A copper(I)-catalyzed asymmetric Mannich reaction of glycine Schiff bases with isatin-derived ketimines: enantioselective synthesis of 3-substituted 3-aminooxindoles†
Jing-Yan
Zhu
,
Wu-Lin
Yang
*,
Yang-Zi
Liu
,
Shao-Jing
Shang
and
Wei-Ping
Deng
 *
*
School of Pharmacy and Shanghai Key Laboratory of New Drug Design, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: ywlecust@gmail.com; weiping_deng@ecust.edu.cn
First published on 20th September 2017
Abstract
A Cu(I)/Ph-Phosferrox complex catalyzed asymmetric Mannich reaction of glycine Schiff bases with isatin-derived ketimines has been described. This strategy can provide direct access to chiral 3-substituted 3-aminooxindoles bearing vicinal quaternary–tertiary carbon stereocenters in high yields (up to 99%), excellent enantioselectivities (up to >99% ee) and moderate to high diastereoselectivities (up to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr).
2 dr).
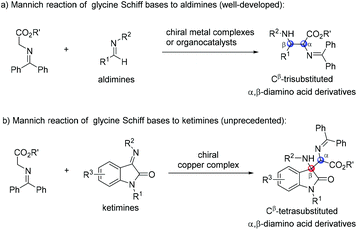
Glycine Schiff bases have been recognized as ideal precursors in the synthesis of optically active unnatural α-amino acids.1 Among them, the asymmetric Mannich reaction of glycine Schiff bases to imines is an efficient methodology for the preparation of enantioenriched α,β-diamino acids.2 In the past few decades, a highly enantioselective Mannich reaction of glycine Schiff bases with aldimines was reported by using chiral metal complexes,3 as well as organocatalysts (Scheme 1a).4 Nevertheless, the asymmetric Mannich reaction of glycine Schiff bases with ketimines to construct Cβ-tetrasubstituted α,β-diamino acids remains a daunting challenge.
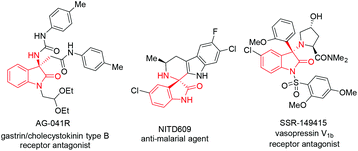
The oxindole skeleton containing a tetrasubstituted carbon stereocenter at the 3-position has long been identified as a “privileged motif” in a large family of bioactive natural products and pharmaceuticals.5 In particular, a chiral 3-substituted 3-aminooxindole scaffold is a key structure present in many drug candidates (Fig. 1), such as the gastrin/cholecystokinin type B receptor antagonist AG-041R,6 the anti-malarial agent NITD609,7 and the orally active nonpeptide vasopressin V1b receptor antagonist SSR-149415.8 In this context, the addition of nucleophiles to isatin-derived ketimines provides a straightforward and powerful route to 3-substituted 3-aminooxindoles. Since the pioneering work reported by Zhou et al. in 2010,9 a variety of catalytic enantioselective addition reactions to isatin-derived ketimines have been developed, including Strecker reactions,10 Mannich reactions,11 aza-Henry reactions,12 aza-Friedel–Crafts reactions,13 Morita–Baylis–Hillman reactions,14 annulation reactions,15 and other asymmetric reactions.16 Nevertheless, the development of a challenging nucleophile in the addition reaction to meet the structural diversity of 3-substituted 3-aminooxindole compounds is still in need. As a part of our continuing interest in developing the asymmetric reaction of glycine Schiff bases,17 we demonstrate herein a copper(I)-catalyzed asymmetric Mannich reaction of glycine Schiff bases with isatin-derived ketimines, affording 3-substituted 3-aminooxindoles with an α-amino acid motif in excellent enantioselectivities (Scheme 1b).
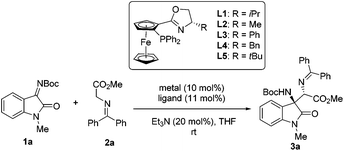
At the outset of this study, isatin-derived N-Boc ketimine 1a was chosen as the model substrate to investigate the feasibility of the Mannich reaction with glycine methyl ester 2a in the presence of a planar-chiral ferrocene P,N-ligand (iPr-Phosferrox L1)/Cu(CH3CN)4BF4 as the catalyst and Et3N as the base in THF at ambient temperature. To our delight, Mannich adduct 3a was obtained as an exclusive product in 92% yield with moderate diastereoselectivity (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 dr) and excellent enantioselectivity (99%/92% ee) (Table 1, entry 1). Screening of other copper salts showed that Cu(CH3CN)4PF6, Cu(CH3CN)4ClO4, and CuOTf gave Mannich adduct 3a in high yields with excellent enantioselectivities (entries 2–4). The yields and ee of adduct 3a declined sharply when using CuOAc, Cu(OTf)2, or Cu(OAc)2 (entries 5–7). Screening of other solvents did not reveal any improvement in diastereoselectivity (see the ESI† for details). Next, we turned our attention to the optimization of ligands, P,N-ligands bearing different substituents on the oxazoline ring provided excellent yields and enantioselectivities (entries 8–11), and Ph-Phosferrox L3 was the optimal ligand in terms of diastereoselectivity (85
61 dr) and excellent enantioselectivity (99%/92% ee) (Table 1, entry 1). Screening of other copper salts showed that Cu(CH3CN)4PF6, Cu(CH3CN)4ClO4, and CuOTf gave Mannich adduct 3a in high yields with excellent enantioselectivities (entries 2–4). The yields and ee of adduct 3a declined sharply when using CuOAc, Cu(OTf)2, or Cu(OAc)2 (entries 5–7). Screening of other solvents did not reveal any improvement in diastereoselectivity (see the ESI† for details). Next, we turned our attention to the optimization of ligands, P,N-ligands bearing different substituents on the oxazoline ring provided excellent yields and enantioselectivities (entries 8–11), and Ph-Phosferrox L3 was the optimal ligand in terms of diastereoselectivity (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 dr, entry 9). The diastereoisomeric ratio was increased to 92
15 dr, entry 9). The diastereoisomeric ratio was increased to 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 when the temperature was decreased to −20 °C (entry 12). Further lowering the temperature to −40 °C resulted in a considerable improvement in diastereoselectivity without the loss of enantioselectivity (97
8 when the temperature was decreased to −20 °C (entry 12). Further lowering the temperature to −40 °C resulted in a considerable improvement in diastereoselectivity without the loss of enantioselectivity (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr, 99% ee, entry 13). The diastereoselectivity decreased slightly when the catalyst loading was reduced to 5 mol% (94
3 dr, 99% ee, entry 13). The diastereoselectivity decreased slightly when the catalyst loading was reduced to 5 mol% (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr, entry 15).
6 dr, entry 15).
| Entry | Metal | Ligand | Yieldb (%) | drc | eed (%) | t (h) |
|---|---|---|---|---|---|---|
| a All reactions were carried out with 0.1 mmol of 1a and 0.11 mmol of 2a in 1.5 mL of THF; CuBF4 = [Cu(CH3CN)4BF4]; CuPF6 = [Cu(CH3CN)4PF6]; CuClO4 = [Cu(CH3CN)4ClO4]; CuOTf = [(CuOTf)2·C6H6]; Cu(OAc)2 = Cu(OAc)2·H2O. b Combined yield of two diastereomers. c Determined by 1H NMR spectroscopy and chiral HPLC analysis. d Determined by chiral HPLC analysis. e Reaction conducted at −20 °C. f Reaction conducted at −40 °C. g Reaction conducted at −78 °C. h 5 mol% catalyst was used. | ||||||
| 1 | CuBF4 | L1 | 92 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 61 |
99/92 | 0.5 |
| 2 | CuPF6 | L1 | 98 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
99/99 | 0.5 |
| 3 | CuClO4 | L1 | 98 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
96/90 | 0.5 |
| 4 | CuOTf | L1 | 99 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
92/92 | 0.5 |
| 5 | CuOAc | L1 | 35 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
14/62 | 1 |
| 6 | Cu(OTf)2 | L1 | 66 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 |
75/85 | 8 |
| 7 | Cu(OAc)2 | L1 | 52 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
2/36 | 24 |
| 8 | CuBF4 | L2 | 98 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
94/84 | 0.5 |
| 9 | CuBF4 | L3 | 98 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
98/55 | 0.5 |
| 10 | CuBF4 | L4 | 98 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
96/86 | 0.5 |
| 11 | CuBF4 | L5 | 98 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
98/85 | 0.5 |
| 12e | CuBF4 | L3 | 99 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99/88 | 1 |
| 13f | CuBF4 | L3 | 98 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
99/94 | 4 |
| 14g | CuBF4 | L3 | 34 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
84/15 | 24 |
| 15f,h | CuBF4 | L3 | 98 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
98/76 | 7 |
After optimization of the reaction conditions, the substrate scope for this catalytic asymmetric Mannich reaction was investigated. Initially, the ester groups of glycine Schiff bases 2 were varied (Table 2). The results indicated that the reactivities and stereoselectivities of the Mannich reaction were highly dependent on the steric hindrance of ester groups. As glycine ethyl ester was used, the yield and the diastereoselectivity were maintained, while the enantioselectivity of the major diastereomer was slightly declined (entry 2), whereas the diastereoselectivities decreased obviously as the steric bulk group iso-propyl or benzyl was introduced (entries 3 and 4). Even more, glycine tert-butyl ester Schiff base 2e was inactive under the optimal conditions due to the steric encumbrance (entry 5).
| Entrya | R | Yieldb (%) | drc | eed (%) | t (h) |
|---|---|---|---|---|---|
| a All reactions were carried out with 0.1 mmol of 1a and 0.11 mmol of 2 in 1.5 mL of THF at −40 °C. b Combined yield of two diastereomers, nr = no reaction. c Determined by 1H NMR spectroscopy and chiral HPLC analysis, nd = not determined. d Determined by chiral HPLC analysis. | |||||
| 1 | Me (2a) | 98 (3a) | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
99/94 | 4 |
| 2 | Et (2b) | 98 (3b) | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
93/nd | 4 |
| 3 | Bn (2c) | 98 (3c) | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
95/68 | 10 |
| 4 | iPr (2d) | 74 (3d) | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
98/45 | 24 |
| 5 | tBu (2e) | nr | nd | nd | 24 |
Subsequently, the potential of this catalytic asymmetric Mannich reaction with a variety of isatin-derived ketimines was further evaluated (Table 3). The results revealed that the N-protecting group of isatin is crucial to the conversion. When ketimines derived from N-benzyl and N-acetyl isatins were introduced into this reaction, the diastereoselectivities were disappointing (entries 1 and 2). Ketimine 1d prepared from isatin without a protecting group at the N1 position also could not give the Mannich adduct (entry 3). Gratifyingly, N-Cbz-1-methyl ketimine 1e also performed well with excellent enantioselectivity (>99% ee), though the diastereoselectivity was slightly lower (entry 4). Subsequently, N-Boc ketimines derived from diverse substituted N-methyl isatins were evaluated. The electronic nature and the position of the substituents on isatin backbones had little influence on the enantioselectivities (>99% ee in most cases), whereas they had an obvious influence on the diastereoselectivities (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45–96
45–96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dr). For example, the 5-OMe-substituted isatin imine 1f gave adduct 3i in excellent diastereoselectivities (96
4 dr). For example, the 5-OMe-substituted isatin imine 1f gave adduct 3i in excellent diastereoselectivities (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dr) (entry 6), while the 6-OMe-substituted substrate 1l only gave 85
4 dr) (entry 6), while the 6-OMe-substituted substrate 1l only gave 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 dr of the product (entry 11). Furthermore, the substrates bearing electron-withdrawing groups at the 6-position all worked well and afforded the products in high yields and stereoselectivities (entries 12 and 13). In contrast, an electron-withdrawing substituent at the 5-position provided the decreased diastereoselectivities (entries 9 and 10). Particularly, ketimine 1i with a fluorine atom at the 5-position led to a desirable outcome of both yield and stereoselectivity (entry 8). In addition, both electron-donating and electron-withdrawing groups at the 7th position of isatins were harmful for the diastereoselectivities (entries 14 and 15).
15 dr of the product (entry 11). Furthermore, the substrates bearing electron-withdrawing groups at the 6-position all worked well and afforded the products in high yields and stereoselectivities (entries 12 and 13). In contrast, an electron-withdrawing substituent at the 5-position provided the decreased diastereoselectivities (entries 9 and 10). Particularly, ketimine 1i with a fluorine atom at the 5-position led to a desirable outcome of both yield and stereoselectivity (entry 8). In addition, both electron-donating and electron-withdrawing groups at the 7th position of isatins were harmful for the diastereoselectivities (entries 14 and 15).
| Entry | R1/R2/R3 | Yieldb (%) | drc | eed (%) | t (h) |
|---|---|---|---|---|---|
| a All reactions were carried out with 0.1 mmol of 1 and 0.11 mmol of 2a in 1.5 mL of THF at −40 °C. b Combined yield of two diastereomers, nr = no reaction. c Determined by 1H NMR spectroscopy and chiral HPLC analysis, nd = not determined. d Determined by chiral HPLC analysis. | |||||
| 1 | Bn/Boc/H (1b) | 55 (3e) | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
87/74 | 24 |
| 2 | Ac/Boc/H (1c) | 90 (3f) | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
98/99 | 22 |
| 3 | H/Boc/H (1d) | nr | nd | nd | 24 |
| 4 | Me/Cbz/H (1e) | 98 (3g) | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
>99/>99 | 1 |
| 5 | Me/Boc/5-Me (1f) | 99 (3h) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
99/nd | 3 |
| 6 | Me/Boc/5-OMe (1g) | 99 (3i) | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
>99/nd | 1.5 |
| 7 | Me/Boc/5-OCF3 (1h) | 98 (3j) | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
>99/>99 | 5.5 |
| 8 | Me/Boc/5-F (1i) | 99 (3k) | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
>99/nd | 3 |
| 9 | Me/Boc/5-Cl (1j) | 90 (3l) | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
>99/>99 | 2.5 |
| 10 | Me/Boc/5-I (1k) | 98 (3m) | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
>99/83 | 2 |
| 11 | Me/Boc/6-OMe (1l) | 96 (3n) | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
>99/>99 | 2 |
| 12 | Me/Boc/6-Cl (1m) | 99 (3o) | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
>99/nd | 2.5 |
| 13 | Me/Boc/6-Br (1n) | 92 (3p) | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
>99/nd | 2 |
| 14 | Me/Boc/7-Cl (1o) | 79 (3q) | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
>99/>99 | 6.5 |
| 15 | Me/Boc/7-Me (1p) | 98 (3r) | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
98/94 | 5.5 |
To explore the practicality of the current methodology, a gram-scale experiment was carried out under optimized conditions. The corresponding product 3m was obtained in 89% yield, 99% ee and 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 dr. The absolute configuration of compound 3m was determined by single crystal X-ray diffraction analysis (Scheme 2).18 Besides, the transformations of the Mannich adduct 3a was explored. Oxindole-based Cβ-tetrasubstituted α,β-diamino acid ester 4 could be obtained via hydrolysis and deprotection in high yield (83%) without the loss of enantioselectivity (99% ee) (Scheme 3a). The Mannich adduct could be conveniently converted to biologically important spirooxindole compounds196 and 720 in excellent enantioselectivities (99% ee) (Scheme 3b).
13 dr. The absolute configuration of compound 3m was determined by single crystal X-ray diffraction analysis (Scheme 2).18 Besides, the transformations of the Mannich adduct 3a was explored. Oxindole-based Cβ-tetrasubstituted α,β-diamino acid ester 4 could be obtained via hydrolysis and deprotection in high yield (83%) without the loss of enantioselectivity (99% ee) (Scheme 3a). The Mannich adduct could be conveniently converted to biologically important spirooxindole compounds196 and 720 in excellent enantioselectivities (99% ee) (Scheme 3b).
In summary, we have developed the diastereo- and enantioselective Mannich reaction of glycine Schiff bases 2 with isatin N-Boc ketimines 1 catalyzed by the Cu(I)/Ph-Phosferrox complex. A wide range of 3-substituted 3-aminooxindole compounds 3 were produced in high yields (up to 99%) with excellent enantioselectivities (>99% ee in most cases) and moderate to high diastereoselectivities (up to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr). Notably, this study disclosed for the first time the enantioselective Mannich reaction of glycine Schiff bases with ketimines furnishing Cβ-tetrasubstituted α,β-diamino acid precursors efficiently. Furthermore, the synthetic utility of this protocol was illustrated by a gram-scale experiment and transformations of Mannich adduct 3a, and biologically important α,β-diamino acid ester 4 and spirooxindole derivatives 6 and 7 were obtained conveniently without the loss of enantioselectivity (99% ee).
2 dr). Notably, this study disclosed for the first time the enantioselective Mannich reaction of glycine Schiff bases with ketimines furnishing Cβ-tetrasubstituted α,β-diamino acid precursors efficiently. Furthermore, the synthetic utility of this protocol was illustrated by a gram-scale experiment and transformations of Mannich adduct 3a, and biologically important α,β-diamino acid ester 4 and spirooxindole derivatives 6 and 7 were obtained conveniently without the loss of enantioselectivity (99% ee).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 21372074 and 21572053).Notes and references
- (a) M. J. O'Donnell, Acc. Chem. Res., 2004, 37, 506 CrossRef PubMed; (b) T. Hashimoto and K. Maruoka, Chem. Rev., 2007, 107, 5656 CrossRef CAS PubMed; (c) S. Shirakawa and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 4312 CrossRef CAS PubMed.
- (a) R. G. Arrayás and J. C. Carretero, Chem. Soc. Rev., 2009, 38, 1940 RSC; (b) R. Narayan, M. Potowski, Z.-J. Jia, A. P. Antonchick and H. Waldmann, Acc. Chem. Res., 2014, 47, 1296 CrossRef CAS PubMed; (c) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS PubMed.
- (a) L. Bernardi, A. S. Gothelf, R. G. Hazell and K. A. Jørgensen, J. Org. Chem., 2003, 68, 2583 CrossRef CAS PubMed; (b) M. M. Salter, J. Kobayashi, Y. Shimizu and S. Kobayashi, Org. Lett., 2006, 8, 3533 CrossRef CAS PubMed; (c) J. Hernández-Toribio, R. G. Arrayás and J. C. Carretero, J. Am. Chem. Soc., 2008, 130, 16150 CrossRef PubMed; (d) D. Shang, Y. Liu, X. Zhou, X. Liu and X. Feng, Chem. – Eur. J., 2009, 15, 3678 CrossRef CAS PubMed; (e) G. Liang, M.-C. Tong, H. Tao and C.-J. Wang, Adv. Synth. Catal., 2010, 352, 1851 CrossRef CAS; (f) J. Hernández-Toribio, R. G. Arrayás and J. C. Carretero, Chem. – Eur. J., 2010, 16, 1153 CrossRef PubMed; (g) K. Imae, K. Shimizu, K. Ogata and S.-i. Fukuzawa, J. Org. Chem., 2011, 76, 3604 CrossRef CAS PubMed; (h) E. Hernando, R. G. Arrayás and J. C. Carretero, Chem. Commun., 2012, 48, 9622 RSC; (i) T. Arai, A. Mishiro, E. Matsumura, A. Awata and M. Shirasugi, Chem. – Eur. J., 2012, 18, 11219 CrossRef CAS PubMed; (j) A. Cayuelas, L. Serrano, C. Nájera and J. M. Sansano, Tetrahedron: Asymmetry, 2014, 25, 1647 CrossRef CAS; (k) X. Huo, R. He, J. Fu, J. Zhang, G. Yang and W. Zhang, J. Am. Chem. Soc., 2017, 139, 9819 CrossRef CAS PubMed.
- (a) T. Ooi, M. Kameda, J.-i. Fujii and K. Maruoka, Org. Lett., 2004, 6, 2397 CrossRef CAS PubMed; (b) A. Okada, T. Shibuguchi, T. Ohshima, H. Masu, K. Yamaguchi and M. Shibasaki, Angew. Chem., Int. Ed., 2005, 44, 4564 CrossRef CAS PubMed; (c) T. Shibuguchi, H. Mihara, A. Kuramochi, T. Ohshima and M. Shibasaki, Chem. – Asian J., 2007, 2, 794 CrossRef CAS PubMed; (d) S. Kobayashi, R. Yazaki, K. Seki and Y. Yamashita, Angew. Chem., Int. Ed., 2008, 47, 5613 CrossRef CAS PubMed; (e) H. Zhang, S. Syed and C. F. Barbas III, Org. Lett., 2010, 12, 708 CrossRef CAS PubMed; (f) J. S. Bandar and T. H. Lambert, J. Am. Chem. Soc., 2013, 135, 11799 CrossRef CAS PubMed; (g) Z. Tao, A. Adele, X. Wu and L. Gong, Chin. J. Chem., 2014, 32, 969 CrossRef CAS; (h) T. Kano, R. Kobayashi and K. Maruoka, Angew. Chem., Int. Ed., 2015, 54, 8471 CrossRef CAS PubMed.
- (a) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS; (b) K. Shen, X. Liu, L. Lin and X. Feng, Chem. Sci., 2012, 3, 327 RSC; (c) R. Dalpozzo, G. Bartoli and G. Bencivenni, Chem. Soc. Rev., 2012, 41, 7247 RSC; (d) G. M. Ziarani, R. Moradi and N. Lashgari, Tetrahedron: Asymmetry, 2015, 26, 517 CrossRef; (e) R. Dalpozzo, Org. Chem. Front., 2017 10.1039/C7QO00446J.
- (a) M. Ochi, K. Kawasaki, H. Kataoka, Y. Uchio and H. Nishi, Biochem. Biophys. Res. Commun., 2001, 283, 1118 CrossRef CAS PubMed; (b) N. Hara, S. Nakamura, M. Sano, R. Tamura, Y. Funahashi and N. Shibata, Chem. – Eur. J., 2012, 18, 9276 CrossRef CAS PubMed.
- M. Rottmann, C. McNamara, B. K. S. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. E. González-Páez, S. B. Lakshiminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler and T. T. Diagana, Science, 2010, 329, 1175 CrossRef CAS PubMed.
- (a) T. Shimazaki, M. Iijima and S. Chaki, Eur. J. Pharmacol., 2006, 543, 63 CrossRef CAS PubMed; (b) G. Decaux, A. Soupart and G. Vassart, Lancet, 2008, 371, 1624 CrossRef CAS PubMed.
- Y.-L. Liu, F. Zhou, J.-J. Cao, C.-B. Ji, M. Ding and J. Zhou, Org. Biomol. Chem., 2010, 8, 3847 CAS.
- For selected examples: (a) Y.-L. Liu and J. Zhou, Chem. Commun., 2013, 49, 4421 RSC; (b) D. Wang, J. Liang, J. Feng, K. Wang, Q. Sun, L. Zhao, D. Li, W. Yan and R. Wang, Adv. Synth. Catal., 2013, 355, 548 CAS; (c) H. Wang, K. Wang, Y. Ren, N. Li, B. Tang and G. Zhao, Adv. Synth. Catal., 2017, 359, 1819 CrossRef CAS.
- For selected examples: (a) Q.-X. Guo, Y.-W. Liu, X.-C. Li, L.-Z. Zhong and Y.-G. Peng, J. Org. Chem., 2012, 77, 3589 CrossRef CAS PubMed; (b) T. Z. Li, X. B. Wang, F. Sha and X. Y. Wu, J. Org. Chem., 2014, 79, 4332 CrossRef CAS PubMed; (c) J. Zhao, B. Fang, W. Luo, X. Hao, X. Liu, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 241 CrossRef CAS PubMed; (d) K. Zhao, T. Shu, J. Jia, G. Raabe and D. Enders, Chem. – Eur. J., 2015, 21, 3933 CrossRef CAS PubMed; (e) O. D. Engl, S. P. Fritz and H. Wennemers, Angew. Chem., Int. Ed., 2015, 54, 8193 CrossRef CAS PubMed; (f) X. Bao, B. Wang, L. Cui, G. Zhu, Y. He, J. Qu and Y. Song, Org. Lett., 2015, 17, 5168 CrossRef CAS PubMed; (g) F. I. Amr, C. Vila, G. Blay, M. C. Muñoz and J. R. Pedro, Adv. Synth. Catal., 2016, 358, 1583 CrossRef CAS; (h) J. Chen, X. Wen, Y. Wang, F. Du, L. Cai and Y. Peng, Org. Lett., 2016, 18, 4336 CrossRef CAS PubMed; (i) L.-J. Zhou, Y.-C. Zhang, F. Jiang, G. He, J. Yan, H. Lu, S. Zhang and F. Shi, Adv. Synth. Catal., 2016, 358, 3069 CrossRef CAS; (j) C. Cheng, X. Lu, L. Ge, J. Chen, W. Cao, X. Wu and G. Zhao, Org. Chem. Front., 2017, 4, 101 RSC; (k) H. Lin, Z. Zhou, J. Cai, B. Han, L. Gong and E. Meggers, J. Org. Chem., 2017, 82, 6457 CrossRef CAS PubMed.
- For selected examples: (a) Y.-H. Wang, Y.-L. Liu, Z.-Y. Cao and J. Zhou, Asian J. Org. Chem., 2014, 3, 429 CrossRef CAS; (b) A. Kumar, J. Kaur, S. S. Chimni and A. K. Jassal, RSC Adv., 2014, 4, 24816 RSC; (c) T. Arai, E. Matsumura and H. Masu, Org. Lett., 2014, 16, 2768 CrossRef CAS PubMed; (d) M. Holmquist, G. Blay and J. R. Pedro, Chem. Commun., 2014, 50, 9309 RSC; (e) B. Fang, X. Liu, J. Zhao, Y. Tang, L. Lin and X. Feng, J. Org. Chem., 2015, 80, 3332 CrossRef CAS PubMed; (f) M. Holmquist, G. Blay, M. C. Muñoz and J. R. Pedro, Adv. Synth. Catal., 2015, 357, 3857 CrossRef CAS; (g) T. Menapara, R. Tak, E. Chinnaraja, R. I. Kureshy, P. Patel and N. H. Khan, ChemistrySelect, 2017, 2, 4063 CrossRef CAS.
- For selected examples: (a) J. Feng, W. Yan, D. Wang, P. Li, Q. Sun and R. Wang, Chem. Commun., 2012, 48, 8003 RSC; (b) M. M. Magraner, C. Vila, R. Cantón, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, Angew. Chem., Int. Ed., 2015, 54, 6320 CrossRef PubMed; (c) M. M. Magraner, C. Vila, A. R. Patiño, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, ACS Catal., 2016, 6, 2689 CrossRef; (d) X. Zhang, J. Zhang, L. Lin, H. Zheng, W. Wu, X. Liu and X. Feng, Adv. Synth. Catal., 2016, 358, 3021 CrossRef CAS.
- For selected examples: (a) F. L. Hu, Y. Wei, M. Shi, S. Pindi and G. Li, Org. Biomol. Chem., 2013, 11, 1921 RSC; (b) X. Zhao, T.-Z. Li, J.-Y. Qian, F. Sha and X.-Y. Wu, Org. Biomol. Chem., 2014, 12, 8072 RSC; (c) A. Kumar, V. Sharma, J. Kaur, N. Kumar and S. S. Chimni, Org. Biomol. Chem., 2015, 13, 5629 RSC; (d) Y. Yoshida, M. Sako, K. Kishi, H. Sasai, S. Hatakeyama and S. Takizawa, Org. Biomol. Chem., 2015, 13, 9022 RSC.
- For selected examples: (a) H. Lv, B. Tiwari, J. Mo, C. Xing and Y. G. Chi, Org. Lett., 2012, 14, 5412 CrossRef CAS PubMed; (b) F. Shi, G.-J. Xing, R.-Y. Zhu, W. Tan and S. Tu, Org. Lett., 2013, 15, 128 CrossRef CAS PubMed; (c) H. Zheng, X. Liu, C. Xu, Y. Xia, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 10958 CrossRef CAS PubMed; (d) X. Han, W.-L. Chan, W. Yao, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2016, 55, 6492 CrossRef CAS PubMed; (e) M. G. Sankar, M. Garcia-Castro, C. Golz, C. Strohmann and K. Kumar, Angew. Chem., Int. Ed., 2016, 55, 9709 CrossRef CAS PubMed.
- For selected examples: (a) T.-Z. Li, X.-B. Wang, F. Sha and X.-Y. Wu, Tetrahedron, 2013, 69, 7314 CrossRef CAS; (b) J. George, B. Sridhar and B. V. S. Reddy, Org. Biomol. Chem., 2014, 12, 1595 RSC; (c) T. Arai, K. Tsuchiya and E. Matsumura, Org. Lett., 2015, 17, 2416 CrossRef CAS PubMed; (d) C. Beceño, P. Chauhan, A. Rembiak, A. Wang and D. Enders, Adv. Synth. Catal., 2015, 357, 672 CrossRef; (e) Q. He, L. Wu, X. Kou, N. Butt, G. Yang and W. Zhang, Org. Lett., 2016, 18, 288 CrossRef CAS PubMed; (f) T. Chen and C. Cai, Org. Biomol. Chem., 2016, 14, 5019 RSC; (g) W. Guo, Y. Liu and C. Li, Org. Lett., 2017, 19, 1044 CrossRef CAS PubMed.
- (a) Y.-H. Shi, Z. Wang, B. Hu, M. Wang, J. S. Fossey and W.-P. Deng, Org. Lett., 2011, 13, 6010 CrossRef CAS PubMed; (b) M. Wang, Z. Wang, Y.-H. Shi, X.-X. Shi, J. S. Fossey and W.-P. Deng, Angew. Chem., Int. Ed., 2011, 50, 4897 CrossRef CAS PubMed; (c) Z. Wang, X. Yu, B. X. Tian, D. T. Payne, W.-L. Yang, Y.-Z. Liu, J. S. Fossey and W.-P. Deng, Chem. – Eur. J., 2015, 21, 10457 CrossRef CAS PubMed; (d) W.-L. Yang, F.-F. Tang, F.-S. He, C.-Y. Li, X. Yu and W.-P. Deng, Org. Lett., 2015, 17, 4822 CrossRef CAS PubMed; (e) W.-L. Yang, Y.-Z. Liu, S. Luo, X. Yu, J. S. Fossey and W.-P. Deng, Chem. Commun., 2015, 51, 9212 RSC; (f) F.-S. He, J.-H. Jin, Z.-T. Yang, X. Yu, J. S. Fossey and W.-P. Deng, ACS Catal., 2016, 6, 652 CrossRef CAS; (g) W.-L. Yang, C.-Y. Li, W.-J. Qin, F.-F. Tang, X. Yu and W.-P. Deng, ACS Catal., 2016, 6, 5685 CrossRef CAS.
- CCDC 1565164 (3m)† contains the supplementary crystallographic data for this paper.
- (a) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed; (b) M. M. Santos, Tetrahedron, 2014, 70, 9735 CrossRef CAS.
- The relative stereochemistry of compound 7 was assigned by 2D-NOSEY spectroscopic analysis, see the ESI† for details.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1565164. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qo00691h |
| This journal is © the Partner Organisations 2018 |