Ze-Fan Zhu, Jia-Lin Tu and Feng Liu
Chem. Commun., 2019,55, 11478-11481
DOI:
10.1039/C9CC05385A,
Communication
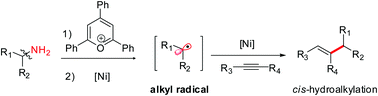
A regioselective cis-hydroalkylation of internal alkynes with readily prepared Katritzky pyridinium salts for the synthesis of tri-substituted alkenes is described. This reaction is the first example of a metal-catalyzed hydroalkylation of an alkyne via C–N bond activation of an amine. The reaction demonstrates broad scope and functional group tolerance, allowing access to desired products with high diversity. Preliminary mechanistic studies indicate that a combination of an SET-initiated radical process and Ni-catalyzed alkylation could engage in the reaction, which makes it possible to bypass the traditional open-shell addition pathway.