Open Access Article
Open Access ArticleAn overview of the uses of per- and polyfluoroalkyl substances (PFAS)†
Juliane
Glüge
 *a,
Martin
Scheringer
*a,
Martin
Scheringer
 a,
Ian T.
Cousins
a,
Ian T.
Cousins
 b,
Jamie C.
DeWitt
c,
Gretta
Goldenman
d,
Dorte
Herzke
b,
Jamie C.
DeWitt
c,
Gretta
Goldenman
d,
Dorte
Herzke
 ef,
Rainer
Lohmann
ef,
Rainer
Lohmann
 g,
Carla A.
Ng
g,
Carla A.
Ng
 h,
Xenia
Trier
i and
Zhanyun
Wang
j
h,
Xenia
Trier
i and
Zhanyun
Wang
j
aInstitute of Biogeochemistry and Pollutant Dynamics, ETH Zürich, 8092 Zürich, Switzerland. E-mail: juliane.gluege@chem.ethz.ch
bDepartment of Environmental Science, Stockholm University, SE-10691 Stockholm, Sweden
cDepartment of Pharmacology & Toxicology, Brody School of Medicine, East Carolina University, Greenville, NC, USA
dMilieu, Brussels, Belgium
eNILU, Norwegian Institute for Air Research, Tromsø, Norway
fDepartment of Arctic and Marine Biology, The Arctic University of Norway (UiT), Hansine Hansens veg 18, Tromsø, NO-9037, Norway
gGraduate School of Oceanography, University of Rhode Island, Narragansett, RI 02882, USA
hDepartments of Civil and Environmental Engineering and Environmental and Occupational Health, University of Pittsburgh, Pittsburgh, PA 15261, USA
iEuropean Environment Agency, Kgs. Nytorv 6, DK-1050 Copenhagen K, Denmark
jChair of Ecological Systems Design, Institute of Environmental Engineering, ETH Zürich, 8093 Zürich, Switzerland
First published on 30th October 2020
Abstract
Per- and polyfluoroalkyl substances (PFAS) are of concern because of their high persistence (or that of their degradation products) and their impacts on human and environmental health that are known or can be deduced from some well-studied PFAS. Currently, many different PFAS (on the order of several thousands) are used in a wide range of applications, and there is no comprehensive source of information on the many individual substances and their functions in different applications. Here we provide a broad overview of many use categories where PFAS have been employed and for which function; we also specify which PFAS have been used and discuss the magnitude of the uses. Despite being non-exhaustive, our study clearly demonstrates that PFAS are used in almost all industry branches and many consumer products. In total, more than 200 use categories and subcategories are identified for more than 1400 individual PFAS. In addition to well-known categories such as textile impregnation, fire-fighting foam, and electroplating, the identified use categories also include many categories not described in the scientific literature, including PFAS in ammunition, climbing ropes, guitar strings, artificial turf, and soil remediation. We further discuss several use categories that may be prioritised for finding PFAS-free alternatives. Besides the detailed description of use categories, the present study also provides a list of the identified PFAS per use category, including their exact masses for future analytical studies aiming to identify additional PFAS.
Environmental significancePer- and polyfluoroalkyl substances (PFAS) are a large group of more than 4700 substances that are used in a wide range of technical applications and consumer products. Releases of PFAS to the environment have caused large-scale contamination in many countries. For an effective management of PFAS, an overview of the use areas of PFAS, the functions of PFAS in these uses, and the chemical identity of the PFAS actually used is needed. Here we present a systematic description of more than 200 uses of PFAS and the individual substances associated with each of them (over 1400 PFAS in total). This large list of PFAS and their uses is intended to support the identification of essential and non-essential uses of PFAS. |
1 Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of thousands of substances1,2 that have been produced since the 1940s and used in a broad range of consumer products and industrial applications.3 Based on concerns regarding the high persistence of PFAS4 and the lack of knowledge on properties, uses, and toxicological profiles of many PFAS currently in use, it has been argued that the production and use of PFAS should be limited.5 However, there are specific uses that make an immediate ban of all PFAS impractical. Some specific uses of PFAS may currently be essential to health, safety or the functioning of today's society for which alternatives so far do not exist. On the other hand, if some uses of PFAS are found to be non-essential, they could be eliminated without having to first find alternatives that provide an adequate function and performance. To determine which uses of PFAS are essential and which are not, the concept of “essential use,” as defined under the Montreal Protocol, has recently been further developed for PFAS, including illustrative case studies for several major use categories of PFAS.6PFAS are costly to produce (e.g. fluorosurfactants are 100–1000 times more expensive than conventional hydrocarbon surfactants per unit volume7) and therefore are often used where other substances cannot deliver the required performance,1 or where PFAS can be used in a much smaller amount and with the same performance as a higher amount of a non-fluorinated chemical. Examples are uses that operate over wide temperature ranges or uses that require extremely stable and non-reactive substances. The C–F bonds in PFAS lead to very stable substances, a feature that also makes the terminal transformation products of PFAS very persistent in the environment. Furthermore, the perfluorocarbon moieties in PFAS are both hydrophobic and oleophobic, making many PFAS effective surfactants or surface protectors.8 PFAS-based fluorosurfactants can lower the surface tension of water from about 72 mN m−1 (ref. 9) to less than 16 mN m−1, which is half of what is attainable by hydrocarbon surfactants.8,10 Likewise, the surfaces of fluorinated polymers have about half the surface tension compared to hydrocarbon surfaces. For instance, a close-packed, uniformly organized array of trifluoromethyl (–CF3) groups creates a surface with a solid surface tension as low as 6 mN m−1.11
Due to these and other desirable properties, PFAS are used in many different applications. A good overview of the range of uses of PFAS as surfactants and repellents is provided in the monograph by Kissa (2001).3 It lists 39 use categories, mostly derived from patents, and describes the functions of PFAS in these use categories. However, the work by Kissa (2001) was published nearly 20 years ago, focused on fluorosurfactants and repellents, and it is not clear which of these uses are still relevant today. In addition to Kissa (2001),3 there are a few other monographs and a number of peer-reviewed scientific articles and reports that have looked into the uses of PFAS.8,12–22 While these articles and reports provide useful information, each of them focuses on the uses of a specific PFAS group (in specific use categories). This is also the case for the reviews from the Persistent Organic Pollutants Review Committee (POPRC), the focuses of which are on perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid (PFHxS), their precursors, and the PFAS that may be or have been introduced as replacements for these PFAS.23–29 The FluoroCouncil30 has provided additional information on uses of PFAS. However, the information is rather generic with limited details about specific uses and substances. Hence, a comprehensive overview that summarizes major current uses is missing.
The present paper, together with the Appendix (Table 4) and the ESI,† aims to provide a broad, but not exhaustive, overview of the uses of PFAS and associated individual substances (note that a working definition of PFAS is used here to define the scope of PFAS considered in this study, which is provided in the Methods section below). The paper addresses the following points: (i) in which use categories have PFAS been employed and for which functions? (ii) Which PFAS have been – and are still – used in a certain category? (iii) What is the extent of the uses in certain parts of the world? Within the European Union (EU), there are discussions underway for restricting PFAS to those uses that are essential,31 and extensive information on many PFAS uses will be needed in this context. The present work also aims to support this process by showing in which specific applications PFAS are used, and in which functions, as a first step toward differentiating essential and non-essential uses of PFAS.
2 Methods
2.1 Which PFAS are addressed?
A first clear definition of PFAS was provided by Buck et al. (2011).1 They defined PFAS as aliphatic substances containing the moiety –CnF2n+1 within their structure, where n is at least 1. The OECD/UNEP Global PFC Group noted that many substances containing other perfluorocarbon moieties (e.g. –CnF2n–) were not commonly recognized as PFAS according to Buck et al. (2011), e.g. perfluorodicarboxylic acids.2 Considering their structural similarities to commonly recognized PFAS with the –CnF2n+1 moiety, the OECD/UNEP Global PFC Group proposed to also include substances that contain the moiety –CnF2n– (n ≥ 1) as PFAS.2 However, the exact definition is still under discussion. The present study is in line with the OECD proposal in several, but not all, respects. In contrast to the definition by Buck et al. (2011), the present study also includes (i) substances where a perfluorocarbon chain is connected with functional groups on both ends, (ii) aromatic substances that have perfluoroalkyl moieties on the side chains, and (iii) fluorinated cycloaliphatic substances.More specifically, the present study focuses on polymeric PFAS with the –CF2– moiety and non-polymeric PFAS with the –CF2–CF2– moiety. It does not include non-polymeric substances that only contain a –CF3 or –CF2– moiety, with the exception of perfluoroalkylethers and per- and polyfluoroalkylether-based substances. For these two PFAS groups, substances with a –CF2OCF2– or –CF2OCFHCF2– moiety are also included.
2.2 Literature sources
The present inventory was started with the risk profiles and risk management evaluations for PFOA, PFOS, PFHxS and their related compounds to obtain an overview of uses of these chemicals.23–29 Reports and books that address fluorosurfactants and fluoropolymers in general were also included.3,8,12,16,20,21,32–43 Literature specific to certain use categories was retrieved for more information either on the substances used, or to understand why PFAS are, or were, necessary for a given use. All specific references are cited in the ESI-1.†In addition, databases, patents, information from PFAS manufacturers and scientific studies that measured PFAS in products were examined. These additional sources are described in more detail in the following subsections. The searches were not exhaustive in any of the sources described, and there are still many more reports, scientific studies, patents, safety data sheets and databases with information on the uses of PFAS than the ones cited here or in the ESI-1.†
The information in the Tables in the ESI-1† from these sources was marked according to its original source. Information from patents (cited in a book, article or report) was marked with “P”, information on PFAS analytically detected in products with “D”, and information on uses or information without additional reference with “U” for “use”, or “U*” for “current use” (which is defined as a use with public record(s) of use from the last 4 years, i.e. 2017 or later).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pounds (11.34 metric tons, t, per year) at a site in the United States (US) between 2012 and 2015 were obliged to report to the US Environmental Protection Agency (US EPA) in 2016 (data for 2016 to 2019 will be reported in 2020). The data reported in 2016 included for each reported substance: the name, Chemical Abstracts Service (CAS) registry number and product categories for consumer and commercial uses and sectors, as well as function categories for industrial processing and use. The masses (tonnages) used and exported also had to be reported; however, they are in most cases confidential business information (CBI). The reported data were filtered according to chemical names containing the word “fluoro”. Non-polymeric substances that did not contain the –CF2CF2– moiety and polymeric substances that did not contain the –CF2– moiety subsequently were removed. This left 39 entries where a specific PFAS was applied in a consumer or commercial use, and around 120 entries where a specific PFAS was applied in an industrial processing or use. The entries are labelled with “U” for “use” in the Tables in the ESI-1 and ESI-3.†
000 pounds (11.34 metric tons, t, per year) at a site in the United States (US) between 2012 and 2015 were obliged to report to the US Environmental Protection Agency (US EPA) in 2016 (data for 2016 to 2019 will be reported in 2020). The data reported in 2016 included for each reported substance: the name, Chemical Abstracts Service (CAS) registry number and product categories for consumer and commercial uses and sectors, as well as function categories for industrial processing and use. The masses (tonnages) used and exported also had to be reported; however, they are in most cases confidential business information (CBI). The reported data were filtered according to chemical names containing the word “fluoro”. Non-polymeric substances that did not contain the –CF2CF2– moiety and polymeric substances that did not contain the –CF2– moiety subsequently were removed. This left 39 entries where a specific PFAS was applied in a consumer or commercial use, and around 120 entries where a specific PFAS was applied in an industrial processing or use. The entries are labelled with “U” for “use” in the Tables in the ESI-1 and ESI-3.†
The data that we used in the present study were extracted for us from the SPIN database by an employee of the Swedish Chemicals Agency (KEMI) and the data included only non-confidential information. However, there is also a substantial amount of confidential information in the SPIN database. This is visible when the substances are accessed via the web interface of the SPIN database.44 It was also pointed out to us that not all substances have available use data due to confidentiality.
The database includes four large data sets with information on uses. Two of the data sets (“UC62” and “National use categories”) contain information on specific use categories, while the other two (“Industrial NACE” and “Industry National”) contain information on sectors of uses. In addition to the use categories and sectors of uses, the data sets also contain information on the quantities of a chemical used in a certain use category or sectors of uses if the reported mass exceeds 0.1 t. The available data cover the time period 2000 to 2017. The four data sets were merged and then (as with the TSCA Inventory data) filtered for chemicals containing the word “fluoro”. Those non-polymeric substances that did not contain the –CF2CF2– moiety and polymeric substances that did not contain the –CF2– moiety subsequently were removed. This left 950 entries. Entries with available data for 2017 were labelled as “current use” (U*) in the Tables in the ESI-1 and ESI-3,† all other entries with “U” for “use”.
2.3 Nomenclature
In the present study, a distinction is made between use categories and subcategories. A use category can, but does not necessarily, have subcategories. An example of a use category for PFAS is sport articles; a subcategory under sport articles is tennis rackets.A distinction is also made between use, function and property. The “use” is the area in which the substances are employed. This can either be the use category or the subcategory. The “function” is the task that the substances fulfil in the use, and the “properties” indicate why PFAS are able to fulfil this function. An example for a use would be chrome plating. In chrome plating, PFAS have the function to prevent the evaporation of hexavalent chromium(VI) vapour, because of the PFAS properties that lower the surface tension of the electrolyte solution and since the PFAS used are stable under strongly acidic and oxidizing conditions.3
In the present study, the term “individual PFAS” always refers to substances with a CAS number, irrespective of whether they are mixtures, polymers or single substances.
2.4 Classification of use categories
The use categories in the present study were developed and refined throughout the course of the project to have as few well-defined use categories as possible that were not too broad. Initially, the use categories as defined by Kissa (2001)3 were employed, but they are very specific and thus broader categories were needed to cover the identified uses. Examples of use categories from Kissa (2001) which were assigned to broader categories are “moulding and mould release” (in the present study a subcategory under “production of plastic and rubber”), “oil wells” (in the present study a subcategory with a slightly different name under “oil & gas”), and “cement additives” (in the present study a subcategory under “building and construction”). In the course of the project, more use categories were defined as additional uses were added. The use categories in the present study were finally divided into “industrial branches” and “other use categories” to make a distinction between use categories that define broad industrial branches such as the “semiconductor industry” or the “energy sector”, and use categories that are more specific such as “personal care products” or “sealants and adhesives”. Note that some of the “other use categories” may be applied to several of the “industry branches”. For example, “wire and cable insulations” may be applied in “aerospace”, “biotechnology”, “building and construction”, “chemical industry” and others. A detailed overview of the use categories and their subcategories is provided in the Appendix (Table 4) of this paper.Overall, the use categories defined in the present study are very similar to the categories of the SPIN database, although some categories of the SPIN database are more specific (and correspond to subcategories in the present study). Some of the categories in the SPIN database could not be assigned to any of the use categories in the present study because they were too general. Examples are “impregnation”, “surface treatment”, “anti-corrosion materials” or “manufacture of other transport equipment”. Although the substances from these categories are not included in the present study, their quantities appear in Fig. 3 under “various”.
2.5 What kind of information can be found where in this article?
The present study comes with an Appendix (Table 4) that lists the functions of the PFAS in the use categories and subcategories that we identified. In addition, we indicate which properties of the PFAS are important for the identified function. The Appendix thus contains the main results of the present study in a condensed form and is therefore part of the main paper and not part of the ESI.†The ESI† of the present study is divided into three parts. ESI-1† is a comprehensive document with over 250 pages. It is available as a pdf, but can also be provided upon request as an MS Word document. ESI-1† is intended to be used as a reference document and contains a detailed description of all uses that were collected here as well as the PFAS employed in these categories with names, structural formulas and CAS numbers. Before reading sections of the ESI-1,† it is recommended to study the first two pages of the ESI-1,† where some of the specific features of the document are explained.
In addition, there is an MS Excel workbook (ESI-2†) that contains all PFAS that appear in ESI-1.† This workbook has a worksheet for each of the most common PFAS groups such as perfluoroalkyl acids (PFAA), perfluoroalkane sulfonyl fluoride (PASF)-based substances, or fluorotelomer-based substances and, thus, offers a good overview of the described PFAS. A list of what is included in the different worksheets is provided in the first worksheet. ESI-2† is primarily intended as a reference for readers who do not have access to SciFindern or other chemical databases or who just want to look up the name or structural formula for a specific CAS number. In addition to name, CAS number, and structural formula, ESI-2† also contains the identified uses of each PFAS. In contrast to ESI-1, ESI-2† assigns the uses to the PFAS (and not the PFAS to the uses).
The third part of the ESI-3† is also an Excel workbook that provides a separate worksheet for each use category. These worksheets list the PFAS from the ESI-1† with the names, CAS numbers, elemental compositions, and exact monoisotopic masses of the substances. Our intention is that the lists can be added to accurate mass spectrometry libraries and thus help to identify unknown PFAS more easily in the future. For this purpose, it would be helpful to connect the CAS numbers in the ESI-3† with e.g. the Norman SusDat ID of the NORMAN Substance Database79 and perhaps to commercial mass spectrometry libraries in the future.
3 Results
In the present study, more than 200 uses in 64 use categories were identified for more than 1400 individual PFAS. This means that the present study encompasses five times as many uses (counted as use categories plus subcategories) than included in Kissa (2001).3 This shows that our present study goes much further than simply updating this previous work. The following subsections describe the identified use categories and substances, and show and discuss the most important use categories in terms of quantities used, based on the data of the SPIN database and the Chemical Data Reporting database under the TSCA.3.1 In which use categories have PFAS been employed and for which function?
The Appendix to the present study sets forth the use categories identified and answers the question of why PFAS were employed for a specific use. The use categories identified in this study are divided into “industry branches” and “other use categories”, as listed in Table 1. In total, 87 uses within the 21 industry branches and 123 uses within the 43 other use categories were identified. Among the use categories, medical utensils, the semiconductor industry, and the automotive industries have the largest numbers of subcategories. About 15% of the subcategories were identified by patents, and 5% by studies that measured PFAS in products (see ESI-3†). The remaining categories have been mentioned previously in other publications.| Industry branches | |
|---|---|
| Aerospace (7) | Mining (3) |
| Biotechnology (2) | Nuclear industry |
| Building and construction (5) | Oil & gas industry (7) |
| Chemical industry (8) | Pharmaceutical industry |
| Electroless plating | Photographic industry (2) |
| Electroplating (2) | Production of plastic and rubber (7) |
| Electronic industry (5) | Semiconductor industry (12) |
| Energy sector (10) | Textile production (2) |
| Food production industry | Watchmaking industry |
| Machinery and equipment | Wood industry (3) |
| Manufacture of metal products (6) | |
| Other use categories | |
|---|---|
| Aerosol propellants | Metallic and ceramic surfaces |
| Air conditioning | Music instruments (3) |
| Antifoaming agent | Optical devices (3) |
| Ammunition | Paper and packaging (2) |
| Apparel | Particle physics |
| Automotive (12) | Personal care products |
| Cleaning compositions (6) | Pesticides (2) |
| Coatings, paints and varnishes (3) | Pharmaceuticals (2) |
Conservation of books and ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) manuscripts manuscripts |
Pipes, pumps, fittings and liners |
| Cook- and bakingware | Plastic, rubber and resins (4) |
| Dispersions | Printing (4) |
| Electronic devices (7) | Refrigerant systems |
| Fingerprint development | Sealants and adhesives (2) |
| Fire-fighting foam (5) | Soldering (2) |
| Flame retardants | Soil remediation |
Floor covering including carpets and ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) floor polish (4) floor polish (4) |
Sport article (7) |
| Glass (3) | Stone, concrete and tile |
| Household applications | Textile and upholstery (2) |
Laboratory supplies, equipment and ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) instrumentation (4) instrumentation (4) |
Tracing and tagging (5) |
| Leather (4) | Water and effluent treatment |
| Lubricants and greases (2) | Wire and cable insulation, gaskets ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and hoses and hoses |
| Medical utensils (14) |
The identified uses include many uses not previously described in the scientific literature on PFAS. Some examples of those uses are PFAS in ammunition (ESI-1 Section 2.4†), climbing ropes (ESI-1 Section 2.38†), guitar strings (ESI-1 Section 2.24†), artificial turf (ESI-1 Section 1.17†), and soil remediation (ESI-1 Section 2.37†). Also, additional subcategories of PFAS in already described use categories such as in the semiconductor industry were identified. For example, in addition to the subcategories etching agents, anti-reflective coatings, or photoresists, PFAS may also be employed for wafer thinning (patent US20130201635 from 2013)45 and as bonding ply in multilayer printed circuit boards (patent WO2003026371 from 2003) in the semiconductor industry.45 In the energy sector, PFAS are known to be employed in solar collectors and photovoltaic cells, and in lithium-ion, vanadium redox, and zinc batteries. In addition, fluoropolymers are also used to coat the blades of windmills13 and PFAS can be employed in the continuous separation of carbon dioxide in flue gases (patent CN106914122 from 2017)45 and as heat transfer fluids in organic Rankine engines.48 These examples all show that the uses of PFAS are much more extensive than so far reported in the scientific literature.
Altogether, we were able to identify almost 300 functions of PFAS (listed in the Appendix). Examples of those functions are foaming of drilling fluids, heat transfer in refrigerants, and film forming in AFFFs. The properties that led to the use of the PFAS are also identified. These include among others: ability to lower the aqueous surface tension, high hydrophobicity, high oleophobicity, non-flammability, high capacity to dissolve gases, high stability, extremely low reactivity, high dielectric breakdown strength, good heat conductivity, low refractive index, low dielectric constant, ability to generate strong acids, operation at a wide temperature range, low volatility in vacuum, and impenetrability to radiation. In the Appendix (Table 4), these properties are assigned to the specific uses (and functions).
3.2 Which PFAS have been – and are still – used in a certain category?
The ESI-1† to the present study describes or lists those PFAS that have been or are currently employed (or have been patented) for each individual use. In total we have found uses for more than 1400 individual PFAS. About one third of these PFAS are also listed in the OECD list.2 This shows that many of the PFAS listed in the present study are on the market, and that many more PFAS that are not on the OECD list may be used or are already being used.Due to the great variety of uses and the large number of PFAS, it is difficult to make generic statements here. Overall, it was found that the number of different PFAS identified for a certain use mostly depends on the properties required for that use. Some properties, or combinations of properties, are only found in specific groups of PFAS. For example, perfluorocarbons seem to be particularly well suited as vehicles for respiratory gas transport due to the high solubility of oxygen therein. Similarly, anionic PFAS (largely those with a sulfonic acid group) are used as additives in brake and hydraulic fluids due to their ability to alter the electrical potential of the metal surface and thus, protect the metal surface from corrosion through electrochemical oxidation. In contrast, there are also properties that are shared by many different groups of PFAS. Many PFAS are very stable and many can reduce the surface tension of aqueous solutions considerably, improving wetting and rinse-off. Therefore, a typical use in which many different types of PFAS have been or are used is in cleaning compositions. The patented, analytically detected and employed PFAS for this use include PFAAs, PASF-based substances, and fluorotelomer-based substances (see ESI-1 Section 2.6.1†). A similar variety of PFAS (87 substances in total) were identified in patents for photographic materials to control surface tension, electrostatic charge, friction, adhesion, and dirt repellency.
This array of different PFAS may be surprising, but it shows that some properties of PFAS are shared across many PFAS groups. The large number of patented PFAS for the same use raises the question of whether some of these substances offer better performance than others, or whether it does not really matter which PFAS are employed. The latter would indicate that manufacturers can invent new PFAS quite easily to avoid license fees for patents of other manufacturers.
For the majority of uses, however, far fewer PFAS were identified. Fig. 1 highlights the use categories grouped according to the number of PFAS identified. It should be noted that the number of PFAS reflects the number that we have identified in the present study, and not the number of substances on the market or available for a certain use. For half of the use categories, we have identified more than 20 PFAS, and for seven use categories more than 100 PFAS. The use categories with more than 100 identified PFAS are “photographic industry”, “semiconductor industry”, “coatings, paints and varnishes”, “fire-fighting foams”, “medical utensils”, “personal care products”, and “printing”. There are also two categories where no specific substances were identified. These are “ammunition” and “nuclear industry”.
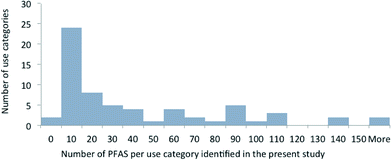 | ||
| Fig. 1 Use categories grouped according to the number of PFAS identified. The use categories are those mentioned in Table 1 without distinction of subcategories. Identified PFAS included PFAS detected analytically in products, patented and employed PFAS. The data show e.g. that 26 use categories contain fewer than 20 PFAS and seven use categories contain more than 100 PFAS. | ||
The most frequently identified PFAS in our literature search are non-polymeric fluorotelomer-based substances, followed by non-polymeric PASF-based substances and PFAAs. Other identified non-polymeric substances are perfluoroalkyl phosphinic acids (PFPIA)-based substances, perfluoroalkyl carbonyl fluoride (PACF)-based substances, cyclic PFAS, aromatic substances with fluorinated side-chains, per- and polyfluoroalkyl ethers, hydrofluoroethers, and other non-polymers. Polymeric substances include fluoropolymers, side-chain fluorinated polymers, and perfluoropolyethers (see also ESI-2†). There is also a variety of substances in the groups themselves, especially among the non-polymeric fluorotelomer-based and PASF-based substances. For many of the substances, only one use (or patent for a use) was identified. For example, one use (or patent) was assigned to 375 fluorotelomer-based substances, two uses (or patents) to 46 fluorotelomer-based substances and three or more uses to 36 fluorotelomer-based substances. The reason why so many PFAS have only one identified use may be that not all the uses were identified for all PFAS. But it also seems that many patents contain “new” PFAS because they work just as well as the established ones.
In contrast to the many PFAS with only one assigned use, some PFAS have many uses. ESI-2† illustrates this point: of the 2400 links between individual PFAS and assigned uses, 16 PFAS have been assigned to 10 or more uses (see Table 2 and Fig. 2). The exact use counts are not important per se, because there may be more uses for these PFAS that have not been included in the present study, but they demonstrate that some PFAS are employed more frequently than others. It has to be noted that the three fluoropolymers in Table 2 are quite different from the other PFAS on the list, as they represent possibly dozens or hundreds of technical products with different grades and molecular sizes.
| Substance | CAS number | Assigned uses |
|---|---|---|
| Ammonium perfluorooctanoate | 3825-26-1 | 14 |
| Potassium perfluorooctane sulfonate | 2795-39-3 | 15 |
| Potassium N-ethyl perfluorooctane sulfonamidoacetate | 2991-51-7 | 22 |
1-Propanaminium, 3-[[(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctyl)sulfonyl]amino]-N,N,N-trimethyl-, iodide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1652-63-7 | 17 |
| 1-Propanaminium, 3-[[(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,9-heptadecafluorooctyl)sulfonyl]amino]-N,N,N-trimethyl-, chloride | 38006-74-5 | 21 |
| Oxirane, 2-[[(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)oxy]methyl]- | 122193-68-4 | 10 |
| 1H-Pentafluoroethane | 354-33-6 | 10 |
| Pentane, 1,1,1,2,2,3,4,5,5,5-decafluoro- | 138495-42-8 | 12 |
| Methyl perfluoropropyl ether | 375-03-1 | 14 |
| Methyl perfluorobutyl ether | 163702-07-6 | 17 |
| Methyl perfluoroisobutyl ether | 163702-08-7 | 17 |
| Ethyl perfluorobutyl ether | 163702-05-4 | 13 |
| Poly(oxy-1,2-ethanediyl), α-[2-[ethyl[(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctyl)sulfonyl]amino]ethyl]-ω-hydroxy- | 29117-08-6 | 11 |
| Polytetrafluoroethylene (PTFE) | 9002-84-0 | 37 |
| Poly(vinylidene fluoride) (PVDF) | 24937-79-9 | 17 |
| Ethylene tetrafluoroethylene copolymer (ETFE) | 25038-71-5 | 10 |
Of the 2400 links between individual PFAS and assigned uses, around 40% were obtained from patents, 26% from studies that detected PFAS in products, and 34% of the links were obtained from publications that reported actual uses.
3.3 What is the extent of the uses in certain areas of the world?
To prioritize PFAS uses in the search for alternatives, it is key to know for which uses PFAS were employed the most. Wang et al.15,17,80 and Boucher et al. 2019 (ref. 14) published global emission inventories for C4–C14 PFCAs and C6–C10 PFSAs. For PFSAs and their precursors, the highest amounts were identified for the use in “apparel/carpet/textile”, followed by “paper and packaging”, “performance” and “after-market/consumers”. There is also information on the quantities of individual fluoropolymers used.40,81 However, a coherent data set with data covering a wide range of uses and at the same time a wide range of PFAS has not been available so far. The following two subsections will show the magnitude of the uses in the Nordic countries and the US based on the data from the SPIN database and the Chemical Data Reporting database under the TSCA, respectively. Data from REACH that would have covered more countries than the data from the SPIN database are not shown, because the tonnage bands in REACH refer to the substances and not to use categories. Accordingly, only in those cases where a substance has only one use would it have been possible to obtain useful information for this study, which would have created a lot of uncertainty in the data.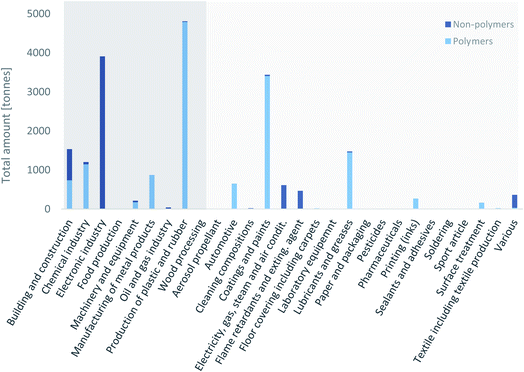 | ||
| Fig. 3 Amount of PFAS employed in the different use categories in Sweden, Finland, Norway and Denmark from 2000 to 2017, as reported in the SPIN database.44 Polymers include fluoropolymers and perfluoropolyethers. Side-chain fluorinated polymers have not been used above 0.2 t in any of the uses. Use categories with dark background are industrial branches, use categories with light grey background are other use categories. | ||
The data illustrate that a large amount of PFAS was used in the production of plastic and rubber, the electronics industry, and coatings and paints (Fig. 3). The production of plastic and rubber does not include the production of fluoropolymers. Between 2000 and 2017, more than 3000 t of PFAS were used in the three categories previously mentioned. Around 1500 t of PFAS were used in building and construction and in lubricants and greases and around 1200 t of PFAS in the chemical industry, respectively. All other uses were below 1000 t.
Non-polymers were mainly used in the electronic industry, in buildings and construction, electricity, gas, steam and air conditioning supply, and flame retardants and extinguishing agents. Of the 6300 t of non-polymers used in the Nordic countries between 2000 and 2017, 5650 t (90%) were the hydrofluorocarbon (and greenhouse gas) 1H-pentafluoroethane (CAS no. 354-33-6). More than 70% (470 t) of the remaining non-polymeric PFAS were used in flame retardants and extinguishing agents. The SPIN database has a combined category for these two use categories, so it was not possible to distinguish them.
Polymers were mostly used in the production of plastic and rubber, coatings and paints, lubricants and greases, and in the chemical industry. At least 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 t of polymers were used in the Nordic countries between 2000 and 2017, and 10
700 t of polymers were used in the Nordic countries between 2000 and 2017, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t (73%) of this was PTFE. This percentage is a bit higher than the numbers published recently by AGC, which stated that 53% of the 320 000 t of fluoroplastics consumed worldwide in 2018 was PTFE.81
000 t (73%) of this was PTFE. This percentage is a bit higher than the numbers published recently by AGC, which stated that 53% of the 320 000 t of fluoroplastics consumed worldwide in 2018 was PTFE.81
| Sector and function | Amount [t] |
|---|---|
| Paint and coating manufacturing – adhesive and sealant chemicals | 0.001 |
| Industrial gas manufacturing – air conditioners/refrigerations | 138 |
| Computer and electronic product manufacturing – solvents for cleaning and degreasing | 1.03 |
| Electrical equipment, appliance, and component manufacturing – functional fluids | 2180 |
| Fabricated metal product manufacturing – solvents for cleaning and degreasing | 0.11 |
| All other chemical product and preparation manufacturing – fire-fighting foam agents | 190 |
| Machinery manufacturing – functional fluids | 2180 |
| Miscellaneous manufacturing – solvents for cleaning and degreasing | 0.10 |
| Oil and gas drilling – surface active agents | 0.022 |
| Paint and coating manufacturing – adhesives and sealant chemicals | 0.31 |
| Paint and coating manufacturing – finishing agents | 0.005 |
| Paper manufacturing – finishing agents | 0.005 |
| Pesticide, fertilizer, and other agricultural chemical manufacturing – surface active agents | 0.07 |
| Miscellaneous manufacturing – plating agents and surface treating chemicals | 1.96 |
| Printing ink manufacturing – processing aids, not otherwise listed | 0.001 |
| All other basic inorganic chemical manufacturing – refrigerants (heat transfer fluids) | 450 |
| Rubber product manufacturing – rubber compounding | 0.13 |
| Soap, cleaning compound, and toilet preparation manufacturing – surface active agents | 0.12 |
| Textile, apparel and leather manufacturing – finishing agents | 0.16 |
The amount of used and exported PFAS was largest for functional fluids in “electrical equipment, appliance, and component manufacturing” and functional fluids in “machinery manufacturing”. The exact same amounts in the two use categories are no coincidence but come from the declaration that 50% of the total amount was used for “electrical equipment, appliance, and component manufacturing” and 50% for “machinery manufacturing”. 1H-Pentafluoroethane (CAS no. 354-33-6) accounted for 100% of the total amount in both cases. The high amounts of 1H-pentafluoroethane employed as functional fluids in “electrical equipment, appliance, and component manufacturing” confirm the data from the SPIN database indicating that the electronic industry is an important purchaser of this hydrofluorocarbon. The high amounts of “functional fluids” in “machinery manufacturing” could be related to refrigerants, air conditioners or other uses, but due to the broadness of the use category, nothing definite can be concluded. Also, as it was found for Europe, no data were available for amounts of non-polymeric PFAS used as processing aids under fluoropolymer production in the US, which may be expected to be a considerable contributor. The same amounts of “finishing agent” in “paint and coating manufacturing” and “paper manufacturing” are again from the declaration of 50% and 50%.
4 Discussion
4.1 Scope of the present study and uncertainties
Another area of uncertainty originates from unidentified uses. We found, for example, that PFAS are used in climbing ropes.82 It therefore cannot be excluded that PFAS are also used in climbing harnesses, but no information was found on this. We did not have the capacity to conduct interviews with industry representatives who might have revealed additional information. We were similarly limited when it came to evaluating the copious amount of information about PFAS uses, for example in reports, scientific papers and patents. Therefore, not all PFAS uses might have been identified in the present study.
In the case of patents in particular, a great amount of information is available, but it should be noted that only some of the PFAS included in patents currently are likely to be used on the market. In addition to these uncertainties, some of the use category-specific information in the SPIN database is CBI, meaning that we may have not seen all categories. It would be desirable if such information was no longer confidential in the future, in order to inform consumers, users, and regulators.
Nevertheless, the SPIN database is a very valuable source of information and it would be much easier to compile such inventories of uses if other countries had product registries like the Nordic countries. Without such product registries, the compilation of uses and the substances used remains difficult and lengthy. It would also be advantageous if the uses under REACH were more precisely named. Current categories like “processing aids at industrial sites” or “manufacture of chemicals” are very broad and thus difficult to include.
An important question is whether the majority of the use categories is covered in the present study or whether important use categories are still missing. It is difficult to answer such a question quantitatively, but a qualitative indication is possible when the use categories of the SPIN database are compared to the categories that were identified independently of the SPIN database. Both categories match very well; only three categories had to be added to accommodate data from the SPIN database in the ESI-1† appropriately. These three categories were “machinery and equipment”, “manufacture of basic metals” and “manufacture of fabricated metal products”. However, with the exception of these three categories, all specific information from the SPIN database could be classified very well into the existing categories of the present study. Overall, we assume that there are no major gaps in the general use categories. However, it is quite possible that subcategories are missing. Among the uses of which we are aware, there may also be some uses where PFAS are no longer employed.
To improve the list of uses in the future, there are several possibilities. Firstly, one could try to get access to product registries of as many countries as possible. Unfortunately, not all product registries are as easily accessible as those of the Nordic countries and many developing countries do not have such a registry. The list could also be extended with information from REACH registration dossiers. These dossiers include information of uses and tonnage bands expected to be used at the time of registration. Interviews with manufacturers of products could also generate more information. However, we know from experiences with past projects that manufacturers often want the interviewers to sign a non-disclosure agreement before the interview, which prevents using the information obtained in publications. The information from such interviews could still provide some indication as to what kind of information to look for in the public domain. The same is true for market reports. They can only provide a clue of what to look for in the public domain (given that they often contain no references). A discouraging factor for researchers who may want to use market reports as data sources is that the companies who generate them often sell them for extortionate sums (i.e. several thousand US dollars) and that most of them are not based on thorough research.83 Another approach could be to use artificial intelligence to systematically search product sales/industry magazines for words or phrases, such as ‘fluor’.
In addition, industry tends to evolve around consumer needs, cost savings, and external factors such as regulatory oversight, and substances used today may no longer be relevant tomorrow. A better overview of the substances being used could be obtained if manufacturers had to list which substances are contained in a product in the safety data sheets. However, except for a few instances (e.g. when uses are authorized for food contact materials in Germany), this is not the case and patents are therefore often the only way to find out what products (might) contain. A better overview of the substances used would also be possible, at least for the US, if substances with tonnages below the reporting threshold of 11.34 t per year were also included in the TSCA Chemical Data Reporting database. In the EU, it would be helpful if the registration dossiers under REACH as well as other legislations were updated regularly with a more detailed breakdown of which quantities of the substances are used in which applications.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year,81 and globally of about 320
000 t per year,81 and globally of about 320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year suggest that a considerable amount of PFAS is used as processing aids in this use category in addition to what is shown in Fig. 3 under “Chemical industry”.
000 t per year suggest that a considerable amount of PFAS is used as processing aids in this use category in addition to what is shown in Fig. 3 under “Chemical industry”.
The data from the US are only partly helpful, because a large part of the reported amounts has been claimed as CBI and only substances manufactured or imported at above 11.34 t per year at a single site have been reported. Although in some use categories large quantities of PFAS are employed, it is difficult to compare the amounts, because the unreported amounts due to CBI could be much larger than the non-confidential reported amounts. The extent of the uncertainties in the SPIN database due to the CBI cannot be estimated with the available data, but could be large. It would be helpful if regulatory agencies, such as the US EPA or the national authorities in the Nordic countries, could create a ranking of the PFAS uses (without stating any numbers) based on the entire datasets they have collected.
4.2 Findings of the present study with regard to uses
The present study is a renewed and expanded effort to systematically compile a wide range of known as well as many overlooked uses of PFAS. Besides describing the uses of PFAS, we also endeavoured to explain which functions the PFAS fulfil in these uses (see Table 4 in the Appendix). The descriptions of the functions and properties of the PFAS employed are especially important for determining “non-essential” use categories and identifying alternatives for those uses currently considered “essential”.However, as can be seen from the question marks in the Appendix it was not always possible to determine why PFAS were used or needed in a particular case. In 4% of the cases we could not clarify which function the PFAS fulfil in the use category or subcategory, and in 21% of the cases we could not clarify which property is needed to fulfil the mentioned function. For example, we do not know exactly why PFAS are employed in the ventilation of respiratory airways, in brake-pad additives, and in resilient linoleum. It would be important to engage with product manufacturers to understand what function the PFAS actually have, in order to identify appropriate replacements. Some of the uses might also be judged as “non-essential” and thus could be eliminated or discontinued.
Our study also shows that in several areas where large quantities of PFAS are employed, discussions concerning alternatives are still not underway in the public domain. In general, in recent years the focus in the search for alternatives for PFAS has been on fire-fighting foams,84,85 paper and packaging,86,87 and textiles.88–91 This focus was certainly appropriate, because these are uses where PFAS are in direct contact with the environment (fire-fighting foam) or with humans (food packaging, textiles). However, our results show that PFAS are also used widely in the production of electronics and in machinery manufacturing, and at least in the Nordic countries in the production of plastic and rubber and in paints and coatings. Measuring and/or reporting emissions along the life cycles of these uses, and the search for alternatives in these use categories should therefore also be prioritized. These uses could for instance be included in the activities for which data have to be reported under the European Pollutant Release and Transfer Registry.
It would also be important to look for alternatives in industry branches that use smaller amounts of PFAS or that are not included in the SPIN database or Chemical Data Reporting database, but produce large amounts of wastewater, exhaust gases or solid waste containing PFAS. More information is needed to prioritize the various use categories, but potentially worrisome categories where environmental contamination has been documented are fluoropolymer production,92–94 the semiconductor industry,95,96 and metal plating.97
Beside the categories mentioned above, there are also uses where humans are in direct contact with PFAS and that have not yet gained much attention regarding alternatives. These include: personal care products and cosmetics (ESI-1 Section 2.28†), pesticides (ESI-1 Section 2.29†), pharmaceuticals (including eye drops) (ESI-1 Section 2.30†), printing inks (ESI-1 Section 2.33†), and sealants and adhesives (ESI-1 Section 2.35†). A search for alternatives would also be important here.
4.3 Findings of the present study with regard to substances
We can ascertain from the SPIN database that two PFAS, 1H-pentafluoroethane and PTFE, account for 75% of the quantities used in the Nordic countries. One explanation is that PTFE and 1H-pentafluoroethane are not used as additives, but as the main products. For example, entire roof structures or coatings are made out of PTFE.30 For 1H-pentafluoroethane (also known as HFC-125), one of the main uses is as a heat transfer fluid and cooling agent,44,98 which could explain the large quantities of that substance used.Other PFAS used as surfactants are utilized in much smaller quantities probably due to their high market price. They may therefore not appear (or at least not in high amounts) in databases such as the SPIN database or the Chemical Data Reporting database, which only report substances (or amounts) above a certain threshold. PFAS used in articles that are manufactured mainly in Asia or other countries outside the EU or the US may also not appear in large amounts in the SPIN or Chemical Data Reporting database, simply because the databases do not contain information on PFAS in articles. The PFAS that we have listed as examples in the ESI-1† are mainly those used in Europe or North America. A recent publication99 lists e.g. seventy PFAS from the Inventory of Existing Chemical Substances Produced or Imported in China (IECSC) that are not in the North American and European chemical inventories. These PFAS are also not in our inventory, because no information on their intended use was provided.
Concerning the currently used PFAS, it was thought – due to the voluntary phase out of all PFAS products derived from perfluorooctane sulfonyl fluoride by 3M100 and the voluntary PFOA Stewardship Program in which eight companies agreed to phase out 95% of uses by 2015 (ref. 101) – that at least ammonium perfluorooctanoate and potassium perfluorooctane sulfonate are no longer in use in the US. However, other companies have not been prevented from taking over the market, and there has been very limited enforcement of the actual phase-out through regulation. A recent article revealed that PFAS that can break down into PFOA and PFOS are still in use in the US.102 Those uses include coatings for medical devices, apparel, and other industries, and equipment in pharmaceutical companies. PFAS that can break down into PFOA and PFOS are also still used by semiconductor and electronics companies.102
4.4 Prioritisation of use categories
Based on the data from the SPIN database, the Chemical Data Reporting under the TSCA and information on the production of wastewater, exhaust gases and solid waste, we propose that the following use categories need to be prioritized for reducing/eliminating the use of PFAS. At the same time, it must be noted that fluoropolymers and hydrofluorocarbons are produced and used in much larger quantities than PFAAs and their precursors. However, PFAAs and their precursors are more critical from a toxicological point of view. Therefore, the proposal for prioritization is made for each of the three PFAS groups individually: PFAAs and precursors, hydrofluorocarbons, and fluoropolymers.4.4.1.1 Fire-fighting foams. PFAS-containing fire-fighting foams are used for extinguishing liquid fires such as fires in oil, jet fuel, other non-water-soluble hydrocarbons, alcohols and acetone. Although relatively small quantities of PFAS are used in fire-fighting foams (class B for extinguishing flammable liquid fires), these foams are an important use category because the foams and the chemicals they contain are released directly into the environment. There are numerous reports about PFAS-contaminated sites where fire-fighting foams have been used (especially for training activities) or spilled.61,63,103,104 Although PFAS-free class B fire-fighting foams have been developed in the meantime, PFAS-containing fire-fighting foams are still widely in use today.65,105,106 For more information, see ESI-1 Section 2.14† and the Appendix.
4.4.1.2 Chemical industry with a special focus on processing aids in the polymerization of fluoropolymers. Important uses of PFAS in the chemical industry are their uses as processing aids in the polymerization of fluoropolymers, the production of chlorine and sodium hydroxide, and the production of other chemicals including solvents. PFAS that are used as processing aids in the polymerization of fluoropolymers are of special concern. This is because the surrounding environments at numerous sites have been heavily contaminated due to the release of the processing aids from the nearby manufacturing plants,92–94 and considerable amounts of fluoropolymers are produced in Europe and worldwide. For more information, see ESI-1 Section 1.4.†
4.4.1.3 Surface protection of textile, apparel, leather, carpets, and paper. Considerable quantities of PFAS, especially of side-chain fluorinated polymers, have been used as surface protectors in textile, apparel, leather, carpets, and paper. These are open and dispersive uses where many consumers come into contact with the PFAS-containing products. It has also been reported that there are high emissions to air, dust, and wastewater from a textile manufacturing plant in China.107 The side-chain fluorinated polymers contain PFAAs as impurities and they may act as important precursors to PFAAs.108 For more information, see ESI-1 Sections 2.5, 2.16, 2.20, 2.26, and 2.40.†
4.4.2.1 Electronic industry. PFAS have been used in electronic devices themselves e.g. in flat panel displays or liquid crystal displays. However, they have also been used for the testing of electronic devices and equipment, as heat transfer fluids/cooling agents, in cleaning solutions, to deposit lubricants and to etch piezoelectric ceramic filters. Based on data from the SPIN database and the Chemical Data Reporting database under the TSCA, the most widely used substance in the electronic industry in the Nordic countries and the US is the hydrofluorocarbon 1H-pentafluoroethane. According to the SPIN database it is mainly used as a heat transferring agent and cooling agent. However, 1H-pentafluoroethane is not only of concern due to its high persistence but also because it has a global warming potential that is 3500 times that of carbon dioxide. Therefore, 1H-pentafluoroethane is one of the substances regulated by the Kigali Amendment of the Montreal Protocol and efforts are being undertaken to reduce the production and consumption of this substance. The search for PFAS-free alternatives is therefore even more important in this use category.
4.4.2.2 Machinery and equipment. The Chemical Data Reporting database under the TSCA lists also high amounts (more than 2000 t per year) of 1H-pentafluoroethane that is used as a “functional fluid” in “machinery manufacturing” in the US. This could be related to refrigerants, air conditioners or other uses, but due to the broadness of the use category, nothing specific can be concluded. Given the high amounts reported, there is an urgent need for more information on where and for which function hydrofluorocarbons, and PFAS in general, are used in this category. For more information, see ESI-1 Section 1.10† and the Appendix.
4.4.3.1 Production of plastic and rubber. The SPIN database reveals that large amounts of fluoropolymers (more than 4000 t between 2000 and 2017) have been used in the production of plastic and rubber in the Nordic countries between 2000 and 2017. PFAS have been used as mould release agents, foam blowing agents, foam regulators, polymer processing aids, in the etching of plastic, as anti-blocking agents for rubber, and as curatives in the production of plastic and rubber. As polymer processing aids, fluoropolymers can increase the processing efficiency and quality of plastic and rubber.109 The use of PFAS in the production of plastic and rubber may explain why PFAS are found, for example, in artificial turf.110 For more information, see ESI-1 Section 2.14† and the Appendix.
4.4.3.2 Coatings, paints and varnishes. The data from the SPIN database show that large amounts of fluoropolymers (more than 3000 t between 2000 and 2017) have been used in coatings and paints in the Nordic countries between 2000 and 2017. Fluoropolymers can be used to impart oil- and water-repellency to the paints or coatings, and fluoropolymers are also used as anti-stick and anticorrosive coatings. For more information, see ESI-1 Section 2.8† and the Appendix.
4.5 Use and implications of the present study
The large number of uses that exist for PFAS, together with the large number of individual substances, makes their regulation and eventual phase-out very challenging. The approach of allowing PFAS only in “essential uses”, as suggested for example in the EU strategy paper “Elements for an EU-strategy for PFAS”,5 will not be easy to implement if regulators try to assess all uses individually. An alternative approach could be to deem all PFAS uses as “non-essential” unless producers or users make a convincing case for essentiality, and that authorities set a sunset clause on “essential uses”.The number of use categories for both non-essential and essential cases is critical to estimate the amount of work that would need to be done, for example, to prepare a restriction proposal under REACH (as planned by five European countries31). The descriptions in the present study of where and why PFAS are used can be used to provide an overview of the uses and may also facilitate an understanding of what alternatives need to be developed and with which priority.
The information in this study may also help regulators and scientists determine which PFAS to measure in contaminated areas, in humans, in surrounding communities, and in products. To facilitate the identification of PFAS in various matrices, we provide the ESI-3 file,† which contains for each use category the name, CAS number, and exact monoisotopic mass of the substance. The ESI-3 file† also includes information on whether PFAS were identified in a patent, detected analytically in products, or reported as employed substances. Laboratories could use modern analytical methods such as suspect-screening analysis utilising accurate mass spectrometry to identify novel and emerging PFAS listed in our ESI-3.†60,111 Patented substances may be less likely to be on the market and could be excluded or given a lower priority or weighting in suspect screening workflows. Similar lists (such as the ESI-3†) are provided by the OECD/UNEP Global PFC Group,2 Zhang et al. (2020),99 the US EPA, the NORMAN Substance Database79 and others. An overview is provided under https://comptox.epa.gov/dashboard/chemical_lists. However, only a few of these lists also contain information on uses.
The ESI-3† may also be valuable for identifying sources of PFAS in the environment. Some uses may impart characteristic PFAS “fingerprints” (i.e. PFAS contamination patterns) to environmental samples that could be used to identify a source, e.g. through statistical methods.112 On the other hand, many environments will be impacted by multiple sources and such fingerprinting methods could be challenging in practice.
5 Conclusions
The present study is the first of its kind to systematically compile a wide range of known as well as poorly documented uses of PFAS. The compilation is not exhaustive, but it still demonstrates that PFAS are used in almost all industry branches and in many consumer products. Some consumer products even have multiple applications of PFAS within the same product. A cell phone for example may contain fluoropolymer-insulated wiring, PFAS in the circuit boards/semiconductors, and a screen coated with a fingerprint-resistant fluoropolymer. The search for alternatives is therefore a challenging and extensive task and is important in all use categories. However, it seems particularly critical to us to replace PFAAs and their precursors in fire-fighting foams, processing aids for the polymerization of fluoropolymers and in the surface protection of textiles, apparel, leather, carpets, and paper. Hydrofluorocarbons seem to be used most in the electronics industry and in machinery and equipment. Replacing them in these categories will therefore be an important but challenging task. A search for alternatives to fluoropolymers will be important in the production of plastic and rubber and in coatings, paints, and varnishes.A matching database of viable alternatives to PFAS would be a logical progression of the present study. It would also be helpful if environmental protection agencies, for example the US EPA, could create a ranking of PFAS uses (without providing tonnages) based on the data they have collected. A ranking without exact figures would still be better than the current situation, in which very little is known about the quantitatively most important use categories due to CBI. The TSCA reform in the US was unfortunately unsuccessful in reducing industry's excessive use of CBI. On the one hand, CBI may protect a specific industry's business, but on the other hand it also results in less protection for consumers, users, and workers from the chemicals. Even regulators are left in the dark about volumes, use categories, and PFAS used, which limits their ability to assess and prevent harm to humans and the environment.
| Use category/subcategory | Function of PFAS | Properties of the PFAS employed |
|---|---|---|
| Industry branch | ||
| Aerospace | ||
| - Phosphate ester-based brake and hydraulic fluids | Corrosion protection | Altering the electrical potential at the metal surface |
| - Gyroscopes | Flotation fluids in gyroscopes | ? |
| - Wire and cable | High-temperature endurance, fire resistance, and high-stress crack resistance | Non-flammable polymers, stable |
| - Turbine-engine | Use as lubricant | Corrosion resistant, stable, non-reactive, operate at a wide temperature range |
| - Turbine-engine | Use as elastomeric seals | Operate at a wide temperature range |
| - Thermal control and radiator surfaces | Reject waste heat | Survival over a wide operating temperature range, low solar absorbance, high thermal emittance, and freedom from contamination by outgassing |
| - Coating | Protect underlying polymers from atomic oxygen attack | Non-reactive, very stable |
| - Propellant system | Elastomers compatible to aggressive fuels and oxidizers | Non-reactive, very stable |
| - Jet engine/satellite instrumentation | Use as lubricant | Long-term retention of viscosity, low volatility in vacuum and their fluidity at extremely low temperatures |
| Biotechnology | ||
| - Cell cultivation | Supply of oxygen and other gases to microbial cells | Great capacity to dissolve gases |
| - Ultrafiltration and microporous membranes | Prevent bacterial growth | ? |
| Building and construction | ||
| - Architectural membranes e.g. in roofs | Resistance to weathering, dirt repellent, light | Oleophobic and hydrophobic, low surface tension, beneficial weight-to-surface ratio |
| - Greenhouse | Transparent to both UV and visible light, resistant to weathering, dirt repellent | Oleophobic and hydrophobic, low surface tension |
| - Cement additive | Reduce the shrinkage of cement | ? |
| - Cable and wire insulation, gaskets & hoses | High-temperature endurance, fire resistance, and high-stress crack resistance | Non-flammable polymers, stable |
| Chemical industry | ||
| - Fluoropolymer processing aid | Emulsify the monomers, increase the rate of polymerization, stabilize fluoropolymers | Fluorinated part is able to dissolve monomers, non-fluorinated part is able to dissolve in water |
| - Production of chlorine and caustic soda (with asbestos diaphragms cells) | Binder for the asbestos-fibre-based diaphragms | ? |
| - Production of chlorine and caustic soda (with fluorinated membranes) | Stable membrane in strong oxidizing conditions and at high temperatures | Stable, non-reactive |
| - Processing aids in the extrusion of high- and liner low-density polyethylene film | Eliminate melt fracture and other flow-induced imperfections | Low surface tension |
| - Tantalum, molybdenum, and niobium processing | Cutting or drawing oil | Non-reactive, stable |
| - Chemical reactions | Inert reaction media (especially for gaseous reactants) | Non-reactive, stable |
| - Polymer curing | Medium for crosslinking of resins, elastomers and adhesives | ? |
| - Ionic liquids | Raw materials for ionic liquids | ? |
| - Solvents | Dissolve other substances | Bipolar character of some of the PFAS |
| Electroless plating | Disperses the pitch fluoride in the plating solution | Low surface tension |
| Electroplating (metal plating) | ||
| - Chrome plating | Prevent the evaporation of chromium(VI) vapour | Lower the surface tension of the electrolyte solution, very stable in strongly acidic and oxidizing conditions |
| - Nickel plating | Non-foaming surfactant | Low surface tension |
| - Nickel plating | Increase the strength of the nickel electroplate by eliminating pinholes, cracks, and peeling | Low surface tension |
| - Copper plating | Prevent haze by regulating foam and improving stability | Low surface tension |
| - Tin plating | Help to produce a plate of uniform thickness | Low surface tension |
| - Alkaline zinc and zinc alloy plating | ||
| - Deposition of fluoropolymer particles onto steel | Supported by fluorinated surfactants | Cationic and amphoteric fluorinated surfactants impart a positive charge to fluoropolymer particles which facilitates the electroplating of the fluoropolymer |
| Electronic industry | ||
| - Testing of electronic devices and equipment | Inert fluids for electronics testing | Non-reactive |
| - Heat transfer fluids | Cooling of electrical equipment | Good heat conductivity |
| - Solvent systems and cleaning | Form the basis of cleaning solutions | Non-flammable, low surface tension |
| - Carrier fluid/lubricant deposition | Dissolve and deposit lubricants on a range of substrates during the manufacturing of hard disk drives | ? |
| - Etching of piezoelectric ceramic filters | Etching solution | Acidic |
| Energy sector | ||
| - Solar collectors and photovoltaic cells | High vapour barrier, high transparency, great weatherability and dirt repellency | Oleophobic and hydrophobic, low surface tension |
| - Photovoltaic cells | Adhesives with PFAS hold mesh cathode in place | Lower the surface tension of the adhesive |
| - Wind mill blades | Coating | High weatherability |
| - Coal-based power plants | Polymeric PFAS filter remove fly ash from the hot smoky discharge | Stable, non-reactive |
| - Coal-based power plants | Separation of carbon dioxide in flue gases | Lower the surface tension of the aqueous solution |
| - Lithium batteries | Binder for electrodes | Almost no reactivity with the electrodes and electrolyte |
| - Lithium batteries | Prevent thermal runaway reaction | Good heat absorption of first layer and good heat conductivity of second layer |
| - Lithium batteries | Improve the oxygen transport of lithium–air batteries | Great capacity to dissolve gases |
| - Lithium batteries | Electrolyte solvents for lithium–sulfur batteries | Bipolar character of some of the PFAS |
| - Ion exchange membrane in vanadium redox batteries | Polymeric PFAS are used as membranes | Resistance to acidic environments and highly oxidizing species |
| - Zinc batteries | Prevent formation of dendrites, hydrogen evolution and electrode corrosion due to adsorption onto the electrode surface | Low surface tension, non-reactive |
| - Alkaline manganese batteries | MnO2 cathodes containing carbon black are treated with a fluorinated surfactant | ? |
| - Polymer electrolyte fuel cells | Polymeric PFAS are used as membranes | Ion conductance |
| - Power transformers | Cooling liquid | Good heat conductivity |
| - Conversion of heat to mechanical energy | Heat transfer fluids | Good heat conductivity |
| Food production | ||
| - Wineries and dairies | Final filtration before bottling with polymeric PFAS | Resist degradation |
| Machinery and equipment | ? | ? |
| Manufacture of metal products | ||
| - Manufacture of basic metals | Inhibit the formation of acid mist during the electrowinning of copper | Lower the surface tension of the aqueous solution |
| - Manufacture of fabricated metal products | ? | ? |
| - Pickling of steel wires | Acid-pickling promoter | ? |
| - Treatment of coating of metal surfaces | Promote the flow of metal coatings, prevent cracks in the coating during drying | Lower the surface tension of the coating |
| - Treatment of coating of metal surfaces | Corrosion inhibitor on steel | Non-reactive |
| - Etching of aluminium in alkali baths | Improving the efficient life of the alkali baths | ? |
| - Phosphating process for aluminium | Fluoride-containing phosphating solutions help to dissolve the oxide layer of the aluminium | ? |
| - Cleaning of metal surfaces | Disperse scum, speed runoff of acid when metal is removed from the bath, increase the bath life | ? |
| - Water removal from processed parts | Solvent displacement | Low surface tension |
| Mining | ||
| - Ore leaching in copper and gold mines | Increase wetting of the sulfuric acid or cyanide that leaches the ore | Low surface tension |
| - Ore leaching in copper and gold mines | Acid mist suppressing agents | Low surface tension |
| - Ore floating | Create stable aqueous foams to separate the metal salts from soil | Low surface tension |
| - Separation of uranium contained in sodium carbonate and/or sodium bicarbonate solutions by nitrogen floatation | Improve the separation | ? |
| - Concentration of vanadium compounds | Destruction of the mineral structure, increases the specific surface area and pore channel thus facilitating vanadium leaching | Acidity |
| Nuclear industry | ||
| - Lubricants for valves and ultracentrifuge bearings in UF6 enrichment plants | PFAS are used as the lubricants | Stable to aggressive gases |
| Oil & gas industry | ||
| - Drilling fluid | Foaming agent | Low surface tension |
| - Drilling – insulating material for cable and wire | Polymeric PFAS are used as insulating material | Withstand high temperatures |
| - Chemical driven oil production | Increase the effective permeability of the formation | Low surface tension |
| - Chemical driven oil production | Foaming agent for fracturing subterranean formations | Low surface tension |
| - Chemical driven oil production | Heavy crude oil well polymer blocking remover | ? |
| - Chemical driven gas production | Change low-permeability sandstone gas reservoir from strong hydrophilic to weak hydrophilic | Hydrophobic and oleophobic properties |
| - Chemical driven gas production | Eliminate reservoir capillary forces, dissolve partial solid, dis-assemble clogging, increase efficiency of displacing water with gas | Lower surface tension of the material |
| - Oil and gas transport | Lining of the pipes is made out of polymeric PFAS | Non-reactive (corrosion resistant) |
| - Oil and gas transport | Reduce the viscosity of crude oil for pumping from the borehole through crude oil-in-water emulsions | Hydrophobic and oleophobic properties |
| - Oil and gas storage | Aqueous layer with PFAS prevents evaporation loss | Lower the surface tension of the aqueous solution |
| - Oil and gas storage | Floating layer of cereal treated with PFAs prevents evaporation loss | Low surface tension |
| - Oil containment (injection a chemical barrier into water) | Prevents spreading of oils or gasoline on water | ? |
| - Oil and fuel filtration | Polymeric PFAS are used as membranes | Non-reactive (corrosion resistant) |
| Pharmaceutical industry | ||
| - Reaction vessels, stirrers, and other components | Use of polymeric PFAS instead of stainless steel | ? |
| - Ultrapure water systems | Polymeric PFAS are used as filter | Low surface tension |
| - Packaging | Polymeric PFAS form moisture barrier film | Hydrophobic |
| - Manufacture of “microporous” particles | Processing aid | ? |
| Photographic industry | ||
| - Processing solutions | Antifoaming agent | Lower the surface tension of the solution |
| - Processing solutions | Prevent formation of air bubbles in the solution | Lower the surface tension of the solution |
| - Photographic materials, such as films and papers | Wetting agents, emulsion additives, stabilizers and antistatic agent | Low surface tension, low dielectric constant |
| - Photographic materials, such as films and papers | Prevent spot formation and control edge uniformity in multilayer coatings | Low surface tension |
| - Paper and plates | Anti-reflective agents | Low refractive index |
| Production of plastic and rubber | ||
| - Separation of mould and moulded material | Mould release agent | Hydrophobic and oleophobic properties |
| - Separation of mould and moulded material | Reduce imperfections in the moulded surface | Low surface tension |
| - Foam blowing | Foam blowing agent | Low surface tension |
| - Polyol foams | Foam regulator | 10.5.3.1.1.1.1 lower the surface tension of the foam |
| - Polymer processing aid | Increase processing efficiency and quality of polymeric compounds | Lower the surface tension of the polymeric products |
| - Etching of plastic | Wetting agent | Low surface tension |
| - Production of rubber | Antiblocking agent | Low surface tension |
| - Fluoroelastomer formulation | Additive in curatives | ? |
| Semiconductor industry | ||
| - Photoresist (itself) | Photoresist matrix, changes solubility when exposed to light | ? |
| - Photoresist (photosensitizer) | Increase the photosensitivity of the photoresist | ? |
| - Photoresist (photo-acid generator) | Generate strong acids by light irradiation | Able to generate strong acids |
| - Photoresist (quencher) | Controlling the diffusion of the acid to unexposed region | ? |
| - Antireflective coating | Provide low reflectivity | Low refractive index |
| - Developer | Facilitate the control of the development process | ? |
| - Rinsing solution | Rinsing the photoresist to remove the developer | Low surface tension |
| - Etching | Wetting agent | Low surface tension |
| - Etching | Reduce the reflection of the etching solution | Low refractive index |
| - Etching | Etching agent in dry etching | Strong acids |
| - Cleaning of silicon wafers | Etch cleaning | Strong acids |
| - Cleaning of integrated circuit modules | Remove cured epoxy resins | ? |
| - Cleaning vapour deposition chamber | Remove dielectric film build up | Generation of reactive oxygen species |
| - Wafer thinning | Non-stick coating composition on carrier wafer | Low surface tension |
| - Vacuum pumps | Working fluid | Stable, non-reactive |
| - Technical equipment in contact with process chemical or reactive plasma | Polymeric PFAS are used in inert moulds, pipes and elastomers | Stable, non-reactive |
| - Multilayer circuit board | Bonding ply composition | Low dielectric constant, low dissipation factor |
| Textile production | ||
| - Dyeing and bleaching of textiles | Wetting agent | Low surface tension |
| - Dyeing process using sulphur dyes | Antifoaming agent | Low surface tension |
| - Dye transfer material | Release agent | Low surface tension |
| - Textile treatment baths | Antifoaming agent | Low surface tension |
| - Fibre finishes | Emulsifying agent | Hydrophobic and oleophobic properties |
| Watchmaking industry | ||
| - Lubricants | Form an oil layer and reduced wear | Non-reactive (do not oxidize, resistant to corrosion) |
| - Drying as production step after aqueous cleaning | Solvents in solvent displacement drying | Low surface tension |
| Wood industry | ||
| - Drum filtration during bleaching | The used coarse fabric is made out of polymeric PFAS | Stable |
| - Coating for wood substrate | Clear coating is made out of polymeric PFAS | Stable, non-reactive |
| - Wood particleboard | Part of adhesive resin | Low surface tension |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Other use areas | ||
| Aerosol propellant | Aerosol propellant | Non-flammable, stable, non-reactive |
| Air conditioning | Working fluid | Non-flammable, stable, non-reactive |
| Antifoaming agent | Prevent foaming | Low surface tension |
| Ammunition | Make the final product rubbery and reduce the likelihood of an unplanned explosion due to shock; enable long-term storage without degradation of the polymer | Long-term stability without degradation |
| Apparel | ||
| - Breathable membranes | Polymeric PFAS are used as membranes | High permeability to water vapour, but resist passage of liquid water |
| - Long-lasting durable water repellent finish | Provide water and oil repellence, stain resistance and soil release | Lower surface tension of the fabric, hydrophobic and oleophobic properties |
| Automotive | ||
| - Car body | Weather resistance paint, no-wax brilliant top coat | Low surface tension |
| - Automotive waxes | Aid spreading, improve the resistance of the polish to water and oil | Lower the surface tension of the wax, oleophobic |
| - Windshield wiper fluid | Prevent icing of the wind shield | ? |
| - Car body | Light, stable | Beneficial weight-to-surface ratio, stable |
| - Engine and steering system | Polymeric PFAS are used as sealants and bearings | Operate at a wide temperature range, non-reactive |
| - Engine oil coolers | Heat transfer fluid | Good heat conductivity |
| - Cylinder head coatings and hoses | Increase the fuel efficiency | ? |
| - Cylinder head coatings and hoses | Reduce the fugitive gasoline vapour emissions | Low surface tension |
| - Electronics | Cables and wires | High-temperature endurance, fire resistance |
| - Fuel lines, steel hydraulic brake tubes | Corrosion protection | Non-reactive, stable |
| - Interior | Dirt repellent in carpets and seats | Low surface tension, oleophobic |
| - Brake pad additives | ? | ? |
| Cleaning compositions | ||
| - Cleaning compositions for hard surfaces | Enhance wettability | Lower the surface tension of the cleaning product |
| - Carpet and upholstery cleaners | Provide stain resistance and repel soil | Low surface tension, oleophobic |
| - Cleaning compositions for adhesives | ? | ? |
| - Dry cleaning fluids | Stabilizer, improve the removal of hydrophilic soil | Hydrophobic and oleophobic, low surface tension |
| - Cleaning of reverse osmosis membranes | Remove calcium sulphate | ? |
| Coatings, paints and varnishes | ||
| - Paints | Emulsifier for the binder, dispersant for the pigments, wetting agent | Hydrophobic and oleophobic, low surface tension |
| - Paints | Enhance the protective properties of anticorrosive paints | Non-reactive |
| - Paints | Antifouling on ships | ? |
| - Paints and coatings | Anti-crater, improved surface appearance, better flow and levelling, reduced foaming, decreased block, open-time extension, oil- and water repellency, dirt pickup resistance | Low surface tension, oleophobic |
| - Paints and coatings | Form second coat on a first coat | Low surface tension |
| - Coatings | Antistick and anticorrosive coatings | Low surface tension, non-reactive |
| - Coatings | Highly durable and weatherable | Stable, non-reactive |
| Conservation of books and manuscripts | Preserve historical manuscripts | Permeability to water vapour, but resist passage of liquid water |
| Cook- and bakingware | Prevent food from sticking to the pan/baking ware | Low surface tension, non-reactive, stable at high temperatures |
| Dispersions | Disperse solutions | Low surface tension |
| Electronical devices | ||
| - Printed circuit boards | Use fibre-reinforced fluoropolymer layer | Low dielectric constant |
| - Capacitors | Separation of high voltage components (dielectric fluid) | High dielectric breakdown strength, non-flammable |
| - Acoustical equipment | Provide an electrical signal in response to mechanical or thermal signals | Piezoelectric and pyroelectric properties |
| - Liquid crystal displays (LCDs) | Provide the liquid crystal with a dipole moment | Dipoles |
| - Liquid crystal displays (LCDs) | Polymeric PFAS provide moisture sensitive coating for displays | Hydrophobic |
| - Light management films in flat panel display | Reduced static electricity build-up and dust attraction during fabrication | Low dielectric constant |
| - Razors | Polymeric PFFAs is used on the razor | ? |
| - Electroluminescent lamps | Polymeric PFAS is used as coating | ? |
| Fingerprint development | Solvent | ? |
| Fire-fighting foam | ||
| - Fluoroprotein (FP) foams | Fuel repellents | Low surface tension |
| - Film-forming fluoroprotein (FFFP) foam | Film formers, foam stabilizers | Lower the surface tension of water |
| - Alcohol-resistant film forming fluoroprotein (AR-FFFP) foam | Film formers, foam stabilizers | Lower the surface tension of water |
| - Aqueous film-forming foams (AFFF) | Film formers | Lower the surface tension of water |
| - Alcohol-resistant aqueous film forming foam (AR-AFFF) | Foam stabilizers | Low surface tension |
| Flame retardants | ||
| - Polycarbonate resin | Flame retardants | Non-flammable |
| - Other plastic | Flame retardants | Non-flammable |
| Floor covering including carpets and floor polish | Improve wetting and levelling | Low surface tension |
| - Soil-release finishes for carpets | Provide water and oil repellence, stain resistance and soil release | Low surface tension, hydrophobic and oleophobic |
| - Aftermarket carpet protection | Provide water and oil repellence, stain resistance and soil release | Low surface tension, hydrophobic and oleophobic |
| - Resilient linoleum | ? | ? |
| - Laminated floor covering | ? | ? |
| - Floor polish | Improve levelling and wetting | Low surface tension |
| Glass | ||
| - Surface treatment | Make glass surfaces hydrophobic and oleophobic | Hydrophobic and oleophobic |
| - Surface treatment | Prevents misting of glass | Hydrophobic |
| - Surface treatment | Dirt-repellent | Low surface tension |
| - Surface treatment | Fire-or weather resistant | Non-flammable, stable |
| - Etching and polishing | Increase the speed of etching, improve wetting | Low surface tension |
| - Drying as production step in glass finishing | Solvents in solvent displacement drying | Low surface tension |
| Household applications | ||
| - Threads and joints | Polymeric PFAS is used for sealing | ? |
| Laboratory supplies, equipment and instrumentation | ||
| - Consumable materials (vials, caps, tape) | Made out of polymeric PFAS | ? |
| - Personal protective equipment (gloves) | ? | ? |
| - Particle filters | Minimize the sorption of compounds to the filter itself | Low surface tension |
| - Solvents | Dissolve other substances | Hydrophobic and oleophobic |
| - LC instruments | Polymeric PFAS are used in the solvent degasser | Non-reactive ? |
| - LC columns | Some columns are based on polymeric PFAS | ? |
| - Reverse phase LC-solvents | can contain PFAS | ? |
| - Seals and membranes in UPLCs, autoclaves and ovens | are made out of polymeric PFAS | Work over a wide temperature range |
| - Oils and greases in pumps | Form a thick oil layer and reduced wear | Non-reactive, non-flammable |
| - Sterilization of an insulated vessel | Sterilization medium | ? |
| - Electro plotting | Protein-sequencing membranes are made out of polymeric PFAS | ? |
| - Analysing the phosphoamino content in proteins | Protein-sequencing membranes are made out of polymeric PFAS | ? |
| Leather | ||
| - Manufacturing of genuine leather | Improve the efficiency of hydrating, pickling, degreasing and tanning | ? |
| - Repellent treatment (genuine leather) | Provide water and oil repellence, stain resistance and soil release | Hydrophobic and oleophobic, low surface tension |
| - Manufacturing of synthetic leather | Polymer melt additives that impart oil and water repellency to the finished fibres | Hydrophobic and oleophobic |
| - Shoe brighteners | Improve the levelling of shoe brighteners | Low surface tension |
| - Impregnation spray | Provide water and oil repellence, stain resistance and soil release | Low surface tension |
| Lubricants and greases | Form a thick oil layer and reduced wear | Non-reactive, non-flammable, operate also at high temperatures, do not form sludge or varnish |
| Medical utensils | ||
| - Electronic devices that rely on high frequency signals (defibrillators, pacemakers, cardiac resynchronization therapy (CRT), positron-emission tomography (PET) and magnetic resonance imaging (MRI) devices) | High dielectric insulators | High dielectric breakdown strength |
| - Video endoscope | Use in charge-coupled device colour filters | ? |
| - Microbubble-based ultrasound contrast agents | Fluorinated gas inner core, which provides osmotic stabilization and contributes to interfacial tension reduction | Low solubility in aqueous media (dissolve more slowly) |
| - X-ray imaging | Contrast enhancement agents | Radio-opaque |
| - Magnetic resonance imaging | Contrast agent | Lack of a 19F endogenous background signal in vivo and high magnetic resonance sensitivity of 19F atoms |
| - Proton and 19F NMR imaging | Contrast agents | Lack of fluorine in organs and tissue |
| - Computed tomography and sonography | Contrast agents | Lack of fluorine in organs and tissue |
| - Radio-opaque materials | Polymeric PFAS has been used | Radio-opaque |
| - Surgical drapes and gowns | Improve water-, oil- and dirt-resistance | Hydrophobic and oleophobic, low surface tension |
| - X-ray films | Wetting agents, emulsion additives, stabilizers and antistatic agent | Low surface tension, low dielectric constant |
| - Dispersant | Facilitate the dispersion of cell aggregates | Low surface tension |
| - Contact lenses | Raw material | |
| - Retinal detachment surgery and proliferative vitreoretinal | Endotamponade gases | High specific gravity, low surface tension, and low viscosity |
| - Retinal detachment surgery and proliferative vitreoretinal | Intraoperative tool during vitreoretinal surgery | High specific gravity, low surface tension, and low viscosity |
| - Eye drops | Delivery agent | Unique combination of apolarity and amphiphility |
| - Filters, tubing, O-rings, seals and gaskets in dialysis machines | Made out of polymeric PFAS | Low surface tension |
| - Dialysis membranes | Made out of polymeric PFAS | Low surface tension |
| - Catheter, stents, and needles | Provide low-friction and clot-resistant coatings | Low surface tension |
| - Surgical patches and vascular catheter | Use of polymeric PFAS | ? |
| - Blood transfer and artificial blood | Oxygen carrier | Great capacity to dissolve gases |
| - Organ perfusion | Oxygen carrier | Great capacity to dissolve gases |
| - Percutaneous transluminal coronary angioplasty | Oxygen carrier | Great capacity to dissolve gases |
| - Toothpaste | Enhances fluorapatite formation and inhibits caries | Low surface tension |
| - Dental floss | Allows the narrow ribbon to slip easily between close-pressed teeth | Low surface tension |
| - UV-hardened dental restorative materials | Improve the wetting of the set materials | Low surface tension |
| - Ventilation of respiratory airway | ? | ? |
| - Anaesthesia | Polymeric PFAS is used to dry or humidify breath | Hydrophobic |
| - Artificial heart pump | Blood compatible and durable | Non-reactive, stable |
| - Wound care | Cleaning burn residues | Dissolve hydrocarbon |
| Metallic and ceramic surfaces | Generates easily removable sludge | Hydrophobic and oleophobic |
| Music instruments | ||
| - Guitar strings | Prevent loss of vibration due to residue build up | ? |
| - Piano keys | Contain polymeric PFAS | ? |
| - Piano | Eliminate squeaks in piano key | ? |
| Optical devices | ||
| - Glass fibre optics | Able to include rare earth in glass fibre optics | ? |
| - Optical lenses | Provide optical lenses with low refractive index and high transparency | Low refractive index |
| Paper and packaging | ||
| - Paper and cardboard | Provide water- and oil repellency | Hydrophobic and oleophobic |
| - Manufacturing of paper | Release agent for paper-coating compositions | Low surface tension |
| Particle physics | ||
| - Particle accelerators | Part of the detection assemblies | Non-reactive, stable, high ionization charge density |
| Personal care products | ||
| - Cosmetics | Emulsifiers, lubricants, or oleophobic agents | Hydrophobic, low surface tension |
| - Cosmetics | Make creams etc. penetrate the skin more easily | |
| - Cosmetics | Make the skin brighter | |
| - Cosmetics | Make the skin absorb more oxygen | Great capacity to dissolve gases |
| - Cosmetics | Make the makeup more durable and weather resistant | Hydrophobic and oleophobic, stable, non-reactive |
| - Hair-conditioning formulations | Enhance wet combing and render hair oleophobic | |
| Pesticides | ||
| - Insecticide against the common housefly and carmine mite | Suffocation of the insect by the adsorbed fluorinated surfactant | ? |
| - Insecticide against ants and cockroaches | ? | ? |
| - Formulation additives | Anti-foaming agent | Low surface tension |
| - Formulation additives | Dispersant, facilitate the spreading of plant protection agents on insects and plant leaves | Low surface tension |
| - Formulation additives | Dispersant, increase uptake by insects and plants | Low surface tension |
| - Formulation additive | Wetting agent for leaves | Low surface tension |
| Pharmaceuticals | ||
| - Active ingredient (fulvestrant) | Estrogen antagonists, inhibits the growth stimulus that the estrogen exert on cells | ? |
| - Active ingredient | Pharmaceutical combination of dabigatran and proton pump inhibitors | ? |
| - Formulation additives | Dispersant in self-propelling aerosol pharmaceuticals | Low surface tension |
| - Formulation additives | Solvent | Hydrophobic and oleophobic |
| Pipes, pumps, fittings and liners | ||
| - Pipes, pipe plugs, seal glands, pump parts, fasteners, fittings and liners | Polymeric PFAS are used for these applications | Stable, non-reactive, low surface tension, hydrophobic and oleophobic |
| - Working fluid for pumps in the electronics industry | Stable to reactive gases and aluminium chloride | Extremely stable, non-reactive |
| Plastic and rubber | ||
| - Plastic | Polymeric PFAS micropowder as additive ? | ? |
| - Thermoplastic | Plasticizer | ? |
| - Bonding of rubber to steel | Allow adhesiveness bonding | Low surface tension |
| - Rubber and plastic | Antistatic agent | Low dielectric constant |
| - Resin | Improve weatherability and elasticity | Non-reactive, stable |
| - Polycarbonate resins | Flame retardant for polycarbonate resins | Non-flammable |
| Printing (inks) | ||
| - Toner and printer ink | Enhance ink flow and levelling, improve wetting, aid pigment dispersion | Low surface tension |
| - Toner and printer ink | Impart water resistance to water-based inks | Hydrophobic |
| - Ink-yet recording heads | Make them ink repellent | Low surface tension |
| - Recording and printing paper | ? | ? |
| - Lithographic printing plates | ? | ? |
| Refrigerant systems | ||
| - Refrigerant fluid system | Heat transfer fluid | Good heat conductivity |
| - Refrigerant compressor | Lubricants | Non-flammable |
| Sealants and adhesives | ||
| - Sealants | Can be made out of polymeric PFAS | Operate at a wide temperature range, non-reactive, stable |
| - Silicone rubber seals | Prevents soiling | Low surface tension, hydrophobic and oleophobic |
| - Adhesives | Improve levelling, spreading, and the penetration of the adhesive into the pore structure of the substrates | Low surface tension |
| - Adhesives | Antistatic agent | Low dielectric constant |
| Soldering | ||
| - Vapour phase fluids in vapour phase soldering | Heat transfer medium | Good heat conductivity |
| - Fluxing agent in solder paste | Low-foaming noncorrosive wetting agent | Non-reactive, stable, low surface tension |
| Soil remediation | ||
| - Vapour barrier material on top of contaminated soil | Evaporation retarder | ? |
| - Surfactants to mobilize pollutants | Surfactants to mobilize soil-bound contaminants in remediation | Stable, non-degradable (during photodegradation) |
| Sport article | ||
| - Ski wax | Highly water repellent | Low surface tension, hydrophobic |
| - (Sailing) boat equipment | Weather protection of textiles; anti-fouling protection of ship hulls | Non-reactive, stable, hydrophobic and oleophobic |
| - Tennis rackets | Used in coatings for tennis rackets | ? |
| - Bicycle | Lubricants | Hydrophobic |
| - Climbing ropes | Provide water repellence, stain resistance and soil release | Low surface tension, hydrophobic |
| - Fishing lines | No water absorption, invisible in water, high knot strength | Hydrophobic |
| - Golf gloves | Antifouling protection for the natural sheep leather of the glove | ? |
| Stone, concrete and tile | Impart oil and water repellency to the surface; delay oxidation and ageing of surface | Low surface tension, hydrophobic and oleophobic |
| Textile and upholstery | ||
| - Surface treatment | Provide water and oil repellence, stain resistance and soil release | Low surface tension, hydrophobic and oleophobic |
| - Waving yarn | Facilitate waving | ? |
| Tracing and tagging | ||
| - Tracking air–borne pollutants | Tracer in air | Non-radioactive, chemically and thermally stable, do not occur naturally, have very low atmospheric background concentrations |
| - Testing ventilation systems | Tracer in air | 〃 |
| - Mapping gas and petroleum reservoirs | Tracer in gas or petroleum | 〃 |
| - Leak detection in cables, pipelines, landfill waste and underground storage tanks | Tracer in leaking material | 〃 |
| - Tracking of marked items | Tracer in the marked item | 〃 |
| Water and effluent treatment | ||
| - Filter membranes | Polymeric PFAS minimize the sorption of compounds to the filter itself | Low surface tension |
| Wire and cable | Provide high-temperature endurance, fire resistance, and high-stress crack resistance | Non-flammable, operate at a wide temperature range |
Conflicts of interest
Jamie DeWitt is serving as a plaintiff's expert witness in several cases related to PFAS.Appendix
Acknowledgements
We thank Stellan Fischer for his help with the data in the SPIN database. J. Glüge acknowledges the financial support of the Swiss Federal Office for the Environmental (FOEN). The authors also thank the Global PFAS Science Panel (GPSP) and the Tides Foundation for supporting this cooperation (grant 1806-52683). In addition, R. Lohmann acknowledges funding from the US National Institute of Environmental Health Sciences (grant P42ES027706); DeWitt from the US Environmental Protection Agency (83948101), the US National Institute of Environmental Health Sciences (1P43ES031009-01) and the North Carolina Policy Collaboratory; C. Ng from the National Science Foundation (grant 1845336) and D. Herzke thanks the Norwegian Strategic Institute Program, granted by the Norwegian Research Council “Arctic, the herald of Chemical Substances of Environmental Concern, CleanArctic”, 117031. We acknowledge contributions from A. Lindstrom (U.S. Environmental Protection Agency), L. Vierke (German Environment Agency), S. Patton (Health and Environment Program, Commonweal) and M. Miller (National Institute of Environmental Health Sciences, US). The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the European Environment Agency.References
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. D. J. Voogt, A. K. Allan, M. Kurunthachalam, S. A. van Leeuwen and P. J. J. Stefan, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins, Integr. Environ. Assess. Manage., 2011, 7(4), 513–541, DOI:10.1002/ieam.258.
- OECD, Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs) – Series on Risk Management Nr. 39, 2018, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7%26doclanguage=en Search PubMed.
- E. Kissa, Fluorinated Surfactants and Repellents, Marcel Dekker AG, 2001 Search PubMed.
- I. T. Cousins, C. Ng, Z. Wang and M. Scheringer, Why is High Persistence Alone a Major Cause of Concern?, Environ. Sci.: Processes Impacts, 2019, 2, 10.1039/C8em00515j.
- Sweden_and_other_EU_countries, Elements for an EU-Strategy for PFASs, 2019 Search PubMed.
- I. T. Cousins, G. Goldenman, D. Herzke, R. Lohmann, M. Miller, C. A. Ng, S. Patton, M. Scheringer, X. Trier, L. Vierke, Z. Wang and J. C. DeWitt, The concept of essential use for determining when uses of PFASs can be phased out, Environ. Sci.: Processes Impacts, 2019, 1–13, 10.1039/c9em00163h.
- R. R. Thomas, Fluorinated surfactants, in Chemistry and Technology of Surfactants, ed. R. J. Farn, Blackwell Publishing, 2006 Search PubMed.
- R. C. Buck, P. M. Murphy and M. Pabon, Chemistry, Properties, and Use of Commercial Fluorinated Surfactants, in The Handbook of Environmental Chemistry – Polyfluorinated Chemicals and Transformation Products, ed. T. P. Knepper and F. T. Lange, Springer Berlin Heidelberg, 2012, vol. 17, pp. 1–24, DOI:10.1007/978-3-642-21872-9.
- Britannica, Surface tension, https://www.britannica.com/science/surface-tension, published 2020, accessed August 26, 2020 Search PubMed.
- S. Alexander, G. N. Smith, C. James, S. E. Rogers, F. Guittard, M. Sagisaka and J. Eastoe, Low-surface energy surfactants with branched hydrocarbon architectures, Langmuir, 2014, 30(12), 3413–3421, DOI:10.1021/la500332s.
- F. M. Fowkes, Contact Angle, Wettability, and Adhesion, Copyright, Advances in Chemistry Series, in Advances in Chemistry, ed. R. F. Gould, Washington DC, 1964, DOI:10.1021/ba-1964-0043.fw001.
- Z. Wang, I. T. Cousins, M. Scheringer and K. Hungerbühler, Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors, Environ. Int., 2013, 60(2013), 242–248, DOI:10.1016/j.envint.2013.08.021.
- B. Ameduri, Fluoropolymers: The Right Material for the Right Applications, Chem.–Eur. J., 2018, 24(71), 18830–18841, DOI:10.1002/chem.201802708.
- J. M. Boucher, I. T. Cousins, M. Scheringer, K. Hungerbühler and Z. Wang, Toward a Comprehensive Global Emission Inventory of C4–C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C6- and C10-Based Products, Environ. Sci. Technol. Lett., 2019, 6(1), 1–7, DOI:10.1021/acs.estlett.8b00531.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part I: production and emissions from quantifiable sources, Environ. Int., 2014, 70, 62–75, DOI:10.1016/j.envint.2014.04.013.
- Z. Wang, I. T. Cousins, U. Berger, K. Hungerbühler and M. Scheringer, Comparative assessment of the environmental hazards of and exposure to perfluoroalkyl phosphonic and phosphinic acids (PFPAs and PFPiAs): current knowledge, gaps, challenges and research needs, Environ. Int., 2016, 89–90, 235–247, DOI:10.1016/j.envint.2016.01.023.
- Z. Wang, J. M. Boucher, M. Scheringer, I. T. Cousins and K. Hungerbühler, Toward a Comprehensive Global Emission Inventory of C4–C10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C8-Based Products and Ongoing Industrial Transition, Environ. Sci. Technol., 2017, 51(8), 4482–4493, DOI:10.1021/acs.est.6b06191.
- Z. Wang, G. Goldenman, T. Tugran, A. McNeil and M. Jones, Per- and Polyfluoroalkylether Substances: Identity, Production and Use, 2020, DOI:10.6027/na2020-901.
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Sources, fate and transport of perfluorocarboxylates, Environ. Sci. Technol., 2006, 40(1), 32–44, DOI:10.1021/es0512475.
- Norwegian Environment Agency, Investigation of Sources of PFBS into the Environment (M-759), 2017 Search PubMed.
- Norwegian Environment Agency, Investigation of Sources to PFHxS in the Environment (M-961), 2018 Search PubMed.
- OECD, Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts, 2002 Search PubMed.
- POPRC, Risk Profile: Perfluorohexane Sulfonic Acid (CAS No: 355-46-4, PFHxS), Its Salts and PFHxS-Related Compounds – Addendum, UNEP/POPS/POPRC.14/6/Add.1, 2018 Search PubMed.
- POPRC, Technical Paper on the Identification and Assessment of Alternatives to the Use of Perfluorooctane Sulfonic Acid, Its Salts, Perfluorooctane Sulfonyl Fluoride and Their Related Chemicals in Open Applications, UNEP/POPS/POPRC.8/INF/17/Rev.1, 2012 Search PubMed.
- POPRC, Consolidated Guidance on Alternatives to Perfluorooctane Sulfonic Acid and Its Related Chemicals, UNEP/POPS/POPRC.12/INF/15/Rev.1, 2016 Search PubMed.
- POPRC, Risk Profile on Pentadecafluorooctanoic Acid (CAS No: 335-67-1, PFOA, Perfluorooctanoic Acid), Its Salts and PFOA-Related Compounds – Addendum, UNEP/POPS/POPRC.12/11/Add.2, 2016 Search PubMed.
- POPRC, Risk Management Evaluation on Pentadecafluorooctanoic Acid (CAS No: 335-67-1, PFOA, Perfluorooctanoic Acid), Its Salts and PFOA-Related Compounds – Addendum, UNEP/POPS/POPRC.13/7/Add.2, 2017 Search PubMed.
- POPRC, Addendum to the Risk Management Evaluation on Perfluorooctanoic Acid (PFOA), Its Salts and PFOA-Related Compounds, UNEP/POPS/POPRC.14/6/Add.2, 2018, http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC14/Overview/tabid/7398/Default.aspx Search PubMed.
- POPRC, Report on the Assessment of Alternatives to Perfluorooctane Sulfonic Acid, Its Salts and Perfluorooctane Sulfonyl Fluoride, UNEP/POPS/POPRC.14/INF/13, 2019 Search PubMed.
- FluoroIndustry, FluoroCouncil, https://fluorocouncil.com/applications/, published 2019, accessed March 22, 2019.
- ECHA, Five European states call for evidence on broad PFAS restriction, https://echa.europa.eu/de/-/five-european-states-call-for-evidence-on-broad-pfas-restriction, published 2020, accessed May 13, 2020 Search PubMed.
- J. Gardiner, Fluoropolymers: Origin, Production, and Industrial and Commercial Applications, Aust. J. Chem., 2015, 68(1), 13–22, DOI:10.1071/ch14165.
- D. Herzke, S. Posner and E. Olsson, Survey, Screening and Analyses of PFCs in Consumer Products, 2009, www.swereaivf.se Search PubMed.
- L. M. Hodgkins, Per- and polyfluoroalkyl substances in the Royal Canadian Navy, 2018 Search PubMed.
- KEMI Swedish Chemical Agency, Occurrence and Use of Highly Fluorinated Substances and Alternatives, 2015, https://www.norden.org/en/publication/and-polyfluorinated-substances-nordic-countries Search PubMed.
- G. H. Millet and J. L. Kosmala, Fluorine-Containing Polymers, Polychlorotrifluoroethylene, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2000, pp. 1–6, DOI:10.1002/0471238961.1615122513091212.a01.
- P. Savu, Fluorine-Containing Polymers, Perfluoroalkanesulfonic Acids, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2000, pp. 1–7, DOI:10.1002/0471238961.1605180619012221.a01.
- Y. Wang, W. Chang, L. Wang, Y. Zhang, Y. Zhang, M. Wang, Y. Wang and P. Li, A review of sources, multimedia distribution and health risks of novel fluorinated alternatives, Ecotoxicol. Environ. Saf., 2019, 182 DOI:10.1016/j.ecoenv.2019.109402.
- Norden, Per- and Polyfluorinated Substances in the Nordic Countries – Use Occurence and Toxicology, 2013, DOI:10.6027/TN2013-542.
- R. E. Banks, B. E. Smart and J. C. Tatlow, Organofluorine Chemistry, ed. Banks R. E., Smart B. E. and Tatlow J. C., Springer US, Boston, MA, 1994, DOI:10.1007/978-1-4899-1202-2.
- M. G. Costello, R. M. Flynn and J. G. Owens, Fluoroethers and Fluoroamines, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2000, vol 11, pp. 1–12, DOI:10.1002/0471238961.0612211506122514.a01.pub2.
- J. E. Dohany, Fluorine-Containing Polymers, Poly(Vinylidene Fluoride), in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2000, DOI:10.1002/0471238961.1615122504150801.a01.
- S. Ebnesajjad and L. G. Snow, Fluorine-Containing Polymers, Poly(Vinyl Fluoride), in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2000, DOI:10.1002/0471238961.1615122505021405.a01.
- Norden, SPIN – Substances in preparations in Nordic countries, http://www.spin2000.net/spinmyphp/, published 2020, accessed April 8, 2020 Search PubMed.
- CAS, SciFindern, https://scifinder-n.cas.org/, published 2019, accessed November 5, 2019.
- Google Patents, https://patents.google.com, published 2019, accessed December 5, 2019.
- P. Peters, Personal Communication, 2020 Search PubMed.
- F2_Chemicals, Fluorocarbons, http://www.f2chemicals.com/full_range.html, published 2019, accessed September 25, 2019 Search PubMed.
- 3M, 3M™ Novec™ 7000 Engineered Fluid – Product Information, 2014, http://multimedia.3m.com/mws/mediawebserver?66666UuZjcFSLXTtlXftMxMVEVuQEcuZgVs6EVs6E666666-- Search PubMed.
- 3M, 3M™ Novec™ 7100 Engineered Fluid – Product Information, 2009 Search PubMed.
- 3M, 3M™ Novec™ 7500 Engineered Fluid – Product Information, 2008 Search PubMed.
- 3M, 3M™ Novec™ 7200 Engineered Fluid – Product Information, 2009, https://multimedia.3m.com/mws/media/199819O/3mtm-novectm-7200-engineered-fluid.pdf Search PubMed.
- Chemours, Vertrel™ X-DF – Drying Agent, 2019, https://www.chemours.com/en/brands-and-products/vertrel/products/xdf Search PubMed.
- P.-E. Schulze and H. Norin, Fluorinated Pollutants in All-Weather Clothing, Friends of the Earth Norway, 2006 Search PubMed.
- G. F. Peaslee, J. T. Wilkinson, S. R. McGuinness, M. Tighe, N. Caterisano, S. Lee, A. Gonzales, M. Roddy, S. Mills and K. Mitchell, Another Pathway for Firefighter Exposure to Per- and Polyfluoroalkyl Substances: Firefighter Textiles, Environ. Sci. Technol. Lett., 2020, 5, DOI:10.1021/acs.estlett.0c00410.
- R. M. Janousek, S. Lebertz and T. P. Knepper, Previously unidentified sources of perfluoroalkyl and polyfluoroalkyl substances from building materials and industrial fabrics, Environ. Sci.: Processes Impacts, 2019, 21(11), 1936–1945 RSC.
- H. Zhu and K. Kannan, A pilot study of per- and polyfluoroalkyl substances in automotive lubricant oils from the United States, Environ. Technol. Innov., 2020, 19, 100943, DOI:10.1016/j.eti.2020.100943.
- A. W. Nørgaard, P. Wolkoff and F. R. Lauritsen, Characterization of nanofilm spray products by mass spectrometry, Chemosphere, 2010, 80(11), 1377–1386, DOI:10.1016/j.chemosphere.2010.06.004.
- A. W. Nørgaard, J. S. Hansen, J. B. Sørli, M. Levin, P. Wolkoff, G. D. Nielsen, D. Gunnar and S. T. Larsen, Pulmonary toxicity of perfluorinated silane-based nanofilm spray products: solvent dependency, Toxicol. Sci., 2014, 137(1), 179–188, DOI:10.1093/toxsci/kft225.
- K. A. Barzen-Hanson, S. C. Roberts, S. Choyke, K. Oetjen, A. McAlees, N. Riddell, R. McCrindle, P. L. Ferguson, C. P. Higgins and J. A. Field, Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater, Environ. Sci. Technol., 2017, 51(4), 2047–2057, DOI:10.1021/acs.est.6b05843.
- X. Dauchy, V. Boiteux, C. Bach, C. Rosin and J. F. Munoz, Per- and polyfluoroalkyl substances in firefighting foam concentrates and water samples collected near sites impacted by the use of these foams, Chemosphere, 2017, 183, 53–61, DOI:10.1016/j.chemosphere.2017.05.056.
- B. J. Place and J. A. Field, Identification of novel fluorochemicals in aqueous film-forming foams used by the US military, Environ. Sci. Technol., 2012, 46(13), 7120–7127, DOI:10.1021/es301465n.
- W. J. Backe, T. C. Day and J. A. Field, Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. military bases by nonaqueous large-volume injection HPLC-MS/MS, Environ. Sci. Technol., 2013, 47(10), 5226–5234, DOI:10.1021/es3034999.
- A. Rotander, A. Kärrman, L. M. L. Toms, M. Kay, J. F. Mueller and M. J. Gómez Ramos, Novel fluorinated surfactants tentatively identified in firefighters using liquid chromatography quadrupole time-of-flight tandem mass spectrometry and a case-control approach, Environ. Sci. Technol., 2015, 49(4), 2434–2442, DOI:10.1021/es503653n.
- M. Mumtaz, Y. Bao, L. Liu, J. Huang, G. Cagnetta and G. Yu, Per- and Polyfluoroalkyl Substances in Representative Fluorocarbon Surfactants Used in Chinese Film-Forming Foams: Levels, Profile Shift, and Environmental Implications, Environ. Sci. Technol. Lett., 2019, 6(5), 259–264, DOI:10.1021/acs.estlett.9b00154.
- W. A. Gebbink, S. Ullah, O. Sandblom and U. Berger, Polyfluoroalkyl phosphate esters and perfluoroalkyl carboxylic acids in target food samples and packaging-method development and screening, Environ. Sci. Pollut. Res., 2013, 20(11), 7949–7958, DOI:10.1007/s11356-013-1596-y.
- I. K. Dimzon, X. Trier, T. Frömel, R. Helmus, T. P. Knepper and P. De Voogt, High Resolution Mass Spectrometry of Polyfluorinated Polyether-Based Formulation, J. Am. Soc. Mass Spectrom., 2016, 27(2), 309–318, DOI:10.1007/s13361-015-1269-9.
- M. Kotthoff, J. Müller, H. Jürling, M. Schlummer and D. Fiedler, Perfluoroalkyl and polyfluoroalkyl substances in consumer products, Environ. Sci. Pollut. Res., 2015, 22(19), 14546–14559, DOI:10.1007/s11356-015-4202-7.
- J. Bečanová, L. Melymuk, Š. Vojta, K. Komprdová and J. Klánová, Screening for perfluoroalkyl acids in consumer products, building materials and wastes, Chemosphere, 2016, 164, 322–329, DOI:10.1016/j.chemosphere.2016.08.112.
- C. Blom and L. Hanssen. Analysis of Per- and Polyfluorinated Substances in Articles (M-360), 2015 Search PubMed.
- F. Xiao, S. A. Golovko and M. Y. Golovko, Identification of novel non-ionic, cationic, zwitterionic, and anionic polyfluoroalkyl substances using UPLC-TOF-MS E high-resolution parent ion search, Anal. Chim. Acta, 2017, 988, 41–49, DOI:10.1016/j.aca.2017.08.016.
- D. Borg and J. Ivarsson, Analysis of PFASs and TOF in Products, 2017, http://urn.kb.se/resolve?urn=urn:nbn:se:norden:org:diva-4901 Search PubMed.
- K. V. Vejrup and B. Lindblom, Survey of Chemical Substances in Consumer Products – Analysis of Perfluorooctanesulfonate Compounds in Impregnating Agents, Wax and Floor Polish Products, 2002 Search PubMed.
- M. J. A. Dinglasan-Panlilio and S. A. Mabury, Significant residual fluorinated alcohols present in various fluorinated materials, Environ. Sci. Technol., 2006, 40(5), 1447–1453, DOI:10.1021/es051619.
- S. Fiedler, G. Pfister and K. W. Schramm, Poly- and perfluorinated compounds in household consumer products, Toxicol. Environ. Chem., 2010, 92(10), 1801–1811, DOI:10.1080/02772248.2010.491482.
- GMI, Fluorotelomers Market Report, 2016 Search PubMed.
- GMI, Fluorochemicals Market Report, 2018 Search PubMed.
- GMI, Perfluoropolyether Market Report, 2019, https://www.gminsights.com/industry-analysis/perfluoropolyether-market Search PubMed.
- NORMAN, Norman Substance Databse, https://www.norman-network.com/nds/susdat/susdatSearchShow.php.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: the remaining pieces of the puzzle, Environ. Int., 2014, 69, 166–176, DOI:10.1016/j.envint.2014.04.006.
- AGC, Fluoroplastics, https://www.agcce.com/fluoroplastics/, published 2018, accessed June 6, 2020 Search PubMed.
- Edelrid, Das weltweit erste PFC freie Kletterseil, https://www.edelrid.de/de/microsite/kletterseil-swift-eco-dry.php, published 2020, accessed April 24, 2020 Search PubMed.
- Opinion.org, Fake Market Research, https://opinion.org/market-research-industry-clogged-with-spam/, published 2018, accessed February 13, 2020 Search PubMed.
- ECHA, Fighting fire with fluorine-free foams, https://echa.europa.eu/fluorine-free-foams, accessed June 18, 2020 Search PubMed.
- DOD, DOD Funds Firefighting Foam Research for a PFAS-Free Alternative, https://www.defense.gov/Explore/News/Article/Article/2018096/dod-funds-firefighting-foam-research-for-a-pfas-free-alternative/, published 2019, accessed June 18, 2020 Search PubMed.
- ChemSec, PFC-free food packaging tray, https://marketplace.chemsec.org/Alternative/PFC-free-food-packaging-tray-354, accessed June 18, 2020 Search PubMed.
- ChemicalWatch, Denmark announces advisory ban on PFCs in food packaging, https://chemicalwatch.com/36900/denmark-announces-advisory-ban-on-pfcs-in-food-packaging, published 2015, accessed June 18, 2020 Search PubMed.
- Rudolf, BIONIC-FINISH®ECO, https://www.rudolf.de/en/technology/bionic-finish-eco/, accessed June 18, 2020 Search PubMed.
- SympaTex, Our solution: Sympatex avoids harmful chemistry, https://www.sympatex.com/en/sustainability/our-solution-closing-the-loop/reduction-in-the-use-of-chemicals/, accessed June 18, 2020 Search PubMed.
- DEPA, Alternatives to Perfluoroalkyl and Polyfluoro- Alkyl Substances (PFAS) in Textiles, 2015, http://www2.mst.dk/Udgiv/publications/2015/05/978-87-93352-16-2.pdf Search PubMed.
- S. Schellenberger, P. Gillgard, A. Stare, A. Hanning, O. Levenstam, S. Roos and I. T. Cousins, Facing the rain after the phase out: performance evaluation of alternative fluorinated and non-fluorinated durable water repellents for outdoor fabrics, Chemosphere, 2018, 193, 675–684, DOI:10.1016/j.chemosphere.2017.11.027.
- Y. Pan, H. Zhang, Q. Cui, N. Sheng, L. W. Y. Yeung, Y. Guo, Y. Sun and J. Dai, First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern, Environ. Sci. Technol., 2017, 51(17), 9553–9560, DOI:10.1021/acs.est.7b02259.
- W. A. Gebbink, L. Van Asseldonk and S. P. J. Van Leeuwen, Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands, Environ. Sci. Technol., 2017, 51(19), 11057–11065, DOI:10.1021/acs.est.7b02488.
- A. B. Lindstrom, J. E. Galloway and M. J. Strynar, D. Knappe, M. Sun, S. Newton and L. K. Weavers, Emerging Per- and Polyfluoroalkyl Substances (PFAS), Highly Fluorinated Compounds Social and Scientific Discovery Northeastern, University Social Science Environmental Health Research Institute, Boston, 2017 Search PubMed.
- A. Y. C. Lin, S. C. Panchangam and C. C. Lo, The impact of semiconductor, electronics and optoelectronic industries on downstream perfluorinated chemical contamination in Taiwanese rivers, Environ. Pollut., 2009, 157(4), 1365–1372, DOI:10.1016/j.envpol.2008.11.033.
- C. Y. Tang, Q. S. Fu, A. P. Robertson, C. S. Criddle and J. O. Leckie, Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater, Environ. Sci. Technol., 2006, 40(23), 7343–7349, DOI:10.1021/es060831q.
- H. Hauser, L. Füglister and T. Scheffelmaier, Verwendung von Fluortensiden in Der Galvanikbranche, 2020 Search PubMed.
- NIH, Pubchem, U.S. National Library of Medicine National Center for Biotechnology Information, https://pubchem.ncbi.nlm.nih.gov/, published 2019, accessed September 25, 2019.
- X. Zhang, X. Sun, R. Jiang, E. Y. Zeng, E. M. Sunderland and D. C. G. Muir, Screening New Persistent and Bioaccumulative Organics in China's Inventory of Industrial Chemicals, Environ. Sci. Technol., 2020, 54, 7398–7408 CrossRef CAS.
- CSRwire, 3M Phasing Out Some of its Specialty Materials, https://www.csrwire.com/press_releases/25065-3M-Phasing-Out-Some-of-its-Specialty-Materials, published 2000, accessed June 6, 2020 Search PubMed.
- USEPA, Fact Sheet: 2010/2015 PFOA Stewardship Program, https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program, accessed June 8, 2020 Search PubMed.
- BloombergLaw, Older PFAS That EPA Thought Obsolete Still Used, Agency Told, 2020, https://news.bloomberglaw.com/environment-and-energy/older-pfas-that-epa-thought-obsolete-still-used-agency-told Search PubMed.
- I. T. Cousins, R. Vestergren, Z. Wang, M. Scheringer and M. S. McLachlan, The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater, Environ. Int., 2016, 94, 331–340, DOI:10.1016/j.envint.2016.04.044.
- G. Munoz, M. Desrosiers, S. V. Duy, P. Labadie, H. Budzinski, J. Liu and S. Sauvé, Environmental Occurrence of Perfluoroalkyl Acids and Novel Fluorotelomer Surfactants in the Freshwater Fish Catostomus commersonii and Sediments Following Firefighting Foam Deployment at the Lac-Mégantic Railway Accident, Environ. Sci. Technol., 2017, 51(3), 1231–1240, DOI:10.1021/acs.est.6b05432.
- A. R. Angusfire, FFFP – Alcohol resistant film forming fluoroprotein, http://angusfire.co.uk/products/foam-concentrates/product-range/ar-fffp/, published 2019, accessed October 23, 2019 Search PubMed.
- S. H. Korzenioswski, R. C. Buck, D. M. Kempisty and M. Pabon, Fluorosurfactants in Firefighting Foams: Past and Present (Chapter 1), in Perfluoroalkyl Substances in the Environment, ed. Kempisty D. M., Xing Y. and Racz L., CRC Press, 2019 Search PubMed.
- F. Heydebreck, J. Tang, Z. Xie and R. Ebinghaus, Emissions of Per- and Polyfluoroalkyl Substances in a Textile Manufacturing Plant in China and Their Relevance for Workers' Exposure, Environ. Sci. Technol., 2016, 50(19), 10386–10396, DOI:10.1021/acs.est.6b03213.
- H. Holmquist, S. Schellenberger, I. van der Veen, G. M. Peters, P. E. G. Leonards and I. T. Cousins, Properties, performance and associated hazards of state-of-the-art durable water repellent (DWR) chemistry for textile finishing, Environ. Int., 2016, 91, 251–264, DOI:10.1016/j.envint.2016.02.035.
- SpecialChem, What are Fluoropolymers-based PPA?, https://polymer-additives.specialchem.com/centers/fluoropolymers-as-polymer-processing-aid, published 2020, accessed June 4, 2020 Search PubMed.
- Unknown, Polyethylene composition for artificial turf, patent EP1672020, 2006 Search PubMed.
- Y. Wang, N. Yu, X. Zhu, H. Guo, J. Jiang, X. Wang, W. Shi, J. Wu, H. Yu and S. Wei, Suspect and Nontarget Screening of Per- and Polyfluoroalkyl Substances in Wastewater from a Fluorochemical Manufacturing Park, Environ. Sci. Technol., 2018, 52(19), 11007–11016, DOI:10.1021/acs.est.8b03030.
- J. W. Washington, C. G. Rosal, J. P. McCord, M. J. Strynar, A. B. Lindstrom, E. L. Bergman, S. M. Goodrow, H. K. Tadesse, A. N. Pilant, B. J. Washington, M. J. Davis, B. G. Stuart and T. M. Jenkins, Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils, Science, 2020, 368(6495), 1103–1107, DOI:10.1126/science.aba7127.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0em00291g |
| This journal is © The Royal Society of Chemistry 2020 |