Degradable silver-based nanoplatform for synergistic cancer starving-like/metal ion therapy†
Yifan
Zhang‡
,
Yichen
Yang‡
,
Shanshan
Jiang‡
,
Fan
Li
,
Jing
Lin
 ,
Tianfu
Wang
and
Peng
Huang
,
Tianfu
Wang
and
Peng
Huang
 *
*
Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, Laboratory of Evolutionary Theranostics, School of Biomedical Engineering, Health Science Center, Shenzhen University, Shenzhen 518060, China. E-mail: peng.huang@szu.edu.cn
First published on 23rd October 2018
Abstract
Developing a simple and degradable synergistic therapy nanosystem based on glucose oxidase (GOx) remains a challenge. Inspired by the chain reactions among silver nanoparticles, GOx and intratumoral glucose, a degradable GOx-conjugated silver nanocube (AgNC–GOx) for synergistic cancer starving-like/metal ion therapy is presented. The combination of glucose depletion, toxic H2O2 and Ag ions effectively inhibits tumor growth with minimal adverse effects.
Conceptual insightsCurrently, it is still a great challenge to develop an easily degradable nanosystem for synergistic cancer therapy. Herein, we have demonstrated a new concept of synergy between cancer starving-like therapy mediated by glucose oxidase (GOx) and metal ion therapy mediated by silver nanocube (AgNC), in which the chain reactions among intratumoral glucose, GOx and AgNC generate toxic H2O2 and Ag ions to effectively kill tumor cells. In contrast to existing research, which often uses inorganic or macropolymer nanocarriers to load synergistic therapeutic agents, AgNC undergoes dissolution and gets completely oxidized to Ag ions at the tumor sites, thus avoiding the potential risks of drug metabolism and easing the patients' extra body burden. As a proof of concept, such degradable synergistic nanosystem exhibits efficient tumor growth inhibition with minimal adverse effects. This study might present meaningful insights for exploring highly degradable and easily-prepared nanosystems for synergistic cancer therapy. |
The trend of cancer therapy has shifted from monotherapy to synergistic therapy, which could result in synergistic therapeutic effects.1–4 Among various therapeutic modalities, cancer starving-like therapy that uses glucose oxidase (GOx) to catalyze the oxidization of glucose into gluconic acid and toxic H2O2 has attracted increasing interest, as GOx is inherently biocompatible, degradable and has high catalytic efficiency against β-D-glucose.5–9 More interestingly, the generated H2O2 possesses effective oxidization and photolysis activity and can be easily combined with many other treatment modalities for cancer synergistic therapy.10–12 For instance, our group conjugated GOx with L-arginine (L-Arg) loaded hollow mesoporous organosilica nanoparticles (L-Arg-HMON-GOx) for synergistic cancer starving-like/gas therapy.13 In this case, the high concentration of glucose in the tumor tissues was depleted and oxidized into gluconic acid and H2O2 under the catalysis of GOx, and then L-Arg was oxidized into NO gas by H2O2. In another case, Kataoka et al. utilized GOx-loaded therapeutic vesicular nanoreactors to achieve synergistic cancer starving-like/quinone methide therapy.14 More recently, Cai et al. developed GOx-loaded porous hollow Prussian Blue nanoparticles that were coated with hyaluronic acid via redox-cleavable bond for synergistic cancer starving-like/low-temperature photothermal therapy.15 However, their synergistic agents involved inorganic or macropolymer nanocarriers that brought extra body burden for elimination, which imposes a considerable demand for an easily degradable nanosystem for cancer synergistic therapy.
Metals and metal complexes have long been used in cancer treatment.16–19 For instance, platinum anticancer agents including cisplatin, carboplatin and oxaliplatin appear in a myriad of chemotherapy regimens.20,21 Silver nanoparticles (AgNPs), due to the well-documented antimicrobial properties of Ag ions, have become one of the most fascinating commercialized nanomaterials in the biomedical fields.22 Recently, the therapeutic applications of AgNPs have garnered enormous interest, especially in cancer treatment.23 For instance, many studies proved that Ag ions have cytotoxicity on various cancer cell lines through the induction of oxidative stress, mitochondrial damage, and autophagy.24–26 Soenen et al. found that AgNPs at subcytotoxic concentrations could activate the immune system and cause inflammation via the NFκB pathway.27 Therefore, the exploration of AgNP-based metal ion therapy represents an intriguing avenue towards cancer treatment.
In the light of the degradation of AgNPs into Ag ions in an acidic environment,28 herein, we reported a strategy that combined GOx and silver nanocubes (AgNC) (denoted as AgNC–GOx) to realize synergistic cancer starving-like/metal ion therapy (Scheme 1). The high concentration of intratumoral glucose was oxidized into gluconic acid and toxic H2O2 for the subsequent dissolution of AgNC into Ag ions by the two-step reactions as follows:
 | (1) |
| 2Ag + H2O2 + 2H+ → 2Ag+ + 2H2O | (2) |
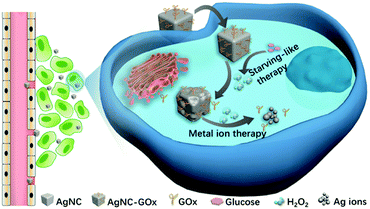 | ||
| Scheme 1 Schematic illustration of the application of AgNC–GOx for synergistic cancer starving-like/metal ion therapy. | ||
This synergy of glucose depletion, H2O2 oxidation and H2O2-accelerated AgNC degradation into toxic Ag ions could achieve a superadditive antitumor effect. Different from the existing research often with inorganic or macropolymer nanocarriers to load synergistic therapeutic agents, AgNC undergoes self-immolation and is completely oxidized into Ag ions at the tumor sites, thus avoiding the potential risks of drug metabolism and easing the patients' extra body burden. In addition, GOx was covalently conjugated to the surface of AgNC, assuring the close contact and adequate reactions between AgNC and H2O2 generated from eqn (1). Furthermore, this two-step reaction happened spontaneously in the acidic pH microenvironment of the tumor, avoiding the complex process of external triggering. Therefore, this synergistic starving-like/metal ion therapy nanosystem could provide a promising treatment paradigm in the battle against cancer.
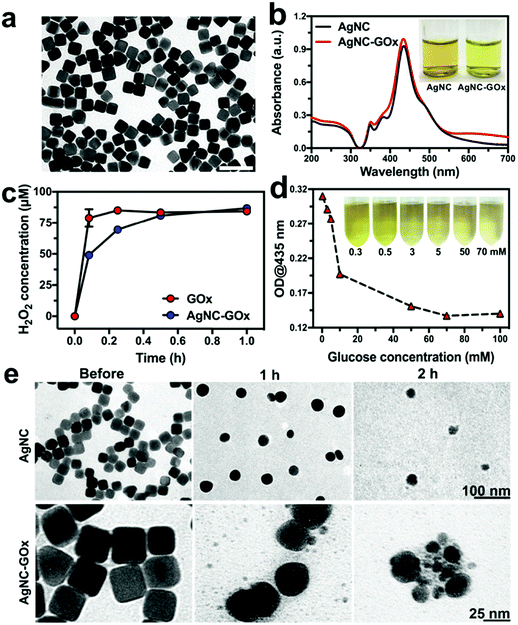
To synthesize the AgNC–GOx, AgNC was firstly fabricated according to a seeded growth method.29 Thioctic acid (TA) was used to modify AgNC through the Ag–S bond. Afterward, the GOx was covalently conjugated with the carboxyl group of TA through the amide bond (Fig. S1, ESI†). During the conjugation process, AgNC remained in cubic shape, indicating its good stability (Fig. S2, ESI†). The as-prepared AgNC–GOx also exhibited the cubic morphology of AgNC with high dispersity and a narrow size distribution at ∼49.2 nm (Fig. 1(a)). Dynamic light scattering (DLS) measurements also provided similar results with TEM imaging, and the polydispersity index (PDI) of AgNC–GOx was 0.267, further confirming its good size homogeneity (Fig. S3(a), ESI†). The UV-visible-Near-Infrared (UV-Vis-NIR) spectra indicated that AgNC–GOx had a characteristic AgNC peak at 435 nm, further validating the stability of AgNC (Fig. 1(b)). As shown in the inset, the AgNC–GOx solution turned brighter yellow than the original solution due to the conjugation of light yellow GOx. As circular dichroism (CD) spectra demonstrated, GOx maintained its secondary structure after conjugation to AgNC, implying the reservation of its catalytic function (Fig. S3(b), ESI†). As shown in Fig. S4(a) (ESI†), the zeta potential of AgNC–TA (−28.1 mV) was much lower than that of AgNC (−10.53 mV) due to the existence of numerous carboxyl groups on its surface. After the conjugation of GOx, the zeta potential of AgNC–GOx changed into −23.3 mV, which suggested that GOx was successfully conjugated with AgNC–TA. Fourier-transform infrared spectroscopy (FTIR) curves and SDS-PAGE further consolidated the stable binding between GOx and AgNC, strongly suggesting a covalent linkage (Fig. S4(b) and S5, ESI†). Using the BCA protein assay and coupled plasma mass spectroscopy (ICP-MS), the loading efficiency of GOx was measured to be 20.5%. As shown in Fig. S6 (ESI†), the GOx of AgNC–GOx was gradually released with the dissolution of AgNC in 1 h.
The sequential chain reactions between AgNC–GOx and glucose were then evaluated in vitro. H2O2 and gluconic acid (reflected by pH) generated in the GOx and glucose mixed solution reached a plateau within 1 h, confirming the excellent catalytic function of GOx (Fig. S7, ESI†). H2O2 could oxidize AgNC into Ag ions, as demonstrated by the gradual decrease in absorption of AgNC at 435 nm (Fig. S8(a), ESI†), which is the premise of silver ion therapy. The rate of decrease in absorption of AgNC in an acidic environment was faster than that at pH 7.0, indicating the promotion effect of acid on the AgNC–H2O2 reaction (Fig. S8(b), ESI†). Moreover, the pure mixing of AgNC and GOx could not cause the rapid decrease in absorption of AgNC, even at high concentrations of GOx, which indicated that covalent conjugation might be the premise of efficient chain reactions between AgNC and GOx (Fig. S8(c), ESI†). H2O2 and acid generated from the AgNC–GOx-catalyzed decomposition reaction increased with glucose concentration, suggesting that GOx retained its catalytic function after conjugation (Fig. 1(c) and Fig. S9, ESI†). Based on the above results, it was reasonable to infer that glucose could accelerate the decomposition of AgNC within AgNC–GOx. As shown in Fig. 1(d), the absorption of AgNC–GOx at 435 nm decreased with the increase in glucose concentration. Meanwhile, the color of the AgNC–GOx solution changed from bright yellow to light green-yellow. To further demonstrate the dissolution of AgNC, transmission electron microscopy (TEM) of the AgNC/H2O2 and AgNC–GOx/glucose solutions was performed. As shown in Fig. 1(e), cubic AgNC in both samples became elliptic and fragmentary 2 h after reaction with H2O2, further verifying the dissolution of AgNC into Ag ions. Similar gradual dissolution of AgNC was also observed inside 4T1 cells during 24 h incubation (Fig. S10, ESI†). These experiments consolidated the synergistic reactions between AgNC–GOx and glucose. According to the literature, the shape of the Ag nanoparticles has no influence on their cytotoxicity to human mesenchymal stem cells.30 Our experiments showed that round-shaped AgNP-GOx underwent similar self-immolative activity to AgNC–GOx in vitro (Fig. S11, ESI†), further confirming that AgNPs with different shapes could perform this synergistic cancer therapy.
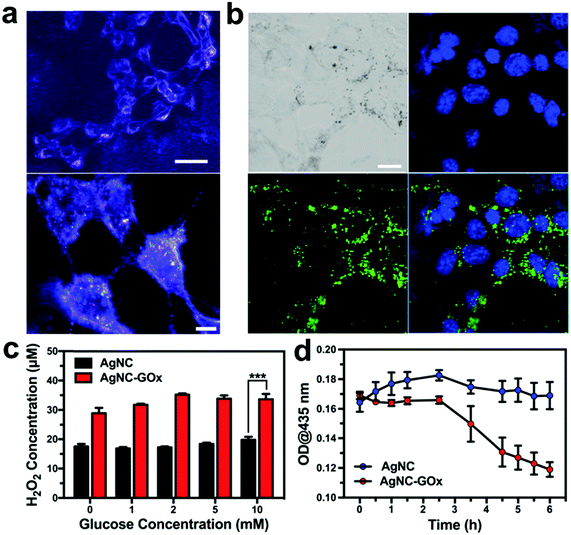
We then investigated the synergistic starving-like/metal ion therapy on 4T1 and A375 tumor cells. The efficient intracellular transport of AgNC via a typical endocytosis process was directly observed under dark-field microscopy (Fig. 2(a)). By conjugation with 5-aminofluorescein (5-AF), confocal laser scanning microscopic (CLSM) imaging further indirectly demonstrated the cellular uptake of AgNC after 6 h incubation (Fig. 2(b)). As shown in Fig. 2(c), the H2O2 generation profile in 4T1 cells treated with AgNC–GOx was positively correlated with glucose concentration in the range of 0–2 mM, while the amount of H2O2 generation in 4T1 cells treated with AgNC was much lower, which demonstrated the GOx-catalyzed glucose decomposition reaction. When the concentration of glucose was higher than 2 mM, H2O2 generation reached a plateau due to the direct oxidization of glucose by H2O2.31 Meanwhile, the absorbance peak of AgNC–GOx at 435 nm inside 4T1 cells decreased over time, indicating that AgNC could be degraded into Ag ions by H2O2 in an intracellular environment (Fig. 2(d)).
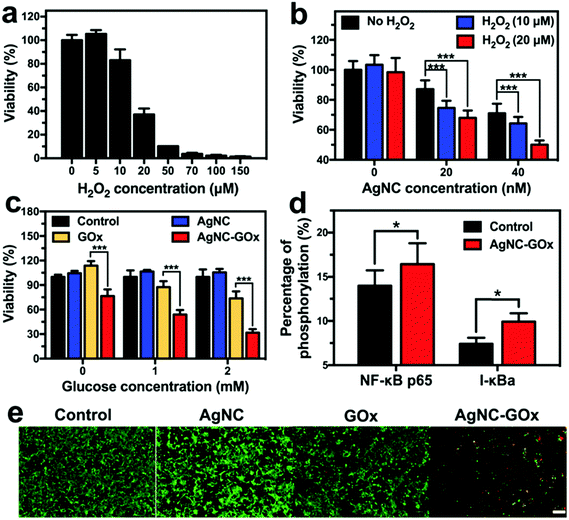
On the one hand, glucose serves as an energy supplier to promote cancerous cell proliferation, so the oxidization of glucose cut off the essential energy supply of cancer cells (Fig. S12(a) and S13(a), ESI†). On the other hand, while a low amount of H2O2 stimulates the proliferation of cancer cells, a high concentration of H2O2 as such causes significant cell death (Fig. 3(a) and Fig. S14(a), ESI†). Therefore, starving-like therapy via the GOx-catalyzed decomposition of glucose into toxic H2O2 produced a strong anticancer effect. Although AgNC could react with intracellular oxidizing agents including H2O2 to produce Ag ions and cause marginal cell death (Fig. S12(b) and S13(b), ESI†), many more Ag ions were generated at higher H2O2 concentrations and caused severe cancer cell death (Fig. 3(b)), thus yielding an enhanced metal ion therapeutic effect. Therefore, the synergistic starving-like/metal ion therapy mediated by AgNC–GOx exhibited a more efficient glucose concentration-dependent cell killing effect than single metal ion therapy mediated by AgNC or starvation-like therapy mediated by GOx (Fig. 3(c) and Fig. S14(b), ESI†). This synergistic therapeutic effect was also superior to that of the AgNC + GOx mixture group, further indicating that covalent conjugation may allow more adequate contact between H2O2 and AgNC (Fig. S12(c), ESI†). The activation of the NFκB pathway in 4T1 cells treated with AgNC–GOx at subcytotoxic concentration was observed (Fig. 3(d)), which was in agreement with previous studies.27 NFκB plays a crucial role in both ROS-mediated cellular stress signaling mediation and the onset of inflammation.32 Therefore, the therapeutic effect of AgNC–GOx might be associated with immune competence. AgNC–GOx induced a similar synergistic therapeutic effect, which was further verified by live/dead cell staining assay (Fig. 3(e) and Fig. S14(c), ESI†).
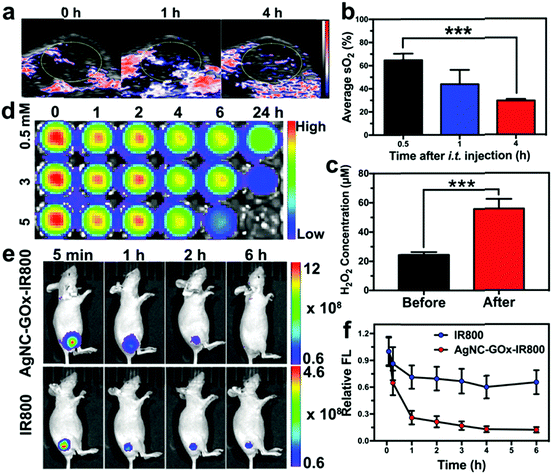
Inspired by the in vitro results, we evaluated the synergistic therapeutic effect of AgNC–GOx on the 4T1 tumor-bearing mice. The GOx-catalyzed glucose decomposition reaction would consume a large amount of oxygen. The intratumoral blood oxygen saturation (sO2) of mice quickly dropped from ∼65% to ∼28% within 4 h post-injection of AgNC–GOx (Fig. 4(a) and (b)), while the mice in the PBS group maintained their original sO2 levels (Fig. S15, ESI†). This rapid decrease in oxygen saturation was due to oxygen consumption during the glucose decomposition reaction catalyzed by GOx. After the injection of AgNC–GOx, the intratumoral H2O2 concentration increased about 2.5-fold (Fig. 4(c)). To visualize the generation of H2O2, a near-infrared (NIR) fluorescence dye IR800 was conjugated to AgNC (Fig. S16, ESI†), which could be degraded by H2O2, followed by fluorescence bleaching (Fig. 4(d)). After intratumoral injection, the fluorescence of AgNC–GOx-IR800 decreased much more quickly than that of AgNC-IR800, further demonstrating the generation of H2O2 in the tumors (Fig. 4(e) and (f)).
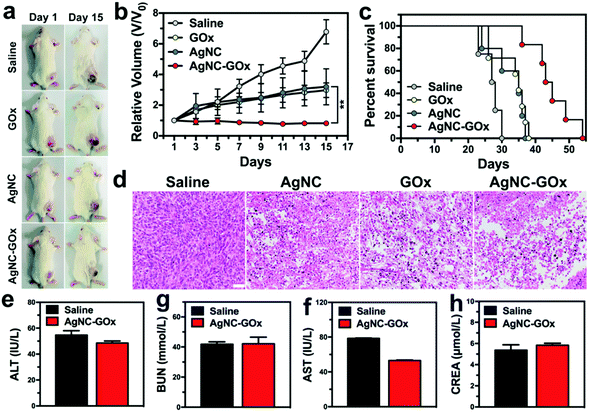
24 h post-injection of GOx or AgNC solutions, the tumor tissues started to ulcerate from the core portion, but the surrounding area kept growing (Fig. 5(a)). Conversely, AgNC–GOx exhibited complete tumor growth inhibition due to the synergistic effect of both the enhanced H2O2 concentration arising from the GOx-catalyzed decomposition of intratumoral glucose and the accelerated generation of Ag ions due to the oxidation by H2O2/H+ (Fig. 5(b) and (c)). Moreover, AgNC–GOx resulted in significant tumor apoptosis and necrosis (Fig. 5(d)), indicating the improved synergistic starving-like/metal ion therapeutic effects, much better than either single treatment alone. Negligible body weight change was observed in each treatment group (Fig. S17, ESI†). After treatment with AgNC–GOx, the normal tissue morphology showed no obvious changes, and the excretion level of serum glutamic-pyruvic transaminase (ALT), glutamic oxalacetic transaminase (AST), creatinine (CREA) and blood urea nitrogen (BUN) in healthy mice were normal (Fig. 5(e–h) and Fig. S18, ESI†). These results indicated that AgNC–GOx does not cause acute toxicity to normal tissues and has good biocompatibility.
In summary, we have successfully constructed a degradable nanosystem by conjugating GOx to AgNC for synergistic cancer starving-like/metal ion therapy. According to our experiments, GOx could assist the conversion of glucose into gluconic acid and toxic H2O2 to kill tumor cells directly and cut off their energy supply, thus achieving more efficient cancer treatment than conventional starving therapy. Moreover, in situ generated H+ and H2O2 can accelerate the oxidization of AgNC into toxic Ag ions for enhanced metal ion therapy. The combination of glucose depletion, toxic H2O2 and Ag ions effectively inhibits tumor growth with minimal adverse effects. Through the two-step reaction, the synergistic treatment paradigm provides a new insight into the application of metal ions-based cancer synergistic treatment, which could be applied to other metal ions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study is financially supported by the National Natural Science Foundation of China (31771036, 51573096, 51703132), the Basic Research Program of Shenzhen (JCYJ20170412111100742, JCYJ20160422091238319), the Guangdong Province Natural Science Foundation of Major Basic Research and Cultivation Project (2018B030308003), the Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (161032), and the China Postdoctoral Science Foundation (2018M633138).Notes and references
- W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed.
- L. Wang, T. Zhang, P. Li, W. Huang, J. Tang, P. Wang, J. Liu, Q. Yuan, R. Bai, B. Li, K. Zhang, Y. Zhao and C. Chen, ACS Nano, 2015, 9, 6532–6547 CrossRef CAS PubMed.
- Y. Chao, L. Xu, C. Liang, L. Feng, J. Xu, Z. Dong, L. Tian, X. Yi, K. Yang and Z. Liu, Nat. Biomed. Eng., 2018, 2, 611–621 CrossRef.
- K. Lu, C. He, N. Guo, C. Chan, K. Ni, G. Lan, H. Tang, C. Pelizzari, Y.-X. Fu, M. T. Spiotto, R. R. Weichselbaum and W. Lin, Nat. Biomed. Eng., 2018, 2, 600–610 CrossRef.
- L. H. Fu, C. Qi, J. Lin and P. Huang, Chem. Soc. Rev., 2018, 47, 6454–6472 RSC.
- O. Ben-Yoseph and B. D. Ross, Br. J. Cancer, 1994, 70, 1131–1135 CrossRef CAS PubMed.
- C. F. Nathan and Z. A. Cohn, J. Exp. Med., 1981, 154, 1539–1553 CrossRef CAS PubMed.
- Q. Liu, A. M. Rauth, J. Liu, K. Babakhanian, X. Wang, R. Bendayan and X. Y. Wu, Pharm. Res., 2009, 26, 2343–2357 CrossRef CAS PubMed.
- W. Zhao, J. Hu and W. Gao, ACS Appl. Mater. Interfaces, 2017, 9, 23528–23535 CrossRef CAS PubMed.
- C. Wang, Y. Ye, G. M. Hochu, H. Sadeghifar and Z. Gu, Nano Lett., 2016, 16, 2334–2340 CrossRef CAS PubMed.
- C. Zhang, D. L. Ni, Y. Y. Liu, H. L. Yao, W. B. Bu and J. L. Shi, Nat. Nanotechnol., 2017, 12, 378–386 CrossRef CAS PubMed.
- K. Chang, Z. Liu, X. Fang, H. Chen, X. Men, Y. Yuan, K. Sun, X. Zhang, Z. Yuan and C. Wu, Nano Lett., 2017, 17, 4323–4329 CrossRef CAS PubMed.
- W. Fan, N. Lu, P. Huang, Y. Liu, Z. Yang, S. Wang, G. Yu, Y. Liu, J. Hu, Q. He, J. Qu, T. Wang and X. Chen, Angew. Chem., Int. Ed., 2017, 56, 1229–1233 CrossRef CAS PubMed.
- J. Li, A. Dirisala, Z. Ge, Y. Wang, W. Yin, W. Ke, K. Toh, J. Xie, Y. Matsumoto, Y. Anraku, K. Osada and K. Kataoka, Angew. Chem., Int. Ed., 2017, 56, 14025–14030 CrossRef CAS PubMed.
- J. Zhou, M. Li, Y. Hou, Z. Luo, Q. Chen, H. Cao, R. Huo, C. Xue, L. Sutrisno, L. Hao, Y. Cao, H. Ran, L. Lu, K. Li and K. Cai, ACS Nano, 2018, 12, 2858–2872 CrossRef CAS PubMed.
- K. H. Thompson and C. Orvig, Science, 2003, 300, 936–939 CrossRef CAS PubMed.
- T. W. Hambley, Science, 2007, 318, 1392–1393 CrossRef CAS PubMed.
- A. Bergamo and G. Sava, Chem. Soc. Rev., 2015, 44, 8818–8835 RSC.
- M. Frezza, S. Hindo, D. Chen, A. Davenport, S. Schmitt, D. Tomco and Q. P. Dou, Curr. Pharm. Des., 2010, 16, 1813–1825 CrossRef CAS PubMed.
- T. C. Johnstone, K. Suntharalingam and S. J. Lippard, Chem. Rev., 2016, 116, 3436–3486 CrossRef CAS PubMed.
- A. M. Florea and D. Busselberg, Cancers, 2011, 3, 1351–1371 CrossRef CAS PubMed.
- B. Calderon-Jimenez, M. E. Johnson, A. R. Montoro Bustos, K. E. Murphy, M. R. Winchester and J. R. Vega Baudrit, Front. Chem., 2017, 5, 6 Search PubMed.
- L. Wei, J. Lu, H. Xu, A. Patel, Z. S. Chen and G. Chen, Drug Discovery Today, 2015, 20, 595–601 CrossRef CAS PubMed.
- S. J. Soenen, W. J. Parak, J. Rejman and B. Manshian, Chem. Rev., 2015, 115, 2109–2135 CrossRef CAS PubMed.
- M. I. Sriram, S. B. Kanth, K. Kalishwaralal and S. Gurunathan, Int. J. Nanomed., 2010, 5, 753–762 CAS.
- S. Gurunathan, J. W. Han, V. Eppakayala, M. Jeyaraj and J. H. Kim, BioMed Res. Int., 2013, 2013, 535796 Search PubMed.
- B. B. Manshian, J. Jimenez, U. Himmelreich and S. J. Soenen, Adv. Healthcare Mater., 2017, 6, 1601099 CrossRef PubMed.
- K. Peynshaert, B. B. Manshian, F. Joris, K. Braeckmans, S. C. De Smedt, J. Demeester and S. J. Soenen, Chem. Rev., 2014, 114, 7581–7609 CrossRef CAS PubMed.
- Q. Zhang, W. Li, C. Moran, J. Zeng, J. Chen, L. P. Wen and Y. Xia, J. Am. Chem. Soc., 2010, 132, 11372–11378 CrossRef CAS PubMed.
- J. Helmlinger, C. Sengstock, C. Groß-Heitfeld, C. Mayer, T. A. Schildhauer, M. Köller and M. Epple, RSC Adv., 2016, 6, 18490–18501 RSC.
- Y. M. Mao, J. Food Process. Preserv., 2016, 41, e12742 CrossRef.
- C. J. Wright, F. Agboke, M. Muthu, K. A. Michaelis, M. A. Mundy, P. La, G. Yang and P. A. Dennery, J. Biol. Chem., 2012, 287, 6230–6239 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8mh00908b |
| ‡ Equal contribution authors. |
| This journal is © The Royal Society of Chemistry 2019 |