Open Access Article
Open Access ArticleIsolation and structural characterisation of rhodium(III) η2-fluoroarene complexes: experimental verification of predicted regioselectivity†
Matthew R.
Gyton‡
 ,
Amy E.
Kynman‡
,
Amy E.
Kynman‡
 ,
Baptiste
Leforestier
,
Baptiste
Leforestier
 ,
Angelo
Gallo
,
Angelo
Gallo
 ,
Józef R.
Lewandowski
,
Józef R.
Lewandowski
 and
Adrian B.
Chaplin
and
Adrian B.
Chaplin
 *
*
Department of Chemistry, University of Warwick, Gibbet Hill Road, Coventry, CV4 7AL, UK. E-mail: a.b.chaplin@warwick.ac.uk
First published on 21st April 2020
Abstract
The isolation and solid-state characterisation of complexes featuring partially coordinated benzene, fluorobenzene and all three isomers of difluorobenzene are described. Supported by a DFT analysis, this well-defined homologous series demonstrates the preference for η2-coordination of fluoroarenes via the HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH sites adjacent to a fluorine substituent.
CH sites adjacent to a fluorine substituent.
Partially fluorinated benzenes are chemically robust and weakly coordinating substrates, for which there is a paucity of late transition metal π-complexes.1 Whilst well-defined examples can be found in the literature they are almost exclusively limited to half sandwich formulations, where the arene adopts an η6-coordination mode.1,2 The formation of η2-arene complexes is notably invoked in C–H bond oxidative addition of partially fluorinated benzenes to late transition metals (Scheme 1),3,4 but to the best of our knowledge isolation of mononuclear species of this nature is limited to coinage metal examples.5 Computational studies indicate a coordination site preference in the order HC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH > HC
CH > HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF > FC
CF > FC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF, with the strongest η2-arene complexes formed at the HC
CF, with the strongest η2-arene complexes formed at the HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH positions adjacent to a fluorine substituent.6 Building on our work employing the high trans-influence 2,2′-biphenyl (biph) ancillary ligand,7,8 we herein present the synthesis and solid-state characterisation of rhodium(III) pincer complexes [Rh(CNC-Me)(biph)(η2-arene)]+ (Scheme 1; arene = C6H6, 1a; FC6H5, 1b; 1,2-F2C6H4, 1c; 1,3-F2C6H4, 1d; 1,4-F2C6H4, 1e) that corroborate this conclusion experimentally.
CH positions adjacent to a fluorine substituent.6 Building on our work employing the high trans-influence 2,2′-biphenyl (biph) ancillary ligand,7,8 we herein present the synthesis and solid-state characterisation of rhodium(III) pincer complexes [Rh(CNC-Me)(biph)(η2-arene)]+ (Scheme 1; arene = C6H6, 1a; FC6H5, 1b; 1,2-F2C6H4, 1c; 1,3-F2C6H4, 1d; 1,4-F2C6H4, 1e) that corroborate this conclusion experimentally.
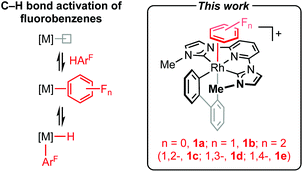 | ||
| Scheme 1 Intermediacy of η2-adducts in the C–H bond activation of fluoroarenes. [B(3,5-(CF3)2C6H3)4]− anion omitted for clarity. | ||
To enable systematic synthesis of the target complexes, [Rh(CNC-Me)(biph)(κ1-ClCH2Cl)]+2 was ultimately identified as the most convenient well-defined precursor and prepared using a silver-based transmetallation procedure involving reaction of [Ag(CNC-Me)]+ with [Rh(biph)Cl(tBu2PCH2PtBu2)] and halide abstraction in CH2Cl2 (80% yield; see ESI† for solid-state structure, Rh–Cl = 2.5932(7) Å).† Dichloromethane is labile and not retained on dissolution of 2 in CD2Cl2 or neat fluoroarene, with the organometallic displaying time averaged C2v symmetry at 298 K consistent with formulation as a five-coordinate complex in solution and rapid pseudorotation of the biphenyl ligand on the NMR time scale (ΔH‡ = 75 ± 1 kJ mol−1, ΔS‡ = +80 ± 5 J K−1 mol−1, ΔG‡298 K = 52 ± 3 kJ mol−1 in CD2Cl2).6 In the latter case, selective removal of CH2Cl2in vacuo and subsequent recrystallisation from the neat fluoroarene enabled isolation of the corresponding η2-arene complexes 1b–e in 61–81% yield. Benzene is a poor solvent for cationic species of this nature, but 1a was prepared in a similar manner using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
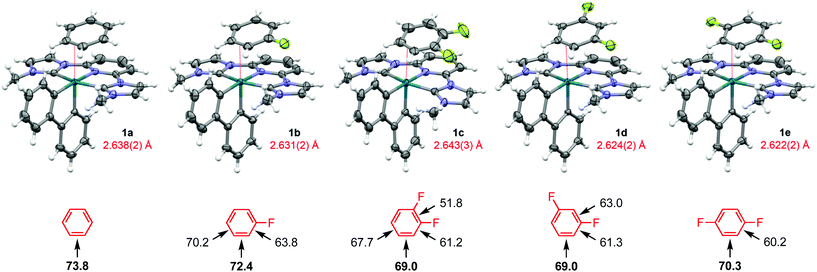
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of benzene – 1,2-difluorobenzene in 77% yield. Crystals suitable for analysis by X-ray diffraction were obtained in all cases (Fig. 1), with bulk purity confirmed using a combination of combustion analysis, solid-state 19F MAS NMR spectroscopy and dissolution in CD2Cl2; with one equivalent of the respective free arene observed by 1H and 19F NMR spectroscopy (see ESI).†
1 molar mixture of benzene – 1,2-difluorobenzene in 77% yield. Crystals suitable for analysis by X-ray diffraction were obtained in all cases (Fig. 1), with bulk purity confirmed using a combination of combustion analysis, solid-state 19F MAS NMR spectroscopy and dissolution in CD2Cl2; with one equivalent of the respective free arene observed by 1H and 19F NMR spectroscopy (see ESI).†
The rigid chelates of the biph and CNC pincer ligands provide a framework for pseudo-octahedral metal geometries in 1a–e, where η2-arene coordination [Rh–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) = 2.622(2)–2.643(2) Å] completes the coordination sphere and enables attainment of 18 VE configurations. The observed selectivity for coordination of the fluoroarenes via the HC
C) = 2.622(2)–2.643(2) Å] completes the coordination sphere and enables attainment of 18 VE configurations. The observed selectivity for coordination of the fluoroarenes via the HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH sites adjacent to a fluorine substituent, notably vindicates computational trends in binding energy previously established for neutral rhenium cyclopentadienyl fragments6 and those determined as part of this study for 1a–e at the ωB97X-D3/def2-TZVP(-f) level of theory (Fig. 1). The absolute magnitudes of the calculated arene binding energies are considerably lower than the corresponding rhenium systems (69.0–73.8 vs. 87.0–99.3 kJ mol−1 for the lowest energy regioisomers), consistent with the cationic nature of 1 and reconciling the entropically unfavourable coordination inferred in solution. Moreover, the relative binding energies of 1a/c are supported by the aforementioned (competition) experiment involving dissolution of 2 in a 1
CH sites adjacent to a fluorine substituent, notably vindicates computational trends in binding energy previously established for neutral rhenium cyclopentadienyl fragments6 and those determined as part of this study for 1a–e at the ωB97X-D3/def2-TZVP(-f) level of theory (Fig. 1). The absolute magnitudes of the calculated arene binding energies are considerably lower than the corresponding rhenium systems (69.0–73.8 vs. 87.0–99.3 kJ mol−1 for the lowest energy regioisomers), consistent with the cationic nature of 1 and reconciling the entropically unfavourable coordination inferred in solution. Moreover, the relative binding energies of 1a/c are supported by the aforementioned (competition) experiment involving dissolution of 2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of benzene – 1,2-difluorobenzene, yielding exclusively 1a. DFT-based energy decomposition analysis of the metal-arene bonding interactions using the ETS-NOCV method, as implemented in ORCA 4.1.2,9 suggests these interactions are dominated by arene to metal σ-donation with only minor metal to arene π-backbonding contributions (see ESI).† The former are sufficient to explain the observed regioselectivity for all but 1c, where subtle differences in π-backbonding are decisive.
1 molar mixture of benzene – 1,2-difluorobenzene, yielding exclusively 1a. DFT-based energy decomposition analysis of the metal-arene bonding interactions using the ETS-NOCV method, as implemented in ORCA 4.1.2,9 suggests these interactions are dominated by arene to metal σ-donation with only minor metal to arene π-backbonding contributions (see ESI).† The former are sufficient to explain the observed regioselectivity for all but 1c, where subtle differences in π-backbonding are decisive.
In summary, we have exploited a planar NHC-based pincer ligand and the high trans-influence 2,2′-biphenyl ancillary to prepare an unprecedented homologous series of rhodium(III) complexes featuring η2-coordinated benzene and fluoroarenes. Supported by a DFT analysis, these complexes provide evidence for preferential η2-coordination of fluoroarenes via the HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH sites adjacent to a fluorine substituent; an important finding relevant to the selective C–H activation of these valuable fluoroaryl synthons.
CH sites adjacent to a fluorine substituent; an important finding relevant to the selective C–H activation of these valuable fluoroaryl synthons.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by European Research Council (ERC, grant agreements 637313 and 639907) and Royal Society (UF100592, UF150675). Computing facilities were provided by the Scientific Computing Research Technology Platform of the University of Warwick.Notes and references
- S. D. Pike, M. R. Crimmin and A. B. Chaplin, Chem. Commun., 2017, 53, 3615–3633 RSC.
- For recent rhodium examples see: (a) A. I. McKay, J. Barwick-Silk, M. Savage, M. C. Willis and A. S. Weller, Chem. – Eur. J., 2020, 26, 2883–2889 CrossRef CAS PubMed; (b) A. L. Colebatch, A. I. McKay, N. A. Beattie, S. A. Macgregor and A. S. Weller, Eur. J. Inorg. Chem., 2017, 4533–4540 CrossRef CAS; (c) S. D. Pike, I. Pernik, R. Theron, J. S. McIndoe and A. S. Weller, J. Organomet. Chem., 2015, 784, 75–83 CrossRef CAS; (d) I. Pernik, J. F. Hooper, A. B. Chaplin, A. S. Weller and M. C. Willis, ACS Catal., 2012, 2, 2779–2786 CrossRef CAS; (e) A. B. Chaplin, J. F. Hooper, A. S. Weller and M. C. Willis, J. Am. Chem. Soc., 2012, 134, 4885–4897 CrossRef CAS PubMed.
- O. Eisenstein, J. Milani and R. N. Perutz, Chem. Rev., 2017, 117, 8710–8753 CrossRef CAS PubMed.
- For spectroscopic evidence see: J. J. Carbó, O. Eisenstein, C. L. Higgitt, A. H. Klahn, F. Maseras, B. Oelckers and R. N. Perutz, Dalton Trans., 2001, 1452–1461 RSC.
- For representative examples see: (a) A. Martens, P. Weis, M. C. Krummer, M. Kreuzer, A. Meierhöfer, S. C. Meier, J. Bohnenberger, H. Scherer, I. Riddlestone and I. Krossing, Chem. Sci., 2018, 9, 7058–7068 RSC; (b) M. M. D. Roy, M. J. Ferguson, R. McDonald and E. Rivard, Chem. Commun., 2018, 54, 483–486 RSC; (c) R. Ramírez-Contreras and O. V. Ozerov, Dalton Trans., 2012, 41, 7842–7844 RSC; (d) G. Santiso-Quinones, A. Higelin, J. Schaefer, R. Brückner, C. Knapp and I. Krossing, Chem. – Eur. J., 2009, 15, 6663–6677 CrossRef CAS PubMed.
- E. Clot, B. Oelckers, A. H. Klahn, O. Eisenstein and R. N. Perutz, Dalton Trans., 2003, 4065–4074 RSC.
- T. M. Hood, B. Leforestier, M. R. Gyton and A. B. Chaplin, Inorg. Chem., 2019, 58, 7593–7601 CrossRef CAS PubMed.
- (a) T. M. Hood, M. R. Gyton and A. B. Chaplin, Dalton Trans., 2020, 49, 2077–2086 RSC; (b) J. Emerson-King, I. Prokes and A. B. Chaplin, Chem. – Eur. J., 2019, 25, 6317–6319 CrossRef CAS PubMed; (c) M. R. Gyton, B. Leforestier and A. B. Chaplin, Organometallics, 2018, 37, 3963–3971 CrossRef CAS PubMed; (d) R. C. Knighton, J. Emerson-King, J. P. Rourke, C. A. Ohlin and A. B. Chaplin, Chem. – Eur. J., 2018, 24, 4927–4938 CrossRef CAS PubMed.
- (a) A. Altun, F. Neese and G. Bistoni, J. Chem. Theory Comput., 2019, 15, 215–228 CrossRef CAS PubMed; (b) M. P. Mitoraj, A. Michalak and T. Ziegler, J. Chem. Theory Comput., 2009, 5, 962–975 CrossRef CAS PubMed; (c) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed; (d) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–77 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental and computational details, including NMR and IR spectra and ETS-NOCV deformation density plots (PDF), and optimised geometries (XYZ). CCDC 1988128–1988133. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0dt01137a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |