Open Access Article
Open Access ArticleZinc phthalocyanine activated by conventional indoor light makes a highly efficient antimicrobial material from regular cellulose†
Natalia E.
Grammatikova
 a,
Lijo
George
a,
Lijo
George
 b,
Zafar
Ahmed
b,
Zafar
Ahmed
 b,
Nuno R.
Candeias
b,
Nuno R.
Candeias
 b,
Nikita A.
Durandin
b,
Nikita A.
Durandin
 b and
Alexander
Efimov
b and
Alexander
Efimov
 *b
*b
aG. F. Gause Institute of New Antibiotics, Bolshaya Pirogovskaya 11, Moscow, Russian Federation
bFaculty of Engineering and Natural Sciences, Tampere University, Korkeakoulunkatu 8, Tampere, Finland. E-mail: alexandre.efimov@tuni.fi
First published on 21st June 2019
Abstract
Zn phthalocyanine with improved synthesis suitable for bulk production shows extremely high antimicrobial efficacies even under weak indoor light. The dye-impregnated cellulose material inactivates over 99.996% of drug-resistant C. albicans, S. aureus and E. faecalis in just one hour exposure with consumer-grade fluorescent lamps and diodes.
1. Introduction
Pathogens of various taxonomies have become resistant rapidly even to novel antibiotics and have claimed millions of lives annually, not to mention the burden of trillions of US dollars to the global economy.1 Global mobility has resulted in the spread of, e.g., the Severe Acute Respiratory Syndrome coronavirus (SARS),2 the multidrug-resistant bacterium S. aureus (MRSA),3 and more recently the C. auris fungus.4 Newly arriving pathogen species can be the carriers of new antibiotic-resistant genes that, together with growing resistance to commonly prescribed antimicrobial drugs, can result in new generations of “superbugs”.5–8 The existence of pathogens resistant to “last-resort” antibiotics, i.e. colistin, is a cause for alarm.9 The circumscription of recently emerging Candida auris is another global health threat that needs to be tackled opportunely.4,10,11 Notwithstanding the somewhat efficacy of conventional antiseptics and disinfectants, the high volatility of their components drastically limits the desired long-term effects.12 Therefore, preventive methods that are easy to apply and capable of targeting simultaneously several classes of pathogens without offering them a possibility to develop resistance are of utmost importance.Self-disinfecting materials (SDMs) such as fabrics, films, and coatings can play a vital role in preventing the transmission of pathogens from, e.g., hospital surfaces to patients, and from patient to patient. Ideally, self-sterilizing surfaces should be non-specific to pathogens, easy-to-make, scalable and robust. To this end, materials utilizing principles of photodynamic therapy represent a promising approach to combat pathogenic species, including antibiotic-resistant ones.13–15 Photodynamic antimicrobial chemotherapy (PACT) relies on the cytotoxic action of reactive oxygen species (ROS), such as singlet oxygen and superoxide radicals, generated by a photosensitizer (PS) upon light excitation.16,17 The absence of any antimicrobial resistance mechanisms towards ROSs, spatiotemporal precision, controllability and non-invasive properties make PACT a method of choice in hampering the evolution of drug resistant bacteria and controlling the spread of existing ones. Of ultimate importance, photodynamic inactivation does not induce resistivity in microorganisms.18–20
Incorporation of photosensitizers (metal nanoparticles and/or small organic dyes, like phenothiazines) into polymers has already provided a potent class of antimicrobial surfaces.14,15 In particular, SDMs made of polysiloxane with methylene blue and gold nanoparticles resulted in more than 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction of methicillin-resistant S. aureus after 5 min of exposure to 250 mW laser light (660 nm).21 Notwithstanding their efficacy, such systems require either tricky covalent linkage or labour intensive “swell–encapsulation–shrink” methods to load dyes/nanoparticles into polymer matrixes. Metal nanoparticles tend to be condition- and pathogen-specific, while phenothiazines are prone to photobleaching, which together with the other above-mentioned limitations profoundly hinders their applicability.22–25
log10 reduction of methicillin-resistant S. aureus after 5 min of exposure to 250 mW laser light (660 nm).21 Notwithstanding their efficacy, such systems require either tricky covalent linkage or labour intensive “swell–encapsulation–shrink” methods to load dyes/nanoparticles into polymer matrixes. Metal nanoparticles tend to be condition- and pathogen-specific, while phenothiazines are prone to photobleaching, which together with the other above-mentioned limitations profoundly hinders their applicability.22–25
Porphyrinoids can be an excellent option to abrogate pathogenic microorganisms via a PACT mechanism.26 In particular, cationic metallated phthalocyanines exhibit significant phototoxicity because of their high yield of ROS generation.27,28 Broad absorption bands together with high molar absorption coefficients and long triplet state lifetimes allow phthalocyanines to generate high ROS concentrations even while using low intensity light.
Therefore, over 99% eradication efficiencies of pathogens have been achieved under cheap LED source illumination, white light or even sunlight instead of expensive laser setups.29–31 Although the hydrophobicity of phthalocyanines is an issue, their strong resistance against photobleaching makes them good candidates for PACT-based materials with prolonged life durability.30
2. Experimental
2.1. Materials and methods
Reagents and solvents were purchased from TCI Europe, Sigma Aldrich Co. or VWR and were used without further purifications unless otherwise mentioned. NMR spectra were recorded using a JEOL JNM-ECZ500R 500 MHz spectrometer using TMS as an internal standard. HRMS measurements were performed with a Waters LCT Premier XE ESI-TOF benchtop mass spectrometer. Lock-mass correction (leucine enkephalin as a reference compound), centering and calibration were applied to raw data to obtain accurate mass. Paper samples containing 80 and 160 mg m−2 of 3 were prepared as described earlier.29 An OSRAM Star PAR16 80 W 575 lm GU10 diode lamp was used to generate 270 lux emittance. Illumination power density was measured with TKA-PKM(09) apparatus that allows monitoring luminous emittance (E, lux) in the visible spectral range (380–760 nm), percent flicker (PF, %) and luminance (L, cd m−2) with the overlapping method of extended self-luminous objects (self-luminous objects with an extended spatial distribution) in the visible range of the spectrum (380–760 nm).2.2. Synthesis of compounds and PACT paper preparation
The compounds 2,3-dicyanophenyl trifluoromethanesulfonate and 4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)pyridine were synthesized according to the literature procedure.32,33 Pyridine boronate ester (0.726 g, 3.78 mmol), triflate phthalonitrile (1.0 g, 3.78 mmol), PdCl2(dppf)·DCM (0.092 g, 0.1134 mmol) and K3PO4 (2.4 g, 11.31 mmol) were dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and toluene (40 ml). The reaction mixture was stirred at room temperature and saturated by bubbling with argon for 15 minutes. After saturation, the mixture was heated with vigorous stirring at 90 °C for 2 h under argon. The reaction mixture was cooled to room temperature, extracted with CHCl3, washed with brine, and dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure to yield a crude product. Re-precipitating the crude product from a 9
1 mixture of water and toluene (40 ml). The reaction mixture was stirred at room temperature and saturated by bubbling with argon for 15 minutes. After saturation, the mixture was heated with vigorous stirring at 90 °C for 2 h under argon. The reaction mixture was cooled to room temperature, extracted with CHCl3, washed with brine, and dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure to yield a crude product. Re-precipitating the crude product from a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CHCl3/hexane yielded 0.603 g of brown solid as pure product, yield 77%. The analytical data (NMR, MS, etc.) were identical to previously reported values.29 Further synthesis of phthalocyanine 2 and its cationic form 3 was performed as reported in our earlier paper.29
1 mixture of CHCl3/hexane yielded 0.603 g of brown solid as pure product, yield 77%. The analytical data (NMR, MS, etc.) were identical to previously reported values.29 Further synthesis of phthalocyanine 2 and its cationic form 3 was performed as reported in our earlier paper.29
A stock solution was prepared by dissolving 1.0 mg of the dye in 200 μl of water. For PACT paper with 80 mg m−2 dye load, 20 μl of this stock solution was diluted in 1 ml of water and taken into a Petri dish. Filter paper (Qualitative Grade 413 from VWR, 3.5 cm × 3.5 cm) was soaked into the aqueous solution for approximately 5 minutes. The paper became evenly light green in colour, leaving behind transparent water, indicating complete loading of the dye on the paper. Similarly, to prepare 160 mg m−2 dye load paper, 40 μl of stock solution in 1 ml of water was used to impregnate 3.5 cm × 3.5 cm filter paper. The papers were dried in air for 24 h protected from direct sunlight before use. The papers were stored at r.t. in the dark. The 6 mm disks used for inactivation experiments were cut with a hole punch. The preparation process is illustrated in the ESI,† page S6.
2.3. Cell culture
Clinical isolates of Staphylococcus aureus strain 88 (MRSA), Enterococcus faecalis 583 (vancomycin-resistant) and Candida albicans 604M, resistant to fluconazole, were used for the study. The microorganisms were kept at −75 °C in casein–soymeal–peptone broth with 10–15% glycerol. Before the experiment a bacterial strain was activated from cryoconservation by seeding it onto CASO Agar medium (“Sifin”, Germany) and incubated at 35 ± 2 °C for 18–20 hours. Candida albicans 604M was seeded onto Sabouraud Dextrose Agar medium (Becton Dickinson, Germany) and incubated at 35 ± 2 °C for 48 hours.Overnight bacterial strains were seeded onto Mueller–Hinton Broth (“Sifin”, Germany) and adjusted to have 0.5 McFarland standard turbidity. Microorganism suspension turbidity was determined by using a DEN-1 (densitometer (suspension turbidity detector) BioSan, Riga, Latvia).
The microorganisms were incubated further at 35 ± 2 °C to obtain 1 McF optical turbidity. Then, they were precipitated by centrifugation and washed with phosphate buffered saline (PBS). The cell densities were adjusted with PBS to reach 0.5 McF and were equal to ca. 1.5 × 108 CFU per ml (cells per ml).
To perform an experiment with Candida albicans 604M, individual colonies were collected from Sabouraud Agarose medium, suspended in PBS, pH 7.2–7.6 (Eco Service, Russia), precipitated by centrifugation and washed twice with PBS. The cell density was adjusted in PBS solution to reach 3 McF turbidity and was equal to 3 × 107 CFU per ml.
2.4. Photoinactivation experimental conditions
Each 6 mm disk of the paper impregnated with zinc phthalocyanine 3 was placed into a Petri dish (40 mm diameter) and covered with 25 μl of cell suspension of each microorganism suspended in PBS. The calculated infection dose was equal to ca. 3.7 × 106 (log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5) for bacterial strains and ca. 7.5 × 105 (log10
6.5) for bacterial strains and ca. 7.5 × 105 (log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.87) for Candida albicans.
5.87) for Candida albicans.
The experiment included the following experimental variants:
1. Control measurements under 4000 lux illumination;
2. Dark toxicity control measurements;
3. Photoinactivation of microorganisms under 270 lux illumination;
4. Photoinactivation of microorganisms under 4000 lux illumination.
Illumination of the samples was performed for either 30 or 60 minutes. The distance between the illumination source and the PACT paper disk was kept constant (14 cm) to get 4000 lux. Office light was utilized to obtain a luminous emittance of 270 lux. The black background below the Petri dish was implemented to avoid the diffusely-reflecting effect on the results of the experiments.
A 2–3 lux illumination was provided at the end of all experimental variants. A series of dilutions was prepared having already the paper disk inside the Petri dish. Ten-fold dilutions were already performed in vials. An aliquot of 0.1 ml of the corresponding dilution was introduced into a Petri dish 90 mm in diameter. All Petri dishes, including the first one with the paper disk, were filled with selective media: for S. aureus – BD™ Mannitol Salt Agar (BD, France), for Enterococcus faecalis – BBL™ Enterococcosel™ Agar (BD, France), for Candida albicans – BBL Sabouraud Dextrose Agar with Chloramphenicol (BD, France), and were left to harden the medium. They were incubated for 24–96 hours at 35 ± 2 °C.
Each experimental variant was reproduced on different days in triplicate.
Microorganisms’ maximal CFU values were achieved after 72 hours of incubation for bacterial strains and after 96 hours for Candida. A further increase in CFU values wasn’t observed. Each experimental variant was analyzed in terms of CFU average values in Petri dishes, recalculated with respect to dilution and converted into log10 values (log10 CFU per ml).
2.5. Statistical analysis
SPPS (SPSS: An IBM Company, USA) was employed for statistical analysis. Figures were plotted by using OriginPro 2018 software (OriginLab Corp., USA).Briefly, testing for normality distribution for repeated measurements or for intergroup comparison in terms of log10 CFU per ml values was performed by using Shapiro–Wilk's test. The average mean (M) and standard deviation (SD) values were used to analyze the data with normal distribution. ANOVA dispersion analysis with a posteriori tests for multiple comparison by using Tukey's criterion for repeated measurements was utilized to compare the values of log10 CFU per ml with respect to both luminous emittance and exposure time. Testing of statistical hypotheses was performed by using a critical significance level of 95%, i.e. the difference was statistically significant when p < 0.05. Figures represent average mean values, medians, and standard mean squared errors.
Evaluation of the difference between controls and experimental groups was carried out by using the Mann–Whitney U-test when the distribution of the values was different from normal (see the ESI†). Hence, the median values were used for graphical representation of the data.
3. Results and discussion
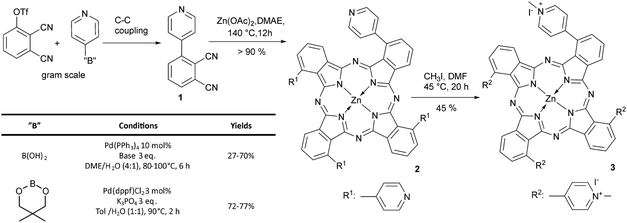
We have recently reported the synthesis of pharmacologically relevant tetrapyridinyl phthalocyanine 2 and its water soluble cationic form 3 and shown their activity against Gram-negative microbes E. coli and A. baylyi under LED light.29,30 Moving a step forward, in the present work we report an unusually high efficacy of a self-disinfecting material based on tetracation 3 against drug-resistant Gram-positive bacteria and fungi. Of exceptional importance, efficient antimicrobial activities of this material are achieved even under weak indoor light of 270 lux.While appropriate for milligram scale synthesis, the previously reported procedure had a bottleneck of the Suzuki–Miyaura coupling between triflate phthalonitrile and 4-pyridyl boronic species.29 Unreproducible yields were obtained in the gram scale preparation of 1. It should be noted that a general method for the Pd-catalysed C–C coupling of pyridyl boronic derivatives remains undeveloped, despite previous methods reported for 2-heterocyclic boronates and aryl bromides.34,35 In order to overcome this inconsistency and to reliably obtain reasonable yields during the preparation of pyridylphthalonitrile 1, we screened different boronic components (acid and ester), Pd catalysts (Pd(PPh3)4 and Pd(dppf)Cl2), bases (K2CO3, Na2CO3, Cs2CO3 and K3PO4), solvents and reaction temperatures. Surprisingly, different batches of Pd(PPh3)4 produced inconsistently variable yields. A closer examination by NMR and IR revealed that they contained remarkably different proportions of phosphine and oxide ligands with no clear dependence on the reaction yield (see the ESI†). However, by modifying the previously reported procedure, i.e. by reducing the amount of Pd(dppf)Cl2 to 3 mol%, and heating the reaction mixture at 90 °C for 2 hours in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 toluene:water mixture and with K3PO4 as base, we were able to obtain the desired compound 1 in 72–77% yield after purification. Importantly, saturation of the reaction mixture by bubbling it with argon for 15 minutes prior to heating was essential for reproducibility and improved yields in gram scale reactions. Condensation of 1 in zinc acetate/dimethylaminoethanol provided metallated complex 2 in excellent yield, which was further methylated to provide tetracationic phthalocyanine 3 for antimicrobial studies (Scheme 1).
1 toluene:water mixture and with K3PO4 as base, we were able to obtain the desired compound 1 in 72–77% yield after purification. Importantly, saturation of the reaction mixture by bubbling it with argon for 15 minutes prior to heating was essential for reproducibility and improved yields in gram scale reactions. Condensation of 1 in zinc acetate/dimethylaminoethanol provided metallated complex 2 in excellent yield, which was further methylated to provide tetracationic phthalocyanine 3 for antimicrobial studies (Scheme 1).
In our quest towards self-disinfecting surfaces, zinc phthalocyanine 3 was incorporated into cellulose matrix (PACT paper). Cellulose is a material of choice due to its biocompatibility, physical stability, high loading capacity for cationic molecules, low-cost and flexibility.22,36–39 Unlike polysiloxane or polyurethane matrix, this paper doesn’t block the interaction between the PS triplet state and molecular oxygen because of its porous structure that in general facilitates the formation of ROS.40
The impregnation of cellulose with phthalocyanine 3 was remarkably simple and efficient. Qualitative Grade 413 filter paper (VWR) was immersed into aqueous solutions of 3 for 10 minutes; the concentration of phthalocyanine was calculated according to the paper area to obtain 80 or 160 mg m−2 dye loading. In a few minutes, the deeply coloured solution became colourless, while all the dye was adsorbed onto the paper, forming a visually even colouring (see the ESI†). After drying in air at room temperature, the desired PACT paper was ready to be used in antimicrobial testing. The strong interaction between the positively charged photosensitizer and cellulose fibres makes the PACT paper resistant to leaching and bleaching. Hence, this method is extremely simple and versatile since dye loading can be easily controlled.30 A unique advantage of this system over other photodynamic materials is that the PACT surface can be easily renewed just by soaking or spraying it with a fresh aqueous dye solution, which is of vital importance for practical applications. Moreover, as previously published, 64 h illumination with sunlight intensity bleaches out the PACT paper only by 13% and does not change its activity. The PACT paper was further examined against clinical isolates of antibiotic-resistant pathogens Staphylococcus aureus strain 88 (MRSA), vancomycin-resistant Enterococcus faecalis 583 (VRE) and Candida albicans 604M resistant to fluconazole. MRSA and VRE belong to the ESKAPE group of pathogens and take a leadership in the list of bacteria causing severe Healthcare Associated Infections (HAIs).41 MRSA is the cause of skin and soft tissue infections, nosocomial pneumonia and bacteraemia.42 VRE is mostly associated with HAIs, but public healthcare recently expressed concerns about wide spreading of VRE elsewhere.43 Moreover, VRE strains are the source of vancomycin-resistant plasmids, which were detected already in MRSA strains.44C. albicans is a saprophyte fungus that can, however, cause various infections of any organ and tissue when the immune status is compromised.4,45 The calculated infection dose was equal to either ca. 3.7 × 106 colony-forming units per ml (CFU per ml) (ca. log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5) for bacterial strains or ca. 7.5 × 105 CFU per ml (ca. log
6.5) for bacterial strains or ca. 7.5 × 105 CFU per ml (ca. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.87) for C. albicans.
5.87) for C. albicans.
Modern photodynamic agents suffer from the drawback of requiring intense light irradiation to be effective.21,46 In contrast, we performed our photoinactivation experiments at light intensities typical of indoor living/office space: 270 lux or 4000 lux (40 or 586 μW cm−2) light intensities with 30 min or 60 min exposure time. The value of 270 lux is so small that it falls outside the normal sensitivity of common (laser) light power meters, which justifies the use of lux units throughout this study. Additionally, lux is a well known unit for construction/architecture/work safety bodies and allows connecting the efficacy of photodynamic antimicrobial agents with demands of light levels in living spaces. In our study, a fluorescent lamp was utilized to obtain a luminous emittance of 270 lux, while a LED lamp was used to generate 4000 lux light intensity.
Control experiments on non-dyed papers showed that such low intensity light has no effect on the studied microorganisms regardless of the exposure time, and the same values were obtained for the viability of microorganisms. Dark controls with 80 mg m−2 PACT paper showed no difference with respect to non-dyed paper samples. Therefore, all control values were combined into one mean value (see the ESI†).
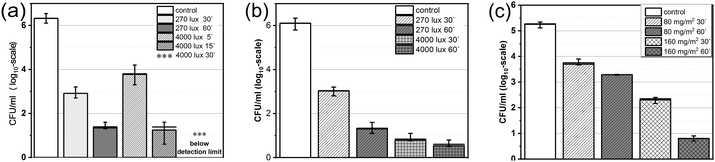
Under light irradiation, a very strong antibacterial effect was observed for the dyed papers (Fig. 1). Indoor light 270 lux on 80 mg m−2 paper resulted in a significant reduction of MRSA 88. The values of 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 and 4.8
log10 and 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU per ml reduction of cell viability with respect to control (log10
log10 CFU per ml reduction of cell viability with respect to control (log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2) were obtained for 30 and 60 min irradiation, respectively. An increase in luminous emittance up to 4000 lux led to the total eradication of the cell population after 30 minutes of illumination (Fig. 1a). The antimicrobial efficiency against the VRE 583 strain under 270 lux was similarly high. The reduction of CFU per ml values with respect to the control (log10
6.2) were obtained for 30 and 60 min irradiation, respectively. An increase in luminous emittance up to 4000 lux led to the total eradication of the cell population after 30 minutes of illumination (Fig. 1a). The antimicrobial efficiency against the VRE 583 strain under 270 lux was similarly high. The reduction of CFU per ml values with respect to the control (log10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.1) was as high as 4.88 and 5.4
6.1) was as high as 4.88 and 5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 after 30 minutes and 60 minutes of treatment, respectively (Fig. 1b). However, 4000 lux illumination did not result in complete elimination of the microorganism; with either exposure time a small number of microbes survived (0.9 and 0.6
log10 after 30 minutes and 60 minutes of treatment, respectively (Fig. 1b). However, 4000 lux illumination did not result in complete elimination of the microorganism; with either exposure time a small number of microbes survived (0.9 and 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU per ml for 30 and 60 min, respectively). The photoantimicrobial activity of 80 mg m−2 PACT paper using 270 lux illumination against the fungus C. albicans was lower. We did not observe significant differences between the control group and the experimental groups under 270 lux light applied for 30 or 60 minutes (p = 0.915 and 0.323, respectively). Treatment with 4000 lux for 30 and 60 minutes resulted in CFU value reduction by 2 and 1.6
log10 CFU per ml for 30 and 60 min, respectively). The photoantimicrobial activity of 80 mg m−2 PACT paper using 270 lux illumination against the fungus C. albicans was lower. We did not observe significant differences between the control group and the experimental groups under 270 lux light applied for 30 or 60 minutes (p = 0.915 and 0.323, respectively). Treatment with 4000 lux for 30 and 60 minutes resulted in CFU value reduction by 2 and 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 (Fig. 1c).
log10 (Fig. 1c).
However, the paper sample with a 160 mg m−2 load of dye 3 under 4000 lux illumination deactivated C. albicans significantly, and only 2.3 and 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU per ml survived. Reduction of 2.97 and 4.4
log10 CFU per ml survived. Reduction of 2.97 and 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 in cell viability (p < 0.001) was achieved after 30 and 60 minutes of light exposure, respectively (Fig. 1c). The control experiments were not dependent on the exposure time (p = 0.881); however, there was a profound effect for both 80 and 160 mg m−2 PACT paper samples with respect to the time of illumination (p < 0.001).
log10 in cell viability (p < 0.001) was achieved after 30 and 60 minutes of light exposure, respectively (Fig. 1c). The control experiments were not dependent on the exposure time (p = 0.881); however, there was a profound effect for both 80 and 160 mg m−2 PACT paper samples with respect to the time of illumination (p < 0.001).
The direct comparison of PACT with existing antimicrobial coatings is always difficult, as there is no standardized procedure for measuring the efficacy of light-activated materials. In addition, commercial companies tend to mention just “Tested according to ISO”. The procedures used in the present work are similar to ISO221961 (antimicrobial plastic surfaces and coatings), which typically gives 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in 24 hours for silver-based paints. Another benchmark could be AATCC 100-2004 or EN ISO 20743 (antimicrobial textiles), which can give 5–6
log reduction in 24 hours for silver-based paints. Another benchmark could be AATCC 100-2004 or EN ISO 20743 (antimicrobial textiles), which can give 5–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in 1 hour, though with a load of silver/copper of ca. 10 g m−2.49
log reduction in 1 hour, though with a load of silver/copper of ca. 10 g m−2.49
It is also worth mentioning that the PACT papers used in this study were stored before use for different periods of time and still preserved their antimicrobial activity. Thus, the 80 mg m−2 paper used against MRSA 88 and VRE 583 was either 6 days, or 110 days, or 140 days old, and there was no observable difference in efficacy. The 80 mg m−2 paper used against C. albicans was 14 and 36 days old, and 160 mg m−2 PACT paper was 442 and 453 days old. The paper was stored at room temperature in a plastic bag, in a desk drawer.
4. Conclusion
In conclusion, a scalable and robust PACT material based on affordable components has been developed. We have already demonstrated its efficacy against drug-resistant Gram-negative strains, and with this work we extend its applicability to eradicate Gram-positive microorganisms and fungi of clinical isolates resistant to antibiotics. At light intensities and with light sources typical for indoor spaces (270 lux in offices, hospitals, and living rooms) PACT paper with 80 mg m−2 dye load is able to inactivate more than 99.998% of MRSA and VRE. A higher dye load (160 mg m−2) allows inactivating 99.996% of C. albicans within an hour, though brighter 4000 lux light is beneficial.Noteworthily, the ability of this self-disinfecting material to work efficiently under low intensity indoor light sources presents a considerable leap forward in the field of antimicrobial materials. The vast majority of known photosensitizers perform well only under strong laser or white light, and thus are suitable for therapeutic purposes or for smaller devices where the use of a focused light/laser beam of high power is feasible.7,8,25,46–48 In contrast, our dye permits rendering self-disinfecting large areas, and requires no special equipment. This makes possible quick disinfection of surfaces in isolation wards, waiting rooms, field surgery tents, etc. High antimicrobial efficacy upon indoor light exposure, an ability to eradicate simultaneously fungi, Gram+, and Gram− bacteria, and a unique option for renewing and reapplying the coating as needed makes PACT material based on tetracationic phthalocyanine 3 the substance of choice for real life applications. It can be utilized to control the spreading of emerging infection diseases in hospitals or crowded areas such as airports, for disinfection of pathological waste, as a coating or treatment for personal protection items or clothes with disinfecting modalities upon indoor light illumination.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors gratefully acknowledge Business Finland (decision 5595/31/2018), the Academy of Finland (decision no. 326487) and Janne and Aatos Erkko Foundation (Finland) for the financial support.Notes and references
- J. O'Neill, The Wellcome Trust and HM Government, Tackling Drug-resistant Infections Globally: Final Report And Recommendations, 2016, pp. 1–84 Search PubMed.
- E. de Wit, N. van Doremalen, D. Falzarano and V. J. Munster, Nat. Rev. Microbiol., 2016, 14, 523–534 CrossRef CAS PubMed.
- A. S. Lee, H. de Lencastre, J. Garau, J. Kluytmans, S. Malhotra-Kumar, A. Peschel and S. Harbarth, Nat. Rev. Dis. Primers, 2018, 4, 18033 CrossRef PubMed.
- M. C. Fisher, N. J. Hawkins, D. Sanglard and S. J. Gurr, Science, 2018, 360, 739–742 CrossRef CAS PubMed.
- M. Vouga and G. Greub, Clin. Microbiol. Infect., 2016, 22, 12–21 CrossRef PubMed.
- M. Souli, I. Galani and H. Giamarellou, Eurosurveillance, 2008, 13, 19045 Search PubMed.
- K. J. Forsberg, A. Reyes, B. Wang, E. M. Selleck, M. O. Sommer and G. Dantas, Science, 2012, 337, 1107–1111 CrossRef CAS PubMed.
- H. Hasman, A. M. Hammerum, F. Hansen, R. S. Hendriksen, B. Olesen, Y. Agersø, E. Zankari, P. Leekitcharoenphon, M. Stegger, R. S. Kaas and L. Cavaco, Eurosurveillance, 2015, 20, 1–5 CrossRef PubMed.
- J. Li, C. R. Rayner, R. L. Nation, R. J. Owen, D. Spelman, K. E. Tan and L. Liolios, Antimicrob. Agents Chemother., 2006, 50, 2946–2950 CrossRef CAS PubMed.
- A. Ruiz-Gaitán, A. M. Moret, M. Tasias-Pitarch, A. I. Aleixandre-López, H. Martínez-Morel, E. Calabuig, M. Salavert-Lletí, P. Ramírez, J. L. López-Hontangas, F. Hagen and J. F. Meis, Mycoses, 2018, 61, 498–505 CrossRef PubMed.
- S. Tsay, A. Kallen, B. R. Jackson, T. M. Chiller and S. Vallabhaneni, Clin. Infect. Dis., 2017, 66, 306–311 CrossRef PubMed.
- J. H. Macias, V. Arreguin, J. M. Munoz, J. A. Alvarez, J. L. Mosqueda and A. E. Macias, Am. J. Infect. Control, 2013, 41, 634–637 CrossRef CAS PubMed.
- S. Noimark, C. W. Dunnill and I. P. Parkin, Adv. Drug Delivery Rev., 2013, 65, 570–580 CrossRef CAS PubMed.
- K. Page, M. Wilson and I. P. Parkin, J. Mater. Chem., 2009, 19, 3818–3831 RSC.
- M. Q. Mesquita, C. J. Dias, M. G. P. M. S. Neves, A. Almeida and M. A. F. Faustino, Molecules, 2018, 23, 2424 CrossRef PubMed.
- M. Wainwright, J. Antimicrob. Chemother., 1998, 42, 13–28 CrossRef CAS PubMed.
- M. Wainwright, Drugs Future, 2004, 29, 85–93 CrossRef CAS.
- N. C. M. Gomes, J. A. S. Cavaleiro, E. Alves, A. C. Tomé, Â. Cunha, A. Tavares, J. P. C. Tomé, M. G. P. M. S. Neves, A. Almeida, C. M. B. Carvalho and M. A. Faustino, Mar. Drugs, 2010, 8, 91–105 CrossRef PubMed.
- N. Kashef and M. R. Hamblin, Drug Resist. Updates, 2017, 31, 31–42 CrossRef PubMed.
- Â. Cunha, A. C. Tomé, J. P. C. Tomé, J. A. S. Cavaleiro, A. Almeida, L. Costa, M. G. P. M. S. Neves, M. A. F. Faustino and N. C. M. Gomes, Antiviral Res., 2011, 91, 278–282 CrossRef PubMed.
- S. Perni, C. Piccirillo, J. Pratten, P. Prokopovich, W. Chrzanowski, I. P. Parkin and M. Wilson, Biomaterials, 2009, 30, 89–93 CrossRef CAS PubMed.
- B. L. Carpenter, F. Scholle, H. Sadeghifar, A. J. Francis, J. Boltersdorf, W. W. Weare, D. S. Argyropoulos, P. A. Maggard and R. A. Ghiladi, Biomacromolecules, 2015, 16, 2482–2492 CrossRef CAS PubMed.
- P. Chauhan and N. Yan, RSC Adv., 2016, 6, 32070–32073 RSC.
- C. Piccirillo, S. Perni, J. Gil-Thomas, P. Prokopovich, M. Wilson, J. Pratten and I. P. Parkin, J. Mater. Chem., 2009, 19, 6167–6171 RSC.
- W. J. Peveler, S. Noimark, H. Al-Azawi, G. B. Hwang, C. R. Crick, E. Allan, J. B. Edel, A. P. Ivanov, A. J. MacRobert and I. P. Parkin, ACS Appl. Mater. Interfaces, 2018, 10, 98–104 CrossRef CAS PubMed.
- B. S. T. Peddinti, F. Scholle, R. A. Ghiladi and R. J. Spontak, ACS Appl. Mater. Interfaces, 2018, 10, 25955–25959 CrossRef CAS PubMed.
- A. Sindelo, N. Kobayashi, M. Kimura and T. Nyokong, J. Photochem. Photobiol., A, 2019, 374, 58–67 CrossRef CAS.
- A. Galstyan, R. Schiller and U. Dobrindt, Angew. Chem., Int. Ed., 2017, 56, 10362–10366 CrossRef CAS PubMed.
- L. George, A. Müller, B. Röder, V. Santala and A. Efimov, Dyes Pigm., 2017, 147, 334–342 CrossRef CAS.
- L. George, A. Hiltunen, V. Santala and A. Efimov, J. Inorg. Biochem., 2018, 183, 94–100 CrossRef CAS PubMed.
- R. Jia, W. Tian, H. Bai, J. Zhang, S. Wang and J. Zhang, Adv. Healthcare Mater., 2019, 8, 1801591 CrossRef PubMed.
- J. Ranta, T. Kumpulainen, H. Lemmetyinen and A. Efimov, J. Org. Chem., 2010, 75, 5178–5194 CrossRef CAS PubMed.
- T. Maki, K. Ishihara and H. Yamamoto, Org. Lett., 2005, 7, 5043–5046 CrossRef CAS PubMed.
- P. R. Parry, C. Wang, A. S. Batsanov, M. R. Bryce and B. Tarbit, J. Org. Chem., 2002, 67, 7541–7543 CrossRef CAS PubMed.
- K. L. Billingsley and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 4695–4698 CrossRef CAS PubMed.
- T.-T. Tsai, N. Y.-J. Ho, C.-F. Chen, T.-H. Huang, C.-J. Chang and Y.-T. Tseng, Sci. Rep., 2017, 7, 3155 CrossRef PubMed.
- R. Bonnett and A. Galia, Biotechnol. Biotechnol. Equip., 1994, 8, 68–74 CrossRef.
- Q. Wei, Y. Cai, J. Dong, Q. Wang and R. A. Ghiladi, Nanotechnology, 2018, 29, 265601 CrossRef PubMed.
- V. Decraene, J. Pratten and M. Wilson, Appl. Environ. Microbiol., 2006, 72, 4436–4439 CrossRef CAS PubMed.
- M. A. Filatov, S. Baluschev and K. Landfester, Chem. Soc. Rev., 2016, 45, 4668–4689 RSC.
- S. T. Cole, Philos. Trans. R. Soc., B, 2014, 369, 20130430 CrossRef PubMed.
- R. V. Rasmussen, V. G. Fowler, R. Skov and N. E. Bruun, Future Microbiol., 2011, 6, 43–56 CrossRef CAS PubMed.
- M.-C. Kim, M.-H. Cha, J.-G. Ryu and G.-J. Woo, Foodborne Pathog. Dis., 2017, 14, 195–201 CrossRef CAS PubMed.
- I. T. Paulsen, L. Banerjei, G. S. A. Hyers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty and C. M. Fraser, Science, 2003, 299, 2071–2074 CrossRef CAS PubMed.
- M. A. Jabra-Rizk, E. F. Kong, C. Tsui, M. H. Nguyen, C. J. Clancy, P. L. Fidel and M. Noverr, Infect. Immun., 2016, 84, 2724–2739 CrossRef CAS PubMed.
- Y. Qiao, F. Ma, C. Liu, B. Zhou, Q. Wei, W. Li, D. Zhong, Y. Li and M. Zhou, ACS Appl. Mater. Interfaces, 2018, 10, 193–206 CrossRef CAS PubMed.
- S. Noimark, C. W. Dunnill, M. Wilson and I. P. Parkin, Chem. Soc. Rev., 2009, 38, 3435–3448 RSC.
- J. Gil-Tomás, S. Tubby, I. P. Parkin, N. Narband, L. Dekker, S. P. Nair, M. Wilson and C. Street, J. Mater. Chem., 2007, 17, 3739–3746 RSC.
- H. Haase, L. Jordan, L. Keitel, C. Keil and B. Mahtlig, PLoS One, 2017, 12(11), e0188304 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tb01095e |
| This journal is © The Royal Society of Chemistry 2019 |