Blue-shifting tuning of the selective reflection of polymer stabilized cholesteric liquid crystals†
Kyung
Min Lee
ab,
Vincent P.
Tondiglia
ab,
Nicholas P.
Godman
ab,
Claire M.
Middleton
ab and
Timothy J.
White
 *a
*a
aAir Force Research Laboratory, Materials and Manufacturing Directorate, Wright-Patterson Air Force Base, Ohio 45433-7750, USA. E-mail: Timothy.White.24@us.af.mil; Tel: +1-937-255-9551
bAzimuth Corporation, 4027 Colonel Glenn Hwy, Beavercreek, Ohio 4543, USA
First published on 8th August 2017
Abstract
We report on electrically-induced, large magnitude (>300 nm), and reversible tuning of the selective reflection in polymer stabilized cholesteric liquid crystals (PSCLCs) prepared from negative dielectric anisotropy nematic liquid crystal hosts. The electrically-induced blue shift in the selective reflection of the PSCLCs is distinguished from our prior reports of bandwidth broadening and red-shifting tuning of the selective reflection in PSCLCs. The dominant factor in delineating the electro-optic response of the PSCLCs detailed here are the preparation conditions. Specifically, long exposure to UV intensity exceeding 250 mW cm−2. Other factors are shown to contribute to the response, including the type and concentration of photoinitiator.
Introduction
The cholesteric liquid crystal phase self-organizes into a helicoidal structure.1,2 The periodicity of this phase produces a selective reflection, with the center wavelength (λ) and bandwidth (Δλ) defined as λ0 = navg × P0 and Δλ = Δn × P0, where navg is the average refractive index of the liquid crystal, P0 is the cholesteric pitch length, and Δn is the birefringence of the liquid crystal media. A simple way to prepare the CLC phase is to add a chiral dopant into a nematic liquid crystal mixture. Through chirality transfer, the pitch length (reflection wavelength) of the CLC is readily adjusted by the concentration of the chiral dopants. The selective reflection of these materials has been subject to popular use as temperature sensors and “mood” rings. Ongoing research efforts continue to examine these materials targeting applications ranging from displays to apparel.Dynamic control of the selective reflection of cholesteric liquid crystals has been and continues to be a topic of intense research. Various external stimuli have been used including thermal, optical, and electrical inputs.3–6 Optical control via application of an electric field is the most desirable for many applications. Since the work of Yang and coworkers, polymer stabilization of cholesteric liquid crystals has been recognized as a means to extend or enhances the electro-optic response of these materials.7–13 Dynamic electro-optic responses, such as electrical control of bandwidth,11–14 reflection wavelength position (tuning),15–17 or scatter,18 in PSCLCs formulated with nematic liquid crystal hosts exhibiting negative dielectric anisotropy (−Δε) have been reported. Control studies have been undertaken in nematic and twisted nematic orientations to confirm the importance of polymer stabilization.12–14 These examinations indicate that the polymer stabilizing network is delocalized by application of a DC bias and the direction of the displacement is determined by the polarity of the DC bias.12,13 A subsequent study has confirmed that the polymer stabilizing network formed in the presence of the low-molar mass CLC mixture retains a “structural chirality” that is strong enough that it can overrule the chemically-derived chirality transfer that occurs within low-molar mass CLC mixtures.14 The potential to utilize these electro-optic responses as smart windows have been recently subject to examination.19
Electro-optic experiments indicate strong correlation between the magnitude of the response with the ion concentration of the host. Commercial liquid crystal mixtures contain ionic impurities (109–1014 ions per cm3), attributable to synthetic and/or purification steps (catalysts, salts, moisture, and dust), alignment layers,20 and/or degradation of the LC molecules.21–24 We have proposed that application of a DC bias to PSCLCs exhibiting “structural chirality” primarily act upon the native ionic impurities in the mixture that are trapped on or within the polymer stabilizing network.12,14 Due to the polarity of the DC bias and informed by the control experiments detailed hereto, we have hypothesized that the polymer network is subject to a directional electromechanical force that distorts the structural chirality.12,13,17 This deformation then affects the anchoring of the low-molar mass CLC mixture resulting in broadening of the reflection band due to nonuniformity in the pitch length of the CLC across the cell gap. To date, employing Δε < 0 nematic liquid crystal hosts is thought to be critical in enabling this mechanism as the low-molar mass liquid crystalline molecules do not reorient at low to moderate field strengths.
Our recent investigations have focused on electrically-induced tuning in PSCLCs prepared from Δε < 0 nematic liquid crystals with comparatively higher polymer concentrations (up to 20 wt%).15 Due to the large polymer concentration employed in our initial work, the optical properties of the PSCLCs both before and during reflection wavelength tuning exhibit substantial haze and poor transmission. Our recent studies have yielded devices with large magnitude and high optical quality reflection wavelength tuning in PSCLCs also formulated with Δε < 0 nematic liquid crystal hosts by employing polymer concentrations ranging from 3.5–10 wt%. Confocal imaging confirmed that the tuning is a result of nonuniform pitch displacement across the cell gap.17
In this contribution, we report on the realization of yet another distinct electro-optic response in these materials systems. The selective reflection of the PSCLCs examined here are blue-shifting upon application of a DC field. We report a systematic examination of contributing factors influencing the response including the polymer concentration as well as the type and concentration of photoinitiator. Several commercial photoinitiators and various exposure conditions (UV intensity and curing time) are employed and complimented with characterization of the electro-optic response, polymerization kinetics, and ion concentration.
Results
We report here on blue-shifting tuning of the selective reflection of polymer stabilized cholesteric liquid crystals (PSCLCs), the unexpected result of adjusting the exposure conditions during the preparation of these materials. The blue-shifting of the selective reflection of the PSCLCs that is the focus of this manuscript is illustrated in Fig. 1a and Video 1 (ESI†). The mixture is formulated such that the selective reflection emanating from the CLC phase is centered at 700 nm (red/IR). Upon application of DC field at strengths ranging from 0–35 V DC, the minimum in the reflection of these materials is shifted to 430 nm. The correlation between the applied field and the center of the reflection wavelength is plotted in Fig. 1b. Insets into Fig. 1b are photographs of the color of the selective reflection. Reflection wavelength tuning is observed across the entirety of the visible region of the electromagnetic spectrum. As evident in Video 1 (ESI†), the blue shift in the reflection notch is completely reversible and the optical properties restore upon removal of the DC field.The chemical structures of the photoinitiators, liquid crystal monomers, and chiral dopants employed in this examination are shown in Scheme 1. The sample examined in Fig. 1 was formulated by mixing two right handed chiral dopants, R811 and R1011, with the negative dielectric anisotropy (Δε < 0) nematic liquid crystal MLC-2079 (Δn of 0.15 and Δε of −6.1). A photoinitiator (1 wt% Irgacure 369) and monomer (6 wt% RM82) were added to this formulation, and the sample was polymerized with 365 nm UV light at 700 mW cm−2 intensity for 30 min. It should be noted that all samples were rotated during the UV exposure process to limit heat accumulation (thermal polymerization). This mixture is identical in composition to our previous examinations12,14,17,25,26 prepared under different exposure conditions (typically 100 mW cm−2 intensity for 3 min), in which the selective reflection of the PSCLC red-shifts to applied DC field.17 As illustrated in Fig. 2, the intensity and duration of UV light exposure are the primary variable to distinguish whether this formulation exhibits red-shifting tuning or blue-shifting tuning to an applied DC field. In Fig. 2a, the samples are prepared with 250 mW cm−2 of 365 nm light. The duration of the photopolymerization is increased from 3 min to as long as 30 min. For this particular composition, the PSCLC exhibits red-shifting tuning if polymerized for 3 min, symmetric bandwidth broadening as in ref. 14 if polymerized for 5 min or 10 min, and blue-shifting tuning similar to Fig. 1 if polymerized for 15–30 min. In Fig. 2b, the samples were prepared with UV exposures of 700 mW cm−2 of 365 nm light. At this intensity, the selective reflection of the PSCLC red-shifts to an applied DC field in samples prepared with exposure times less than 5 min and blue-shifts to an applied DC field in samples prepared with exposure times of 10 min or more.
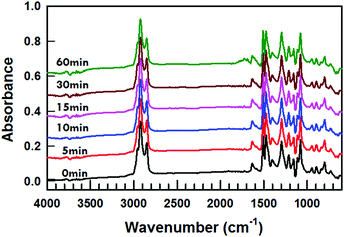
Liquid crystal molecules can degrade to UV light.21–24Fig. 3 presents FTIR spectra of the MLC-2079 at the exposure of 700 mW cm−2 UV light for 0 min to 60 min. The mixture was sandwiched between two potassium bromide (KBr) plates and was rotated at an angular velocity of 2 Hz while subjected to UV light. The only noticeable change in FTIR spectra is the development of a small peak centered around 1700 cm−1 after 60 min exposure. However, blue shifting tuning is observed after 15 min of exposure of 700 mW cm−2 365 nm UV in Fig. 2b and the FTIR spectra show no discernable degradation of the MLC-2079 at the same UV exposure condition.
The formulation employed here incorporates an exceptionally high concentration of photoinitiator relative to the monomer concentration as well as a very high light intensity. It is expected that the photopolymerization reaction is complete in 3 min or less.11,12,14,17 To confirm this assumption and inform the discussion herein, we examined the photopolymerization kinetics with real-time infrared spectroscopy, monitoring the acrylate double bond conversion as a function of time over the range of UV intensities (70–350 mW cm−2) examined in Fig. 4. To obtain the acrylate double bond conversion, 15 μm thick glass rod spacers are added into the mixture to prevent the shrinkage of the mixture during photopolymerization. The acrylate bond conversion was monitored at 986 cm−1 and 1636 cm−1.27–29 The variation in the peak area at 986 cm−1 was used to calculate conversion, as:
 | (1) |
Recently, we have reported on the photosensitivity of the electro-optic response that is observed specifically when morpholino-keto type initiators such as Irgacure 369 or 907 are used to prepare the polymer stabilizing networks.25 The association of the electro-optic response with the photoinitiator evident in this prior work could also be a contributor to the dependence revealed here. To develop this correlation, samples were prepared over a wide range of I-369 concentrations (from 0.1–5 wt%) and were exposed to 250 mW cm−2 of 365 nm light for 30 min. As evident in Fig. 5, the application of a DC field to a PSCLC prepared with 0.1 wt% I-369 exhibits red-shifting tuning. The samples prepared with 0.5–5 wt% I-369 all exhibit blue-shifting tuning. This indicates that the concentration of I-369 is a strong contributor and can even define the electro-optic response of a given composition.
To further isolate the correlation, we prepared PSCLCs of identical composition but replaced I-369 with the common photoinitiator Irgacure 651 (DMPA, I-651). I-651 does not contain a polar morpholino unit (Scheme 1). In a prior examination, PSCLCs prepared with I-651 did not exhibit any photosensitivity to UV irradiation.25 As evident in Fig. 6a, PSCLCs prepared with 1 wt% I-651 and polymerized from 5–60 min all exhibit red-shifting tuning. Further, as evident in Fig. 6b, PSCLCs prepared with concentrations ranging from 0.1–5 wt% I-651 also exhibit red-shifting tuning to applied DC fields. Thus, evident in the direct comparison presented in Fig. 6c, the prevalence of blue-shifting tuning in PSCLCs may be strongly influenced and even enabled by the use of I-369 as photoinitiator.
The ion density of selected mixtures was examined, to illustrate the contribution of the photoinitiator to this important variable. The ion density was measured with the so-called “transient current method”, with 1 Vpeak AC applied at 3 Hz.25 The ion density was then calculated using the triangle method. Ion density values are summarized in the scatter plot presented in Fig. 7a. The liquid crystal MLC-2079 has an average ion density of 2.3 × 1013 ions per cm3. For reference, the common liquid crystal mixture E7 has an order of magnitude larger ion density, at 2.1 × 1014 ions per cm3. Adding the photoinitiator I-369 increases the concentration, as can be seen in Fig. 7a-ii–v. Comparatively, the photoinitiator I-651 does not appear to strongly affect the ion density measured in MLC-2079 (Fig. 7a-vi). Similar to our prior report,25 the chemically similar photoinitiator I-907 can also result in a sizable increase in ion density in Fig. 7a-vii. As apparent in Fig. 7b, UV light exposure increases the ion density of MLC-2079. Notably, adding I-651 to MLC-2079 does not strongly affect the photoinduced change in ion density.
Per the data in Fig. 5–7, it seems clear that the blue-shifting tuning reported here is largely related to PSCLCs prepared with I-369 or related photoinitiators. From Fig. 4, we know that in the conditions employed here, the acrylate groups of the RM82 monomer are almost completely converted in approximately 10 seconds regardless of the initiator concentration. However, the data in Fig. 7 indicate that the photoinitiator has a strong influence on both the starting and post-irradiation ion densities. Prior studies have indirectly confirmed that the polymer stabilizing network maintains a “structural chirality” that can be distorted.14 We have proposed that ions, embedded within or attached onto the polymer stabilizing network, sensitize the polymer stabilizing network to a DC bias (which has a polarity).
To confirm that this influence extends to a range of monomer concentrations as well as types, Fig. 8a reports the electro-optic response of PSCLCs prepared with 250 mW cm−2 of 365 nm light for 30 min exposure of mixtures including 1 wt% I-369 with 2.5–8 wt% RM82. As in our prior examinations, polymerization of just 2.5 wt% RM82 results in a network that is easily damaged by applied DC field and is not shown here. From Fig. 8a, it is clear that despite an increase in monomer concentration from 4–8 wt%, all samples exhibit blue-shifting tuning and maintain similar relationship between reflection wavelength and applied field. Blue-tuning similar to that observable in the samples prepared from achiral liquid crystal monomer RM82 (Fig. 1a and Fig. 8a) is observable in formulations prepared with other chiral (SLO4151, Fig. 8b) and achiral (RM257, Fig. 8c) liquid crystal monomers. Thus, the chirality of the liquid crystal monomer nor the composition of the achiral liquid crystal monomer are distinguishing factors in realizing blue shifting tuning in PSCLCs.
Similarly, the blue-shifting tuning is not explicitly dependent on the use of MLC-2079 as the nematic liquid crystal host. We formulated and prepared a PSCLC with the nematic liquid crystal MLC-2037. This liquid crystal MLC-2037 has a birefringence (Δn) of 0.0649 and a dielectric anisotropy (Δε) of −3.1. The reduction in the birefringence reduces the bandwidth to 40 nm. As evident in Fig. 9a, full spectrum tuning (>350 nm) is observed that also includes the entire visible region of the electromagnetic spectrum. The blue-shifting tuning of the selective reflection is summarized in Fig. 9b.
Discussion
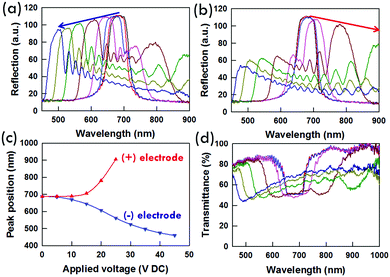
Why do some PSCLC samples exhibit bandwidth broadening, other samples exhibit red-shifting tuning, and still other samples exhibit blue-shifting tuning? To further elucidate the nature of the electro-optic response in the PSCLCs examined here, reflection spectra were collected and are presented in Fig. 10a and b. The direction (positive or negative) of the applied DC bias was reversed in the two measurements. Comparing the spectra in Fig. 10a and b confirms that as in our previous examinations, the reflection spectra are sensitive to the direction of the DC bias.17 In the case of Fig. 10a, the reflection data are taken as a function of voltage with negative bias (e.g. negative electrode nearest white light probe). The maximum reflectivity, arising from pitches near the surface, indicates that the blue-shifting side of the reflection is attributable to movement towards the negative electrode. Fig. 10b presents reflection spectra taken as a function of voltage with positive bias (e.g. positive electrode nearest the white light probe). Here, the maximum reflectivity is red-shifting and quickly moves out of the spectral sampling region. However, a smaller blue-shifting peak is evident that matches the position reported (in Fig. 10a) for these voltages in Fig. 10b. From the shape of the reflection (and transmission in Fig. 10d) spectra, the pitch clearly is heterogeneously distributed across the cell gap.It has been clear from similar experiments that the symmetric broadening of the reflection is attributable to heterogeneity in the pitch across the sample thickness.12,13 We believe a similar heterogeneity is the source of the “tuning” we have reported previously17 as well as the manifestation detailed here (summarized in Fig. 10c). In the set of conditions reported in this manuscript, the pitch compresses near the negative electrode and seems to expand near the positive electrode. Evident in the transmission spectrum reported in Fig. 10d, it seems clear that the majority of pitches are contracting in length and that many of them are doing so in a regular fashion.
While it is generally accurate to describe the manifestation of the electro-optic response as red-shifting, blue-shifting, or bandwidth broadening, the distinguishing feature discussed hereto as well as in part in our prior efforts, is the preparation conditions. Variables such as the type and concentration of photoinitiator, monomer concentration, and exposure conditions affect the composition, homogeneity (in crosslink density or inclusion of photoinitiator fragments) as well as potentially the morphology of the stabilizing polymer network. Further, the preparation conditions also seem to strongly influence the sensitivity of the network to ion-mediated electromechanical deformation.
In this way, it makes sense that the manifestations of the electro-optic response reported here and in our prior studies can be observed in a single formulation. In fact, the transmission spectra in Fig. 10d show as much – in this particular sample, initially symmetric bandwidth broadening is observed followed by spectra in which distinct reflection notches are split (red and blue) at which time the depth and position of the red-shifting notch (in this instance) are no longer identifiable at higher applied DC voltages, and only the blue-shifting notch is observed.
The electro-optic response of samples prepared with morpholino-type initiators (I-369, I-907) are particularly sensitive to preparation conditions. Detailed study is necessary to further elucidate the role of this functional group in enhancing and distinguishing the electro-optic response in the PSCLCs examined to date. At the initiator concentrations and light intensity used here, the number of initiating radicals is exceptionally high, resulting in a comparatively large number of initiating sites that incorporate this moiety into the polymer networks. With a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 wt
6 wt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt ratio of initiator to monomer in most of these and our prior studies, the kinetic chain length of the polymer stabilizing liquid crystalline network will be low. The comparatively larger concentration of these functional groups could act as ionic contaminants (and traps). Further, the residual initiator could be post-functionalizing the polymer network through back-biting reactions during the extended irradiation conditions. This would explain the sensitivity to this particular type of initiator as well as the time dependence.
wt ratio of initiator to monomer in most of these and our prior studies, the kinetic chain length of the polymer stabilizing liquid crystalline network will be low. The comparatively larger concentration of these functional groups could act as ionic contaminants (and traps). Further, the residual initiator could be post-functionalizing the polymer network through back-biting reactions during the extended irradiation conditions. This would explain the sensitivity to this particular type of initiator as well as the time dependence.
Furthermore, from Fig. 7, it is clear that the relative change in ion density with UV light exposure is commensurate in comparing MLC-2079 and MLC-2079 mixed with photoinitiator – indicating that although an ionic event is observable in the host formulation, our hypothesis is that the very high light intensity is producing a distinctive polymer network that may be decorated with additional initiator fragments. The polar heterocycle morpholino units in I-369 are known to be effective ion traps (particularly to cations),30–32 due to the sterically constrained shape of the amino heterocycle. The resulting network seems to be particularly sensitive to positive ionic species, as per our prior examinations.
Materials and methods
Preparation of Δε < 0 PSCLCs
Alignment cells were prepared from ITO-coated glass slides (Colorado Concepts). The glass slides were cleaned and spin-coated with a polyimide alignment layer. The alignment layers were rubbed with a cloth, and the cell was constructed to yield planar alignment conditions. The cell gap was controlled by mixing 15 μm thick glass rod spacers into an optical adhesive. Samples were prepared by formulating 0.1–5 wt% of the photoinitiator Irgacure 369, two right-handed chiral dopants (5 wt% R1011 and 5 wt% R811, Merck), 0–10 wt% of an achiral liquid crystal monomer (RM82, Merck), and −Δε achiral nematic liquid crystal (MLC-2079, Merck). The polymer stabilizing network was formed within the samples by photoinitiated polymerization with 50–700 mW cm−2 of 365 nm light (Exfo) for 1–120 min. To ensure homogeneous curing conditions and also prevent heat accumulation, the cell was rotated at an angular velocity of 2 Hz during polymerization. All materials were used as received without any purification.Experimental setup and measurements
Transmission spectra were collected with a fiber optic spectrometer. Unless otherwise mentioned, the right handed circularly polarized (RH CPL) light probe was used. Transmission spectra were collected before, during, and after application of dc fields with the scanning rate of 1 V s−1 or directly applied to the target voltage.The ion density of the mixtures was measured with a commercial instrument from LC Vision in the homeotropic alignment cells. During the experiment, the samples were subjected to a 1 Vp bias at a frequency of 3 Hz. The ion density values are the average of five measurements. There is no switching response of liquid crystals because the applied alternating voltage (1 Vp) is below the threshold voltage. The ion density is calculated from current measurements with the triangle method.
Real time FTIR (RTIR) measurements were performed on a FTIR (Nicolet iS50 FTIR, Thermo Scientific) at room temperature. Acrylate conversion was measured as the decrease in the peak area of 986 cm−1 or 1636 cm−1. Series scans were taken 2 scan per second. The sample with 15 μm thick glass rod spacers was pressed in between sodium chloride plates. Light exposure was done with a 365 nm LED light (Omnicure LED 365 nm, LX 500).
Conclusions
We report on the preparation of polymer stabilized cholesteric liquid crystals (PSCLCs) in which the selective reflection is blue-shifted by an applied DC bias. Conversion of acrylate double bonds is complete in 10 seconds at various UV exposures and photoinitiator concentrations. The blue-shifting tuning reported here is observable in identical formulations examined in our prior reports that have shown either bandwidth broadening or red-shifting tuning. The electro-optic response of these materials is shown to be delineated by the intensity and duration of UV exposure as well as the photoinitiator type and concentration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding from the Materials and Manufacturing Directorate of the Air Force Research Laboratory.Notes and references
- H.-S. Kitzerow and C. Bahr, Chirality in Liquid Crystals, Springer-Verlag, New York, 2001 Search PubMed.
- S. T. Wu and D.-K. Yang, Reflective Liquid Crystal Displays, Wiley, West Sussex, UK, 2001 Search PubMed.
- T. J. White, R. L. Bricker, L. V. Natarajan, V. P. Tondiglia, L. Green, Q. Li and T. J. Bunning, Opt. Express, 2010, 18, 173–178 CrossRef CAS PubMed.
- T. J. White, M. E. McConney and T. J. Bunning, J. Mater. Chem., 2010, 20, 9832–9847 RSC.
- M. E. McConney, V. P. Tondiglia, J. M. Hurtubise, T. J. White and T. J. Bunning, Chem. Commun., 2011, 47, 505–507 RSC.
- M. E. McConney, V. P. Tondiglia, J. M. Hurtubise, L. V. Natarajan, T. J. White and T. J. Bunning, Adv. Mater., 2011, 23, 1453–1457 CrossRef CAS PubMed.
- D.-K. Yang, J. W. Doane, Z. Yaniv and J. Glasser, Appl. Phys. Lett., 1994, 64, 1905–1907 CrossRef CAS.
- D.-K. Yang, J. L. West, L.-C. Chien and J. W. Doane, J. Appl. Phys., 1994, 76, 1331–1333 CrossRef CAS.
- D.-K. Yang, J. Disp. Technol., 2006, 2, 32–37 CrossRef.
- M. Xu and D.-K. Yang, Appl. Phys. Lett., 1997, 70, 720–722 CrossRef CAS.
- V. P. Tondiglia, L. V. Natarajan, C. A. Bailey, M. M. Duning, R. L. Sutherland, D.-K. Yang, A. Voevodin, T. J. White and T. J. Bunning, J. Appl. Phys., 2011, 110, 053109 CrossRef.
- V. P. Tondiglia, L. V. Natarajan, C. A. Bailey, M. E. McConney, K. M. Lee, T. J. Bunning, R. Zola, H. Nemati, D.-K. Yang and T. J. White, Opt. Mater. Express, 2014, 4, 1465 CAS.
- H. Nemati, S. Liu, R. S. Zola, V. P. Tondiglia, K. M. Lee, T. J. White, T. J. Bunning and D.-K. Yang, Soft Matter, 2015, 11, 1208–1213 RSC.
- K. M. Lee, V. P. Tondiglia, M. E. McConney, L. V. Natarajan, T. J. Bunning and T. J. White, ACS Photonics, 2014, 1, 1033 CrossRef CAS.
- M. E. McConney, V. P. Tondiglia, L. V. Natarajan, K. M. Lee, T. J. White and T. J. Bunning, Adv. Opt. Mater., 2013, 1, 417–421 CrossRef.
- T. J. White, K. M. Lee, M. E. McConney, V. P. Tondiglia, L. V. Natarajan and T. J. Bunning, SID Int. Symp. Dig. Tech. Pap., 2014, 45, 555–558 CrossRef.
- K. M. Lee, V. P. Tondiglia, T. Lee, I. I. Smalyukh and T. J. White, J. Mater. Chem. C, 2015, 3, 8788 RSC.
- K. M. Lee, V. P. Tondiglia and T. J. White, MRS Commun., 2015, 5, 223 CrossRef CAS.
- H. Khandelwal, M. G. Debije, T. J. White and A. P. H. J. Schenning, J. Mater. Chem. A, 2016, 4, 6064–6069 CAS.
- L. Lu, V. Sergan and P. J. Bos, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 86, 051706 CrossRef PubMed.
- W. Lee, C.-T. Wang and C.-H. Lin, Display, 2010, 31, 160–163 CrossRef CAS.
- B. Gosse and J. P. Gosse, J. Appl. Electrochem., 1978, 6, 515–519 CrossRef.
- C.-H. Wen, S. Gauza and S. T. Wu, Liq. Cryst., 2004, 31, 1479–1485 CrossRef CAS.
- P.-T. Lin, S. T. C. Wu, C.-Y. Chang and C.-S. Hsu, Mol. Cryst. Liq. Cryst., 2004, 411, 243–253 CrossRef.
- K. M. Lee, V. P. Tondiglia and T. J. White, Soft Matter, 2016, 12, 1256 RSC.
- B. Worth, K. M. Lee, V. P. Tondiglia, J. Myers, S. Mou and T. J. White, Appl. Opt., 2016, 55, 7134–7137 CrossRef PubMed.
- K. M. Lee, T. H. Ware, V. P. Tondiglia, M. K. McBride, X. Zhang, C. N. Bowman and T. J. White, ACS Appl. Mater. Interfaces, 2016, 8, 28040–28046 CAS.
- N. B. Cramer and C. N. Bowman, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 3311–3319 CrossRef CAS.
- T. J. White, W. B. Liechty and C. A. Guymon, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4062–4073 CrossRef CAS.
- P. Kirsch, W. Binder, A. Hahn, K. Jährling, M. Lenges, L. Lietzau, D. Maillard, V. Meyer, E. Poetsch, A. Ruhl, G. Unger and R. Fröhlich, Eur. J. Org. Chem., 2008, 3479–3487 CrossRef CAS.
- P. Kirsch and K. Tarumi, Angew. Chem., Int. Ed., 1998, 37, 484–489 CrossRef CAS.
- S. N. Naemura, Y. Nakazono, K. Nishikawa, A. Sawada, P. Kirsch, M. Bremer and K. Tarumi, Mater. Res. Soc. Symp. Proc., 1998, 508, 235–240 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sm01190c |
| This journal is © The Royal Society of Chemistry 2017 |