Unraveling the mechanisms of ketene generation and transformation in syngas-to-olefin conversion over ZnCrOx|SAPO-34 catalysts†
Abstract
Ketene was identified as an intermediate in syngas-to-olefin (STO) conversion catalyzed by metal oxide–zeolite composites, which sparked a hot debate regarding its formation mechanism and catalytic roles. Here, we employed large-scale atomic simulations using global neural network potentials to explore the STO reaction pathways and microkinetic simulations to couple the reaction kinetics in ZnCrOx|SAPO-34 composite sites. Our results demonstrate that the majority of ketene (86.1%) originates from the methanol carbonylation-to-ketene route 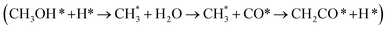 via nearby zeolite acidic sites, where methanol is produced through conventional syngas-to-methanol conversion on the Zn3Cr3O8 (0001) surface, while the minority of ketene (13.9%) arises from a direct CHO*–CO* coupling pathway (CHO* + CO* + H* → CHOCO* + H* → CH2CO + O*) on Zn3Cr3O8. The presence of the ketene pathway significantly alters the catalytic performance in the zeolite, as methanol carbonylation to ketene is kinetically more efficient in competing with conventional methanol-to-olefins (MTO) conversion and thus predominantly drives the product to ethene. Based on our microkinetic simulation, it is the methanol carbonylation activity in the zeolite that dictates the performance of STO catalysts.
via nearby zeolite acidic sites, where methanol is produced through conventional syngas-to-methanol conversion on the Zn3Cr3O8 (0001) surface, while the minority of ketene (13.9%) arises from a direct CHO*–CO* coupling pathway (CHO* + CO* + H* → CHOCO* + H* → CH2CO + O*) on Zn3Cr3O8. The presence of the ketene pathway significantly alters the catalytic performance in the zeolite, as methanol carbonylation to ketene is kinetically more efficient in competing with conventional methanol-to-olefins (MTO) conversion and thus predominantly drives the product to ethene. Based on our microkinetic simulation, it is the methanol carbonylation activity in the zeolite that dictates the performance of STO catalysts.

- This article is part of the themed collections: 15th anniversary: Chemical Science community collection and 2025 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...