Cells and proteins at interfaces
Abstract
Interfaces are present any time cells, tissue, proteins or molecules interact with each other. Even the function of something as small-scale as an individual molecule or protein is substantially altered at the interface to which it binds.
 | ||
| Fig. 1 The interface between biology and new materials enables the tailoring of complex interactions inherent in biological processes. | ||
At the microscopic scale, the interactions between cells and tissue of a given molecular composition and rigidity regulate cell function and fate (U. Hersel, C. Dahmen and H. Kessler, Biomaterials, 2003, 24, 4385–4415; V. Vogel and M. Sheetz, Nat. Rev. Mol. Cell Biol., 2006, 7, 265–275; D. E. Discher, P. Janmey and Y. L. Wang, Science, 2005, 310, 1139–1143; E. Zamir and B. Geiger, J. Cell Sci., 2001, 114, 3583–3590). Replacement of the natural tissue by a synthetic matrix provides control over cell matrix properties such as chemical composition, structure and rigidity. One persuasive example of this phenomenon was demonstrated by Janmey and Georges (P. C. Georges and P. A. Janmey, J. Appl. Physiol., 2005, 98, 1547–1553), who discovered that neuronal cells adhere to and grow well on soft synthetic supports. In general, tissue cells prefer to be in contact with rather stiff interfaces. The stiffness rigidity of the cell substrate even proved to impact guidance of stem cell differentiation as described in A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689. The impact of material rigidity on cellular processes is described in this issue in two articles (I. Levental, P. C. Georges and P. A. Janmey, Soft Matter, 2007, 3, 299, and U. Schwarz, Soft Matter, 2007, 3, 263).
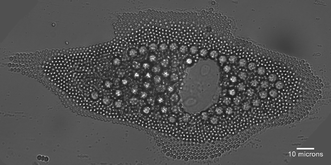 | ||
| Fig. 2 Cell-assisted colloidal assembly. From Curtis et al., p. 337. | ||
Apart from substrate rigidity, the impact of spatial positioning and controlled clustering of single signaling molecules such as proteins, protein fragments, peptides or sugar derivatives along cell substrates has lately been identified as a major regulator of cellular signalling (G. Maheshwari, G. Brown, D. A. Lauffenburger, A. Wells and L. G. Griffith, J. Cell Sci., 2000, 113, 1677–1686; M. Arnold, E. A. Cavalcanti-Adam, R. Glass, J. Blummel, W. Eck, M. Kantlehner, H. Kessler and J. P. Spatz, ChemPhysChem, 2004, 5, 383–388; L. Kiessling, J. Gestwicki and L. Strong, Angew. Chem., Int. Ed., 2006, 45, 2348–2368). Materials science techniques for the spatial positioning of biomolecules, as well as for controlling the formation of molecular clusters, are described by Girard et al. in this issue (P. P. Girard, E. A. Cavalcanti-Adam, R. Kemkemer and J. P. Spatz, Soft Matter, 2007, 3, 307). The sensitivity displayed by cells to molecular distances between transmembrane receptors or sugar derivatives could be anticipated from the fact that major components of cell tissue such as collagen present a periodicity at the nanometre-length scale (P. Fratzl, K. Misof, I. Zizak, G. Rapp, H. Amenitsch and S. Bernstorff, J. Struct. Biol., 1997, 122, 119–122). Along that same line, Humphries et al. (M. D. Bass, M. R. Morgan and M. J. Humphries, Soft Matter, 2007, 3, 372) discuss in this issue the recruitment of two major transmembrane receptors; i.e., integrin and syndecan-4, to cluster upon cell contact formation with specific interfaces and its strong involvement in cell signaling. The question of how these initial cell contacts are formed is addressed by Geiger et al. in this issue (M. Cohen, D. Joester, I. Sabanay, L. Addadi and B. Geiger, Soft Matter, 2007, 3, 327).
 | ||
| Fig. 3 Outside cover of this theme issue (image by Nicholas Melosh, Matthew Footer and Ian Wong). | ||
Cells appear to be sensitive to multiple environmental cues; therefore, the proper design of adhesive cellular environments is of critical biological and medical importance. The design of surfaces with specific bioactivity requires multiple inputs from experimentalists, theoreticians, chemists, physicists, biologists and engineers. Surface features that must be addressed include: surface chemistry, single or multiple adhesive epitopes, surface topography, and surface rigidity. This topic is discussed by both Girard et al. and Offenhäusser et al. in this issue (P. P. Girard, E. A. Cavalcanti-Adam, R. Kemkemer and J. P. Spatz, Soft Matter, 2007, 3, 307 and A. Offenhäusser, S. Böcker-Meffert, T. Decker, R. Helpenstein, P. Gasteier, J. Groll, M. Möller, A. Reska, S. Schäfer, P. Schulte and A. Vogt, Soft Matter, 2007, 3, 290).
The impact of such well-defined model interfaces is twofold. First, well defined synthetic interfaces reduce the complexity present at a natural interface such as tissue. Applying synthetic surfaces of tailored complexity as a technical tool, researchers can more readily understand the processes regulated by the interactions of cells and surfaces at the interface. Second, such tailored interfaces are useful materials for implant technology, or as a nanotechnology platform as demonstrated by Vogel and co-workers in this issue (R. K. Doot, H. Hess and V. Vogel, Soft Matter, 2007, 3, 349). These platforms make use of biological molecules or proteins in distinct conformations and hierarchic networks for biotechnological gain (Fig. 4).
 | ||
| Fig. 4 Seeded microtubules growing in channels. From Vogel et al., p. 349. | ||
Joachim Spatz
Guest Editor of the Theme Issue on Proteins and Cells at Functional Interfaces and Soft Matter Editorial Board Member
| This journal is © The Royal Society of Chemistry 2007 |

