A robust molecular unit nanogrid servicing as network nodes via molecular installing technology†
Guangwei
Zhang‡
a,
Ying
Wei‡
a,
Jishu
Wang‡
a,
Yuyu
Liu
a,
Linghai
Xie
*a,
Long
Wang
a,
Baoyi
Ren
b and
Wei
Huang
*ac
aCenter for Molecular Systems and Organic Devices (CMSOD), Key Laboratory for Organic Electronics and Information Displays & Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamlhxie@njupt.edu.cn
bCollege of Applied Chemistry, Shenyang University of Chemical Technology, No. 11 Street, Shenyang Economic and Technological Development Area, Shenyang 110142, China
cKey Laboratory of Flexible Electronics (KLOFE) & Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing 211816, China. E-mail: wei-huang@njtech.edu.cn
First published on 9th August 2016
Abstract
Organic square nanogrid units have been designed in order to create molecular system nodes and their networks. A nanogrid that consists of four fluorenes and two carbazoles has been synthesized by molecular installing technology from an L-shaped synthon at a total yield of 21%. The fluorene-based nanogrids showed excellent thermal, electrochemical and spectral stability. Nanogrids will be one robust platform to polynodes for the exploration of molecular consciousness, intelligence and robotics.
It is of great importance to explore inter-connectable and addressable molecular nodes with special functions for various future technologies at the era of consciousness for artificial intelligence.1 Molecular polynodes and the internet could activate molecular consciousness/awareness by means of complex adaptive systems. Before molecular manufacturing technology,2 in order to achieve the final targets, the starting step is to fabricate the covalent nanobuilding blocks with multiple motion complexes which could serve as molecular nodes with multifunction operation systems and to explore the methodology of the internet or network. In general, nanobuilding blocks are called either nanoatoms by physicists or nanoclusters by chemists. Indeed, some nanobuilding blocks have been synthesized such as C60,3 polyhedral oligomeric silasesquioxane (POSS),4 and inorganic nanoclusters such as polyoxometalates and CdS clusters.5 However, most of them can either be easily connected through the strongly covalent bonds or noncovalent supramolecular bonds.6 It is crucial to create molecular nodes by establishing state-of-the-art molecular installing technology (MIT).
A molecular grid platform has been proposed inspired by traditional Chinese culture, symbols, and window patterns.7 The grid is an alternative to the cycle unit which can generate complex motifs and patterns. These molecular nano-architectures obtained by polygrids, and two-dimensional polymerization, as well as three-dimensional interconnection are not only aesthetically pleasing but should also be in accordance with mathematically encodable and addressable geometry. Fluorene derivatives are perfect candidates for the construction of grid shapes to set up MIT rules by means of several merits as follows: (1) persistent shape and backbones, a special advantage over the tetraphenyl counterparts (carbon, silicon, boron, and other cores) without fusing;8 (2) 9-substituent mode with alkyloxy side chains ensures the solubility of the complex polygrid;9 (3) vertical extensibility by means of combining the carbon sp3 linkage sites with sp2 linkage sites for polygrids, poly(ladders), and poly(arrays) as well as polycube metamolecules. In fact, fluorenes act as building blocks, and there are three basic types of linkage patterns, including sp2-mode (type-I),10 sp3-mode (type-II)11 and sp2–sp3-mixture-modes (type-III).7 The type III allows for the fabrication of square-like grids that are essentially different from the cycles or macrocycles owing to the orthodox expandability, fused backbones and site-selective functionalization. In addition, the type III paradigm of fluorenes can be planted for the template of other similar building blocks such as carbazole,12 cyclopentadithiophene,13 heterofluorenes,14 and diazafluorenes.15 (4) Furthermore, various C–C coupling reactions are suitable for the connection between fluorene in a flexible linkage manner, including sp2–sp2 linkage via palladium-catalyzed Suzuki reaction and sp3–sp2 linkage via Friedel–Craft (F–C) reaction16 and direct arylation of C–H activation/functionalization.17 Nevertheless, it is the start to setting up a systemic platform of molecular grids for encouraging top-issue exploration of complex molecular systems and consciousness. Herein, we demonstrate the MIT of a unit grid with a near square  shape (Fig. 1). We make a retrosynthetic analysis of the grid unit with four fluorenes and two carbazoles. Finally, an efficient molecular welding and joining protocol has been developed for the bottom-up fabrication of
shape (Fig. 1). We make a retrosynthetic analysis of the grid unit with four fluorenes and two carbazoles. Finally, an efficient molecular welding and joining protocol has been developed for the bottom-up fabrication of  shape nanogrids via an F–C based self-gridization of an L-shaped synthon.
shape nanogrids via an F–C based self-gridization of an L-shaped synthon.
 | ||
| Fig. 1 A schematic model of a unit grid as nanomolecules together with an H shape and three-rung ladders with a fused digrid. | ||
Retrosynthetic analysis of the  shape nanogrid is shown in Fig. 2. Retrosynthetic analysis reveals I-shape, L-shape, and U-shape synthons as potential key building blocks for the projected four possible synthetic routes. First, the disconnection of bond 1, bond 2, bond 3, and bond 4 gives an I-shaped synthon (route I). An I-shaped synthon is simpler by a one-pot procedure, avoiding the complicated process of intermediates and laborious purification. Then, the disconnection of bond 1 and bond 4 affords an L-shaped synthon (route II). Unimolecular self-gridization could be effective if the shape facilitates ring closure reactions. Finally, we disconnect either bond 1 and bond 2 or bond 1 and bond 3, giving a U-shaped synthon (routes III and IV). However, the cyclization and separation of a U-shaped synthon could be difficult because it contains several configurational isomers. In addition, the
shape nanogrid is shown in Fig. 2. Retrosynthetic analysis reveals I-shape, L-shape, and U-shape synthons as potential key building blocks for the projected four possible synthetic routes. First, the disconnection of bond 1, bond 2, bond 3, and bond 4 gives an I-shaped synthon (route I). An I-shaped synthon is simpler by a one-pot procedure, avoiding the complicated process of intermediates and laborious purification. Then, the disconnection of bond 1 and bond 4 affords an L-shaped synthon (route II). Unimolecular self-gridization could be effective if the shape facilitates ring closure reactions. Finally, we disconnect either bond 1 and bond 2 or bond 1 and bond 3, giving a U-shaped synthon (routes III and IV). However, the cyclization and separation of a U-shaped synthon could be difficult because it contains several configurational isomers. In addition, the  shape nanogrid can be accessed from these synthons via F–C reaction, C–H activation reaction and Suzuki coupling reactions, respectively.
shape nanogrid can be accessed from these synthons via F–C reaction, C–H activation reaction and Suzuki coupling reactions, respectively.
In previous work, we established a BF3·EtO2-based fluorenol's F–C protocol between tertiary alcohols and electron-rich arenes in our research.16 The molecular welding and joining technology has obvious advantages in terms of C–H functionalization, compatibility of functional groups, and site-selectivity. Complex diarylfluorenes (CDAFs) were fabricated by an intermolecular F–C C–C coupling bottom-up synthesis with H shapes and a fused digrid with a three-rung ladder at a relatively high yield (Fig. 1).7a,16 In addition, the regular bifluorene tertiary alcohols (I-shaped synthon) configurations have been designed by the backbone of diarylfluorenes according to our previous observations.9 Electron-rich 9-hexyl-carbazole was chosen as the crosspieces of the  shaped nanogrid due its high reactivity in the F–C reaction. As a result, we decided to adopt route I to synthesize a fluorene-based nanogrid via the F–C reaction.
shaped nanogrid due its high reactivity in the F–C reaction. As a result, we decided to adopt route I to synthesize a fluorene-based nanogrid via the F–C reaction.
With this idea in mind, the precursor bifluorene tertiary alcohol (I-1) was synthesized by the Suzuki coupling of 2-bromo-9-phenyl-fluorenol 1 and 9-phenyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolanyl)fluorenol 2 with yields of 90%. Finally, the BF3·Et2O-mediated F–C reaction of I-1 and 9-hexyl-carbazole was used to synthesize Grid. An unsatisfactory result was obtained for the desired Grid in 10% yield (Scheme 1, black arrow). We next checked routes III, and IV and were disappointed by our inability to improve the yield of Grid. As shown in route III (Scheme 1, blue arrow), I-1 underwent the BF3·Et2O-mediated F–C reaction with 9-hexyl-carbazole to give U-1 in 95% yield, which was reacted with I-1 in the presence of BF3·Et2O to afford Grid in 4.3% yield through two steps. As outlined in route IV (Scheme 1, green arrow), our synthesis commenced from the intermediate 4 (90%), prepared via the BF3·Et2O-mediated F–C reaction of 2-bromo-9-phenyl-fluorenol 1 with 9-hexyl-carbazole, which underwent the Suzuki coupling reaction with 2 to give U-2 (51%). Treatment of U-2 with 9-hexyl-carbazole in the presence of BF3·Et2O in CH2Cl2 at room temperature furnished Grid in 6% yield. Finally, evaluation indicated that route II is a suitable reaction pathway, as Grid could be obtained in 21% yield (Scheme 1, red arrow). With route II in hand, we began to synthesize L-1. To this end, Suzuki coupling of 3 with 2 afforded the desired L-1 (58%), which subsequently underwent a self-cyclization with L-1 to give Grid under BF3·Et2O condition. In addition, we found that a low concentration is favorable for intramolecular closures that probably are attributed to avoiding polymerization. Grid was moderately soluble in CH2Cl2, CHCl3, and THF, slightly soluble in CH3CN, and insoluble in toluene, EtOH, and n-hexane.
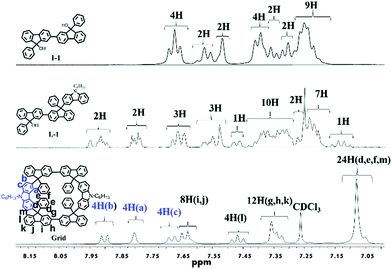
The comparisons of 1H NMR spectra of I-1, L-1, and Grid are illustrated in Fig. 3. As anticipated, the 1H NMR spectra of these products showed significant differences in their chemical shift patterns. The 1H NMR spectra of I-1 and L-1 showed a signal corresponding to the hydroxyl proton at 2.49 ppm. However, in the 1H NMR spectra of the Grid, the characteristic peak of hydroxyl groups (2.49 ppm) disappears and the peak at 4.94 ppm from sp3 carbon of fluorene was not observed, suggesting cyclization. The peaks at 7.89–7.91 ppm (doublet), 7.80 ppm (singlet) and 7.67–7.69 ppm (doublet) can be assigned to the protons b, a and c of carbazole unit, while peaks at 7.63–7.65 ppm (doublet), 7.45–7.49 ppm (triplet), 7.32–7.36 ppm (multiplet) and 7.05–7.08 ppm (multiplet, 4H) were attributed to the protons of the fluorene unit. The aryl linked to the fluorene shows a broad peak at 7.05–7.08 ppm (multiplet, 20H). The chemical shift of aryl protons of Grid will shift upfield, and aryl protons of I-1 or L-1 will not do that. The 1H NMR signals of aryl protons were broadened under the influence of the ring. With regard to the 13C NMR spectra of Grid, the disappearance of the peak at 83.7 ppm also suggested the disappearance of the –OH group, indicating effective transformation of C-9 of flurorenol in I-1 into C-9 of diarylfluorenes (see ESI†). MALDI-TOF MS permits accurate determination of molecular weights. The Grid exhibited isotopic distribution with a molecular ion mass of m/z = 1458.6 that is consistent with a hydrocarbon of the molecular formula of C112H86N2, which completely confirms cyclization (Fig. S8, ESI†).
In order to confirm the stable grid stereoisomer with optical energy, we began by optimizing possible stereoisomers of Grid by DFT calculation at the B3LYP/6-31G(d) level, which led to the discovery of more than 12 different kinds of grid stereoisomers with relatively stable conformations and configuration. In this study, we list five stereoisomers with relatively stable configuration without discussion of the enantiomers (Fig. 4). As a result, isomer 5 having three phenyl groups with the same direction as the alkyl groups is reasonably stable compared with the other isomers. And its geometry optimization, frontier molecular orbitals and electrostatic potential (ESP) have also been calculated. The target Grid has the size of about the length × width of 1.7 nm × 1.2 nm, with a cave of about 0.77 nm × 0.73 nm. From analysing the isosurfaces of wave functions of the frontier orbitals, the HOMO and LUMO of Grid are localized at different sites. The HOMO is predominantly localized at the crosspiece carbazole units, whereas the LUMO is localized by one of the 2,2′-bifluorene chains. The electrostatic potential analysis shows that the electrostatic potential of Grid is more negative on the fluorene chain and more positive on the carbazole moiety. The strong steric repulsion between carbazole and phenyl at the 9-position of fluorine is observed.
The optical properties of Grid were investigated by UV-vis absorption spectra, and photoluminescence (PL) spectra as shown in Fig. 5a. Grid exhibits almost the same UV with absorption bands located at 304 nm and 331 nm in dilute CHCl3 solution and in film. Grid in dilute CHCl3 solution exhibits emission spectra with peaks at 367 and 380 nm. Grid in the thin film exhibits emission peaks at 399 nm. One striking property of Grid is that the PL spectra are remarkably stable upon annealing, showing very little change after annealing in N2 at 200 °C for 4 h. The above results indicate that the grid molecules avoid aggregation between molecules and impart the material with a good random phase.
 | ||
| Fig. 5 (a) Absorption spectra and emission spectra of Grid; (b) TGA curves of Grid; (c) CV curves of Grid (10 successive scans for anode processes); (d) DSC curves of Grid. | ||
Cyclic voltammetry (CV), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) of Grid was performed. CV measurements were used to investigate the oxidation and reduction behaviors of Grid and estimate their HOMO and LUMO energy levels (Fig. 5b). The HOMO energy level of Grid was −5.58 eV according to the onset oxidation potential (Eox) of 0.87 eV. The LUMO energy level of Grid was −2.20 eV according to the onset reduction potential (Ered) of −2.51 eV. As a result, the electrochemical band gap is 3.38 eV. Grid showed good thermal stability as measured by TGA. The decomposition temperatures were 458.2 °C for Grid (Fig. 5c). The DSC of Grid shows no clear glass transition temperature below 250 °C, indicating that the nanogrids exhibit stable amorphous states (Fig. 5d). To investigate the electroluminescence (EL) properties of Grid, the non-doped OLED was fabricated using Grid as the emission layer. Without further structure optimization, a luminance up to 621.3 cd m−2, turn-on voltage of 5 V and maximum current efficiency of 0.8 cd A−1 were successfully achieved in the device. The obtained EL performance reveal that Grid opens up interesting perspectives for the development of promising OLEDs in the future.18
In summary, the total synthesis of a  shaped nanogrid has been achieved with four separate approaches. Finally, we have found route II to be a suitable method for the synthesis of an
shaped nanogrid has been achieved with four separate approaches. Finally, we have found route II to be a suitable method for the synthesis of an  shaped nanogrid by a one-pot self-cyclization reaction of an L-shaped synthon (21% overall yield). The current synthetic method would be applicable to the syntheses of other molecular grid systems. The unit grid with a single square shape could be easily modified as nanomonomers with the potential application of one, two, three-dimensional polygrids and polynodes. The unit grids are versatile building blocks not only in nanonode-based networks, but also supramolecular host–guest systems such as catexane and rotaxanes. Polygrids will provide an advanced platform for optoelectronic nanopolymers not only in organic electronics, but also in biosensors, imaging, as well as medicine. If the grid systems have the function of the molecular or quantum rule, they could act as molecular system nodes for smart polynodes and networks. Future work will explore these possibilities, as well as further expand the substrate scope and challenge the methodology to create more complex nanogrids, polygrids, and their supramolecular nanopolymers.
shaped nanogrid by a one-pot self-cyclization reaction of an L-shaped synthon (21% overall yield). The current synthetic method would be applicable to the syntheses of other molecular grid systems. The unit grid with a single square shape could be easily modified as nanomonomers with the potential application of one, two, three-dimensional polygrids and polynodes. The unit grids are versatile building blocks not only in nanonode-based networks, but also supramolecular host–guest systems such as catexane and rotaxanes. Polygrids will provide an advanced platform for optoelectronic nanopolymers not only in organic electronics, but also in biosensors, imaging, as well as medicine. If the grid systems have the function of the molecular or quantum rule, they could act as molecular system nodes for smart polynodes and networks. Future work will explore these possibilities, as well as further expand the substrate scope and challenge the methodology to create more complex nanogrids, polygrids, and their supramolecular nanopolymers.
Acknowledgements
The project was supported by the National Natural Science Funds for Excellent Young Scholar (21322402), National Natural Science Foundation of China (U1301243, 21274064, 21144004, 61177029, 20974046, 51273092), National Key Basic Research Program of China (973) (2015CB932200), Doctoral Fund of Ministry of Education of China (20133223110007), Excellent science and technology innovation team of Jiangsu Higher Education Institutions (2013), Natural Science Foundation of Jiangsu Province (BM2012010, BK20150832), and Open Project from State Key Laboratory of Supramolecular Structure and Materials at Jilin University (klssm201612). Project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, PAPD.References
- (a) L. H. Xie and W. Huang, Advances in Science, Science Network, Canada, 2013, ch. 4, vol. 1, p. 104 Search PubMed; (b) L. H. Xie and W. Huang, Advances in Optoelectronics Research, Nova Science Publishers, USA, 2014, ch. 2, p. 29 Search PubMed; (c) L. H. Xie and W. Huang, Supramolecular Steric Hindrance at Bulky Organic/Polymer Semiconductors and Devices, in Non-covalent Interactions in the Synthesis and Design of New Compounds, ed. A. M. Maharramov, K. T. Mahmudov, M. N. Kopylovich and A. J. L. Pombeiro, John Wiley & Sons, Inc., Hoboken, NJ, 2016, p. 443 Search PubMed.
- (a) K. E. Drexler, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 5275–5278 CrossRef CAS PubMed; (b) D. R. Forrest, IEEE Instrum. Meas. Mag., 2001, 4, 11–20 CrossRef; (c) L. H. Xie, S. H. Yang, J. Y. Lin, M. D. Yi and W. Huang, Philos. Trans. R. Soc. London, Ser. A, 2013, 371, 20120337 CrossRef PubMed.
- (a) H. W. Kroto, A. W. Allaf and S. P. Balm, Chem. Rev., 1991, 91, 1213–1235 CrossRef CAS; (b) H. W. Kroto, J. R. Heath, S. C. ÒBrien, R. F. Curl and R. E. Smalle, Nature, 1985, 318, 162–163 CrossRef CAS; (c) L. T. Scott, M. M. Boorum, B. J. McMahon, S. Hagen, J. Mack, J. Blank, H. Wegner and A. De. Meijere, Science, 2002, 295, 1500–1503 CrossRef CAS PubMed.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
- (a) N. Zheng, X. Bu, H. Lu, Q. Zhang and P. Feng, J. Am. Chem. Soc., 2005, 127, 11963–11965 CrossRef CAS PubMed; (b) G. A. DeVries, M. Brunnbauer, Y. Hu, A. M. Jackson, B. Long, B. T. Neltner, O. Uzun, B. H. Wunsch and F. Stellacci, Science, 2007, 315, 358–361 CrossRef CAS PubMed; (c) Q. Zhang, T. Wu, X. Bu, T. Tran and P. Feng, Chem. Mater., 2008, 20, 4170–4172 CrossRef CAS; (d) D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 CrossRef CAS PubMed; (e) J. Gao, X. Liu, Y. Liu, L. Yu, Y. Feng, H. Chen, Y. Li, G. Rakesh, C. H. A. Huan, T. C. Sum, Y. Zhao and Q. Zhang, Dalton Trans., 2012, 41, 12185–12191 RSC; (f) J. Gao, S. Cao, Q. Tay, Y. Liu, L. Yu, K. Ye, P. C. S. Mun, Y. Li, G. Rakesh, S. C. J. Loo, Z. Chen, Y. Zhao, C. Xue and Q. Zhang, Sci. Rep., 2013, 3, 1853 Search PubMed.
- G. A. DeVries, M. Brunnbauer, Y. Hu, A. M. Jackson, B. Long, B. T Neltner, O. Uzun, B. H. Wunsch and F. Stellacci, Science, 2007, 315, 358–361 CrossRef CAS PubMed.
- (a) L. Wang, G. W. Zhang, C. J. Ou, L. H. Xie, J. Y. Lin, Y. Y. Liu and W. Huang, Org. Lett., 2014, 16, 1748–1751 CrossRef CAS PubMed.
- (a) L. H. Xie, X. Y. Hou, C. Tang, Y. R. Hua, R. J. Wang, R. F. Chen, Q. L. Fan, L. H. Wang, W. Wei, B. Peng and W. Huang, Org. Lett., 2006, 8, 1363–1366 CrossRef CAS PubMed; (b) J. Song, F. Wei, W. Sun, K. Li, Y. Tian, C. Liu, Y. Li and L. H. Xie, Org. Lett., 2015, 17, 2106–2109 CrossRef CAS PubMed.
- G. W. Zhang, L. Wang, L. H. Xie, J. Y. Lin and W. Huang, Int. J. Mol. Sci., 2013, 14, 22368–22379 CrossRef PubMed.
- S. C. Simon, B. Schmaltz, A. Rouhanipour, H. J. Raeder and K. Muellen, Adv. Mater., 2009, 21, 83–85 CrossRef CAS.
- (a) M. Y. Song, H. K. Na, E. Y. Kim, S. J. Lee, K. I. Kim, E. M. Baek, H. S. Kim, D. K. An and C. H. Lee, Tetrahedron Lett., 2004, 45, 299–301 CrossRef CAS; (b) Q. Kong, D. Zhu, Y Quan, Q. Chen, J. Ding, J. Lu and Y. Tao, Chem. Mater., 2007, 19, 3309–3318 CrossRef CAS.
- R. R. Liu, Z. Q. Lin, N. E. Shi, J. F. Zhao, X. Y. Hou, Y. Qian, L. H. Xie and W. Huang, Chem. Lett., 2010, 39, 522–523 CrossRef CAS.
- L. H. Xie, X. Y. Hou, Y. R. Hua, Y. Q. Huang, B. M. Zhao, F. Liu, B. Peng, W. Wei and W. Huang, Org. Lett., 2007, 9, 1619–1622 CrossRef CAS PubMed.
- B. Pal, W. C. Yen, J. S. Yang, C. Y. Chao, Y. C. Hung, S. T. Lin, C. H. Chuang, C. W. Chen and W. F. Su, Macromolecules, 2008, 41, 6664–6671 CrossRef CAS.
- J. F. Zhao, L. Chen, P. J. Sun, X. Y. Hou, X. H. Zhao, W. J Li, L. H. Xie, Y. Qian, N. E. Shi, W. Y. Lai, Q. L. Fan and W. Huang, Tetrahedron, 2011, 67, 1977–1982 CrossRef CAS.
- (a) L. H. Xie, X. Y. Hou, Y. R. Hua, C. Tang, F. Liu, Q. L. Fan and W. Huang, Org. Lett., 2006, 8, 3701–3704 CrossRef CAS PubMed; (b) Y. Yang, J. F. Zhao, R. R. Liu, J. W. Li, M. D. Yi, G. H. Xie, L. H. Xie, Y. Z. Chang, C. R. Yin, X. H. Zhou, Y. Zhao, Y. Qian and W. Huang, Tetrahedron, 2013, 69, 6317–6322 CrossRef CAS; (c) Y. Z. Chang, Q. Shao, L. Y. Bai, C. J. Ou, J. Y. Lin, L. H. Xie, Z. D. Liu, X. Chen, G. W. Zhang and W. Huang, Small, 2013, 9, 3218–3223 CAS.
- X. Cao, W. Yang, C. Liu, F. Wei, K. Wu, W. Sun, J. Song, L. H. Xie and W. Huang, Org. Lett., 2013, 15, 3102–3105 CrossRef CAS PubMed.
- See ESI.† The EL spectra, L–V and LE–V characteristics of device prepared from Grid.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qm00004e |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2017 |