Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Five-coordinate MII-semiquinonate (M = Fe, Mn, Co) complexes: reactivity models of the catechol dioxygenases†
Peng
Wang
,
Glenn P. A.
Yap
and
Charles G.
Riordan
*
Department of Chemistry & Biochemistry, University of Delaware, Newark, DE 19716, USA. E-mail: riordan@udel.edu; Tel: +1 302 831 1073
First published on 23rd April 2014
Abstract
A series of five-coordinate MII-semiquinonate (M = Fe, Mn, Co) complexes were synthesized and characterized, including the first example of a mononuclear FeII-semiquinonate. Intermediates were observed in the reactions of MII-phenSQ (M = Fe, Co) with O2. Evidence for the relevance of these intermediates to the intradiol catechol dioxygenases was obtained by characterization of the oxidized semiquinone-derived product, muconic anhydride, resulting from the reaction of [PhTttBu]CoII(3,5-DBSQ) with O2.
Intradiol catechol dioxygenases are non-heme iron enzymes that catalyze the oxidative cleavage of the C1–C2 bond of catechols.1 The state of the enzyme that reacts with O2 contains a five-coordinate metal site. The activity of the enzyme and its synthetic analogs has been attributed to the partial FeII-semiquinonate (SQ) character within the FeIII-catecholate species,2 which results in the formation of a FeIII-alkylperoxy intermediate upon addition of O2. The extradiol catechol dioxygenases contain iron or manganese active sites and catalyze the oxidative cleavage of C2–C3 bond of catechols.1,3 During catalytic turnover, superoxo-FeII-semiquinonate and FeII-alkylperoxy intermediates have been detected.4 Interestingly, comparable extradiol-cleaving activities were obtained by substituting the native iron with manganese or cobalt suggesting the intermediacy of superoxo-MII-semiquinonate species (M = Fe, Mn, Co) in the catalytic cycles.5 Moreover, reactions of redox-active ligand complexes with dioxygen have received recent attention due to their potential utility in stoichiometric and catalytic transformations.6
In spite of the implications of the FeII-semiquinonate species in both intradiol and extradiol dioxygenases, to the best of our knowledge, well characterized mononuclear FeII-semiquinonate complexes are unknown. Related complexes were reported recently by Fiedler and co-workers, including a mononuclear FeII-(imino)semiquinonate complex7 and a semiquinonate-bridged diiron(II) complex.8 Herein, we report the first well characterized mononuclear FeII-semiquinonate complex and its MnII and CoII analogues – [PhTttBu]M(phenSQ) (M = Fe, Mn, Co, PhTttBu = phenyltris((tert-butylthio)methyl)borate, phenSQ = 9,10-phenanthrenesemiquinonate). The suitability of these complexes in modelling catalytic intermediates of the intradiol dioxygenases was evaluated by O2 reactivity studies. A related complex, [PhTttBu]Co(3,5-DBSQ) (3,5-DBSQ = 3,5-di-tert-butyl-1,2-semiquinonate) exhibited the intradiol reactivity, suggesting the relevance of the observed intermediates to the intradiol catechol dioxygenases.
[PhTttBu]M(phenSQ) were synthesized using two complementary preparative routes, Scheme 1. Metathesis of [PhTttBu]MI (M = Fe, Mn, Co) with Tl(phenSQ) yielded [PhTttBu]M(phenSQ) in excellent yields (89–95%). A similar method was applied to the synthesis of [PhTttBu]Co(3,5-DBSQ) by replacing Tl(phenSQ) with Tl(3,5-DBSQ). Alternatively, the oxidative addition of phenQ to monovalent metal precursors, [PhTttBu]M(PMe3) (M = Fe, Co)9 afforded [PhTttBu]M(phenSQ) (M = Fe, Co) in good yields (65–70%). High resolution mass spectroscopy (HRMS) data combined with 1H NMR spectral analyses confirmed the composition and purity of the [PhTttBu]MII-(SQ) complexes (Fig. S4–S7, S11–S14, ESI†).
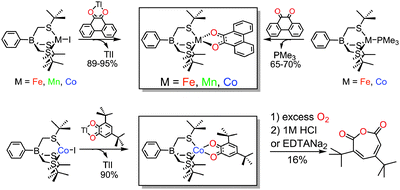 | ||
| Scheme 1 Synthetic routes to [PhTttBu]M(SQ) complexes (M = Fe, Mn, Co) and O2 reactivity of [PhTttBu]Co(3,5-DBSQ). | ||
The electronic spectra of the [PhTttBu]M(SQ) complexes are contained in Fig. 1. [PhTttBu]Fe(phenSQ) shows two features of low intensity at 600 nm (ε = 868 M−1 cm−1) and 935 nm (ε = 539 M−1 cm−1), the latter being consistent with a typical ligand field transition for five-coordinate, high-spin FeII complexes.10 No apparent ligand field transition was observed for [PhTttBu]Mn(phenSQ), indicating a high-spin MnII center. [PhTttBu]Co(phenSQ) exhibits two features at 683 (ε = 1310 M−1 cm−1) and 803 (ε = 1290 M−1 cm−1), both of which agree well with the ligand field transitions of five-coordinate, high-spin CoII.11 Unlike the previously reported [TpCum,Me]Co(3,5-DBSQ),12 the ligand field transitions of [PhTttBu]Co(3,5-DBSQ) were not observed in the spectrum presumably due to the overlap with the intense band at 784 nm (ε = 5470 M−1 cm−1).13
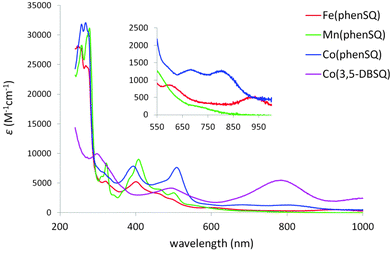 | ||
| Fig. 1 Electronic spectra of the [PhTttBu]M(SQ) complexes. The insert highlights ligand field transitions. | ||
The description of the metal complex electronic structures as MII-SQ is supported by their infrared spectra. In contrast to the IR spectra of the respective [PhTttBu]MI complexes, [PhTttBu]M(phenSQ) exhibit intense bands between 1500 cm−1 and 1600 cm−1, which are consistent with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches of phenSQ (Fig. S15–S17, ESI†).14 Although [PhTttBu]CoI also shows two bands in the 1400–1450 cm−1 range, the much more intense band at 1462 cm−1 of [PhTttBu]Co(3,5-DBSQ) is tentatively assigned to the ν(C
O stretches of phenSQ (Fig. S15–S17, ESI†).14 Although [PhTttBu]CoI also shows two bands in the 1400–1450 cm−1 range, the much more intense band at 1462 cm−1 of [PhTttBu]Co(3,5-DBSQ) is tentatively assigned to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of 3,5-DBSQ (Fig. S18, ESI†).15
O) of 3,5-DBSQ (Fig. S18, ESI†).15
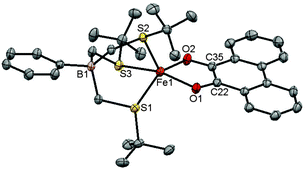
All the complexes are five-coordinate as deduced by X-ray diffraction analyses (Fig. S20, ESI†). The coordination geometry of the [PhTttBu]Mn(phenSQ) lies between trigonal-bipyramidal and square pyramidal (τ5 = 0.58),16 whereas the geometries of [PhTttBu]Fe(phenSQ) (τ5 = 0.14), [PhTttBu]Co(phenSQ) (τ5 = 0.01) and [PhTttBu]Co(3,5-DBSQ) (τ5 = 0.17) are best described as distorted square pyramids. Key bond lengths support the redox state assignment of the bidentate ligand as semiquinonate, Table 1 and Fig. S20 (ESI†). For the MII-phenSQ complexes, the C–O distances are in the range of 1.28–1.30 Å and C–C distances are in the range of 1.41–1.44 Å. These bond distances are characteristic of a bound phenSQ ligand.14a,17 For [PhTttBu]Co(3,5-DBSQ), the average C–O distance is 1.314(2) Å. This distance is certainly among the longest C–O distances for 3,5-DBSQ, but is not unprecedented.18 Furthermore, the “four long/two short” quinoid distortion in the semiquinonate ring further supports its electronic structure description. All complexes are air-sensitive in solution as demonstrated by O2 reactivity studies, vide infra, which can be rationalized by the MII-SQ charge distribution.2a–c Space filling models (Fig. S21, ESI†) indicate O2 accessibility to both the metal centers and the semiquinonate ligands.
The MII-SQ complexes showed paramagnetic 1H NMR spectra. Their effective magnetic moments measured in solution by the Evans method are [PhTttBu]Mn(phenSQ) and [PhTttBu]Co(3,5-DBSQ) μeff = 5.01(6) μB and 2.91(2) μB, respectively. These values are very close to the spin-only values for S = 2 and S = 1 systems, indicating strong antiferromagnetic coupling between high-spin divalent metal centers and the SQ radicals. [PhTttBu]Fe(phenSQ) and [PhTttBu]Co(phenSQ) display μeff = 4.65(2) μB and 3.43(3) μB, respectively. These values are higher than the spin-only values for S = 3/2 and S = 1 systems, but lower than expected for non-spin coupled systems,12 suggesting either weaker antiferromagnetic coupling or more likely, non-negligible spin–orbit coupling.
The cyclic voltammograms (CV) were measured in THF to evaluate the redox characteristics of the MII-SQ complexes (Fig. S22, ESI†). [PhTttBu]Co(phenSQ) and [PhTttBu]Co(3,5-DBSQ) exhibit reversible reduction events at −0.97 V and −0.82 V (vs. Fc+/0) which are assigned as ligand-centered reductions, phenSQ/phenCat and 3,5-DBSQ/DBCat, respectively. These redox potentials match very well with the values reported for Me4cyclam supported CoII-semiquinonate complexes (−1.00 V for phenSQ/phenCat and −0.85 V for 3,5-DBSQ/DBCat).19 The redox events for [PhTttBu]M(phenSQ) (M = Mn, Fe) are irreversible on the electrochemical timescale, exhibiting Ec values of −1.17 V for [PhTttBu]Fe(phenSQ) and −1.11 V for [PhTttBu]Mn(phenSQ), assigned as phenSQ/phenCat reductions. The trend in reduction potentials among the [PhTttBu]M(phenSQ) species, Fe < Mn < Co, indicates the Co complex is most readily reduced. The oxidations of [PhTttBu]Co(phenSQ) and [PhTttBu]Co(3,5-DBSQ) at 0.26 V and 0.47 V, respectively are also irreversible.
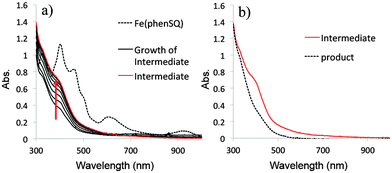
To evaluate the utility of five-coordinate MII-SQ complexes to model putative intermediates in catechol dioxygenase catalysis, electronic spectroscopy was employed to monitor the reaction of [PhTttBu]M(phenSQ) with O2.20 Even at very low temperature (−90 °C) rapid spectroscopic changes were observed upon addition of O2 to [PhTttBu]M(phenSQ) (M = Fe, Co), producing intermediates which decay to the thermodynamic products in 5–10 minutes upon warming to higher temperatures (Fig. 2 and Fig. S28, ESI†).21 Indirect evidence for the relevance of these synthetic intermediates to the intradiol dioxygenases was obtained using [PhTttBu]Co(3,5-DBSQ). Upon reaction with O2 in THF for 16 hours, [PhTttBu]Co(3,5-DBSQ) produced the intradiol cleavage product, muconic anhydride in 16% yield, Scheme 1 (see ESI† for details). This result, together with a previous discovery that the five coordinate complex [TpiPr2]Mn(3,5-DBSQ) also produces the intradiol product,22 suggests that the semiquinonate character of the ligand may contribute to the intradiol cleaving reactivity, even for different metals. To the best of our knowledge, this is the first example of intradiol reactivity of a CoII(3,5-DBSQ) complex.23
In summary, a series of five-coordinate MII-semiquinonate (M = Fe, Mn, Co) complexes supported by the tris(thioether) ligand [PhTttBu] were synthesized and characterized, including the first example of a mononuclear FeII-semiquinonate complex. While [PhTttBu]Co(3,5-DBSQ) was found to be a reactivity model for the intradiol catechol dioxygenases, [PhTttBu]M(phenSQ) (M = Fe, Co) serve as potential precursors to model the putative intermediates in intradiol dioxygenase catalysis. Our current efforts are focused on additional spectroscopic and structural characterization of the intermediates produced from the low temperature reactions of [PhTttBu]M(phenSQ) with O2. Also under investigation are the reactions of [PhTttBu]M(phenSQ) with superoxide to model intermediate(s) of relevance in extradiol dioxygenase catalysis.
The US National Science Foundation supported this research program via CHE-1112035 to CGR. The X-ray diffractometer (CHE-1048367) and LIFDI mass spectrometer (CHE-1229234) acquisitions were supported by NSF.
Notes and references
-
(a) T. D. H. Bugg and S. Ramaswamy, Curr. Opin. Chem. Biol., 2008, 12, 134–140 CrossRef CAS PubMed
; (b) M. Costas, M. P. Mehn, M. P. Jensen and L. Que, Jr., Chem. Rev., 2004, 104, 939–986 CrossRef CAS PubMed
.
- For experimental work, see:
(a) D. D. Cox and L. Que, Jr., J. Am. Chem. Soc., 1988, 110, 8085–8092 CrossRef CAS
; (b) H. G. Jang, D. D. Cox and L. Que, Jr., J. Am. Chem. Soc., 1991, 113, 9200–9204 CrossRef CAS
; (c) T. Funabiki, A. Fukui, Y. Hitomia, M. Higuchia, T. Yamamotoa, T. Tanaka, F. Tani and Y. Naruta, J. Inorg. Biochem., 2002, 91, 151–158 CrossRef CAS
. For theoretical work, see: ; (d) T. Funabiki and T. Yamazaki, J. Mol. Catal. A: Chem., 1999, 150, 37–47 CrossRef CAS
; (e) N. Nakatani, Y. Hitomi and S. Sakaki, J. Phys. Chem. B, 2011, 115, 4781–4789 CrossRef CAS PubMed
.
- J. D. Lipscomb, Curr. Opin. Struct. Biol., 2008, 18, 644–649 CrossRef CAS PubMed
.
- E. G. Kovaleva and J. D. Lipscomb, Science, 2007, 316, 453–457 CrossRef CAS PubMed
.
-
(a) J. P. Emerson, E. G. Kovaleva, E. R. Farquhar, J. D. Lipscomb and L. Que, Jr., Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7347–7352 CrossRef CAS PubMed
; (b) A. J. Fielding, E. G. Kovaleva, E. R. Farquhar, J. D. Lipscomb and L. Que, Jr., JBIC, J. Biol. Inorg. Chem., 2011, 16, 341–355 CrossRef CAS PubMed
.
-
(a) S. Mukherjee, T. Weyhermuller, E. Bothe, K. Wieghardt and P. Chaudhuri, Dalton Trans., 2004, 3842–3853 RSC
; (b) C. A. Lippert, S. A. Arnstein, C. D. Sherrill and J. D. Soper, J. Am. Chem. Soc., 2010, 132, 3879–3892 CrossRef CAS PubMed
; (c) C. J. Rolle, III, K. I. Hardcastle and J. D. Soper, Inorg. Chem., 2008, 47, 1892–1894 CrossRef PubMed
.
- M. M. Bittner, S. V. Lindeman and A. T. Fiedler, J. Am. Chem. Soc., 2012, 134, 5460–5463 CrossRef CAS PubMed
.
- A. E. Baum, S. V. Lindeman and A. T. Fiedler, Chem. Commun., 2013, 49, 6531–6533 RSC
.
-
(a) M. T. Mock, C. V. Popescu, G. P. A. Yap, W. G. Dougherty and C. G. Riordan, Inorg. Chem., 2008, 47, 1889–1891 CrossRef CAS PubMed
; (b) J. A. Dupont, G. P. A. Yap and C. G. Riordan, Inorg. Chem., 2008, 47, 10700–10707 CrossRef CAS PubMed
.
- E. I. Solomon, T. C. Brunold, M. I. Davis, J. N. Kemsley, S. K. Lee, N. Lehnert, F. Neese, A. J. Skulan, Y. S. Yang and J. Zhou, Chem. Rev., 2000, 100, 235–349 CrossRef CAS PubMed
.
-
(a) M. Ciampolini, N. Nardi and G. P. Speroni, Coord. Chem. Rev., 1966, 1, 222–233 CrossRef CAS
; (b) Z. Dori and H. B. Gray, Inorg. Chem., 1968, 7, 889–892 CrossRef CAS
.
- M. Ruf, B. C. Noll, M. D. Groner, G. T. Yee and C. G. Pierpont, Inorg. Chem., 1997, 36, 4860–4865 CrossRef CAS PubMed
.
- Analogous to [PhTttBu]Co(3,5-DBSQ), an intense band at 818 nm was observed for [PhTttBu]Co(phenSQ) at −90 °C.
-
(a) A. Caneschi, A. Dei, F. Fabrizi de Biani, P. Gutlich, V. Ksenofontov, G. Levchenko, A. Hoefer and F. Renz, Chem. – Eur. J., 2001, 7, 3926–3930 Search PubMed
; (b) S. Roy, B. Sarkar, D. Bubrin, M. Niemeyer, S. Zalis, G. K. Lahiri and W. Kaim, J. Am. Chem. Soc., 2008, 130, 15230–15231 Search PubMed
.
-
(a) F. Hartl and A. Vlcek, Jr., Inorg. Chem., 1996, 35, 1257–1265 Search PubMed
; (b) P. A. Wicklund, L. S. Beckmann and D. G. Brown, Inorg. Chem., 1976, 15, 1996–1997 Search PubMed
.
- A. W. Addison, T. N. Rao, J. Reedijk, J. Vanrijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 Search PubMed
.
- C. G. Pierpont and R. M. Buchanan, Coord. Chem. Rev., 1981, 38, 45–87 Search PubMed
.
- M. Graf, G. Wolmershäser, H. Kelm, S. Demeschko, F. Meyer and H.-J. Krüger, Angew. Chem., Int. Ed., 2010, 49, 950–953 Search PubMed
.
- A. Caneschi, A. Dei, D. Gatteschi and V. Tangoulis, Inorg. Chem., 2002, 41, 3508–3512 Search PubMed
.
- A (triphos)Ir(O2)(phenSQ) complex modeling the intradiol dioxygenase intermediate was formed from the reaction of [(triphos)Ir(phenCat)]BPh4 with O2. See: P. Barbaro, C. Bianchini, C. Mealli and A. Meli, J. Am. Chem. Soc., 1991, 113, 3181–3183 Search PubMed
.
- No intermediate was observed upon reaction of [PhTttBu]Mn(phenSQ) with O2 at −90 °C.
- H. Komatsuzaki, A. Shiota, S. Hazawa, M. Itoh, N. Miyamura, N. Miki, Y. Takano, J. Nakazawa, A. Inagaki, M. Akita and S. Hikichi, Chem. – Asian J., 2013, 8, 1115–1119 Search PubMed
.
- A tetramine-CoII(3,5-DBSQ) complex reacted with O2, leading to the ring cleavage of the catechol. However, the organic products were not unambiguously characterized as intradiol or extradiol products. See: S. Nakashima, H. Ohya-Nishiguchi, N. Hirota, S. Tsuboyama and T. Chijimatsu, Bull. Chem. Soc. Jpn., 1992, 65, 1225–1232 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, new compound characterization data and crystallographic data, etc. CCDC 969713 ([PhTttBu]Fe(phenSQ)), 969714 ([PhTttBu]Co(phenSQ)), 969715 ([PhTttBu]CoI), 969716 ([PhTttBu]MnI), 969717 ([PhTttBu]Mn(phenSQ)) and 992704 ([PhTttBu]Co(3,5-DBSQ)). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc49143a |
| This journal is © The Royal Society of Chemistry 2014 |