Thermoresponsive polymers with lower critical solution temperature: from fundamental aspects and measuring techniques to recommended turbidimetry conditions†
Qilu
Zhang
ab,
Christine
Weber
cd,
Ulrich S.
Schubert
 cd and
Richard
Hoogenboom
cd and
Richard
Hoogenboom
 *a
*a
aSupramolecular Chemistry Group, Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281 S4, B-9000 Ghent, Belgium. E-mail: richard.hoogenboom@ugent.be
bState Key Laboratory for Mechanical Behavior of Materials, Xi'an Jiaotong University, 710049, Xi'an, P. R. China
cLaboratory of Organic and Macromolecular Chemistry (IOMC), Friedrich Schiller University Jena, Humboldtstr. 10, 07743 Jena, Germany
dJena Center for Soft Matter (JCSM), Friedrich Schiller University Jena, Philosophenweg 7, 07743 Jena, Germany
First published on 20th January 2017
Abstract
Thermoresponsive polymers that undergo reversible phase transition by responding to an environmental temperature change, in particular polymers showing lower critical solution temperature (LCST), are frequently used as smart materials that have found increasing applications. Recently, there has been a rapid growth in interest on LCST polymers and many new research groups are entering the field from a wide range of application areas. While it is great to see more researchers working on LCST polymers, the downside of this rapid growth is that the fundamentals of the LCST phase transition behavior are not always clearly known and respected. Hence, this focus article provides a systematic discussion of the key aspects of the LCST behavior of polymers starting from fundamentals of LCST behavior to practical determination of cloud point temperature (Tcp). Finally, we offer a basic set of recommended measuring conditions for determination of Tcp (10 mg mL−1; 0.5 °C min−1; 600 nm) to facilitate the comparison of the LCST behavior and Tcp values of polymers developed and studied in different laboratories around the globe, which is nowadays nearly impossible since various techniques and parameters are being utilized for the measurements. It should be noted that these recommended conditions serve as a robust tool for turbidimetry, which is one out of the many characterization techniques one should utilize to fully understand LCST behavior of polymers.
1. Introduction
Stimuli-responsive polymers that undergo a property change in response to variation in the environmental conditions are of great interest for advanced applications as smart materials.1–11 Amongst the various stimuli applicable, temperature is the most extensively exploited in the field of ‘smart’ polymers due to the important role temperature plays in nature.12–19 Furthermore, temperature can externally be applied in a non-invasive manner to control the properties of the thermoresponsive polymers, and the behavior is often completely reversible.The reason why thermoresponsive polymers are appealing ‘smart materials’ is the fact that they apparently precipitate from a solution when the temperature is increased or decreased. This behavior is caused by a miscibility gap in the phase diagram in the binary polymer/solvent mixture, accompanied by phase separation. If elevation of temperature leads to the formation of two immiscible liquid phases with different polymer concentrations, the mixture exhibits lower critical solution temperature (LCST) behavior.16,19 The LCST is defined as the temperature of the minimum of the binodal (or the coexistence curve) of the phase diagram, as depicted in Fig. 1. The concentration at the minimum of the binodal is termed the lower critical solution concentration (LCSC). If the two liquid phases are formed upon decrease of temperature, the binary mixture exhibits upper critical solution temperature (UCST) behavior.15 Therefore, in order to determine the LCST of a polymer in water, the binodal of the phase diagram has to be constructed to identify the minimum phase separation temperature. This can be carried out by dissolving the polymer in water at a low temperature and annealing the mixture at a temperature above the phase separation temperature. The concentrations of both the formed phases at the given temperature represent points on the left and right side of the binodal and can be assessed experimentally, e.g., from the refractive index or UV absorption of the high and low polymer concentration phases using a calibration curve. This experiment has to be performed at a range of temperatures in order to obtain a number of points on the binodal to find its minimum temperature (LCST).
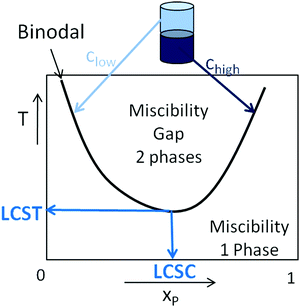 | ||
| Fig. 1 Phase diagram for a binary mixture exhibiting an LCST. Reprinted from ref. 12. | ||
In particular polymers that reveal LCST behavior in water are of tremendous interest for many applications due to the opportunity to profit from their altering hydrophilicity upon temperature variation.3,6 The sudden change from hydrophilic to hydrophobic behavior of the same polymer is based on hydrogen bonds that are present between the polymer and surrounding water molecules at low temperatures. Hence, the polymer chains are hydrated and solubilized resulting in a one-phase system. At increased temperature, the hydrogen bonds are weakened, and the polymer chains are partially dehydrated and cannot be solubilized anymore leading to polymer aggregation.
The “weaker” hydrogen bonding at high temperature can be explained when the varying contributions of entropy and enthalpy to the free energy of mixing are deduced: the binding of the water molecules to the polymer chain results in a favorable enthalpy of mixing (ΔHidmix = 0, ΔHexmix < 0) but also leads to an enhanced ordering, which contributes unfavorably to the entropy of mixing (ΔSidmix > 0, ΔSexmix < 0). At higher temperatures, the entropy term TΔS becomes predominant and the free energy of mixing turns positive, which is manifested in phase separation. As such, it is evident that the LCST phase transition is entropy-driven.
The hydrated polymer chains exhibit a hydrated coiled conformation at low temperature but minimize their contact with surrounding water by changing towards a globular conformation at high temperature, which is expressed in the common catchphrase “coil to globule transition” (Fig. 2). In short, it can be stated that the LCST phase transition is an entropy driven event governed by the water molecules that enhance the entropy of the system by dehydration of the polymer chains at a critical temperature. As such, the LCST transition represents a first order phase transition (see Section 3.1).
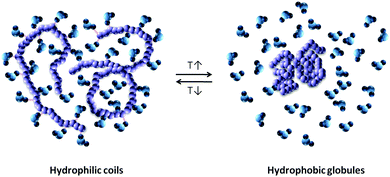 | ||
| Fig. 2 Coil to globule transition of a polymer in aqueous solution. Note that the remaining water molecules in the phase separated polymer phase and the remaining polymer chains in the water phase are not shown.12 | ||
The vast amount of water-soluble polymers exhibiting LCST behavior in water makes it relatively easy to develop smart materials based on LCST polymers, and also to design and develop new classes of polymers that reveal LCST behavior in water. In fact, every polymer with the appropriate hydrophilic–hydrophobic balance will show LCST behavior. However, this rather easy access to the area of thermoresponsive LCST polymers has led to rapid growth of this research area with many new research groups entering the field from a wide range of application areas. Of course, it is great to see the increasing research activities and development of exciting new applications, but the downside of this rapid growth is that the fundamentals of the LCST phase transition behavior are not always known and respected. The most common example and mistake in the area is that the term LCST is used interchangeably with cloud point temperature (Tcp), which is incorrect per definition as shown in Fig. 1. This will become even clearer in Section 2 of this manuscript that describes the use of Tcp as an important parameter for the phase transition temperature of a solution of an LCST polymer, which can experimentally be assessed in an easy manner. A similar observation was recently also discussed by Halperin et al.19 when reviewing the LCST behavior of PNIPAM, where the authors also mentioned that the wide variety of measurement conditions in combination with the characteristics of PNIPAM, leads to diversity in reported LCST and Tcp values of PNIPAM.
In this focus article, we will discuss the effect of the measurement technique on diversity in the determination of Tcp values of thermoresponsive polymers, both from a fundamental and practical aspect aiming to facilitate the comparison of Tcp values reported based on different techniques. With turbidimetry being the most common method for the determination of Tcp, we have experimentally evaluated and optimized the measurement conditions as will be discussed in Section 3, finally resulting in a set of recommended settings and conditions for the robust determination of Tcp by turbidimetry.
The overall aim of this focus article is to provide a fundamental basis for researchers interested in LCST polymers as well as to offer a basic set of measuring conditions for determination of Tcp, hopefully facilitating future comparison of the LCST behavior and Tcp values of polymers developed and studied in different laboratories around the globe, which is nearly impossible nowadays due to the fact that a wide variety of different measurement conditions are being used. Note that even though reliable turbidimetric data are important for comparison of the Tcp values, for a complete understanding of the LCST behavior of a polymer more in depth studies should also be performed with other characterization techniques, such as NMR spectroscopy, dynamic light scattering and/or calorimetry.
2. Cloud point temperature (Tcp)
For the application of thermoresponsive polymers in a particular condition, the phase transition temperature, or Tcp, is one of the most important parameters of a thermoresponsive polymer in solution. Tcp refers to the temperature at which the phase transition of a polymer solution at a specific concentration occurs from the soluble state to the collapsed aggregated state, accompanied by clouding of the solution. As shown in Fig. 1, the Tcp is a phase transition temperature at a specific polymer concentration, which can be located at any position of the binodal curve and, therefore, the polymer concentration during Tcp determination has to be specified. Importantly, Tcp is not equivalent with the LCST as the LCST is the minimal temperature value of the binodal. In other words, the LCST is the lowest value of Tcp in the phase diagram, whereby it should be noted that the cloud point curve in the entire phase diagram does not exactly coincide with the binodal curve.20 This difference in Tcp and the binodal curve is related to kinetic aspects of determining the Tcpversus the thermodynamic binodal curve as well as the limitations of turbidimetry as it only determines polymer agglomerates that are large enough and sufficiently dehydrated to scatter the incident light.The Tcp of a polymer in solution can easily be varied by chemical strategies such as copolymerization21–25 and end group modification26 to tune the hydrophilic–hydrophobic balance of the polymer chains, or physical strategies, like mixing different polymers,27 concentration28 and ionic strength29,30 to control the polymer–polymer, polymer–solvent and solvent–solvent interactions. On the one hand this tunability provides the possibility to accurately control the Tcp for specific applications while, on the other hand, it makes it very difficult, if not impossible, to compare the thermoresponsive behavior and Tcp values of polymers reported by different research groups. In addition, the determination of the Tcp values using different techniques, such as turbidimetry,241H NMR spectroscopy31 and dynamic light scattering (DLS)32–34 also results in deviations of Tcp depending on the method used. For instance, turbidimetry determines the Tcp as the transition from a homogeneous solution into a heterogeneous milky phase with a concentrated polymer phase dispersed in a diluted polymer solution phase. In contrast, DLS allows more sensitive determination of the onset of the phase transition by the appearance of aggregates even when they do not yet cause clouding of the solution.
3. Characterization of the phase transition of thermoresponsive LCST polymers
Several techniques have been employed to characterize the phase transition temperature of thermoresponsive LCST polymers. Various physical or physico-chemical properties of the polymer solution, for instance polymer chain conformation, hydrogen bonding, chain mobility and optical properties undergo drastic changes when crossing the phase transition temperature during heating or cooling. Such sharp changes of properties can be followed by related techniques, i.e. light scattering, calorimetry, NMR spectroscopy and turbidimetry, respectively, to identify the phase transition temperature, mostly referred to as Tcp. In this section, the techniques mentioned above will be discussed. Some other techniques, like fluorescence spectroscopy,35,36 pressure perturbation calorimetry15 and Fourier transform infrared spectroscopy (FTIR),37–39 have also been applied to investigate the phase transition behavior of thermoresponsive polymer solutions. However, these techniques are less commonly used and hence will not be covered in this contribution.3.1 Dynamic light scattering (DLS)
DLS measures the translational self-diffusion coefficient (D) of the polymers in solution, which can be correlated with the size of the polymeric particles according to the Stokes–Einstein equation:| d(H) = kT/(3πηD) |
DLS measurements of the polymer solutions can be performed at different temperatures to follow the LCST phase transition (Fig. 3).32,40,41 Below the Tcp the polymer chains exist as individually dissolved polymer chains (coils) with a small hydrodynamic radius, although often a minor fraction of large loose aggregates is also observed by DLS, in particular when examining the scattering intensity as a function of size, as a minor fraction of larger objects can significantly contribute to the scattering. Upon the coil to globule transition, the polymer chains are partially dehydrated leading to collapse and agglomeration to form particles of a larger size, also referred to as mesoglobules. Compared to other techniques, DLS provides direct information on the particle size of the polymers allowing accurate determination of the onset of the phase separation by the appearance of polymer agglomerates even when they do not yet cause clouding of the solution or give a significant change in NMR signals. Furthermore, the size determination by DLS provides direct information on the size of the formed mesoglobules, being the high concentration polymer phase dispersed in the low polymer concentration solution, which is strongly dependent on the polymer concentration as well as the heating rate.
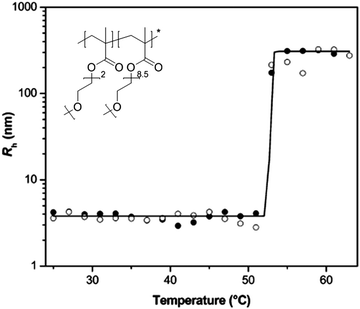 | ||
| Fig. 3 Change in the hydrodynamic radius of a thermoresponsive LCST copolymer in aqueous solution upon coil to globule transition. Reprinted from ref. 40. | ||
The combination of DLS and turbidimetry has been widely used to characterize the thermoresponsive behavior of LCST polymers and usually provides similar Tcp results for LCST polymers when using conditions that lead to a sharp phase transition.32,33,37,41 Deviation between DLS and turbidimetry may be detected if, for instance, small particles are formed due to gradual dehydration during heating of LCST polymers, which allows the in depth analysis of the phase transition behavior of the polymers by DLS while such smaller objects may simply be overlooked by turbidimetry.33,37
The change of intensity in scattering light is another parameter that could be followed by DLS during heating or cooling of the polymer solution. A sharp increase in the intensity of scattered light can be detected during the coil to globule transition upon heating due to the fact that the intensity of the scattered light is proportional to d6, where d is the diameter of a particle, according to the Rayleigh approximation. The partial dehydration leads to an increase in differential refractive index between the two phases, i.e. the concentrated, mostly dispersed, phase and the dilute phase. In contrast, the minor difference in refractive index of the hydrated polymer chains and the bulk water causes significantly less light scattering below the Tcp. Typically, the intensity of the scattered light increases by several orders of magnitude during the coil to globule transition.41
3.2 Calorimetry
Due to loss of the specific hydrogen bonds between the polymer and the water molecules, the coil to globule transition of a thermoresponsive LCST polymer represents an endothermic process. As such it can be examined by calorimetric methods, such as (high sensitivity) differential scanning calorimetry (DSC).35,42 This technique is based on the measurement of differences in heat capacity between a sample and a reference cell. Representative thermograms are depicted in Fig. 4. DSC provides information about the temperature at which the phase separation occurs, as well as about the enthalpy of the phase transition. In particular the latter represents an interesting value since it enables the estimation of the number of hydrogen bonds that are broken during the phase transition process. In this way, it is possible to gain more quantitave information on the number of water molecules that leave the polymer chains during the phase transition, either per repeating unit or per total macromolecule, as well as the amount of water that is retained in the collapsed polymer globules.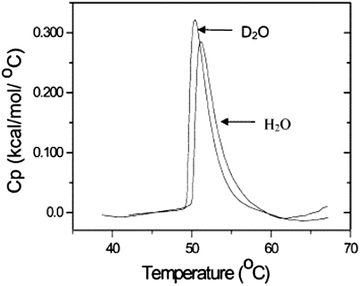 | ||
| Fig. 4 Calorimetric investigation of aqueous solutions of poly(2-iso-propyl-2-oxazoline) illustrating the endotherms measured by high sensitivity DSC. Reprinted from ref. 43. | ||
3.3 Proton nuclear magnetic resonance (1H NMR) spectroscopy in D2O
1H NMR spectroscopy in deuterated water is able to provide insights into the thermoresponsive behavior of polymers on the molecular level.38,44–47 As long as the temperature is below Tcp, the polymer chains are well hydrated and thus mobile resulting in sharp peaks in the 1H NMR spectrum (Fig. 5). As a result of the decreased chain mobility of the partially dehydrated polymer globules, the peaks become broadened (and may even disappear in the baseline of the spectrum) when the coil to globule transition temperature of the polymer is exceeded. In cases, where the thermoresponsive polymer contains also highly hydrophilic moieties, the collapsed globules will retain more hydrating water molecules, and broad peaks may remain visible even above Tcp, thereby providing accurate information on the local hydration of different parts of the polymer chains. A clear example is provided in Fig. 5 for the LCST phase transitions of poly(methoxydiethylene glycol methacrylate) (PmDEGMA) and poly(methoxytriethylene glycol methacrylate) (PmTEGMA). While for PmDEGMA, all signals are lost in the 1H NMR spectrum above the Tcp at 35 °C, the side chain signals of PmTEGMA remain visible in the spectrum above the Tcp at 65 °C. This is a clear indication that the collapsed globules of PmTEGMA contain more water and that the hydrophobic backbone of PmTEGMA is more efficiently dehydrated than the hydrophilic side chains. During interpretation of such NMR measurements one should be aware of the fact that Tcp is slightly different in D2O than in H2O since in D2O “deuterium bonding” between solvent and polymer is affected by temperature changes instead of hydrogen bonding, as is also evident in Fig. 4.48,49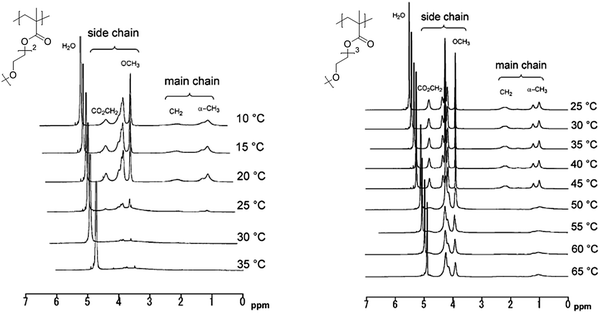 | ||
| Fig. 5 1H NMR spectra of thermosensitive polymers in D2O below and above Tcp. Reprinted from ref. 50. | ||
3.4 Turbidimetry: general considerations
Turbidimetry represents the method that is most widely used to determine the Tcp of thermoresponsive polymer solutions. Besides special turbidimetry instruments, the measurement can be performed on a simple UV-vis spectrometer with temperature control, which is a standard piece of equipment in most laboratories explaining the popularity of this method. For Tcp determination, a solution of the polymer is prepared in water, filled into a suitable cuvette and placed in the spectrometer. A temperature program is applied to heat the solution inside the spectrometer, and the transmittance of light through the solution is constantly measured. Upon heating above the Tcp, droplets consisting of the concentrated phase are dispersed in a solution of the dilute phase. These concentrated phase droplets scatter the incident light leading to a rapid decrease in the transmittance. Both phases re-mix upon cooling, which results in an increase in transmittance. As can be seen from Fig. 6, often a hysteresis between the transmittance curves of the heating and cooling runs is obtained. Since an important prerequisite for a useful application of the thermoresponsive polymer is the reversibility of its coil to globule transition, more than one heating–cooling cycle is often carried out as shown in Fig. 6B. If the turbidimetry is carried out as described here, it represents a dynamic method since the temperature is constantly changed. That is why the temperature ramp of the measurements may also influence the results that are obtained from the measurements. Although the used temperature ramp is much smaller in some studies, nowadays a heating rate of 1 K min−1 has become quite common in order to speed up the measurements. In particular, in the case of a broad turbidity curve it becomes also important to decide which point of the curve is defined as Tcp. Going through the literature one will find several possibilities: onset of the drop in transmittance, 80% or 50% transmittance as well as the inflection point of the turbidity curve.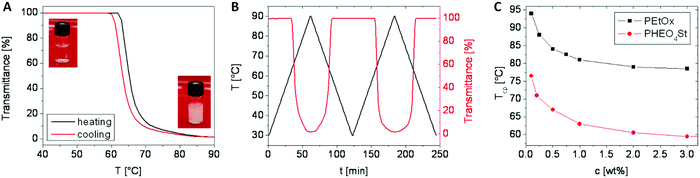 | ||
| Fig. 6 (A and B) Turbidity curves obtained from an aqueous solution of a thermo- responsive polymer. (C) Dependence of Tcp on the polymer concentration. Data taken from ref. 28 and 51. | ||
As shown in the phase diagram in Fig. 1, the phase separation temperature of the solution depends on the polymer concentration. If the Tcp of a polymer in aqueous solution is determined for a range of concentrations, the cloud point curve can be constructed, where the observed Tcp is plotted against the concentration. It is important to note that this cloud point curve will deviate from the binodal of the phase diagram as mentioned earlier already, as has, e.g., been reported for poly(2-ethyl-2-oxazoline)52 as well as for poly[2-(2-ethoxy)ethoxyethyl vinyl ether](PEtEO2V).53 In particular in the diluted concentration regime that is mostly studied during turbidimetry, the observed Tcp usually decreases by several degrees upon increase of polymer concentration (Fig. 6C). Although the authors of a single paper usually keep the concentration constant during their turbidimetry measurements, the used concentrations range from 0.1 to 10 wt% in the literature. In addition, some values are not reported in water, but in phosphate buffered saline (PBS), which is undoubtedly useful with respect to a biological application of the polymer, but leads to an additional salt effect on the Tcp.
3.5 Turbidimetry: recommended conditions
In order to keep the large amount of information that is created throughout the world comparable with one another, it would certainly be desirable to establish a convention regarding the measurement conditions applied in turbidimetry. Therefore, we have undertaken a broad study on the effect of different parameters during turbidimetry on the resulting Tcp as a basis to recommend a set of measurement conditions for robust Tcp determination by turbidimetry as outlined in this section.Two rather different model polymers, namely a defined low molar mass PmDEGMA (Mn ∼ 5.0 kDa; Đ = 1.21) and a high molar mass poly(2-ethyl-2-oxazoline) with broad molar mass distribution (PEtOx; Mw ∼ 50 kDa; Đ ≈ 3–4; Aquazol 50) were employed to analyze the influence of the various measurement parameters. The utilized PEtOx is an ill-defined thermoresponsive polymer, and the Tcp of its aqueous solution is very sensitive to variation of its molar mass.28 On the other hand, the Tcp of aqueous solutions of the defined PmDEGMA is barely influenced by its molar mass.54 Besides, the transition temperature of the two polymers is distinctly different, i.e. the Tcp of PmDEGMA solutions is close to room temperature, while the Tcp of a PEtOx solution is around 65 °C at 0.5 wt%. Several measurement parameters, including polymer concentration, temperature ramp, wavelength of incident light, stirring, cuvette type and method of temperature measurement, were systematically investigated to shed light on their influence on Tcp. With this comprehensive data in hand, we propose a recommended set of measuring conditions allowing robust and accurate determination of Tcp. The full details and discussion of the turbidimetry parameter screening are included in the ESI.† Here, only the main conclusions and recommendations will be discussed.
The raw data obtained by turbidimetry can be plotted as either the transmittance or the absorbance versus the temperature. However, serious deviations could be expected when plotting absorbance versus temperature because the results are strongly dependent on the sensitivity of the optical device used, especially when the absorbance exceeds 1.5. This drawback of plotting absorbance data versus temperature can be easily overcome by converting it to transmittance (1). The resulting value is then plotted as %T.
| %T = 10−A | (1) |
As both the heating rate and the polymer concentration are crucial for determining the Tcp, these parameters were studied in various combinations leading to the conclusion that the most robust Tcp values were obtained for both model polymers with a medium heating rate of 0.5 °C min−1 in combination with a 10 mg mL−1 (1.0 wt%) polymer concentration. Under these conditions, it was found that the determined Tcp is not strongly influenced by variation of the cuvette type or the use (or absence) of stirring during the measurements. Furthermore, the determined Tcp values are close to those obtained with an internal temperature probe.
Regarding the scientific reporting of the Tcp value, it is indispensable to document not only the polymer concentration, but also the heating/cooling rate, the wavelength of incident light absence or presence of stirring, and the method of temperature monitoring as well as the utilized cuvette to allow accurate evaluation and comparison of the data with other reports.
4. Summary
In this focus article, we have provided an overview of the fundamental aspects of thermoresponsive polymers with lower critical solution temperature (LCST) behavior as well as the most commonly employed methods for determination of the cloud point temperature (Tcp), which was triggered by the increasing number of research groups working with LCST polymers and by the loss of knowledge of the basic aspects and definitions. This is in particular true for the often interchangeable and incorrect use of LCST and Tcp, which are fundamentally different values. As such, this article aims to put all scientists working in this area on the same level again regarding terminology. Furthermore, as turbidimetry is the most common method for the determination of Tcp values, we experimentally investigated the influence of a wide range of measurement conditions on the determination of Tcp by turbidimetry to come to a robust set of recommended measurement parameters: an incident wavelength of 600 nm, 10 mg mL−1 (1.0 wt%) polymer concentration and 0.5 °C min−1 heating rate. With these settings and extraction of the Tcp at 50% transmittance, the resulting value is rather independent of the use of stirring, the cuvette type and the temperature monitoring method. It is noteworthy that Halperin, Kroeger, and Winnik19 have recently suggested that experimental data should be reported with care and appropriate details for future reference. We share this opinion and would like to stress that even though the recommended set of conditions for turbidimetry will allow comparison of data from different labs, turbidimetry on its own does not provide a full understanding of the LCST behavior of a polymer. For in-depth research on thermoresponsive polymers, systematic measurements on various aspects of the phase transition behavior are needed using different analytical tools, such as NMR spectroscopy, DLS and/or calorimetry. Nonetheless, utilization of the recommended set of conditions allows robust and accurate Tcp determination by turibidimetry, which will facilitate future comparison of Tcp values that are generated in different laboratories around the world.References
- Y. Li, G. H. Gao and D. S. Lee, Adv. Healthcare Mater., 2013, 2, 388–417 CrossRef CAS PubMed.
- P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25–36 RSC.
- C. D. H. Alarcon, S. Pennadam and C. Alexander, Chem. Soc. Rev., 2005, 34, 276–285 RSC.
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173–1222 CrossRef CAS.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Prog. Polym. Sci., 2010, 35, 278–301 CrossRef CAS.
- E. G. Kelley, J. N. L. Albert, M. O. Sullivan and I. I. I. T. H. Epps, Chem. Soc. Rev., 2013, 42, 7057–7071 RSC.
- L. Zhai, Chem. Soc. Rev., 2013, 42, 7148–7160 RSC.
- J. Zhuang, M. R. Gordon, J. Ventura, L. Li and S. Thayumanavan, Chem. Soc. Rev., 2013, 42, 7421–7435 RSC.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Mueller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- Q. Zhang, Z. Y. Hou, B. Louage, D. Y. Zhou, N. Vanparijs, B. G. De Geest and R. Hoogenboom, Angew. Chem., Int. Ed., 2015, 54, 10879–10883 CrossRef CAS PubMed.
- Q. Zhang and R. Hoogenboom, Prog. Polym. Sci., 2015, 48, 122–142 CrossRef CAS.
- C. Weber, R. Hoogenboom and U. S. Schubert, Prog. Polym. Sci., 2012, 37, 686–714 CrossRef CAS.
- D. Schmaljohann, Adv. Drug Delivery Rev., 2006, 58, 1655–1670 CrossRef CAS PubMed.
- F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468–7483 RSC.
- J. Seuring and S. Agarwal, Macromol. Rapid Commun., 2012, 33, 1898–1920 CrossRef CAS PubMed.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC.
- M. A. Ward and T. K. Georgiou, Polymers, 2011, 3, 1215–1242 CrossRef CAS.
- G. Vancoillie, D. Frank and R. Hoogenboom, Prog. Polym. Sci., 2014, 39, 1074–1095 CrossRef CAS.
- A. Halperin, M. Kröger and F. M. Winnik, Angew. Chem., Int. Ed., 2015, 54, 15342–15367 CrossRef CAS PubMed.
- C. Weber, S. Rogers, A. Vollrath, S. Hoeppener, T. Rudolph, N. Fritz, R. Hoogenboom and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 139–148 CrossRef CAS.
- W. Steinhauer, R. Hoogenboom, H. Keul and M. Moeller, Macromolecules, 2010, 43, 7041–7047 CrossRef CAS.
- W. Steinhauer, R. Hoogenboom, H. Keul and M. Moeller, Macromolecules, 2013, 46, 1447–1460 CrossRef CAS.
- R. Hoogenboom, A.-M. Zorn, H. Keul, C. Barner-Kowollik and M. Moeller, Polym. Chem., 2012, 3, 335–342 RSC.
- Q. Zhang, P. Schattling, P. Theato and R. Hoogenboom, Polym. Chem., 2012, 3, 1418–1426 RSC.
- Q. Zhang, P. Schattling, P. Theato and R. Hoogenboom, Eur. Polym. J., 2015, 62, 435–441 CrossRef CAS.
- F. D. Jochum, L. zur Borg, P. J. Roth and P. Theato, Macromolecules, 2009, 42, 7854–7862 CrossRef CAS.
- N. S. Ieong, M. Hasan, D. J. Phillips, Y. Saaka, R. K. O'Reilly and M. I. Gibson, Polym. Chem., 2012, 3, 794–799 RSC.
- R. Hoogenboom, H. M. L. Thijs, M. J. H. C. Jochems, B. M. van Lankvelt, M. W. M. Fijten and U. S. Schubert, Chem. Commun., 2008, 5758–5760 RSC.
- Y. J. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, J. Am. Chem. Soc., 2005, 127, 14505–14510 CrossRef CAS PubMed.
- M. M. Bloksma, D. J. Bakker, C. Weber, R. Hoogenboom and U. S. Schubert, Macromol. Rapid Commun., 2010, 31, 724–728 CrossRef CAS PubMed.
- Q. Zhang, J.-D. Hong and R. Hoogenboom, Polym. Chem., 2013, 4, 4322–4325 RSC.
- S. Monge, S. Antoniacomi, V. Lapinte, V. Darcos and J.-J. Robin, Polym. Chem., 2012, 3, 2502–2507 RSC.
- Q. Zhang, N. Vanparijs, B. Louage, B. G. De Geest and R. Hoogenboom, Polym. Chem., 2014, 5, 1140–1144 RSC.
- M. Sahn, T. Yildirim, M. Dirauf, C. Weber, P. Sungur, S. Hoeppener and U. S. Schubert, Macromolecules, 2016, 49, 7257–7267 CrossRef CAS.
- J. Niskanen, M. Karesoja, T. Rossi and H. Tenhu, Polym. Chem., 2011, 2, 2027–2036 RSC.
- J. Niskanen, C. Wu, M. Ostrowski, G. G. Fuller, H. Tenhu and S. Hietala, Langmuir, 2012, 28, 14792–14798 CrossRef CAS PubMed.
- L. Hou and P. Wu, Soft Matter, 2014, 10, 3578–3586 RSC.
- T. Li, H. Tang and P. Wu, Soft Matter, 2015, 11, 3046–3055 RSC.
- Y. Maeda, H. Mochiduki and I. Ikeda, Macromol. Rapid Commun., 2004, 25, 1330–1334 CrossRef CAS.
- J. F. Lutz, K. Weichenhan, O. Akdemir and A. Hoth, Macromolecules, 2007, 40, 2503–2508 CrossRef CAS.
- M. Karesoja, E. Karjalainen, S. Hietala and H. Tenhu, J. Phys. Chem. B, 2014, 118, 10776–10784 CrossRef CAS PubMed.
- A. Laukkanen, L. Valtola, F. M. Winnik and H. Tenhu, Polymer, 2005, 46, 7055–7065 CrossRef CAS.
- C. Diab, Y. Akiyama, K. Kataoka and F. M. Winnik, Macromolecules, 2004, 37, 2556–2562 CrossRef CAS.
- F. Zeng, Z. Tong and H. Q. Feng, Polymer, 1997, 38, 5539–5544 CrossRef CAS.
- M. V. Deshmukh, A. A. Vaidya, M. G. Kulkarni, P. R. Rajamohanan and S. Ganapathy, Polymer, 2000, 41, 7951–7960 CrossRef CAS.
- H. Kouřilová, J. Št&astná, L. Hanyková, Z. Sedláková and J. Spěváček, Eur. Polym. J., 2010, 46, 1299–1306 CrossRef.
- J. P. Magnusson, A. Khan, G. Pasparakis, A. O. Saeed, W. X. Wang and C. Alexander, J. Am. Chem. Soc., 2008, 130, 10852–10853 CrossRef CAS PubMed.
- Y. Cho, L. B. Sagle, S. Iimura, Y. Zhang, J. Kherb, A. Chilkoti, J. M. Scholtz and P. S. Cremer, J. Am. Chem. Soc., 2009, 131, 15188–15193 CrossRef CAS PubMed.
- P. Kujawa and F. M. Winnik, Macromolecules, 2001, 34, 4130–4135 CrossRef CAS.
- S. Han, M. Hagiwara and T. Ishizone, Macromolecules, 2003, 36, 8312–8319 CrossRef CAS.
- F. J. Hua, X. G. Jiang, D. J. Li and B. Zhao, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2454–2467 CrossRef CAS.
- F. P. Chen, A. E. Ames and L. D. Taylor, Macromolecules, 1990, 23, 4688–4695 CrossRef CAS.
- Y. Matsuda, Y. Miyazaki, S. Sugihara, S. Aoshima, K. Saito and T. Sato, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 2937–2949 CrossRef CAS.
- S.-I. Yamamoto, J. Pietrasik and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 194–202 CrossRef CAS.
- C. Pietsch, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 5653–5656 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7mh00016b |
| This journal is © The Royal Society of Chemistry 2017 |
