Systematic manipulation of surface chemical reaction on the nanoscale: a novel approach for constructing three-dimensional nanostructures†
Xiuzhu
Wang
,
Dejian
Zhou
,
Trevor
Rayment
and
Chris
Abell
*
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK CB2 1EW. E-mail: ca26@cam.ac.uk; Fax: +44 (0)1223 336362; Tel: +44(0)1223 336405
First published on 20th January 2003
Abstract
Nanoscale patches, created by nanografting a maleimide-terminated thiol into a self-assembled monolayer, were elaborated by sequential chemical reactions. Each stage in the nanofabrication was followed by atomic force microscopy (AFM), providing a controlled approach to the fabrication of novel three-dimensional (3D) surface nanostructures.
The fabrication of nanostructures is of great interest inter alia for its potential application to smaller devices in electronics and computing. Fabrication issues revolve around feature size, simplicity of approach, sophistication of design and reproducibility. It is clear that self-assembly will be important in any bottom-up approach. This is most notable in the use of self-assembled monolayers (SAMs) of long chain alkane thiols, where ω-terminal functionality coupled with techniques such as microcontact printing and photolithography has been used for generating micro to nano-scale surface features.1 The recently developed AFM-based nanolithography methods, nanoshaving and nanografting,2 have allowed the fabrication of nanoscale surface structures of alkane thiols,2 proteins,3 and DNA.4
In principle, scanning probe devices can be used in the fabrication process and for in situ monitoring. Notable pioneering studies focused on using the tip of a STM to induce local surface reactions, e.g. reduction of surface nitriles by a catalyst coated tip,5 and for promoting local polymerisation.6 More recently the tip of an AFM has been used to direct borohydride reduction of imines,7 and for monitoring ester hydrolysis on a surface.8 This communication describes a study where the tip of the AFM was used to remove part of an alkyl thiol SAM, allowing its localised replacement with a SAM terminating in a reactive functional group. This created a site for subsequent chemical elaboration by soluble reagents which were introduced sequentially. The whole fabrication sequence was monitored in situ by AFM. This work significantly develops and extends the pioneering study of Liu et al.2
The Michael addition of a primary amine to a maleimide was chosen for the surface chemistry. In a preliminary study 11,11′-dimaleimidoundecyl disulfide (1) was prepared via Mitsunobu coupling of 11,11′-dihydroxylundecyl disulfide to maleimide,‡ and used to form a SAM on template-stripped gold.4 This was found to have a thickness of 1.9 ± 0.2 nm by ellipsommetry and a water contact angle of 65 ± 2°. Upon treatment of this surface with 1-decylamine (10 mM in 2-butanol) for 30 min the coating thickness increased to 2.9 ± 0.2 nm and the water contact angle to 97 ± 2°, indicating that the maleimide surface had been successfully modified by the decylamine.
This approach was elaborated to build nanoscale features on a surface (Scheme 1). The first step was to prepare a methyl-terminated SAM surface to act as a resist by soaking template stripped gold in a solution of decanethiol in 2-butanol solution (5 mM) overnight. SAMs made from alkyl thiols are relatively chemically inert and give high contrast in AFM friction measurements. A feature (400 × 400 nm2) was created in the alkyl SAM matrix by nanografting,2§ scratching away the alkyl thiol in the presence of a solution of 1. The maleimide feature is seen clearly in the AFM friction image (Fig. 1(a)). It is less pronounced in the topographic image (Fig. 1(b)) where it protruded 0.7 ± 0.2 nm higher than the surrounding decanethiol SAM resist, which was itself 1.1 nm thick.9 Thus the total height is in agreement with ellipsometry measurements made on the original maleimide SAM.
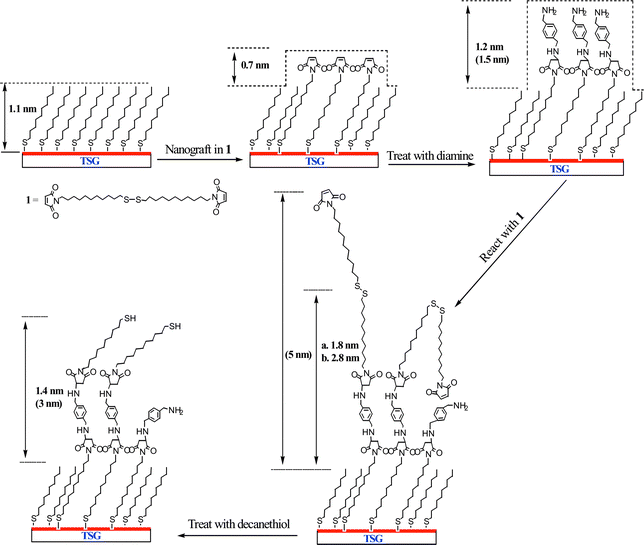 | ||
| Scheme 1 The process for constructing and systematic manipulation of nanoscale maleimide surface by local surface reactions. The height values depicted are experimental averages and corresponding theoretical values are listed in parentheses. In the case of the dimaleimide surface, (a) refers to a shorter reaction time at lower concentration (1.5 h and 5 mM); while (b) refers to a longer reaction time and higher concentration (3 h and 10 mM). | ||
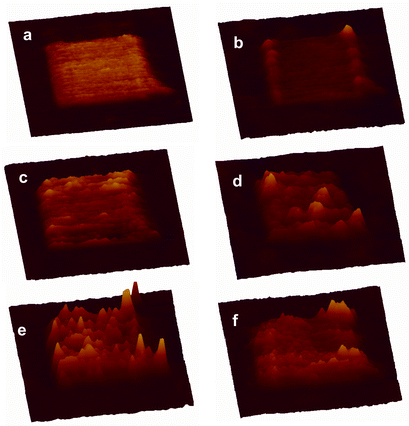 | ||
| Fig. 1 AFM 3D images of the nanofeature at different stages of the chemical manipulation. (a) Friction image of the maleimide nanosquare created by nanografting, (b) topographic image of (a); (c) topographic image after treatment with p-xylylenediamine; (d) topographic image after further treatment with 1 at 5 mM for 1.5 h; (e) topographic image after treating with 1 at 10 mM for 3 h; (f) Topographic image of (e) after treatment with 10 mM decanethiol for 30 min. All images are in the same x, y and z scales, 800 × 800 nm for x and y, and the highest features in (e) have a z value of 5 nm. | ||
The modified surface was then thoroughly washed with 2-butanol to remove the unreacted reagents, and incubated with p-xylylenediamine in 2-butanol (5 mM) for 2 h. p-Xylylenediamine is chosen for its structural rigidity with the expectation that only one of the amino groups would undergo Michael addition to the surface maleimide group leaving the other free for subsequent transformation. The average height of the nanopattern increased to 1.2 nm above the alkyl SAM (Fig. 1(c)), which agrees well with the theoretical value (1.5 nm) considering the effect of tip compression. Furthermore the AFM image section analysis shows a reasonably homogenous increase in height of the whole pattern, indicating a complete reaction across the maleimide feature.
After washing the surface with butanol, a solution of 1 (5 mM in 2-butanol) was re-introduced, this time to react the surface amino groups with a terminal maleimide. N,N-Diisopropylethylamine (10 mM in 2-butanol) was added to deprotonate the surface amino groups. After 1.5 h, the average height of the nanopattern increased to only 1.8 nm (Fig. 1(d)). This is well below the theoretical maximum increase in height (5.0 nm), presumably because of incomplete reaction of the surface functional groups because of the disordered extended structure of 1. There were however several small brighter domains with a height up to 5 nm. It was assumed that these domains were of fully extended SAM structures which had reacted completely in each of the two steps. This conclusion was supported by a subsequent experiment where a similar surface was reacted for longer with a higher concentration of 1 (3 h, 10 mM in 2-butanol). This experiment resulted in significantly more addition of 1 and consequently more extensive taller domains were seen within the whole pattern, and the average height increased to 2.8 nm (Fig. 1(e)).
Two further experiments were carried out to demonstrate how the surface pattern could be manipulated. Taking a surface modified by treatment with 1 as above (1.5 h, 5 mM in 2-butanol), the surface was imaged first in 2-butanol and then water. Respective cross section scans of these features are shown in Fig. 2(a) and (b), respectively. In 2-butanol the extended hydrophobic tails from the 11,11′-dimaleimidoundecyl disulfide are not well packed and spread out laterally. In water they are driven together by the hydrophobic effect to form more rigid aggregates that stand more erect. Finally, decanethiol (10 mM in 2-butanol) was added to the surface for 20 min. This led to disulfide exchange, cleaving off the terminal 11-maleimidoundecyl thiol. The subsequent AFM topographic image showed the disappearance of most of the bright (taller) domains (Fig. 1(f)). Consistent with this interpretation, the average height of the pattern decreased to 1.4 nm.
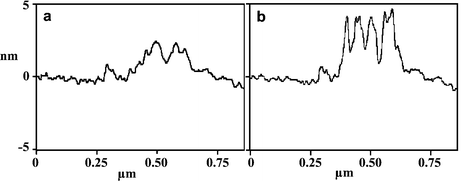 | ||
| Fig. 2 Typical AFM topographic line scan profiles of the nanosquare after treatment with 1 at 5 mM for 1.5 h under 2-butanol (a) and water (b). | ||
To estimate the extent of transformation of the localised surface reactions, we measured the volumes of the nanopatterns before and after each step. These volumes were compared with the volumes that would be required to accommodate the additional structures with sizes calculated using van der Waals radii. These calculations gave yields over approximately 85% for the addition of p-xylylenediamine, and a similar yield for the addition of 1 when added for 3 h using a 10 mM solution. These values are likely to be underestimates because of the lack of uniformity, and hence rigidity in the grafted feature, which will dominate and also exacerbate any tip induced artefacts.
This communication describes an approach to generating and growing nanoscale features on a surface, which is now being generalised to build more complex features with more diverse chemistry. The AFM is seen to be a powerful tool for defining the original feature size and then monitoring the progress and extent of each subsequent chemical step.
We wish to thank the EPSRC and the Leverhulme Trust for financial support.
Notes and references
- Y. Xia, J. A. Rogers, K. E. Paul and G. M. Whitesides, Chem. Rev., 1999, 99, 1823 CrossRef CAS.
- G. Y. Liu, S. Xu and Y. L. Qian, Acc. Chem. Res., 2000, 33, 457 CrossRef CAS; J. F. Liu, S. Cruchon-Dupeyrat, J. C. Garno, J. Frommer and G. Y. Liu, Nano Lett., 2002, 2, 937 CrossRef CAS.
- C. Jang, B. D. Stevens, P. R. Carlier, M. A. Calter and W. A. Ducker, J. Am. Chem. Soc., 2002, 124, 12114 CrossRef CAS.
- D. Zhou, K. Sinniah, C. Abell and T. Rayment, Langmuir, 2002, 18, 8278 CrossRef CAS.
- W. T. Muller, D. L. Klein, T. Lee, J. Clarke, P. L. McEuen and P. G. Schultz, Science, 1995, 268, 272.
- Y. Okawa and M. Aono, Nature, 2001, 409, 683 CrossRef CAS.
- L. K. Blasdel, S. Banerjee and S. S. Wong, Langmuir, 2002, 18, 5055 CrossRef CAS.
- H. Schonherr, V. Chechik, C. J. M. Stirling and G. J. Vancso, J. Am. Chem. Soc., 1999, 122, 3679 CrossRef.
- C. D. Bain, E. B. Troughton, Y. T. Tao, J. Evall, G. M. Whitesides and R. G. Nuzzo, J. Am. Chem. Soc., 1989, 111, 321 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental details. See http://www.rsc.org/suppdata/cc/b2/b211906d/ |
‡ Spectroscopic data for 1: 1H NMR (400 MHz, CDCl3), δ 6.69 (4H, s, maleimide H), 3.50 (4H, t, J 7 Hz, CH2N), 2.67 (4H, t, J 7 Hz, CH2SSCH2), 1.7–1.2 (36H, m, CH2); 13C NMR (100 MHz, CDCl3), δ 170.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 133.8 (C O), 133.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 38.9, 37.7, 29.2, 28.9, 28.8, 28.4, 26.6; HRMS: calc. for C15H25O2NSNa+ (M C), 38.9, 37.7, 29.2, 28.9, 28.8, 28.4, 26.6; HRMS: calc. for C15H25O2NSNa+ (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na)+m/z 306.1504, found 306.1511. Details of the synthesis are shown in the ESI.† Na)+m/z 306.1504, found 306.1511. Details of the synthesis are shown in the ESI.† |
| § AFM topographic images were collected in contact mode under minimum loading force (0.5–1 nN) just enough to obtain a stable image at a scan rate of 2 Hz. Details of the AFM experiment are supplied in the ESI.† |
| This journal is © The Royal Society of Chemistry 2003 |
