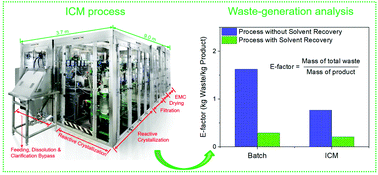
Most drugs are still manufactured by time-intensive and cost-inefficient batch processes; however, the pharmaceutical industry is undergoing a transition to continuous processes to benefit from the reduced lead time, cost, and footprint and improved quality associated with this new methodology. Herein we report some key results of a fully automated end-to-end integrated continuous manufacturing (ICM) pilot plant, and describe the relevant E-factor analysis. The overall yields of the batch and ICM processes are 86.4%, and 88.0%, respectively. The solvent recovery yields for Solvents 1 and 2 are 95.8%, and 94.1% in batch, and are 98.3% and 94.9% in the ICM process. The E-factor value reduced significantly from 1.627 with batch to 0.770 with ICM (∼53% reduction), and after integrating the Solvent Recovery unit operation it decreased from 0.292 for the batch process to 0.210 for ICM (∼30% reduction). The application of a seamless, continuous ICM process could reduce waste generation and lower the E-factor, resulting in a positive outcome for our planet.