Halogen-bonded cocrystals of donepezil with perfluorinated diiodobenzenes†
Vinko
Nemec
 a,
Toni
Vitasović
a,
Toni
Vitasović
 ab and
Dominik
Cinčić
ab and
Dominik
Cinčić
 *a
*a
aDepartment of Chemistry, Faculty of Science, University of Zagreb, Horvatovac 102a, HR-10002 Zagreb, Croatia. E-mail: dominik@chem.pmf.hr
bInterdisciplinary Nanoscience Center (iNANO), Aarhus University, Gustav Wieds Vej 14, DK-8000 Aarhus C, Denmark
First published on 10th August 2020
Abstract
Donepezil, an active pharmaceutical ingredient with several different acceptor sites for halogen bonding has successfully been cocrystallized with two perfluorinated halogen bond donors. The prepared cocrystals provide insight into the halogen bonding potential of organic molecules with increased structural complexity.
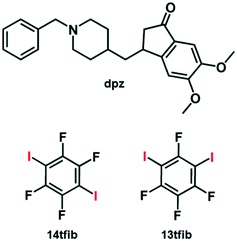
Donepezil (dpz) is an active pharmaceutical ingredient (API) that is usually taken in the form of its chloride salt as a drug for relieving symptoms of Alzheimer's disease.1 To that end, most patents and research on crystal engineering of dpz systems, including the recent study by Lee and coworkers,2 have been focused on chloride salts, polymorphism and hydrogen bonded cocrystals, primarily with organic acids.3 On the other hand, searching for “donepezil cocrystal” or “donepezil co-crystal” resulted in only one match4 in the patent databases used.5 In the CSD (Cambridge Structural Database),6 the donepezil molecule is present in 16 datasets. Six of those correspond to different polymorphs of dpz,7 one dataset corresponds to a hydrate2 and the remaining nine are salts or salt solvates with: fumaric acid (refcode IGIWON),2 mandelic acid (solvate refcode IGIWUT, hemihydrate refcode IGIXEE),2 maleic acid (refcode IGIXAA),2 methanesulfonic acid (refcode KUTQAT),8 benzenesulfonic acid (refcode KUTPUM, hydrate refcode IGOCIT),2,8p-toluenesulfonic acid (refcode KUTPOG),8 and oxalic acid (trihydrate refcode XETKEN).3b
From the point of view of crystal engineering multi-component halogen-bonded materials,9dpz as a molecule presents an interesting multiacceptor target, since it contains the following species that are potential halogen bond10 acceptors: a) carbonyl oxygen atom,11 b) piperidinyl nitrogen atom,12 c) two methoxy oxygen atoms in close proximity, such that they can act as either separate acceptors, or as acceptors of a bifurcated I⋯(Omethoxy)2 halogen bond,13,14 and d) two aromatic ring systems15 (Scheme 1). Additionally, dpz in its unprotonated form lacks strong hydrogen bond donor atoms, so it can be expected that there will be no competition between halogen bonds and potentially weak hydrogen bonds, e.g., C–H⋯O or C–H⋯N. In our work, we selected perfluorinated diiodobenzenes as cocrystal coformers for dpz (Scheme 1): 1,4-diiodotetrafluorobenzene (14tfib) and 1,3-diiodotetrafluorobenzene (13tfib). Both selected isomers are potential ditopic halogen bond donors that differ in their ability to form halogen-bonded cocrystals.16
Cocrystal screening was performed by mechanochemical means, by liquid-assisted grinding (LAG)17 of reaction mixtures with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reactant stoichiometry (dpz
2 reactant stoichiometry (dpz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) donor). Milling was conducted in a Retsch MM200 mill using stainless steel jars under normal laboratory conditions (temperature ca. 25 °C, 40–60% relative humidity, see ESI†). The reactants and products were then characterized by powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC). In order to structurally characterize the new products, mechanochemical experiments were followed by solution crystallization experiments, by dissolving the reactants in an appropriate solvent with heating, followed by letting the obtained solution cool down and evaporate at room temperature (see ESI†). The obtained products were then structurally characterized by single crystal X-ray diffraction (SCXRD).
donor). Milling was conducted in a Retsch MM200 mill using stainless steel jars under normal laboratory conditions (temperature ca. 25 °C, 40–60% relative humidity, see ESI†). The reactants and products were then characterized by powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC). In order to structurally characterize the new products, mechanochemical experiments were followed by solution crystallization experiments, by dissolving the reactants in an appropriate solvent with heating, followed by letting the obtained solution cool down and evaporate at room temperature (see ESI†). The obtained products were then structurally characterized by single crystal X-ray diffraction (SCXRD).
Mechanochemical screening experiments have shown that new products are formed by milling dpz with 13tfib or 14tfib. Single crystals were obtained for both new products, and structural analysis revealed that their formulas are: (dpz)(13tfib) and (dpz)(14tfib)2. The measured PXRD patterns for the mechanochemical products were found to be in good agreement with the patterns calculated from single crystal data (Fig. 1), and the DSC experiments show that both cocrystals were obtained as pure single phases (see ESI†).
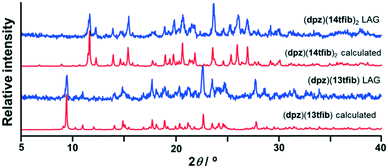 | ||
| Fig. 1 Comparison of experimental powder patterns obtained by LAG with those generated from single crystal data. | ||
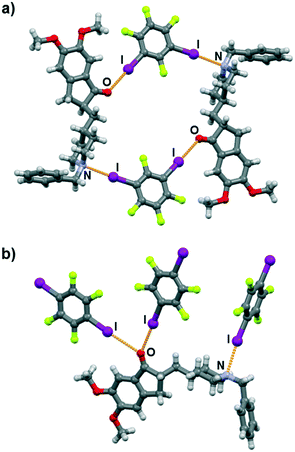
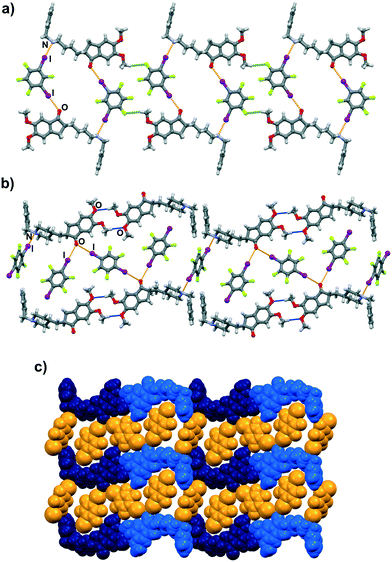
Molecular and crystal structure determination based on single crystal X-ray diffraction has revealed that I⋯N and I⋯O halogen bonds are present in both cocrystals. As expected, both 13tfib and 14tfib molecules act as ditopic halogen bond donors. In the (dpz)(13tfib) cocrystal, each dpz molecule participates in halogen bonding with 13tfibvia I⋯N and I⋯Ocarbonyl halogen bonds (Table 1). This results in the formation of discrete four-membered halogen-bonded assemblies (Fig. 2a). The two methoxy oxygen atoms of dpz do not participate in the formation of either hydrogen or halogen bonds – they are instead “blocked off” by the close packing of the pendant benzyl fragment belonging to an adjacent dpz molecule. The four-membered complexes are connected into chains by C–H⋯F contacts (d(C29⋯F4) = 3.53(1) Å) that are further interconnected in 3D via C–H⋯F (d(C8⋯F1) = 3.327(9) Å, d(C19A⋯F1) = 3.52(1) Å, d(C20B⋯F1) = 3.48(3) Å) and C–H⋯C (d(C18⋯C6) = 3.646(9) Å, d(C20A⋯C24) = 3.53(2) Å) contacts.
Similarly, both the piperidinyl nitrogen and carbonyl oxygen atom participate in halogen bonding in the (dpz)(14tfib)2 cocrystal (Fig. 2b). However, each dpz molecule in this system participates in halogen bonding with three crystallographically independent 14tfib molecules, in such a way that the carbonyl oxygen atom functions as a bifurcated halogen bond acceptor, participating in I⋯Ocarbonyl halogen bonds with two 14tfib molecules (Table 1). All three crystallographically independent 14tfib molecules exhibit different supramolecular bonding. The first 14tfib molecule is a ditopic halogen bond donor that connects two dpz molecules via I⋯Ocarbonyl halogen bonds. In the second crystallographically independent 14tfib molecule one iodine atom participates in I⋯Ocarbonyl halogen bonding with dpz, while the other iodine atom is enveloped by the piperidinyl and benzyl fragments of another dpz molecule. The third crystallographically independent 14tfib molecule is a ditopic halogen bond donor that connects two dpz molecules via I⋯N halogen bonds. The resulting combination of halogen bonds leads to the formation of an intricate molecular chain. Halogen bonding parameters are listed in Table 1. In contrast to the (dpz)(13tfib) cocrystal, in the (dpz)(14tfib)2 cocrystal methoxy oxygen atoms participate in hydrogen bonding, forming an R42(12) supramolecular motif (d(C35⋯O2) = 3.32(2) Å, ∠(C35–H35A⋯O2) = 128°, d(C35⋯O3) = 3.52(2) Å, ∠(C35–H35A⋯O3) = 158°), and in combination with the above-mentioned halogen bonding lead to the formation of a 2D network. Numerous C–H⋯F interactions are present in the network (d(C15⋯F2) = 3.14(2) Å, d(C16⋯F2) = 3.14(2) Å, d(C32⋯F6) = 3.40(2) Å, d(C36⋯F4) = 3.36(1) Å), and also assist in network stacking into 3D (d(C20⋯F1) = 3.17(1) Å).
While the halogen bond dataset obtained in this work is statistically small, it mostly fits previous observations. Halogen bonds with piperidinyl nitrogen atoms in these cocrystals are mostly linear and have a large value of donor⋯acceptor distance shortening (R.S.) with respect to the sum of van der Waals radii18 that is comparable to well-researched halogen bonds with pyridyl nitrogen atoms.9a,19
Halogen bonds with carbonyl oxygen atoms are also quite linear but have a slightly smaller value of R.S. The one exception is the halogen bond with the bridging 14tfib molecule (I2⋯O1) where this value drops down to only 7.1%, which is comparable to weaker Br⋯O halogen bonds with carbonyl oxygen atoms.20 This can be ascribed to supramolecular packing effects in the (dpz)(14tfib)2 structure coupled with acceptor strength. The carbonyl oxygen atom in question is a simultaneous acceptor of two halogen bonds, meaning that it can participate in halogen bonding in two ways: by forming two bonds of relatively equal strength, or by forming one stronger and one weaker bond. Since the non-bridging 14tfib molecule has to fill in the space enveloped by another dpz molecule, it is sterically advantageous for it to form a strong halogen bond. As a result, the oxygen atom weakens as an acceptor in the direction of the bridging 14tfib molecule, which leads to a sterically favorable lengthening of the halogen bond (Fig. 3b). Furthermore, the bridging molecule participates in two halogen bonds with oxygen atoms on two dpz molecules and in supramolecular interactions with nearby weak hydrogen bond donors, meaning that weakening of these individual halogen bonds probably has less of an effect on structural stability than if the bond with the non-bridging molecule were weakened.
Thermal analysis experiments (see ESI†) have shown that both prepared cocrystals have similar thermal stabilities. Their DSC curves exhibit one, well-defined, endothermic peak which corresponds to melting, at 113 °C for (dpz)(13tfib) and 111 °C for (dpz)(14tfib)2. Their melting points are ca. 20 °C higher than that of the pure dpz (91 °C). The similarity in thermal degradation temperature is interesting, considering their different stoichiometries and supramolecular architectures, as well as the fact that the pure halogen bond donors, 13tfib and 14tfib, have a melting point of 23 °C and 108 °C, respectively.
To conclude, our cocrystallization experiments have resulted in two new halogen-bonded cocrystals with the selected API molecule. In line with our previous work, the carbonyl oxygen atom has proven its potential as a good halogen bond acceptor, along with the piperidinyl nitrogen atom, even if they are present in a bulkier molecule such as dpz, which contains a larger variety of functional groups that are potential acceptor sites. Furthermore, the carbonyl oxygen atom as both a monocentric or bifurcated halogen bond acceptor species turned out to be competitive with the piperidinyl nitrogen atom. In keeping with previous comparisons of 13tfib and 14tfib cocrystals, this work reveals similarities in halogen bond lengths and melting points of their cocrystals,16 as well as differences in stoichiometric compositions of cocrystals and the underlying halogen bonding motifs. Finally, we believe that the described results are important in the context of crystal engineering of pharmaceutical materials and in tuning API solid state properties. Further research into halogen bonding might allow for the design of new families of halogen bond donors which could then be used as appropriate coformers in future pharmaceutical cocrystals.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Croatian Science Foundation under the projects IP-2014-09-7367 and IP-2019-04-1868.Notes and references
- (a) H. Sugimoto, H. Ogura, Y. Arai, Y. Iimura and Y. Yamanishi, Jpn. J. Pharmacol., 2002, 89, 7–20 CrossRef CAS PubMed; (b) B. Seltzer, Expert Opin. Drug Metab. Toxicol., 2005, 1, 527–536 CrossRef CAS PubMed; (c) M. P. Murphy and H. LeVine, J. Alzheimer's Dis., 2010, 19, 311–323 Search PubMed.
- J. H. Bae, H. J. Sun, A. Park, D. Kim, J. Yeon, S. K. Kang and E. H. Lee, Cryst. Growth Des., 2020, 20, 2283–2293 CrossRef CAS.
- (a) T. B. Patel, T. R. Patel and B. N. Suhagia, Int. J. Pharma Sci. Res., 2016, 7, 2097–2108 CAS; (b) K. Ravikumar, B. Sridhar, D. G. Sathe, A. V. Naidu and K. D. Sawant, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2006, 62(2006), o681–o683 CrossRef CAS PubMed; (c) W. Somphon, A. Prasertsabw and N. Jarussophon, Mater. Today: Proc., 2019, 17, 1887–1897 CAS; (d) A. Manikowski, B. Ziobro, A. Zaba, M. Makosza, J. Jerkovic, I. Grebenar, E. Mestrovic, Z. M. Samardzic, L. Lerman and K. Kaczorowska, WO2007/015052A1, 2007; (e) T. Mezei, G. Simig, G. Lukács, M. Porcs-Makkay, B. Volk, E. Molnár and V. Hofmanné Fekete, WO2006/030249A1, 2006.
- T. H. Zhang, EP2204364A1, 2010.
- (a) http://www.uspto.gov/ (search date March 30th, 2020); (b) https://worldwide.espacenet.com/patent/ (search date March 30th, 2020); (c) https://www.j-platpat.inpit.go.jp/ (search date March 30th, 2020).
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- (a) M. G. Cardozo, T. Kawai, Y. Iimura, H. Sugimoto, Y. Yamanishi and A. J. Hopfinger, J. Med. Chem., 1992, 35, 590–601 CrossRef CAS PubMed; (b) Y. Park, J. Lee, S. H. Lee, H. G. Choi, C. Mao, S. K. Kang, S.-E. Choi and E. H. Lee, Cryst. Growth Des., 2013, 13, 5450–5458 CrossRef CAS; (c) Y. Park, S. X. M. Boerrigter, J. Yeon, S. H. Lee, S. K. Kang and E. H. Lee, Cryst. Growth Des., 2016, 16, 2552–2560 CrossRef CAS.
- S. H. Lee, J. H. Bae, Y. Park, B. R. Adhikari, C. Mao, D. Kim, K. I. Kim, S. K. Kang and E. H. Lee, Cryst. Growth Des., 2015, 15, 3123–3130 CrossRef CAS.
- (a) G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed; (b) R. W. Troff, T. Mäkelä, F. Topić, A. Valkonen, K. Raatikainen and K. Rissanen, Eur. J. Org. Chem., 2013, 1617 CrossRef CAS; (c) D. Yan, A. Delori, G. O. Lloyd, T. Friščić, G. M. Day, W. Jones, J. Lu, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 50, 12483 CrossRef CAS PubMed; (d) M. A. Sinwell and L. R. MacGillivray, Angew. Chem., Int. Ed., 2016, 55, 3477 CrossRef PubMed; (e) O. S. Bushuyev, T. Friščić and C. J. Barrett, Cryst. Growth Des., 2016, 16, 541 CrossRef CAS.
- (a) A. Priimagi, G. Cavallo, P. Metrangolo and G. Resnati, Acc. Chem. Res., 2013, 46, 2686 CrossRef CAS PubMed; (b) G. R. Desiraju, P. Shing Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711 CAS.
- (a) J.-L. Syssa-Magalé, K. Boubeker and B. Schöllhorn, J. Mol. Struct., 2005, 737, 103–107 CrossRef; (b) M. Zbačnik, M. Pajski, V. Stilinović, M. Vitković and D. Cinčić, CrystEngComm, 2017, 19, 5576–5582 RSC; (c) D. Cinčić and T. Friščić, CrystEngComm, 2014, 16, 10169–10172 RSC; (d) V. Nemec and D. Cinčić, CrystEngComm, 2016, 18, 7425–7429 RSC; (e) G. Bergamaschi, L. Lascialfari, A. Pizzi, M. I. Martinez Espinoza, N. Demitri, A. Milani, A. Gori and P. Metrangolo, Chem. Commun., 2018, 54, 10718–10721 RSC; (f) V. Nemec, L. Fotović, T. Vitasović and D. Cinčić, CrystEngComm, 2019, 21, 3251–3255 RSC; (g) X.-Q. Yang, Z.-Y. Yi, S.-F. Wang, T. Chen and D. Wang, Chem. Commun., 2020, 56, 3539 RSC.
- (a) D. Cinčić, T. Friščić and W. Jones, J. Am. Chem. Soc., 2008, 130, 7524–7525 CrossRef PubMed; (b) H. Deng, A. N. Gifford, A. M. Zvonok, G. Cui, X. Li, P. Fan, J. R. Deschamps, J. L. Flippen-Anderson, S. J. Gatley and A. Makriyannis, J. Med. Chem., 2005, 48, 6386–6392 CrossRef CAS PubMed; (c) P. G. Jones, I. Dix and H. Hopf, CSD Communication, 2015, refcode QUNPAS Search PubMed; (d) E. F. Serantoni, L. Riva di Sanseverino and P. Sabatino, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2473–2476 CrossRef; (e) D. E. Jeffries, J. O. Witt, A. L. McCollum, K. J. Temple, M. A. Hurtado, J. M. Harp, A. L. Blobaum, C. W. Lindsley and C. R. Hopkins, Bioorg. Med. Chem. Lett., 2016, 26, 5757–5764 CrossRef CAS PubMed; (f) P. Tongwa, T. L. Kinnibrugh, G. R. Kicchaiahgari, V. N. Khrustalev and T. V. Timofeeva, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2009, 65, o155–o159 CrossRef CAS PubMed.
- (a) O. Dumele, D. Wu, N. Trapp, N. Goroff and F. Diederich, Org. Lett., 2014, 16, 4722–4725 CrossRef CAS PubMed; (b) H. S. Yathirajan, A. N. Mayekar, B. Narayana, B. K. Sarojini and M. Bolte, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, o2196–o2197 CrossRef CAS; (c) F. D. Cukiernik, A. Zelcer, M. T. Garland and R. Baggio, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2008, 64, o604–o608 CrossRef CAS PubMed; (d) Y. Çetinkaya, A. Menzek, E. Şahin and H. T. Balaydın, Tetrahedron, 2011, 67, 3483–3489 CrossRef; (e) H. Xu, Q. Wang and Y. Guo, Chem. – Eur. J., 2011, 17, 8299–8303 CrossRef CAS PubMed; (f) Y. Tang, R. Han, M. Lv, Y. Chen and P. Yang, Tetrahedron, 2015, 71, 4334–4343 CrossRef CAS; (g) Z. Zhang, L. Chang, S. Wang, H. Wang and Z.-Y. Yao, RSC Adv., 2013, 3, 18446–18452 RSC; (h) M. Chaabene, A. Khatyr, M. Knorr, M. Askri, Y. Rousselin and M. M. Kubicki, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2016, 72, 1167–1170 CrossRef CAS PubMed.
- Similar bifurcated motifs have also been noticed in systems that have ortho-hydroxy and methoxy groups, for example in: (a) A. Carletta, M. Zbačnik, M. Van Gysel, M. Vitković, N. Tumanov, V. Stilinović, J. Wouters and D. Cinčić, Cryst. Growth Des., 2018, 18, 6833–6842 CrossRef CAS; (b) A. Carletta, F. Spinelli, S. d'Agostino, B. Ventura, M. R. Chierotti, R. Gobetto, J. Wouters and F. Grepioni, Chem. – Eur. J., 2017, 23, 5317–5329 CrossRef CAS PubMed.
- (a) A. Verma, K. Tomar and P. K. Bharadwaj, Cryst. Growth Des., 2019, 19, 369 CrossRef CAS; (b) R. Bhowal, S. Biswas, D. P. Adiyeri Saseendran, A. L. Koner and D. Chopra, CrystEngComm, 2019, 21, 1940 RSC; (c) H. Wang and W. J. Jin, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2017, 73, 210 CrossRef CAS PubMed.
- (a) N. Bedeković, V. Stilinović, T. Friščić and D. Cinčić, New J. Chem., 2018, 42, 10584 RSC; (b) L. C. Roper, C. Präsang, V. N. Kozhevnikov, A. C. Whitwood, P. B. Karadakov and D. W. Bruce, Cryst. Growth Des., 2010, 10, 3710 CrossRef CAS.
- (a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC; (b) T. Friščić and W. Jones, Cryst. Growth Des., 2009, 9, 1621–1637 CrossRef.
- van der Waals radii according to: A. J. Bondi, J. Phys. Chem., 1964, 65, 441–451 CrossRef.
- R. B. Walsh, C. W. Padgett, P. Metrangolo, G. Resnati, T. W. Hanks and W. T. Pennington, Cryst. Growth Des., 2001, 1, 165–175 CrossRef CAS.
- V. Nemec, L. Fotović, T. Friščić and D. Cinčić, Cryst. Growth Des., 2017, 17, 6169–6173 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, single crystal and powder X-ray diffraction data. CCDC 2017056 and 2017057 contain crystallographic data for this paper. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ce01065k |
| This journal is © The Royal Society of Chemistry 2020 |