Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Translating solid state organic synthesis from a mixer mill to a continuous twin screw extruder†
Qun
Cao
a,
Joseph L.
Howard
a,
Deborah E.
Crawford
 b,
Stuart L.
James
*b and
Duncan L.
Browne
b,
Stuart L.
James
*b and
Duncan L.
Browne
 *a
*a
aSchool of Chemistry, Main Building, Park Place, Cardiff University, Cardiff, CF10 3AT, UK
bSchool of Chemistry and Chemical Engineering, David Keir Building, Queen's University Belfast, Belfast BT9 5AG, UK. E-mail: dlbrowne@cardiff.ac.uk; s.james@qub.ac.uk
First published on 18th September 2018
Abstract
A study on the translation of a solid-state synthetic reaction from a mechanochemical mixer-mill to a continuous twin-screw extruder is discussed herein. The study highlights some considerations to be made and parameters to be tested in the context of a model fluorination reaction, which is the first organic fluorination to be attempted using extrusion. Upon optimization, which features the first use of grinding auxiliary solids to enable effective synthetic extrusion, the difluorination reaction was successfully translated to the extruder, leading to a 100-fold improvement in Space Time Yield (STY); 29 kg m−3 day−1 in a mixer mill to 3395 kg m−3 day−1 in a twin screw extruder.
Solvent-free or solvent-minimized chemical synthesis is an ideology of sustainable or green chemistry.1 In recent years, mechanochemistry has been explored for its ability to provide solvent-free access to novel polymorphs,2 rapid access to metal–organic-frameworks (MOFs)3 and for its application to organic synthesis.4 Whilst this technique is providing useful discoveries and insights at small scales, scale up of solvent-free processes is less well developed. Developing scalable methods is essential if the potential of solvent-free synthesis for more sustainable manufacturing is to be realised. The scale up of ball-milling mechanochemical approaches has been reported (Knoevenagel condensation up to 300 mmol), but as this method scales, the energy input per unit mass of reactants decreases.5 Indeed, although large scale mills are widely used for processing materials, their applicability to chemical synthesis is yet to be demonstrated. As an alternative to milling, twin-screw extrusion (TSE), used widely for formulation and final form preparation,6 has recently been shown to be successful in the synthesis of MOFs and for organic condensation reactions.7 Herein we describe the translation of an organic fluorination reaction from a mill to an extruder.
Our previous work in a ball-mill demonstrated that mono- and di-fluorination could be carefully controlled in the solid-state using specific conditions and additives. In the presence of a small amount of added liquid (Liquid Assisted Grinding, LAG),8 an improved selectivity for monofluorination versus difluorination was observed.9 Conversely, addition of sodium carbonate as base permitted difluorination within two hours versus 24 hours under solvent conditions.
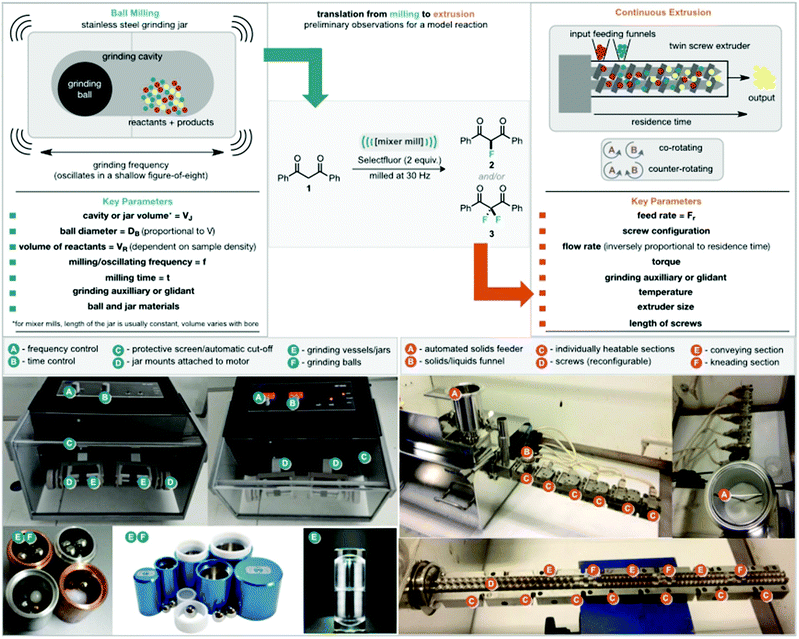
We began by considering extrusion parameters and how these might correlate to milling parameters (Fig. 1). Extrusion typically allows for precise temperature control which is not achievable in an standard mixer mills.7h,11 In the extruder used here, there are six individually addressable heaters along the length of the barrel. Regarding reaction time, in a mixer mill this is set by the operator and normally for the full duration the sample is subjected to impact and shear forces applied by the balls, although resting periods can be included. The reaction time in an extruder is the residence time with 1–2 minutes being typical, during which the reactants are subjected to shear and compression forces. The screws themselves consist of variously configured sections and are customizable. These sections are typically forward-conveying (moving the solids forward), reverse-conveying (retarding the flow of solids) or kneading. Screw elements in the kneading sections can be mutually positioned at angles of 30, 60 or 90° and are responsible for applying the greatest shear during the extrusion process. The screws are normally co-rotating (as here) but can potentially be counter-rotating. In addition to the temperature and screw speed there is also the ability to control the feed input rate using a solid feeder. Finally, the torque of the extrusion process is also a key parameter. The power of the extruder is related to the torque supplied to the screws and when high levels are reached, the extruder cuts-off, highlighting that there are blockages, paste formation or gumming.
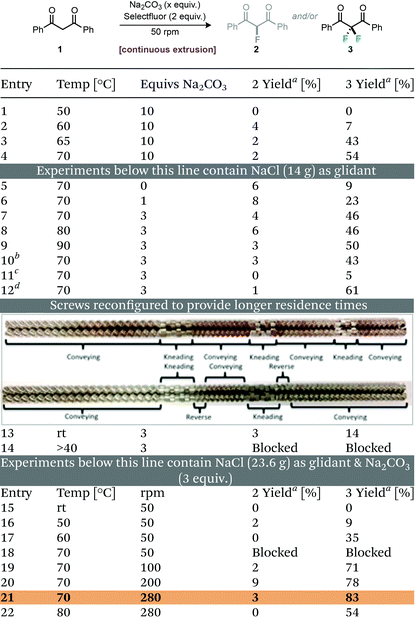
In our initial experiments we looked to explore the difluorination of dibenzoylmethane (1), under basic conditions in the presence of Selectfluor. Using a standard screw-configuration we passed a mixture of materials through the extruder manually at a screw speed of 50 rpm. Initial tests involved manually feeding a solid mixture of dibenzoylmethane (5 mmol, 1.12 g), Selectfluor (10 mmol, 3.54 g), Na2CO3 (50 mmol, 5.30 g) (as base and grinding auxiliary) into the TSE within 2 min at various temperatures (Table 1 entries 1–4). At 50 and 60 °C, there was almost no reaction. However, when the temperature of all sections was increased to 65 °C or 70 °C, the yield of difluorinated product increased to 43% and 54% respectively with only 2% of the monofluorinated product observed. We then explored the addition of sodium chloride rather than an excess of sodium carbonate as grinding auxiliary or glidant.10
The mixture of reagents (20 g), which now contained dibenzoylmethane (5 mmol, 1.12 g), Selectfluor (10 mmol, 3.54 g), Na2CO3 and NaCl was manually fed into the TSE within 2 min (10 g min−1) with a residence time of approximately 1.5 minutes (Table 1, entries 5–12). It can be seen that without the addition of Na2CO3 as base the reaction was slow at 70 °C. By increasing the amount of Na2CO3 from 1 to 3 molar equivalents, the yield of difluorinated product increased to 46% (Table 1, entries 6 & 7). Further increasing the temperature to 80 °C or 90 °C did not increase the yield of desired product, but led to the formation of 2,2-difluoro-1-phenylethanone as a by-product as shown by 19F NMR spectroscopy (see the ESI† for details). With the optimum temperature identified as 70 °C, we then optimised the screw speed, which could affect the degree of mixing and the amount of shear applied, as well as the residence time. It is shown (Table 1, entry 10), that increasing the residence time of the reaction mixture to ca. 2.5 minutes by altering the screw speed to 25 rpm did not improve the yield of difluorinated product. This could be due to reduced shear and mechanical energy input at lower screw speeds. Conversely, when the screw speed was set to 280 rpm, greater mechanical energy was applied, but the residence time was shortened to less than 30 s, which was too short for the completion of reaction (Table 1, entry 11). Thus only 5% difluorinated product was obtained. Pleasingly, we found that the yield of difluorinated product could be increased to 61% by passing the reaction mixture through the extruder three times (Table 1, entry 12 at 50 rpm). These observations led us next to explore modified screw configurations. We switched to a configuration containing only two kneading sections but with reverse conveying segments after each of these sections (Table 1, photographs). These reverse conveying segments can increase the residence time of the reaction mixtures by holding up the material at kneading sections resulting in greater mechanical energy being applied to the reaction mixture.7h During initial attempts to use the modified screw configuration, we found that feeding material with the same speed as the standard screw configuration (∼10 g min−1) resulted in high levels of torque (ca. 12.5 Nm), causing the extruder to cut-off (a safety feature).
To compensate for this, reaction materials were instead fed manually over 8 min (ca. 2.5 g min−1). Results from these conditions are shown in Table 1, entries 13 & 14. Notably, as compared with the standard screw configuration, which gave a residence time of 3 minutes, the modified screw resulted in a residence time of approximately 10 min and an increased yield of the difluorinated product of 14% at room temperature (versus 0% for the previous configuration). Unfortunately, at elevated temperatures, 40–70 °C, blockage occurred at feeding rates of ca. 2.5 g min−1 and 50 rpm screw speed (Table 1, entry 14). This is likely due to the change of rheology of materials at the reverse screw sections where they appear to aggregate and form hard clumps inside the extruder. To prevent this obstruction from occurring the amount of grinding material, NaCl, was increased from 14 g to 23.6 g, providing a total reaction mixture of 30 g which was then manually fed within 8 min (∼3.8 g min−1).11 It was found that increasing the proportion of grinding auxiliary prevented the formation of hard clumps during the extrusion process but led to a decrease in yield due to the dilution of reactants (Table 1, entry 13 vs. entry 15). With the amount of NaCl increased, blockage still occurred at 70 °C and 50 rpm. However, further increasing the screw speed from 50 to 100, 200 and 280 rpm helped break up the clumps inside the extruder and overcame this problem (Table 1, entries 18–21). With an optimal rotation speed of 280 rpm, 83% yield of the difluorinated product was obtained with 3% of the monofluorinated product at 70 °C (Table 1, entry 21).
Previous work from our group reported that liquid-assisted grinding could be used as a powerful tool to control mechanochemically milled organic reactions.9 It was found that milling dibenzoylmethane with Selecfluor (2 equiv.) in the absence of base or any solvent afforded a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of monofluorinated
1 ratio of monofluorinated![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) difluorinated product, whereas the addition of a small amount of acetonitrile (0.125 mL) to the reaction improved monofluorinated selectivity to 50
difluorinated product, whereas the addition of a small amount of acetonitrile (0.125 mL) to the reaction improved monofluorinated selectivity to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
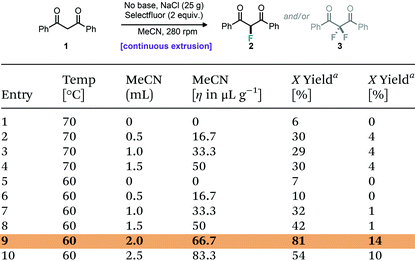
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Herein, we wanted to investigate whether we could observe this phenomenon under continuous flow using a twin screw extruder. Based on the above optimised process for difluorination using TSE, a pre-mixed 30 g solid mixture of dibenzoylmethane (5 mmol, 1.12 g), Selectfluor (10 mmol, 3.54 g), NaCl (23.6 g) and varying amounts of acetonitrile as liquid additive was fed manually into the TSE over an 8 min period (ca. 3.8 g min−1).
1. Herein, we wanted to investigate whether we could observe this phenomenon under continuous flow using a twin screw extruder. Based on the above optimised process for difluorination using TSE, a pre-mixed 30 g solid mixture of dibenzoylmethane (5 mmol, 1.12 g), Selectfluor (10 mmol, 3.54 g), NaCl (23.6 g) and varying amounts of acetonitrile as liquid additive was fed manually into the TSE over an 8 min period (ca. 3.8 g min−1).
Table 2, entry 1 shows that without liquid additive or base, only 6% monofluorinated product was obtained at 70 °C. The addition of acetonitrile (0.5 mL–1.5 mL) improved the yield of monofluorinated product to 30% (Table 2, entries 2–4), but the yield did not very much at different additive quantities at 70 °C. Considering the potential for the small amount of added acetonitrile to be vapourised during the extrusion process at 70 °C (acetonitrile bp 82 °C), a lower temperature of 60 °C was explored. As hoped, the yield of monofluorinated product then increased further with the increasing amount of acetonitrile (Table 2, entries 5–10). When the volume of added acetonitrile was 2 mL (η = 66.7 μL g−1), the conversion of starting material was close to 100% (confirmed by 1H NMR spectroscopy), and the yield of monofluorinated product was 81% with 14% difluorinated product (Table 2, entry 9). Further increasing the acetonitrile to 2.5 mL did not improve the selectivity but lead to a decrease in yield.
| a Yield determined by 19F NMR using trifluorotoluene as internal standard. |
|---|
|
With good conditions identified for producing either monofluorinated or difluorinated products, a continuous process was set up with a twin screw volumetric feeder (Fig. 1) to supply the solid mixture to the twin screw extruder at the desired feeding rate. To demonstrate the potential of using a twin screw extruder under the identified conditions a 90 g reaction mixture of dibenzoylmethane (3.36 g, 15 mmol), Selectfluor (10.62 g, 30 mmol), Na2CO3 (45 mmol, 4.77 g), NaCl (71.3 g) was prepared and fed by the volumetric feeder over 24 min (feeding rate = 3.75 g min−1) into the extruder. Following extrusion of the material at a screw speed of 280 rpm and 70 °C, 45 g of the reacted mixture was collected and washed with water to yield 2.01 g of organic material, 80% of which was the difluorinated product. This material was further purified by recrystallization from cyclohexane to afford a 70% yield of pure difluorinated product (3, 1.370 g). Efforts to use higher feeding rates, e.g. 5 g min−1, led to high torque and blockage. Nonetheless, under these conditions we could obtain a throughput rate of 164 g day−1 for pure difluorinated product, corresponding to a Space Time Yield (STY) of 3395 kg m−3 day−1, whereas, using a mixer mill we were able to produce difluorinated product at a rate of only 5.8 g day−1 with a STY of 29 kg m−3 day−1.9 Similarly, a 90 g reaction mixture of dibenzoylmethane (3.36 g, 15 mmol), Selectfluor (10.62 g, 30 mmol), MeCN (6 mL), NaCl (72 g) were fed by volumetric feeder within 24 min (feeding rate = 3.75 g min−1) to the extruder. Following extrusion of the material at a screw speed of 280 rpm and 60 °C, 45 g of the reacted mixture was collected, purified by washing with water and then dried under high vacuum to afford 90% yield of product (1.789 g, mono![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) difluro = 6
difluro = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), corresponding to a throughput rate (of crude material) of 214 g day−1 and a STY of 4431 kg m−3 day−1.
1), corresponding to a throughput rate (of crude material) of 214 g day−1 and a STY of 4431 kg m−3 day−1.
In conclusion, we have explored the translation of a mixer-mill process into a twin screw extruder, exploring several process parameters and their effects on a fluorination reaction. Notably, the extrusion reaction can be optimized to deliver either mono- or di-fluorinated products and deliver a process that could readily permit kg scale within a few working days without recourse to bulk solvents for the reaction part of the chemical process. The use of grinding auxiliaries in an extrusion process has also been demonstrated and indeed proven necessary in this case for an effective process. The observation that LAG can be used to control selectivity in this organic reaction also appears to translate to the extruder, although the mechanistic origin of this observation remains unclear. Future research will look at broadening the scope of synthetic transformations that can be achieved under extrusion processes.
Notes
Information on the data underpinning the results presented here, including how to access them, can be found in the Cardiff University data catalogue at [http://doi.org/10.17035/d.2018.0055069520].Conflicts of interest
There are no conflicts of interest.Acknowledgements
D. L. B is grateful to the EPSRC for a First Grant (D. L. B. EP/P002951/1), CRD for a studentship award to J. L. H., Queen's University Belfast for a Visiting Research Fellowship and the School of Chemistry at Cardiff University for generous support. S. L. J. is grateful to EPSRC for support (EP/L019655/1).Notes and references
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed; (b) C. Jiménez-González, P. Poechlauer, Q. B. Broxterman, B.-S. Yang, D. am Ende, J. Baird, C. Bertsch, R. E. Hannah, P. Dell'Orco, H. Noorman, S. Yee, R. Reintjens, A. Wells, V. Massonneau and J. Manley, Org. Process Res. Dev., 2011, 15, 900–911 CrossRef; (c) K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025–1074 CrossRef PubMed; (d) B. Rodríguez, A. Bruckmann, T. Rantanen and C. Bolm, Adv. Synth. Catal., 2007, 349, 2213–2233 CrossRef; (e) V. Štrukil, M. D. Igrc, L. Fábián, M. Eckert-Maksić, S. L. Childs, D. G. Reid, M. J. Duer, I. Halasz, C. Mottillo and T. Friščić, Green Chem., 2012, 14, 2462–2473 RSC.
- (a) A. M. Belenguer, G. I. Lampronti, A. J. Cruz-Cabeza, C. A. Hunter and J. K. M. Sanders, Chem. Sci., 2016, 7, 6617–6627 RSC; (b) D. Hasa, E. Miniussi and W. Jones, Cryst. Growth Des., 2016, 16, 4582–4588 CrossRef; (c) A. V. Trask, N. Shan, W. D. S. Motherwell, W. Jones, S. Feng, R. B. H. Tan and K. J. Carpenter, Chem. Commun., 2005, 880–882 RSC; (d) Y. Zhou, F. Guo, C. E. Hughes, D. L. Browne, T. R. Peskett and K. D. M. Harris, Cryst. Growth Des., 2015, 15, 2901–2907 CrossRef.
- (a) D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Chem. Sci., 2015, 6, 1645–1649 RSC; (b) T. Friščić, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC; (c) T. Friščić, J. Mater. Chem., 2010, 20, 7599–7605 RSC; (d) P. J. Beldon, L. Fábián, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Angew. Chem., Int. Ed., 2010, 49, 9640–9643 CrossRef PubMed; (e) K. Užarević, T. C. Wang, S. Y. Moon, A. M. Fidelli, J. T. Hupp, O. K. Farha and T. Friščić, Chem. Commun., 2016, 52, 2133–2136 RSC; (f) M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef.
- (a) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC; (b) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, a G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC; (c) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed; (d) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC; (e) J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef PubMed; (f) D. Tan and T. Friščić, Eur. J. Inorg. Chem., 2018, 2018, 18–33 CrossRef; (g) O. Eguaogie, J. S. Vyle, P. F. Conlon, M. A. Gîlea and Y. Liang, Beilstein J. Org. Chem., 2018, 14, 955–970 CrossRef PubMed; (h) J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC.
- (a) A. Stolle, R. Schmidt and K. Jacob, Faraday Discuss., 2014, 170, 267–286 RSC; (b) R. G. Blair, K. Chagoya, S. Biltek, S. Jackson, A. Sinclair, A. Taraboletti and D. T. Restrepo, Faraday Discuss., 2014, 170, 223–233 RSC; (c) D. W. Peters and R. G. Blair, Faraday Discuss., 2014, 170, 83–91 RSC; (d) X. Ma, G. K. Lim, K. D. M. Harris, D. C. Apperley, P. N. Horton, M. B. Hursthouse and S. L. James, Cryst. Growth Des., 2012, 12, 5869–5872 CrossRef.
- D. E. Crawford and J. Casaban, Adv. Mater., 2016, 28, 5747–5754 CrossRef PubMed.
- (a) S. A. Ross, D. A. Lamprou and D. Douroumis, Chem. Commun., 2016, 52, 8772–8786 RSC; (b) H. G. Moradiya, M. T. Islam, N. Scoutaris, S. A. Halsey, B. Z. Chowdhry and D. Douroumis, Cryst. Growth Des., 2016, 16, 3425–3434 CrossRef; (c) D. Daurio, C. Medina, R. Saw, K. Nagapudi and F. Alvarez-Núñez, Pharmaceutics, 2011, 3, 582–600 CrossRef PubMed; (d) C. Medina, D. Daurio, K. Nagapudi and F. Alvarez-Núñez, J. Pharm. Sci., 2010, 99, 1693–1696 CrossRef PubMed; (e) D. E. Crawford, S. L. James and T. McNally, ACS Sustainable Chem. Eng., 2017, 6, 193–196 CrossRef; (f) D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Chem. Sci., 2015, 6, 1645–1649 RSC; (g) D. E. Crawford, L. A. Wright, S. L. James and A. P. Abbott, Chem. Commun., 2016, 52, 4215–4218 RSC; (h) D. E. Crawford, C. K. G. Miskimmin, A. B. Albadarin, G. Walker and S. L. James, Green Chem., 2017, 19, 1507–1518 RSC; (i) V. Isoni, K. Mendoza, E. Lim and S. K. Teoh, Org. Process Res. Dev., 2017, 21, 992–1002 CrossRef.
- (a) T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418–426 RSC; (b) T. Friščić, A. V. Trask, W. Jones and W. D. S. Motherwell, Angew. Chem., Int. Ed., 2006, 45, 7546–7550 CrossRef PubMed; (c) N. Shan, F. Toda and W. Jones, Chem. Commun., 2002, 2372–2373 RSC.
- (a) J. L. Howard, Y. Sagatov, L. Repusseau, C. Schotten and D. L. Browne, Green Chem., 2017, 19, 2798–2802 RSC; (b) J. L. Howard, Y. Sagatov and D. L. Browne, Tetrahedron, 2018, 74, 3118–3123 CrossRef; (c) J. L. Howard, W. Nicholson, Y. Sagatov and D. L. Browne, Beilstein J. Org. Chem., 2017, 13, 1950–1956 CrossRef PubMed.
- The term ‘grinding auxiliary’, ‘grinding agent’ or ‘grinding material’ is often used in mechanochemistry to describe a material that is added to a reaction vessel to prevent paste or gum formation, and permit efficient mass transfer within the reaction mixture. The term ‘glidant’ is used in to refer to materials added to improve the flowability of a powder or mixture of interest to help it ‘glide’ through the extruder, we use these terms interchangeably in this manuscript.
- For some examples of temperature monitoring and modelling under milling conditions see: (a) K. Užareić, N. Ferdelji, T. Mrla, P. A. Julien, B. Halasz, T. Friščić and I. Halasz, Chem. Sci., 2018, 9, 2525–2532 RSC; (b) H. Kulla, S. Haferkamp, I. Akhmetova, M. Röllig, C. Maierhofer, K. Rademann and F. Emmerling, Angew. Chem., Int. Ed., 2018, 57, 5930–5933 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterization data. See DOI: 10.1039/c8gc02036a |
| This journal is © The Royal Society of Chemistry 2018 |