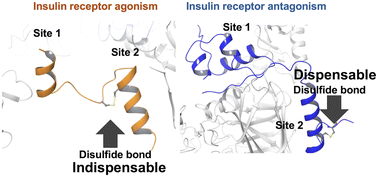
Insulin receptor (IR) activation requires coordinated engagement of two distinct insulin-binding sites, and recent structural insights have highlighted the role of a disulfide bond in IR agonist S597 in the S597-IR complex. In this study, we synthesized and evaluated analogs of S597 and the IR antagonist Ins-AC-S2, replacing their native disulfide bridges with alternative linkages. While these modifications had minimal impact on Ins-AC-S2's antagonistic activity, they significantly reduced the agonistic potency of S597, suggesting that conformational stability is critical for receptor activation. Our findings provide a structural basis for designing non-insulin ligands to selectively activate or inhibit the insulin receptor, with potential therapeutic implications.