Xiaoyu Zhu, Yuai Duan, Po Li, Haiming Fan, Tianyu Han and Xiaonan Huang
Anal. Methods, 2019,11, 642-647
DOI:
10.1039/C8AY02526F,
Paper
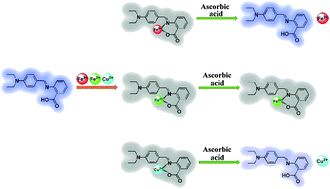
A new and tri-responsive fluorescent Schiff base probe (DBAB) has been designed and developed for the recognition of iron(III) ions (Fe3+), iron(II) ions (Fe2+) and copper(II) ions (Cu2+) simultaneously. This sensor displays a favorable selectivity for Fe3+, Fe2+ and Cu2+ ions over a range of other common metal cations in DMF solution, leading to prominent fluorescence on–off. The detection limits were 2.17 × 10−6 M for Fe3+, 2.06 × 10−6 M for Fe2+ and 2.48 × 10−6 M for Cu2+ respectively. This tri-responsive fluorescent probe for Fe3+, Fe2+ and Cu2+ was distinguished by further reduction of the ascorbic acid. The recognition mechanism of DBAB toward Fe3+, Fe2+ and Cu2+ has been investigated in detail by Job plot measurements and 1H NMR.