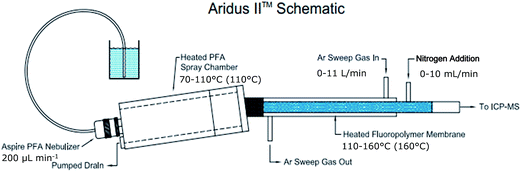
Evaluation of a membrane desolvator for LC-ICP-MS analysis of selenium and platinum species for application to peptides and proteins
Laura Hyrup
Møller
,
Celina Støving
Jensen
,
Tam T. T. N.
Nguyen
,
Stefan
Stürup
and
Bente
Gammelgaard
*
Department of Pharmacy, University of Copenhagen, Denmark. E-mail: bente.gammelgaard@sund.ku.dk
First published on 31st October 2014
Abstract
Analysis of peptides and proteins and their interactions with endogenous elements and metal-based drugs in biological systems demands highly efficient chromatographic systems with the possibility of performing gradient elution to achieve efficient separations. As the detector of choice in metal analysis, the ICP-MS, does not tolerate a high load of organic solvents, these should be removed from the chromatographic eluent prior to the entrance of the ICP-MS detector. The purpose of this study was to evaluate a membrane desolvation (MD) system (Aridus II) for its capability of removing organic solvents from the eluent prior to the ICP-MS introduction and at the same time study the influence on sensitivity and examine if the desolvator system jeopardized the inherent species independent sensitivity of ICP-MS. Selenium and platinum were used as model elements. The MD system was optimized regarding to sweep gas and nitrogen gas flow rates. Sensitivity was highly dependent on the combination of sweep gas flow and N2 addition, and the desolvator system should be optimized for each element. After optimization, 100% methanol and acetonitrile were tolerated by the ICP-MS with an eluent flow rate of 0.2 mL min−1. This opens the possibility of performing LC-ICP-MS analysis by gradient elution with 0–100% organic solvents. Sensitivities were generally increased by employment of the MD system, but the species independent sensitivity of ICP-MS was lost for selenium compounds (trimethylselenonium ion (TMSe), selenomethionine (SeMet), Se-methylselenocysteine (Se-MeSeCys), Se-methylseleno-N-acetyl-galactosamine (SeGalac), selenite and selenate). Sensitivities of the different Se compounds were highly dependent on the desolvator temperature. Different Pt compounds (inorganic Pt-salt, cisplatin and oxaliplatin) showed no species dependent behavior. Linearity was obtained for flow injection analysis of SeMet, TMSe and a selenopeptide in 50% methanol and of inorganic Pt, cisplatin and oxaliplatin in 50% acetonitrile. The optimized system was applied for gradient elution LC-ICP-MS of a cisplatin-albumin adduct and lysate and media samples from a cell uptake study of a selenopeptide.
Introduction
Quantitative analysis of peptides and proteins is becoming increasingly important as the use of these compounds as drugs is steadily increasing.1 In the research area of metallomics, the interactions between endogenous metals as well as metal-based drugs and biomolecules like peptides and proteins are subjects of high importance for elucidating the effects and mechanisms at molecular level. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is the detection method of choice for analysis of the low metal and metalloid levels in biological systems owing to the low detection limits, wide dynamic range and in principle matrix-independent sensitivity of different species of a heteroelement.2 The technique is also increasingly suggested for peptide/protein analysis either exploiting the natural presence of metals or heteroelements as S, P and Se or after tagging the proteins with metals.3 Thus, ICP-MS is often used as a complementary quantitative technique for identification by molecular mass spectrometry. It is therefore desirable to have various LC-methods at disposal which are compatible with both detection systems. Reversed phase high performance liquid chromatography (RP-LC) is the separation method of choice in analysis of drug compounds and biomolecules. The majority of drug molecules and biomolecules have lipophilic properties and efficient separations of the complicated matrices often demands for gradient systems containing up to 90–100% of organic solvent for elution from the chromatographic column. Hence, the general chromatographic separation method for peptides and proteins is RP-LC using a methanol or acetonitrile gradient. While large amounts of organic solvents in the eluent is an advantage in molecular mass spectrometry and cause no problems in UV- and fluorescence detection methods, introduction of high concentrations of organic solvent into the ICP-MS detector is challenging. Using gradient elution, the continuous change in eluent organic solvent concentration may cool the plasma and change the ionization and thereby introduce a risk for changes in sensitivity. Exposure to very high concentrations of organic solvent may even extinguish the plasma. Furthermore, organic solvents may induce carbon build up on the cones in the ICP-MS and decrease sensitivity over time.4,5 Reduction of the solvent load is generally approached either by reducing the flow rate of the eluent or by removing the solvent by cryogenic desolvation or membrane desolvation. Reduced flow rates can be obtained by split flow or use of microbore columns in combination with direct injection nebulizers (DIN), typically operating at eluent flow rates of a few to 50 μL min−1.6,7 However, the signal intensity is influenced by variations in the organic solvent introduced. Cooled spray chambers and addition of oxygen to the nebulizer gas for improved solvent combustion are used routinely in some laboratories.8The ideal approach would be to remove the organic solvent prior to introduction to the ICP-MS. This may be achieved by use of membrane desolvation (MD) systems. Several studies have been performed employing different desolvation systems. By use of an MD concentric nebulizer system, methanol up to 100% and acetonitrile up to 50% with flow rates of 50 μL min−1 and 0.3 mL min−1, respectively have been shown to be tolerated by the ICP-MS.9,10 However, large differences in sensitivity of different analytes of the same element have been reported. Jensen et al. showed loss of bromine, iodine and chloride,9 whereas Bluemlein et al. reported loss of the sulphur containing amino acid methionine.11 In a previous study on a laboratory-build low flow rate MD system running at 45 μL min−1, a constant Se signal for TMSe was obtained in a 10–90% methanol gradient.12 Comparison of 13 selenium species in 50% methanol showed response factors of 0.86–1.13, except for selenite and methaneseleninic acid, for which 95% and 91% were lost, respectively. Different sensitivities of Se compounds have also been observed by use of an ultrasonic nebulizer operating at 120 °C.13
In studies on stability and metabolism in biological systems, the use of the ICP-MS detector is advantageous due to the species independent sensitivity of this technique which makes quantification of unknown degradation products and metabolites possible without access to authentic standards. With focus on such applications, the overall aim of the present work was to evaluate the MD nebulizer system Aridus II for removal of organic solvents in RP-LC-ICP-MS. Partial aims were (i) to optimize the system and evaluate the stability of these optimized settings, (ii) to examine the influence of the desolvation on the sensitivity of different species of the selected elements, (iii) to examine the linearity of selected species and (iv) finally to examine the feasibility of the desolvation system for application in quantitative analysis of low soluble peptides and drug compounds and their reaction products. Selenium and platinum compounds were used as model elements, as different Se compounds have been demonstrated to be differently susceptible to desolvation, while Pt compounds are expected to be unaffected. To our knowledge, this has not been done before.
Experimental
Instrumentation
The desolvation system was equipped with a 0.2 mL min−1 C-flow PFA concentric nebulizer (Cetac Technologies). Unless other stated, spray chamber temperature was 110 °C and desolvator temperature was 160 °C, sweep gas flow was 7 L min−1 and nitrogen gas flow was 5 mL min−1 in methanol (MeOH) and 3 mL min−1 in acetonitrile (MeCN). ICP nebulizer gas flow was 0.9 L min−1.
An Aeris PEPTIDE column, 3.6 μm XB-C18, 100 × 2.1 mm with Security Guard ULTRA C18 (Phenomenex, Torrance, California, USA) was used for Se analysis. The column temperature was 60 °C. An Aeris WIDEPORE column, 3.6 μm XB-C8, 100 × 2.1 mm with Security Guard ULTRA C8 (Phenomenex) was used for Pt analysis. The column temperature was 25 °C. The LC system was coupled to the ICP-MS via the MD system instrument. Data acquisition: dwell time 200 ms, sweeps per reading 1, readings per replicate varying depending on analysis time.
For analysis of the Se compounds lens voltage and ICP RF power were optimized through the desolvation system on a 100 μg L−1 Se standard of TMSe in 5% MeOH + 0.1% CH3COOH. For analysis of the Pt compounds a solution containing 10 μg L−1 Pt in 0.65% HNO3 + 0.1% HCl was used for optimization. Data acquisition: dwell time 200 ms, sweeps per reading 1, readings per replicate: varying depending on analysis time.
Experimental procedures
Optimization of membrane desolvator
Sweep gas and nitrogen gas flows were optimized by direct aspiration (0.2 mL min−1) of standards into the MD unit. Spray chamber and desolvator temperatures were 110 °C and 160 °C, respectively. Se optimization was performed on solutions of TMSe and SeMet (10 μg L−1 Se) in 50% MeOH added 10 μg L−1 Y and Ce. Nitrogen gas flow was maintained constant (0–10 mL min−1) while the sweep gas flow was increased with 1 mL min−1 every second minute in the range of 2–11 L min−1. Mean intensities of Se, Ce and Y were recorded for 2 min.Pt optimization was performed on a solution of cisplatin (6 μg L−1 Pt) in 50% MeCN without N2 addition as described above, except the sweep gas flow was only varied in the range of 6–10 L min−1. Nitrogen optimization was performed with the sweep gas flow maintained at the established optimum by changing the nitrogen gas flow 1 mL min−1 in the range 0–10 mL min−1.
Influence of organic solvent concentration on sensitivity
A solution containing 10 μg L−1 Se as TMSe and elemental standards of Pt, Ce and Y in Milli Q water were analysed by 10 μL flow injections (n = 5) while increasing the MeOH or MeCN concentration in the eluent in steps of 10% in the range 0–100%. Se, Pt, Ce and Y isotopes were monitored and areas calculated.Screening for species dependent sensitivity
10 μg L−1 Se solutions of TMSe, SeMet, Se-MeSeCys, SeGalac and selenite in 30% MeOH + 0.1% CH3COOH, selenate in 20 mM ammonium acetate + 2% MeOH and 10 μg L−1 Pt solutions of cisplatin, oxaliplatin and (NH4)2PtCl6 in 0.65% HNO3 + 0.1% HCl were analysed by 10 μL flow injections with and without the aid of the MD. The FI eluent for Se measurements was 30% MeOH + 0.1% CH3COOH, while the FI eluent for Pt measurements was 5% MeCN in water.Linearity
FI analysis in the range of 10–50 μg L−1 Se and Pt (n = 5) was performed on TMSe, SeMet and PenMSe in an eluent containing 50% MeOH + 0.1% CH3COOH and on cisplatin, oxaliplatin and (NH4)2PtCl6 dissolved in 0.1% HCl + 0.65% HNO3 and an eluent containing 50% MeOH. Linearity of the Se species was further examined in the range 10–1000 μg L−1. Standards were diluted in the eluents.Chromatography
LC-ICP-MS was performed on a solution containing 5 μM PenMSe and SeMet (corresponding to 395 μg L−1 Se) diluted in 2% CH3COOH and a solution containing equal amounts of free cisplatin and cisplatin bound to Human Serum Albumin (12.5 μg L−1 Pt) dissolved in 0.65% HNO3 + 0.1% HCl.A gradient of: 0–2.0 min: 5–50% MeOH, 2.1–3 min: 50% MeOH, 3.1–13.5 min: 5% MeOH in a solution of 0.1% CH3COOH + 0.05% TFA or a gradient of 0–10.0 min: 20–80% MeOH, 10.1–20 min: 20% MeOH in 0.1% CH3COOH + 0.05% TFA were used for Se analysis. A gradient of: 0–5.0 min: 5–95% MeCN, 5.0–5.1 min: 95–5% MeCN, 5.1–13 min: 5% MeCN in a solution of 0.1% TFA was used for Pt analysis. Analysis without the desolvator was performed by cooling the spray chamber to 4 °C.
Results and discussion
Flow rate optimization
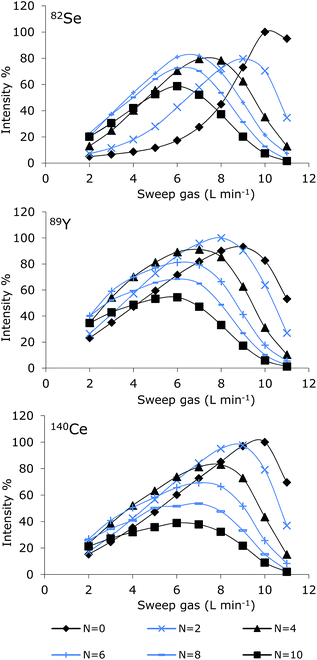
Optimization of the MD system involves optimization of sweep gas flow, nitrogen gas flow, spray chamber temperature and desolvation temperature. The sweep gas is responsible for removal of the organic solvent evaporated and permeated through the membrane. Nitrogen gas is introduced to increase sensitivity due to improved atomization and ionization in the plasma.16 Furthermore, polyatomic interferences caused by the argon plasma may be reduced, and thus reduce the background signal.17 The manufacturer recommends a spray chamber temperature of 110 °C and a desolvator temperature of 160 °C; these settings were used during optimization of the gas flows. Optimization was performed by continuous introduction of solutions containing 50% MeOH with a flow rate of 0.2 mL min−1. Two different selenium solutions containing 10 μg (Se) L−1 of SeMet and TMSe, respectively were applied. Y and Ce (10 μg L−1) were added to both selenium solutions as internal standards. The obtained optimization curves for SeMet are presented in Fig. 2. A similar optimization pattern was obtained for TMSe (not shown).It appears from the figure that for all elements monitored (Se, Y or Ce), decreasing the nitrogen gas flow rate demanded an increased sweep gas flow rate for reaching optimum sensitivity. For nitrogen flow rates in the range 3–10 mL min−1, comparable optimization curve patterns were obtained for all elements (82Se, 89Y and 140Ce). Decreasing the nitrogen flow to 0–2 mL min−1 resulted in much more diverse optimization curves depending on the element monitored. Optimum settings were chosen as the range where minor deviations in settings caused minimum influence on the sensitivity. The optimum sweep gas flow was in general about 7 L min−1, which was following also verified for Pt in an experiment without N2-addition (not shown). The optimum nitrogen gas flow differed depending on the element monitored.
The optimum nitrogen flow rate was 5–6 mL min−1 for Se, 3–4 mL min−1 for Y and 3 mL min−1 for Ce and Pt. Ce and Y were added to the selenium solutions as possible internal standards. However, the signal intensity of 89Y and 140Ce varied differently from the Se intensities and correction by these elements was therefore not possible.
Addition of small amounts of nitrogen to the nebulizer (or the central channel of the plasma) changes the plasma characteristics leading to an improved energy transfer effect of the plasma.18 Adding nitrogen to the plasma has been reported to widen the central channel of the ICP and therefore the sampling depth, applied power and gases flow rates should be optimized for each composition.16 These parameters were not optimized for each addition of nitrogen in this study, thus higher sensitivities may have been obtained after optimization. As the main purpose of this study was to obtain stable plasma after introduction of large amounts of organic solvent rather than achieve maximum sensitivity, it was concluded that a sweep gas flow of 7 L min−1 would fit most purposes and the nitrogen gas flow rate could be optimized for each element.
Influence of organic solvent concentration on sensitivity
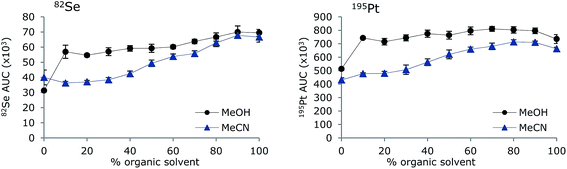
To investigate if the removal of organic solvent by the MD system was sufficient to avoid changes in sensitivity with increasing amounts of organic solvent, a standard containing 10 μg L−1 Se (TMSe), Pt, Ce and Y inorganic standards was analysed by FI-ICP-MS using eluents containing increasing amounts of either MeOH or MeCN (0–100%). The results for Se and Pt are shown in Fig. 3. Employment of the MD permitted introduction of up to 100% of organic modifier, either MeOH or MeCN, without extinguishment of the plasma. Thus, (isocratic) LC-ICP-MS analysis is possible with large amounts of organic solvents in the eluent. However, the sensitivities of both Se and Pt were generally increased when increasing the concentration of MeOH and MeCN, which is in contrast to analysis without MD, where the sensitivity decreases with increasing amounts of organic solvents. The sensitivity is represented by means and standard deviations of areas of FI signals; thus any change in background would be accounted for. The baseline did not change noticeable during the experiment and the low standard deviations (shown as uncertainty bars in the figure) indicate that the increased sensitivity is not due to memory effects. This experimental set-up mimics the use of gradient elution, thus, an increased sensitivity should be expected during gradient elution. Optimization of MD and ICP gas flows for each change in eluent composition may result in constant sensitivity. However, this would not be the situation in LC-ICP-MS analysis, where fixed settings of the MD would be applied.The increase in sensitivity with increasing organic solvent load may be explained by a combination of carbon-enhanced signal response as all organic solvent may not be evaporated in the higher concentrations19 and improved nebulization with higher organic solvent concentrations.20 Furthermore, it has been shown that the sensitivity of volatile Se species is several times larger than the sensitivity of non-volatile compounds.21 The carbon enhancement effect has been shown to be dependent on the ionization potentials of the element and the effect most pronounced for elements with ionization potentials of 9–11 eV.22 As Se and Pt have ionization potentials of 9.8 eV and 9.0 eV, respectively, this could be an explanation for these elements, but the same effect was observed for Ce and Y having ionization potentials of 5.5 eV and 6.2 eV, respectively (results not shown). Thus, carbon enhancement is not the only explanation and improved aerosols may be the main explanation, as the small fraction of organic solvent remaining after passage of the MD will enhance the signal.23
Species dependent sensitivity
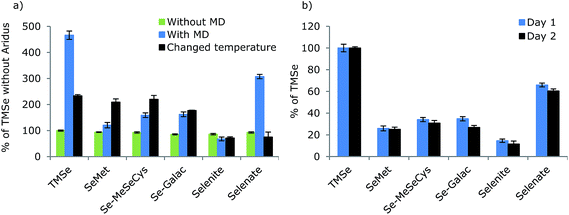
As the MD system was intended for studies on stability and metabolism, species independent sensitivity is important to quantify unknown compounds. In order to examine whether the Se response differed depending on the species introduced, different species with different physical chemical properties were analysed by FI in an eluent containing 30% MeOH, before and after employment of the MD. The results are shown in Fig. 4 together with the day-to-day reproducibility. Before employment of the MD, the signal intensities of the selenium species were equal as RSD between species means was 5.3%. Thus, the species independent sensitivity of the ICP-MS was verified.Employing the desolvator, resulted in increased sensitivity of all selenium species except selenite. However, the sensitivity of the selenium compounds differed remarkably using the MD. The significant differences in responses from the selenium species showed that the favorable species independent quantification characteristic for ICP-MS was lost for selenium species when the MD was applied making quantification of unknown compounds impossible.
The ionic compounds, TMSe and selenite showed large improvement in sensitivity, while the signals from the amino acids, SeMet and Se-MeSeCys and the selenosugar, SeGalac improved to a minor extent.
The different responses may be due to volatilization of some of the selenium compounds induced by the desolvator temperature of 160 °C. Previous experiments have shown loss of selenite by use of a membrane desolvation ultrasonic nebulizer system (U-6000 AT+, Cetac).12 However, the differences between TMSe and SeMet, Se-MeSeCys, SeGalac and selenate observed were not as pronounced as the results presented in Fig. 4a. By use of an identical system (Aridus II), Bluemlein et al. reported loss of methionine, detected as sulphur, using a desolvator temperature of 160 °C.11 As SeMet and Met possess several similar properties, the same observation may be expected from SeMet.
It was attempted to examine the impact of temperature settings of spray chamber and desolvator for optimizing these parameters systematically. This was challenging as the optimal settings and optimization curves varied between days. This may be due to a sub-optimal temperature control of the system. However, it was evident that change of the desolvator and spray chamber temperatures did influence the responses. Results from temperature settings of the spray chamber of 100 °C and the desolvator of 120 °C are shown in Fig. 4 (black).
Lowering of spray chamber and desolvator temperatures changed the relative responses between selenium species remarkably, thus species sensitivities were indeed temperature dependent. While the ionic compounds, TMSe and selenate, benefited from high temperatures, the sensitivity of the amino acids, SeMet and Se-MeSeCys decreased with increasing temperature. It was therefore assessed that similar sensitivities of all species was not obtainable at the temperature combinations investigated. Species independent quantification of low molecular selenium species is therefore not possible using the MD system and calibration with species specific standards is necessary for quantitative analysis. A fixed spray chamber temperature setting of 110 °C and desolvator temperature of 160 °C were applied in all following experiments as the higher temperatures generally resulted in higher sensitivity.
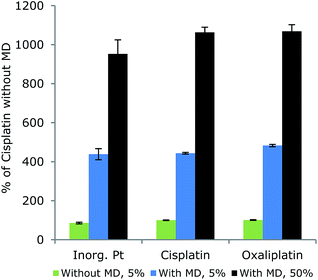
For comparison, the responses of different platinum compounds were examined with and without the aid of the MD (Fig. 5). The platinum signals increased considerably when applying the MD, but the different species responded similarly as the RSD between species means was 9.0% in 5% MeCN without the MD system, and 5.3% and 6.3% in 5% and 50% MeCN, respectively with the MD system. Furthermore, responses increased when the eluent MeCN concentration was increased from 5% to 50% MeCN as the MD was optimized for 50% MeCN in the eluent.
Linearity and precision
FI-ICP-MS analysis in the concentration range 10–50 μg L−1 Se and Pt was performed to test linearity and precision. SeMet and TMSe were included as LMW species and a selenium containing peptide, PenMSe as a representative of larger, more lipophilic compounds. The Pt compounds included the same inorganic salt and drug compounds as used for testing of sensitivity. The analytical figures of merit are shown in Table 1.| Compound | Equation | R 2 | RSD% (10 μg L−1) | RSD% (50 μg L−1) | Estimated LOD (μg L−1) |
|---|---|---|---|---|---|
| SeMet | Y = 2999x − 7355 | 0.9763 | 8.7 | 4.0 | 2.5 |
| PenMSe |
Y = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515x − 6279 515x − 6279 |
0.9962 | 2.0 | 0.5 | 0.6 |
| TMSe |
Y = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 895x − 10 895x − 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 169 169 |
0.9992 | 1.4 | 0.6 | 0.4 |
| Inorg. Pt |
Y = 219![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 145x − 111 145x − 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 151 151 |
0.9982 | 1.6 | 3.2 | 3.4 |
| Oxaliplatin |
Y = 296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093x − 266 093x − 266![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668 668 |
0.9991 | 0.3 | 1.0 | 0.3 |
| Cisplatin |
Y = 254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 796x − 117 796x − 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 939 |
0.9997 | 0.2 | 2.2 | 0.4 |
Satisfactory linearity was obtained for the Pt compounds, TMSe and PenMSe, while the correlation coefficient for SeMet demonstrated poor linearity in this concentration range. Furthermore, the response from the different Se species varied remarkably as observed in the previous screening. It is noticed that the largest standard deviations were observed for the more volatile SeMet. Estimated LODs calculated based on 3 times the standard deviation at 10 μg L−1 (n = 5) are also shown in Table 1. The data for platinum and TMSe are comparable to other data without the use of a MD system, while the LOD for SeMet is higher owing to loss of signal and larger variations. Expanding the linear range to 10–1000 μg L−1 (6 data points) resulted in correlation coefficients of 0.9992, 0.9991 and 0.9999 for SeMet, PenMSe and TMSe, respectively.
Chromatography
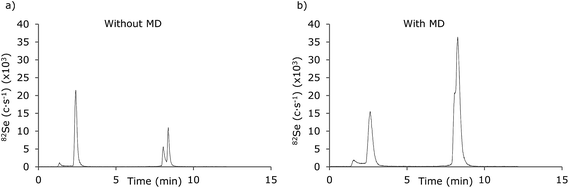
A major advantage in applying an MD system will be the opportunity of hyphenating gradient RP-LC systems to the ICP-MS as this will expand the possibilities in speciation analysis of biomolecules with ICP-MS detection. The chromatographic performances of a gradient LC-ICP-MS system with and without the application of the MD was compared for a sample containing 5 μM of SeMet and a Se-peptide (PenMSe) analysed in an eluent containing 0.1% CH3COOH + 0.05% TFA applying a 5–50% MeOH gradient for the first 3 min (Fig. 6). When ICP-MS is used as detection method, volatile reagents should be chosen. These reagents may influence the signals by e.g. changing the drop size during nebulization. The reagents used in this study were 0.1% acetic acid and 0.05% TFA and were not considered to influence the nebulization noticeably in the methanol gradient of 5–50%. Considerable differences were observed between the chromatogram obtained without MD (Fig. 6a) and with MD (Fig. 6b). The resolution and peak shapes were remarkably better when the MD was not applied. At the same time, the signal intensities of both SeMet and PenMSe were improved while the relative intensity of SeMet and PenMSe was changed as the SeMet signal decreased while the PenMSe signal intensity was improved.The instability of SeMet in the desolvator was further established from the repeatability of the chromatographic method, as the RSD of 5 injections of SeMet was 22.9%, while the RSD for the peptide was 0.8% (n = 5). For comparison, the relative standard deviations were 4.2% and 5.3% (n = 5) when the MD was not applied.
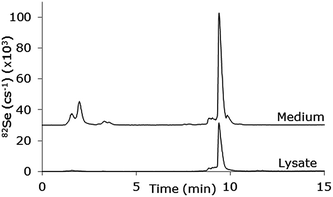
The poorer chromatography obtained with the use of the MD system may be explained by the increase in dead volume when the sample passes through the desolvation membrane. However, this can be improved by adjustment of the gradient. This has been done in Fig. 7 which shows a chromatogram run with a 20–80% MeOH gradient in 0.1% CH3COOH + 0.05% TFA applied during 10 min. A calibration curve for the selenopeptide using this gradient showed linearity in the range 10–500 μg L−1 (R2 = 0.9987), a precision of 7.0% and an estimated LOD of 1.7 μg L−1 determined at the 10 μg L−1 level (N = 5). The column recovery for the selenopeptide was 101.5% ± 1.1% (n = 3). The system was applied for cell uptake studies of a synthetic Se containing peptide15 and the chromatogram shows examples of analysis of cell lysate containing the intact peptide and cell medium containing intact peptide as well as some degradation products eluting around 2 min. The content of the intact peptide in the lysate corresponded to 14 μg L−1 and the content of the cell medium corresponded to 406 μg L−1, but it was not possible to quantify the degradation products due to lack of an authentic standard.
The time based sensitivity was checked each day the system was used for LC-analyses by chromatographic analysis of a standard in the beginning and in the end of the day. The sensitivity decrease during the day was about 2%, and no build-up of carbon on the cones was observed. Thus, the main advantage of the desolvation system is that signal suppression from large amounts of organic solvents is avoided, which results in larger sensitivity for compounds eluting during the gradient.
An example of the use of a 5–95% gradient of MeCN for separation of free cisplatin and protein-bound cisplatin is shown in Fig. 8. The relative standard deviations were 1.5% and 2.6% for free and protein-bound cisplatin, respectively (n = 3). This demonstrates that the LC-MD-ICP-MS system works for the most challenging gradients necessary for separation of large biomolecules.
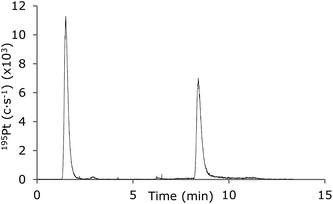 | ||
| Fig. 8 LC-ICP-MS chromatograms of cisplatin and HSA-cisplatin (12.5 μg L−1 Pt). Gradient: 0–5 min 5–95% MeCN in 0.1% TFA. Sweep gas flow: 7 L min−1, N2 flow: 3 mL min−1. | ||
Conclusion
The purpose of this study was to evaluate a membrane desolvation system for its capability of removing organic solvents from the eluent prior to the ICP-MS introduction and at the same time study the influence on sensitivity of different species. Selenium and platinum were used as model elements.After optimization of the MD system, 100% methanol and acetonitrile was tolerated by the ICP-MS at an eluent flow rate of 0.2 mL min−1 for both elements. This opens the possibility of performing LC-ICP-MS analysis by gradient elution with 0–100% organic solvent gradients although a slight increase in sensitivity with increasing amounts of organic solvent was observed. Sensitivity was in general increased by applying the MD system, but LODs were not improved owing to larger variations. The species independent sensitivity of the ICP-MS was lost for selenium, but maintained for platinum showing a 10-fold increase in sensitivity by use of the MD.
In conclusion, the MD-LC-ICP-MS is applicable for platinum speciation as well as selenium speciation using gradient elution. Species unspecific calibration curves could be used for quantitative Pt analysis as different Pt compounds showed similar sensitivities, while authentic Se standards are necessary for quantification of Se compounds as large variations in sensitivity was observed for different Se species. Hence, the major advantage of the MD system is that signal suppression from large amounts of organic solvents is avoided, which results in larger sensitivity for compounds eluting during the gradient.
References
- D. J. Craik, D. P. Fairlie, S. Lira and D. Price, Chem. Biol. Drug Des., 2013, 81, 136–147 CAS.
- D. Pröfrock and A. Prange, Appl. Spectrosc., 2012, 66, 843–868 CrossRef PubMed.
- J. Bettmer, M. Montes-Bayon, J. Encinar, M. L. Fernandez-Sanches, M. D. F. Fernandez de la Campa and A. Sanz-Medel, J. Proteomics, 2014, 72, 989–1005 CrossRef PubMed.
- K. Sutton and J. Caruso, J. Chromatogr. A, 1999, 856, 243–258 CrossRef CAS.
- B. Gammelgaard and B. J. Jensen, J. Anal. At. Spectrom., 2007, 22, 235–249 RSC.
- B. W. Acon, J. A. McLean and A. Montaser, J. Anal. At. Spectrom., 2001, 16, 852–857 RSC.
- M. Wind, A. Eisenmenger and W. D. Lehman, J. Anal. At. Spectrom., 2002, 17, 21–26 RSC.
- A. Polatajko, M. Sliwka-Kaszynska, M. Dernovics, R. Ruzik, J. R. Encinar and J. Szpunar, J. Anal. At. Spectrom., 2004, 19, 114–120 RSC.
- B. P. Jensen, B. Gammelgaard, S. H. Hansen and J. V. Andersen, J. Anal. At. Spectrom., 2003, 18, 891–896 RSC.
- P. Marshall, O. Heudi, S. Mckeown, A. Amour and F. Abou-Shakra, Rapid Commun. Mass Spectrom., 2002, 16, 220–228 CrossRef CAS PubMed.
- K. Bluemlein, E. M. Krupp and J. Feldmann, J. Anal. At. Spectrom., 2009, 24, 108–113 RSC.
- L. Bendahl and B. Gammelgaard, J. Anal. At. Spectrom., 2005, 20, 410–416 RSC.
- B. Gammelgaard and O. Jøns, J. Anal. At. Spectrom., 2000, 15, 499–505 RSC.
- S. J. Foster and H. E. Ganther, Anal. Biochem., 1984, 137, 205–209 CrossRef CAS.
- L. H. Møller, C. Gabel-Jensen, H. Franzyk, J. Bahnsen, S. Stürup and B. Gammelgaard, Metallomics, 2014, 6, 1639–1647 RSC.
- G. L. Scheffler and D. Pozebon, Anal. Methods, 2014, 6, 6170–6182 RSC.
- S. F. Durrant, Fresenius. J. Anal. Chem., 1993, 347, 389–392 CrossRef CAS.
- Z. Hu, S. Gao, Y. Liu, S. Hu, H. Chen and H. Yuan, J. Anal. At. Spectrom., 2008, 23, 1093–1101 RSC.
- E. H. Larsen and S. Stürup, J. Anal. At. Spectrom., 1994, 9, 1099–1105 RSC.
- J. A. McLean, M. G. Minnich, L. Iacone, H. Liu and A. Montaser, J. Anal. At. Spectrom., 1998, 13, 829–842 RSC.
- D. Juresa, D. Kuehnelt and K. A. Francesconi, Anal. Chem., 2006, 78, 8569–8574 CrossRef CAS PubMed.
- P. Allain, L. Jaunault, J. Mermet and T. Delaporte, Anal. Chem., 1991, 63, 1497–1498 CrossRef CAS.
- S. J. Kumar and S. Gangadharan, J. Anal. At. Spectrom., 1999, 14, 967–971 RSC.
| This journal is © The Royal Society of Chemistry 2015 |