization, J. Clin. Med., 2019, 9(1), E68 CrossRef PubMed.
- N. A. Franken, H. M. Rodermond, J. Stap, J. Haveman and C. van Bree, Clonogenic assay of cells in vitro, Nat. Protoc., 2006, 1(5), 2315–2319 CrossRef CAS PubMed.
- M. Cojoc, C. Peitzsch, I. Kurth, F. Trautmann, L. A. Kunz-Schughart and G. D. Telegeev, et al., Aldehyde Dehydrogenase Is Regulated by beta-Catenin/TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells., Cancer Res., 2015, 75(7), 1482–1494 CrossRef CAS PubMed.
- J. Mihatsch, M. Toulany, P. M. Bareiss, S. Grimm, C. Lengerke and R. Kehlbach, et al., Selection of radioresistant tumor cells and presence of ALDH1 activity in vitro, Radiother. Oncol., 2011, 99(3), 300–306 CrossRef CAS PubMed.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti and S. O. Gollnick, et al., Photodynamic therapy of cancer: an update, Ca-Cancer J. Clin., 2011, 61(4), 250–281 CrossRef PubMed.
- K. Berg, S. Nordstrand, P. K. Selbo, D. T. Tran, E. Angell-Petersen and A. Hogset, Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization, Photochem. Photobiol. Sci., 2011, 10(10), 1637–1651 RSC.
- J. L. Merlin, S. Azzi, D. Lignon, C. Ramacci, N. Zeghari and F. Guillemin, MTT assays allow quick and reliable measurement of the response of human tumour cells to photodynamic therapy, Eur. J. Cancer, 1992, 28A(8-9), 1452–1458 CrossRef CAS PubMed.
- W. L. Yip, A. Weyergang, K. Berg, H. H. Tonnesen and P. K. Selbo, Targeted delivery and enhanced cytotoxicity of cetuximab-saporin by photochemical internalization in EGFR-positive cancer cells, Mol. Pharmacol., 2007, 4(2), 241–251 CrossRef CAS PubMed.
- P. K. Selbo, A. Weyergang, A. Bonsted, S. G. Bown and K. Berg, Photochemical internalization of therapeutic macromolecular agents: a novel strategy to kill multidrug-resistant cancer cells, J. Pharmacol. Exp. Ther., 2006, 319(2), 604–612 CrossRef CAS PubMed.
- P. K. Selbo, A. Weyergang, M. S. Eng, M. Bostad, G. M. Maelandsmo and A. Hogset, et al., Strongly amphiphilic photosensitizers are not substrates of the cancer stem cell marker ABCG2 and provides specific and efficient light-triggered drug delivery of an EGFR-targeted cytotoxic drug, J. Controlled Release, 2012, 159(2), 197–203 CrossRef CAS PubMed.
- C. E. Olsen, K. Berg, P. K. Selbo and A. Weyergang, Circumvention of resistance to photodynamic therapy in doxorubicin-resistant sarcoma by photochemical internalization of gelonin, Free Radical Biol. Med., 2013, 65, 1300–1309 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2020 |