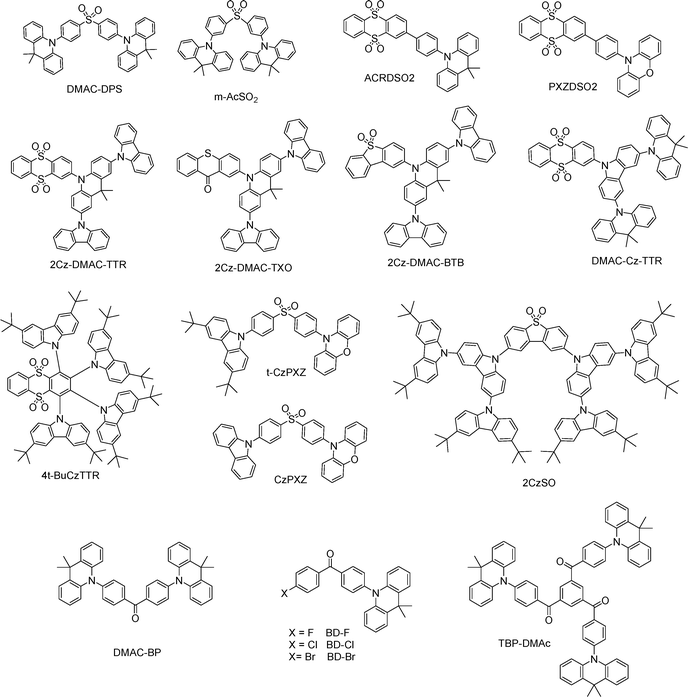
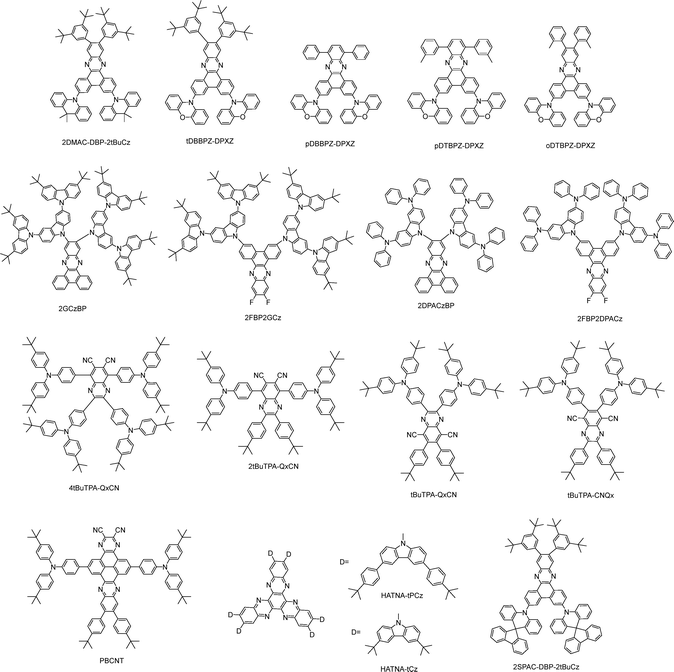
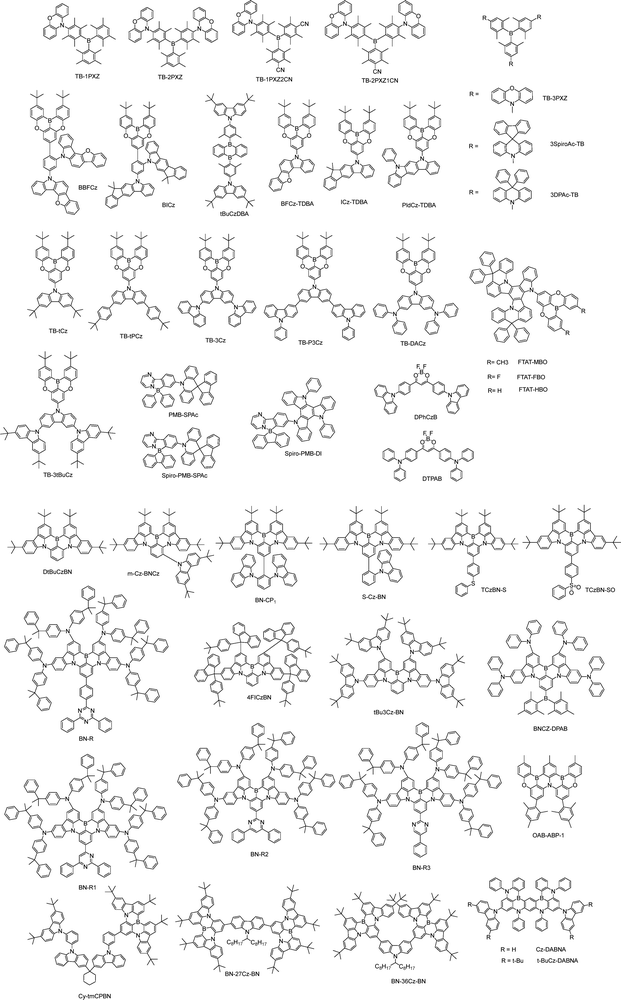
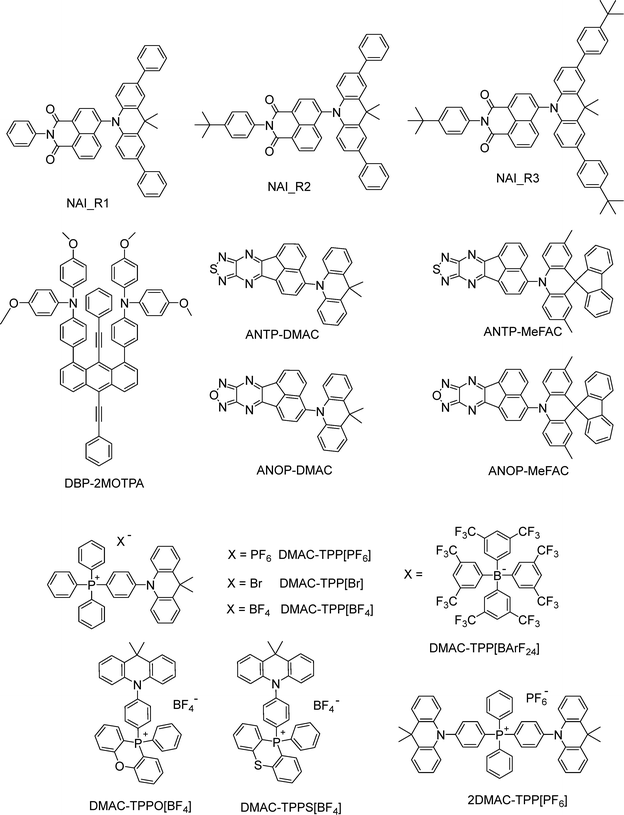
Recent advances in highly efficient small-molecule TADF emitters for solution-processed OLEDs
Yongxia
Ren
and
Shi-Jian
Su
 *
*
Guangdong Basic Research Center of Excellence for Energy and Information Polymer Materials, State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: mssjsu@scut.edu.cn
First published on 20th September 2025
Abstract
Solution-processable thermally activated delayed fluorescence (TADF) emitters have demonstrated strong competitiveness and significant development potential in display applications, due to their intrinsic merits of low cost, high efficiency, and compatibility with large-area fabrication. This comprehensive review highlights recent advancements in small-molecule solution-processable TADF emitters across the full-color spectrum, including near infrared, red, green, blue, and purple. It delves into their molecular design and electrical properties, with a particular focus on efficiency, color purity, and device stability. Ultimately, this review aims to provide valuable guidance for the development of high-efficiency, narrowband-emission, and stable solution-processed organic light-emitting diodes.
1. Introduction
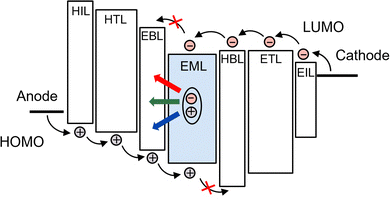
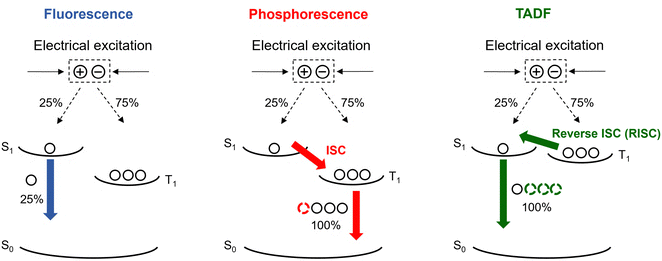
Since the pioneering work conducted by Tang and VanSlyke in 1987,1 organic light-emitting diodes (OLEDs) have experienced explosive development, rapidly emerging as a core technology in modern display and lighting applications. Their distinct advantages, including high contrast ratios, wide viewing angles, lightweight structure, and compatibility with flexible and transparent substrates, have enabled widespread commercialization in smartphones, televisions, and wearable devices. A typical OLED consists of a multilayer stack of organic semiconductors (Fig. 1), among which the emission layer (EML) plays a pivotal role in determining the device performance. In most cases, the EML comprises a host–guest system, where the emitter is doped into a wide-bandgap host material to confine excitons and facilitate efficient energy transfer. Fluorescent emitters were initially used in OLEDs due to their high color purity and stable emission characteristics. However, their internal quantum efficiency (IQE) is inherently limited to 25%, as they can utilize only the singlet excitons (25%) generated upon electrical excitation, while the remaining 75% triplet excitons are non-radiatively lost as heat (Fig. 2). Subsequently, phosphorescent materials garnered considerable attention from researchers due to their ability of achieving 100% exciton utilization.2 These compounds are typically heavy-metal complexes based on elements such as Ir, Pt, Cu, or Au and exhibit strong spin–orbit coupling (SOC). This facilitates rapid intersystem crossing (ISC) from the lowest excited singlet state (S1) to the lowest excited triplet state (T1), followed by radiative decay from T1 to the ground state (S0). Through this mechanism, phosphorescent emitters can attain IQEs approaching 100% (Fig. 2), thereby greatly improving OLED performance. However, their reliance on scarce and expensive noble metals raises concerns regarding economic feasibility, large-scale manufacturing and long-term sustainability. These limitations have driven the exploration of alternative triplet-harvesting mechanisms. Among them, thermally activated delayed fluorescence (TADF) has emerged as a particularly promising strategy, enabling 100% exciton utilization solely through organic molecular design.3 TADF operates by converting non-radiative triplet excitons into emissive singlet excitons via thermally induced reverse ISC (RISC), followed by radiative decay from S1 to S0, enabling the harvesting of all excitons without any heavy metals.4 Since their inception, significant progress has been made in molecular design strategies, including donor–acceptor (D–A) structures, multiple resonance (MR) frameworks, and rigidified skeletons, leading to superior device efficiencies.With an equal importance, the device fabrication method can significantly affect the efficiency, operational stability, and production cost of OLEDs. Broadly, OLEDs can be classified into two types, vacuum-deposited and solution-processed, depending on whether the EML is formed via thermal evaporation or solution-processed techniques. Vacuum deposition offers precise control over film thickness and uniformity and readily enables the formation of complex multilayer architectures, thereby delivering the record efficiencies in many state-of-the-art TADF OLEDs. However, it suffers from high operational costs, poor material utilization, and device size limitations imposed by the vacuum chamber, all of which hinder scalability to large-area manufacturing. In contrast, solution-processed TADF OLEDs represent a promising alternative, as they dispense with high-vacuum conditions and deposition chambers, enabling low-cost, scalable fabrication. Nevertheless, they typically exhibit lower efficiency and more pronounced efficiency roll-off than vacuum-deposited counterparts. Multiple functional layers are required to balance charge injection and transport, thereby fully exploiting the emitters’ potential, but the scarcity of mutually orthogonal solvent systems makes such multilayer stacks difficult to fabricate. As a result, solution-processed OLEDs tend to employ simplified architectures, introducing injection barriers and carrier-mobility mismatches that foster charge accumulation and enhance nonradiative loss channels, thus stronger efficiency roll-off. In addition, achieving uniform, defect-free layers and interfaces by the solution process is challenging because solute precipitation, recrystallization, and aggregation readily occur during film formation, promoting interfacial charge accumulation and annihilation that degrade efficiency and operational stability. To overcome these limitations, efforts span molecular and device engineering. Molecular engineering of solution-processable TADF emitters focuses on (i) minimizing the singlet–triplet energy gap (ΔEST) to accelerate RISC while retaining high oscillator strength for high photoluminescence quantum yield (PLQY); (ii) enhancing spin–orbit coupling (SOC) to boost RISC and suppress efficiency roll-off at the high-brightness region; (iii) increasing rigidity (spiro/bridged/locked torsions) to reduce nonradiative loss; (iv) adopting bulky substituents and alkyl/dendritic groups to suppress aggregation and improve solubility. Device engineering includes (i) optimizing the hole injection layer (HIL), hole transport layer (HTL), and electron transport layer (ETL) for balanced charge injection and transport, as well as exciton confinement; (ii) selecting high-T1, high-mobility hosts (small-molecule, dendrimeric, or polymer hosts), mixed-host and interfacial exciplex systems to improve charge balance; and (iii) adopting TADF-sensitized (hyperfluorescence) architectures to maximize exciton utilization and reduce efficiency roll-off. In what follows, we place emphasis on solution-processable emitters, complemented by research on device engineering aimed at improving their performance.
TADF emitters can be categorized into three main types based on their molecular architectures: small molecules, dendrimers, and polymers. Among these, small molecules are the most extensively investigated and well-established class, particularly in high-performance vacuum-deposited OLEDs. Given their prominent role, a comprehensive understanding of recent advances in small-molecule TADF emitters is essential for the continued advancement of molecular design and device performance. This review systematically summarizes recent developments in highly efficient solution-processable small-molecule TADF emitters, across the full emission spectrum from violet to near-infrared. We highlight the molecular design strategies, key optoelectronic parameters (e.g., PLQY, kRISC, and τDF), device performance metrics (e.g., EQE, emission wavelength, color purity, and efficiency roll-off), and the resulting structure–property relationships. Finally, we conclude by identifying critical challenges and future opportunities for achieving high-efficiency, high-color-purity, and stable solution-processed TADF OLEDs.
2. Molecular design criteria for solution-processable TADF emitters
Highly efficient solution-processable TADF emitters should not only possess intrinsically efficient TADF behavior (as will be discussed later), but also meet several specific criteria for solution processing. These include, (i) adequate solubility in common organic solvents to form uniform, pinhole-free films; (ii) a relatively high glass transition temperature to avoid crystallization during solution processing, thereby ensuring favorable film morphology; (iii) balanced charge injection and transport abilities to ensure high performance using a simplified device architecture. Altogether, these properties are essential for realizing stable, high-efficiency solution-processed OLEDs.As shown in Fig. 2, RISC plays a pivotal role in determining the intrinsic efficiencies of TADF emitters. Efficient RISC is essential for maximizing exciton harvesting and minimizing triplet exciton accumulation, which would otherwise lead to several non-radiative exciton losses. According to Fermi's golden rule, a fast RISC requires a small ΔEST and sufficient SOC between T1 and S1.5 A common strategy is to construct molecules with highly twisted D–A architecture, which spatially separate the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) on D and A, respectively. This separation reduces the electron exchange energy and thereby offering a small ΔEST.6 In addition, D–A molecules exhibit excellent structural flexibility due to the wide variety of selectable donor and acceptors. They are also easily synthesized and readily modifiable, allowing for the optimization of solubility and energy level alignment. These advantages make D–A architectures one of the most extensively studied and well-established classes of TADF emitters.7 However, this molecular skeleton exhibits three primary inherent limitations, which warrant thorough consideration in future molecular design. First, such a large orbital separation diminishes the HOMO–LUMO overlap, which leads to a slower radiative decay. As a result, a portion of S1 excitons will go back to T1via a fast ISC and accumulate in the ISC/RISC cycle until they emit or lost non-radiatively. This results in inefficient exciton utilization and ultimately reduced device efficiency. Second, SOC is inherently weak in purely organic D–A molecules, yielding typical kRISCs ∼ 106 s−1 and limiting further improvement, even with a negligible ΔEST.8 Incorporating heavier organic atoms, such as S and Se, is effective to SOC via the heavy atom effect, affording a larger kRISC of >107 s−1. However, the simultaneously accelerated ISC possibly causes exciton accumulation within the ISC/RISC cycle, again compromising exciton utilization. Third, these materials typically exhibit broad charge-transfer (CT)-type emissions and low spectral purity, which fall far short of the high color purity and resolution requirements for advanced display applications.
Another class of TADF emitters, MR-type molecules, can partially circumvent several drawbacks of D–A molecules. Pioneered by Hatakeyama and co-workers,9 the MR concept leverages the precise placement of electron-donating atoms (e.g., nitrogen) and electron-accepting atoms (e.g., boron and the carbonyl group) in an alternating pattern within a rigid, polycyclic π-conjugated framework. This unique structural arrangement induces alternately patterned HOMO–LUMO distributions, resulting in a small ΔEST and locally excited (LE)-type S1 states. These features collectively enable efficient RISC, narrowband emission and a high radiative decay rate, making MR-TADF emitters highly attractive for high-performance and high-purity applications. However, these emitters also exhibit several limitations. MR-TADF molecules generally possess relatively large ΔEST, affording a typical kRISC ∼ 104 s−1, which is a primary cause of the pronounced efficiency roll-off in their devices. In addition, their rigid and planar structures, while beneficial for narrowband emission, often lead to poor solubility and limited processability, thereby restricting their applications in solution-processed OLEDs. Furthermore, strong intermolecular interactions arising from the planar structures can induce severe aggregation in the solid state, ultimately comprising emission purity.10 All the above necessitates meticulous molecular design to ensure high PLQY, high color purity and superior device performance.
3. Solution-processable TADF emitters
For ease of reference, recently reported efficient solution-processable small-molecule TADF emitters were categorized according to their molecular skeletons, with the most efficient representative of each category highlighted at the end of each section in the accompanying figure.3.1 CzCN derivatives for solution-processable TADF emitters
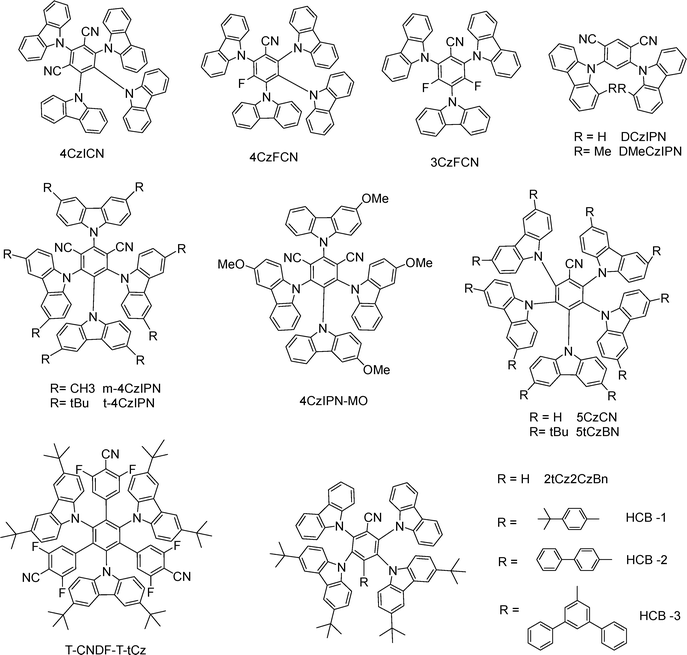
Carbazolyl-cyano (CzCN) derivatives, featuring carbazole (Cz) donors and a cyano (CN) acceptor, represent a versatile class of TADF emitters due to their well-defined D–A architecture and tunable photophysical properties.4CzIPN has served as a benchmark green TADF emitter, and numerous groups have optimized device architectures to maximize its solution-processed device performance. Inserting a self-organized buffer HIL between ITO and the EML increases the hole-injection capability and blocks the exciton quenching at the HIL/EML interface, delivering an EQEMAX of 24%.11 Yang and co-workers doped InCl3 into PEDOT:PSS layer, enhancing hole injection and also achieving an EQEMAX of 24%.12 Jou and co-workers developed a series of HTMs, possessing high hole mobility, well-aligned HOMO/LUMO energy levels, and adequately high T1 energy, thus enabling comparable or even superior device performance to the conventional HTMs.13 Lee and co-workers introduced tert-butyl groups into 4CzIPN, yielding t4CzIPN. It shows enhanced solubility and improved film morphology, thereby reaching a high EQEMAX of 18.3%.14 Choi and co-workers reported several novel host materials, including mCPDPO,15 APC,16 Py2Cz, Py2BFCz, and Py2ICz,17 CzPy2TCz and CzPy3TCz,18 BBCzC, BTDC, BCzTC,19 Cy-mCP, and Cy-mCBP,20 along with a polymer/small molecule exciplex system,21 markedly boosting the performance of t4CzIPN in solution-processed OLEDs with an EQEMAX of 18.8–31.8% (Fig. 3). The elevated efficiencies are chiefly attributable to the emitter's high PLQY and the excellent balance of hole and electron transport in the EML. They also developed a novel HTM, PmCP, featuring a high T1 and superior hole mobility in comparison to the conventional HTM poly(N-vinylcarbazole) (PVK). Incorporating PmCP into a t4CzIPN-based, solution-processed OLED achieved a high EQEMAX of 29.6%, surpassing the 21.1% in the PVK-based device.22
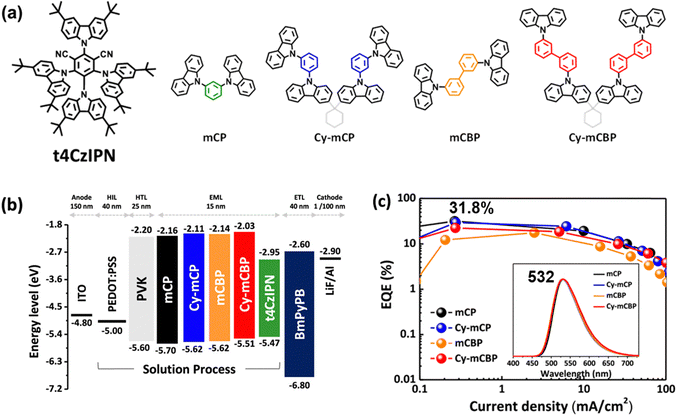 | ||
| Fig. 3 (a) Structures of emitter/hosts, (b) device configurations, and (c) performance of the most efficient solution-processed CzCN-based TADF OLEDs. Reproduced with permission from ref. 20. Copyright © 2023 American Chemical Society. | ||
Replacing the CN of 4CzIPN with F produces 4CzFCN, which exhibits unity PLQY and blue electroluminance (EL) at 471 nm with an EQEMAX of 20%. Further replacing Cz with F affords 3CzFCN, which shows decreased PLQY and a longer τDF, resulting in a lower EQEMAX of 17.8%.23 Host optimization can further elevate 4CzFCN-based device performance.24 Wang and co-workers employed an interfacial exciplex host (T1 = 3.15 eV) formed by mCP/mSiTRZ, driving 4CzFCN's EQEMAX to 22.9% with low efficiency roll-off (EQE1000 = 18.3%).25 Lee and co-workers substituted one CN of 4CzIPN with Cz to produce 5CzCN, which possesses an enlarged dihedral angle between Cz and phenyl moieties, thereby enhancing steric hindrance and promoting solvent penetration. As a result, 5CzCN exhibits a higher solubility in toluene (0.5 wt%) than 4CzIPN (0.1 wt%). In solution-processed OLEDs, 5CzCN achieves an EQEMAX of 18.7%, representing the first reported solution-processed blue TADF OLEDs with efficiency comparable to their vacuum-deposited counterpart.26 Pairing 5CzCN with a solution-processable DPOBBPE (T1 = 3.15 eV) yields an high EQEMAX of 25.8%.27 5TCzBN, a tert-butyl-substituted 5CzCN, reported by Duan and co-workers,28 is well suited to solution-processed OLEDs. Using a dendritic host mCDtCBPy,29 a small-molecule host material DMBN-PTC,30 and a polymer host P4CzCN-PA,31 5TCzBN gives EQEMAX values of 14.1%, 20.8%, and 20.9%, respectively. Notably, a DMBN-PTC-based OLED simultaneously delivers high EQEMAX, low efficiency roll-off (EQE1000 = 20.1%), and a long lifetime (T50 = 398.3 h at 500 cd m−2). Sun and co-workers constructed a non-doped green TADF emitter, 4CzPhIPN-MO, by introducing phenyl bridges between carbazole units and the central benzene core, along with methoxy substituents. Remarkably, its non-doped solution-processed OLED achieved a maximum luminance (LMAX) of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 682 cd m−2, a high EQEMAX of 14.5% and minimal efficiency roll-off (EQE1000 = 13.9%).32 Tang and co-workers developed four efficient and non-doped blue TADF emitters by adjusting the positions of four donors and the CN moiety around a benzene core. Among them, 2Cz2tCzBn and 2tCz2CzBn realize high EQEMAX values of 25.8% and 24.5%, respectively.33 They further introduced bulky phenyl units into 2tCz2CzBn to generate additional blue TADF emitters, HCB-1, HCB-2 and HCB-3.34 Yang and co-workers developed a blue non-doped TADF emitter, T-CNDF-T-tCz, featuring both through-space and through-bond charge transfer characters. Consequently, it achieves an EQEMAX of 21.0% and an LMAX of over 5200 cd m−2.35
682 cd m−2, a high EQEMAX of 14.5% and minimal efficiency roll-off (EQE1000 = 13.9%).32 Tang and co-workers developed four efficient and non-doped blue TADF emitters by adjusting the positions of four donors and the CN moiety around a benzene core. Among them, 2Cz2tCzBn and 2tCz2CzBn realize high EQEMAX values of 25.8% and 24.5%, respectively.33 They further introduced bulky phenyl units into 2tCz2CzBn to generate additional blue TADF emitters, HCB-1, HCB-2 and HCB-3.34 Yang and co-workers developed a blue non-doped TADF emitter, T-CNDF-T-tCz, featuring both through-space and through-bond charge transfer characters. Consequently, it achieves an EQEMAX of 21.0% and an LMAX of over 5200 cd m−2.35
The DCzIPN scaffold is viable for solution-processable blue TADF;36 however, even after device-structure optimization, the solution-processed OLED attains a low EQEMAX of 9.5%. Introducing a methyl substituent in DCzIPN reduces ΔEST from 0.19 to 0.07 eV, accelerates kRISC by ∼35 times, and shortens τDF from 30.8 to 3.66 μs. The resulting emitter, DMeCzIPN, exhibits blue EL at 478 nm with a high EQEMAX of 21.6% and extremely low efficiency roll-off (EQE1000/EQE10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 = 19.6%/12.7%) in its solution-processed OLED.37 All the aforementioned molecules are summarized in Fig. 4. In summary, adjusting the type, number, and position of substituents on CzCN derivatives, together with optimal device-engineering tactics, enables their solution-processed OLEDs with exceptional performance: an EQEMAX of 25.8% for the non-doped one and EQEMAX of 31.8% for the doped case.
000 = 19.6%/12.7%) in its solution-processed OLED.37 All the aforementioned molecules are summarized in Fig. 4. In summary, adjusting the type, number, and position of substituents on CzCN derivatives, together with optimal device-engineering tactics, enables their solution-processed OLEDs with exceptional performance: an EQEMAX of 25.8% for the non-doped one and EQEMAX of 31.8% for the doped case.
3.2 Triazine acceptor-adopted solution-processable TADF emitters
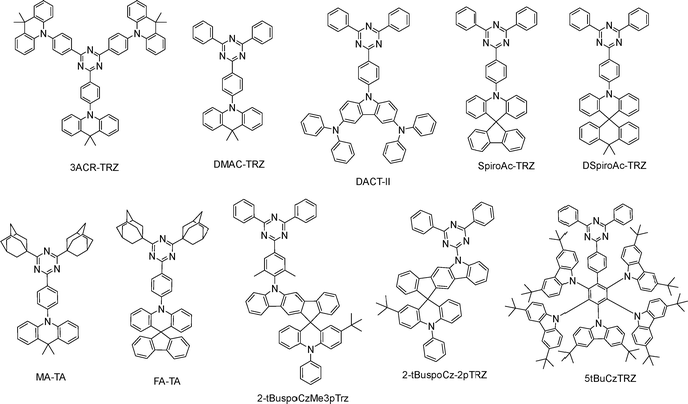
Triazine acceptors likewise constitute a versatile platform for creating highly efficient, solution-processable TADF emitters,38 as shown in Fig. 6. 3ACR-TRZ yields an EQEMAX of 18.6% and green EL (∼510 nm) in the doped device,39 while DMAC-TRZ exhibits an PLQY of 84% in the neat film, delivering an EQEMAX of 17.6% and blue EL in the non-doped device.40 Chen and co-workers developed a novel cross-linkable HTM, X-DCDPA, featuring a high T1 of 2.89 eV. An X-DCDPA-included double-HTL architecture shows enhanced hole injection and improved charge balance, enabling the DMCz-TRZ-based doped device to reach an EQEMAX of 27.2% and an LMAX of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. The same strategy applied to DACT-II delivers green EL at 530 nm with an EQEMAX of 30.8% and an LMAX of 81
000 cd m−2. The same strategy applied to DACT-II delivers green EL at 530 nm with an EQEMAX of 30.8% and an LMAX of 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 cd m−2 (Fig. 5).41 Wang and co-workers reported two hybridized local and charge-transfer emitters, 2-tBuspoCz-2pTrz and 2-tBuspoCzMe3pTrz, that generate exciplex-type TADF systems when paired with TAPC. Such exciplex systems exhibit green EL at 518 nm and 520 nm, with EQEMAX values of 10.1% and 16.6%, respectively. Doping into these systems, BN5 shows a high EQEMAX of 22.1% and narrow green EL at 504 nm.42 SpiroAC-TRZ, with its rigid spiro-donor scaffold and optimized D–A architecture, has been implemented as a TADF emitter in solution-processed OLEDs. Wang and co-workers developed adamantane-cored and cyclohexane-cored dendritic host materials, Ad-4D243 and CH-2D2.44 Paring with these hosts, spiroAC-TRZ achieved a high EQEMAX of 18.3% and 17.8% with blue EL at 482 nm and 476 nm, respectively. Zhao and co-workers incorporated PVK into the EML to modulate interfacial carrier transport and improve film morphology. A ternary blend of 15 wt% spiroAC-TRZ: 10 wt% PVK: mCBP provides an impressive EQEMAX of 25.1%.45 DspiroAc-TRZ displays an high EQEMAX of 26.8% in the doped solution-processed OLED.46 Liao and co-workers developed two through-space charge-transfer TADF emitters, 2tDMG and 3tDMG. In solution-processed OLEDs, 2tDMG exhibits blue EL at 486 nm with an EQEMAX of 16.2%. 3tDMG exhibits enhanced solubility and processability due to tert-butyl units, thus achieving a higher EQEMAX of 20.2% and green EL at 504 nm.47 Kaji and co-workers replaced the two phenyl substituents on the triazine core of DMAC-TRZ with adamantyl groups, affording MA-TA. This change weakens the acceptor and suppresses non-radiative decay, leading to a high PLQY of ∼99% and thus a high EQEMAX of 22.1%.48
100 cd m−2 (Fig. 5).41 Wang and co-workers reported two hybridized local and charge-transfer emitters, 2-tBuspoCz-2pTrz and 2-tBuspoCzMe3pTrz, that generate exciplex-type TADF systems when paired with TAPC. Such exciplex systems exhibit green EL at 518 nm and 520 nm, with EQEMAX values of 10.1% and 16.6%, respectively. Doping into these systems, BN5 shows a high EQEMAX of 22.1% and narrow green EL at 504 nm.42 SpiroAC-TRZ, with its rigid spiro-donor scaffold and optimized D–A architecture, has been implemented as a TADF emitter in solution-processed OLEDs. Wang and co-workers developed adamantane-cored and cyclohexane-cored dendritic host materials, Ad-4D243 and CH-2D2.44 Paring with these hosts, spiroAC-TRZ achieved a high EQEMAX of 18.3% and 17.8% with blue EL at 482 nm and 476 nm, respectively. Zhao and co-workers incorporated PVK into the EML to modulate interfacial carrier transport and improve film morphology. A ternary blend of 15 wt% spiroAC-TRZ: 10 wt% PVK: mCBP provides an impressive EQEMAX of 25.1%.45 DspiroAc-TRZ displays an high EQEMAX of 26.8% in the doped solution-processed OLED.46 Liao and co-workers developed two through-space charge-transfer TADF emitters, 2tDMG and 3tDMG. In solution-processed OLEDs, 2tDMG exhibits blue EL at 486 nm with an EQEMAX of 16.2%. 3tDMG exhibits enhanced solubility and processability due to tert-butyl units, thus achieving a higher EQEMAX of 20.2% and green EL at 504 nm.47 Kaji and co-workers replaced the two phenyl substituents on the triazine core of DMAC-TRZ with adamantyl groups, affording MA-TA. This change weakens the acceptor and suppresses non-radiative decay, leading to a high PLQY of ∼99% and thus a high EQEMAX of 22.1%.48
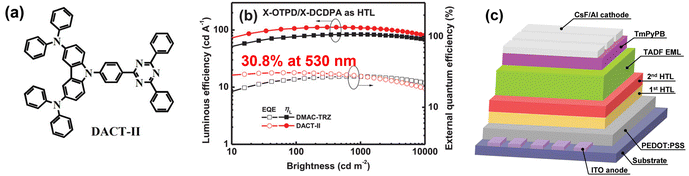 | ||
| Fig. 5 (a) Structures of emitters, (b) device performance, and (c) device configurations of the most efficient solution-processable TRZ-based TADF emitter. Reproduced with permission from ref. 41. Copyright © 2019, Wiley-VCH. | ||
In summary, solution-processed OLEDs based on TRZ derivatives have exhibited impressive performance, with an EQEMAX of 17.6% for the non-doped device and an EQEMAX of 30.8% for the doped case. These highlight the great potential of TRZ-based emitters as promising candidates for efficient, low-cost, and scalable solution-processed OLED applications.
3.3 Sulfone-/carbonyl group-based solution-processable TADF emitters
Sulfone units have also emerged as effective electron-acceptor motifs in the design of solution-processable TADF materials, as listed in Fig. 7. The archetypal blue emitter, DMAC-DPS, with a D–A–D configuration, exhibits blue EL at 480 nm and an EQE of 19.5% at 100 cd m−2 and 14.6% at 1000 cd m−2 in non-doped OLEDs.49 The meta-linked analogue, m-AC-SO2, exhibits a slightly red-shifted EL at 486 nm and a similar EQEMAX of 17.2%.50 Our group reported two D–A TADF emitters, ACRDSO2 and PXZDSO2, achieving a high EQEMAX of 17.5% and 15.2% with green EL at 520 nm and 552 nm, respectively, in doped OLEDs.51 Additionally, host selection strongly influences the performance of PXZDSO2-based solution-processed OLEDs.52 Yang and co-workers reported a D–A–D-type TADF emitter, 2CzSO, featuring a highly twisted molecular configuration, which achieved green EL at 516 nm and a moderate EQEMAX of 10.7% in non-doped OLEDs.53 Zhang and co-workers reported a multi-D/A-type TADF emitter, 4t-BuCzTTR, featuring excellent thermal stability and favorable solution processability. Its optimized doped OLED exhibits EL at 592 nm and a high EQEMAX of 6.2%.54 They further developed a D–D′–A-type TADF emitter, DMAC-Cz-TTR, incorporating both intra- and intermolecular charge-transfer transition pathways, which effectively suppress aggregation-caused quenching typically associated with solution processing. As a result, the optimized doped solution-processed OLED achieved a high EQEMAX of 20.6%.55 Chi and co-workers reported two D–A–D′-type TADF emitters, CzPXZ and t-CzPXZ, exhibiting aggregation-induced emission characteristics. A heavily doped device employing t-CzPXZ as the emitter achieved an EQEMAX of 16.3% with low efficiency roll-off.56 Zhang and co-workers developed three novel TADF emitters using a newly designed electron-donating segment, 2Cz-DMAC-BTB, 2Cz-DMAC-TXO and 2Cz-DMAC-TTR. Their non-doped OLEDs exhibit green, orange and orange-red EL with an EQEMAX of 14.0%, 6.6% and 2.9%, respectively, with the variation in device performance primarily attributed to differences PLQYs.57 In other words, sulfone-based designs, particularly DMAC-DPS, achieve the highest EQE of 19.5% in non-doped solution-processed OLEDs, while DMAC-Cz-TTR reaches up to 20.6% in doped devices. These remain slightly inferior to those of the best-performing CzCN- and TRZ-based counterparts.Another important class of TADF materials are developed using carbonyl groups as electron acceptors. DMAC-BP, a representative green TADF emitter, achieves an impressive EQEMAX of 18.9%, with a high EQE1000 of 18.0% and an LMAX of ≈50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 in a non-doped device.49 Ren and co-workers replaced one DMAC moiety of DMAC-BP with halogens, affording BD-F, BD-Cl and BD-Br. Among them, BD-F exhibits distinctive molecular stacking behaviors, forming dimers and trimers through multiple intermolecular hydrogen bonds, ensuring its superior photophysical properties. Consequently, BD-F-based solution-processed OLEDs achieve green EL at 503 nm, an EQEMAX of 27.13% and small efficiency roll-off (EQE1000 = 24.74%). However, despite reasonable photophysical properties, BD-Br delivers inferior device performance, possibly due to its lower thermal stability.58 TBP-DMAc, developed by our group, combines large steric hindrance between D and A fragments with a flexible, nonplanar acceptor core, affording high solubility and robust compatibility with wet processing. Its solution-processed OLED achieve EQEs at 22.1%, 20.7%, and 13.6% at the luminance of 100, 1000, and 10
000 cd m−2 in a non-doped device.49 Ren and co-workers replaced one DMAC moiety of DMAC-BP with halogens, affording BD-F, BD-Cl and BD-Br. Among them, BD-F exhibits distinctive molecular stacking behaviors, forming dimers and trimers through multiple intermolecular hydrogen bonds, ensuring its superior photophysical properties. Consequently, BD-F-based solution-processed OLEDs achieve green EL at 503 nm, an EQEMAX of 27.13% and small efficiency roll-off (EQE1000 = 24.74%). However, despite reasonable photophysical properties, BD-Br delivers inferior device performance, possibly due to its lower thermal stability.58 TBP-DMAc, developed by our group, combines large steric hindrance between D and A fragments with a flexible, nonplanar acceptor core, affording high solubility and robust compatibility with wet processing. Its solution-processed OLED achieve EQEs at 22.1%, 20.7%, and 13.6% at the luminance of 100, 1000, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, corresponding to 0%, 6.3%, and 38.5% roll-off relative to EQEMAX.59 There are relatively few highly efficient small-molecule TADF emitters bearing carbonyl acceptors (Fig. 7); instead, most high-performing systems in this category are TADF dendrimers,60,61 which benefit from suppressed aggregation and improved film morphology due to their bulky, branched architectures.
000 cd m−2, corresponding to 0%, 6.3%, and 38.5% roll-off relative to EQEMAX.59 There are relatively few highly efficient small-molecule TADF emitters bearing carbonyl acceptors (Fig. 7); instead, most high-performing systems in this category are TADF dendrimers,60,61 which benefit from suppressed aggregation and improved film morphology due to their bulky, branched architectures.
3.4 Quinoxaline acceptor-based solution-processable TADF emitters
While the above series generally exhibit blue to green emission, quinoxaline-based acceptors offer promising features for red emitters, owing to their strong electron-withdrawing character and extended conjugation. For clarity, we classify EL peak wavelengths as 600–620 nm (orange-red), 620–630 nm (red), 630–700 nm (orange-red), and ≥700 nm (near infrared, NIR).Lee, Zhang, and co-workers reported four red solution-processable TADF emitters, tDBBPZ-DPXZ,62pDBBPZ-DPXZ, pDTBPZ-DPXZ, and oDTBPZ-DPXZ,63 sharing the same rigid D–A emissive core but differing in peripheral substitution on the A unit. The introduction of 3,5-di-tert-butylphenyl groups enhances solubility without compromising core photophysical properties, enabling tDBBPZ-DPXZ to deliver red EL at 620 nm with an EQEMAX of 10.1% in a doped OLED. Peripheral substitution modulates excited-state alignment and RISC rates. Notably, oDTBPZDPXZ combines high PLQY with a high kRISC of 0.4 × 106 s−1, achieving an EQEMAX of 18.5% with orange-red EL at 612 nm in doped devices. Zysman-Colman and co-workers systematically examined how donor strength and substitution position tune the optoelectronic properties of phenazine-based emitters. Among them, 2DPACzBP shows the most red-shifted emission and a high kRISC, delivering deep-red EL at 640 nm with an EQEMAX of 7.8% in an mCP-hosted solution-processed OLED. A ternary EML (3 wt% 2DPACzBP: 32 wt% 4CzIPN: mCP) provides a high EQEMAX of 20.0% at 605 nm, owing to efficient exciton harvesting via a hyperfluorescence strategy. The same ternary strategy also boosts other emitters, giving an EQEMAX of 18.7% at 540 nm for 2FBP2GCz and 17.0% at 580 nm for 2FBP2DPACz.64 Kido and co-workers combined the molecular engineering with a sophisticated device to achieve exceptionally high-power efficiency (PE) in solution-processed orange-red TADF OLEDs. 2SPAC-DBP-2tBuCz-based OLEDs achieve an impressive EQEMAX of 23.7% and PEMAX of 48.8 lm W−1, nearly double the previous best (27.1 lm W−1).65 Lee and co-workers introduced CN into the quinoxaline acceptor to strengthen its electron-accepting character for deep-red emission, while bulky tert-butyl substituents improve solubility and suppress intermolecular interactions. As a result, tBuTPA-CNQx,66 2tBuTPA-QxCN, 4tBuTPA-QxCN,67 and tBuTPA-QxCN68 exhibit deep-red EL with an EQEMAX of 662 nm/16.7%, 628 nm/15.5%, 630 nm/11.5%, and 659 nm/10.3%, respectively, in doped solution-processed OLEDs. Wang and co-workers reported an asymmetric pyrene-azaacene derivative, PBCNT, incorporating a strong electron-donating tert-butyl diphenylamine and a CN group. Interestingly, with a MR-TADF host, the solution-processed OLED achieves orange-red EL at 608 nm and an EQEMAX of 28.5% (Fig. 8).69 Yang and co-workers constructed two deep-red TADF emitters, HATNA-tCz and HATNA-tPCz, built on a HATNA acceptor core with a large, rigid π-conjugated framework and strong electron-withdrawing character. The solution-processed OLEDs using these emitters achieved an EQEMAX of 4.8% with the deep-red EL at 692 nm.70
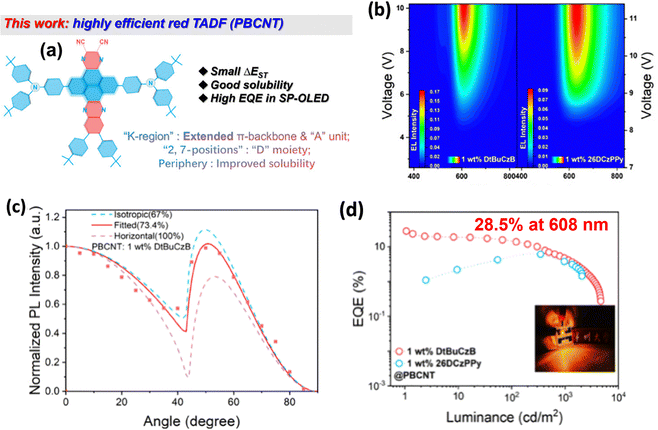 | ||
| Fig. 8 (a) Structures of PBCNT, (b) EL spectra of PBCNT devices at different applied voltages, (c) angle-dependent PL spectra of PBCNT (1 wt%) doped in the DtBuCzB film, (d) device performance of the most efficient quinoxaline acceptor-based solution-processable TADF emitter. Reproduced with permission from ref. 69. Copyright © 2025, Wiley-VCH. | ||
All these results highlight the effectiveness of the quinoxaline acceptor in the development of red and deep-red solution-processable TADF emitters, as summarized in Fig. 9, with a state-of-the-art EQEMAX of 28.5% in the 600–620 nm band in doped devices to date. Nevertheless, compared with the blue and green cases discussed above, quinoxaline-based red emitters show lower efficiency, and truly efficient non-doped red/deep-red systems remain rare. This can be primary attributed to the energy-gap law, where the non-radiative decay increases exponentially as S1–S0 gap narrows, and to the enhanced aggregation/recrystallization of the extended, planar π-conjugated cores during solution processing.
3.5 Boron-based solution-processable TADF emitters
Boron-containing compounds used as solution-processable TADF emitters can be broadly categorized into four main types: triarylboron (TB) acceptor-based compounds, oxygen–boron–oxygen-bridged acceptor-based compounds, nitrogen–boron–nitrogen (N–B–N) MR compounds, and tetracoordinate boron acceptor-based compounds. These four categories are provided in Fig. 10 and will be discussed in detail in the following sections.Leveraging the vacant p orbital of boron, TB acceptors exhibit strong electron-withdrawing character and have been employed in the design of solution-processable TADF emitters. Yang and co-workers reported three TB-based TADF emitters, TB-1PXZ, TB-2PXZ and TB-3PXZ. Among them, TB-3PXZ exhibits the highest PLQY, the smallest ΔEST and the fastest kRISC, delivering green EL at 516 nm, an EQEMAX of 13.9% and a still high EQE1000 of 11.7% in doped OLEDs. In contrast, TB-1PXZ and TB-2PXZ show low device efficiencies mainly due to low PLQYs.71 They introduced one or two CN units into TB-1PXZ and TB-2PXZ, respectively, producing TB-1PXZ2CN and TB-2PXZ1CN, which exhibit 5.6 and 1.6 times higher PLQYs than their CN-free counterparts. Finally, a TB-2PXZ1CN-based OLED achieves a high EQEMAX of 10.2%, outperforming 4.9% of the TB-2PXZ-based device.72 By adopting relatively weak donors instead of PXZ, two TB-based blue emitters, 3DPAc-TB and 3spiroAc-TB, are developed. They exhibit blue EL at 472 nm with an EQEMAX of 12.8%, and at 490 nm with an EQEMAX of 17.3%, respectively, in their optimal doped OLEDs.73
A rigid and symmetric oxygen–boron–oxygen bridged acceptor is typically coupled directly to carbazole-type donors with a D–A configuration, yielding efficient deep-blue TADF emitters with narrowband emission. Choi and co-workers reported several non-doped solution-processable TADF emitters.74,75 Among them, TB-tPCz delivers ultra-deep blue EL at 428 nm with an FWHM of 42.2 nm, as well as a high EQEMAX of 15.8% in a non-doped OLED.76 Wang and co-workers developed three A–π–D type TADF emitters, BFCz-TDBA, ICz-TDBA, and PldCz-TDBA. These emitters retain ultra-deep blue EL emission (424–433 nm) with narrow emission (an FWHM of 41–45 nm) and an EQEMAXs of 12.0–13.0% in doped OLEDs.77 Yan and co-workers adopted 3tBuCz as the donor, however, the resulting emitter, TB-3tBuCz, showed no intrinsic TADF in either solution or neat films. Notably, TADF emerges in the TB-3tBuCz/2,6DCzPPy exciplex system. The corresponding solution-processed devices exhibit ultra-deep-blue EL at 425 nm with an FWHM of 46 nm and a high EQEMAX of 14.6%.78 Wang and co-workers constructed two interfacial exciplex hosts, H2/B4PyMPM and H2/B3PyMPM, for tBuCzDBA-based solution-processed OLEDs, achieving high EQEMAX exceeding 20%. Notably, replacing para-linked B4PyMPM with meta-linked B3PyMPM improves interfacial carrier balance, resulting in a higher EQEMAX of 26.4% (Fig. 11).79 The same group pairs an intramolecularly locked triazatruxene donor with acceptors bearing methyl, hydrogen, or fluorine substituents, affording three blue TADF emitters. In their doped solution-processed OLEDs, FTAT-MBO and FTAT-HBO deliver deep-blue EL with an EQEMAX of 10.2% and 11.4%, respectively, while FTAT-FBO affords sky-blue EL with an EQEMAX of 17.5%. The emission variation is primarily due to the different electron-accepting abilities of different substituents.80 Yin and co-workers developed several spiro boron–nitrogen D–A compounds with tunable emission from deep blue to green based on the acceptor units. TPA-s-Mes*B exhibits deep-blue EL at 454 nm with an EQEMAX of 10.3%, while TPA-s-FMesBF shows green EL at 536 nm with an excellent EQEMAX of 22.1%.81
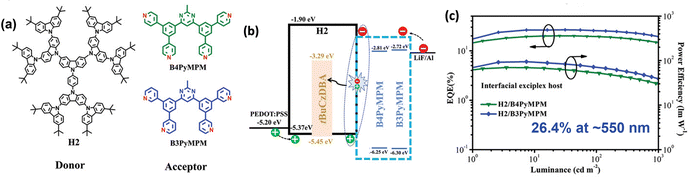 | ||
| Fig. 11 (a) Structures of H2, B4PyMPM and B3PyMPM, (b) device configurations, and (c) device performance of the most efficient solution-processable TB-based and oxygen–boron–oxygen-bridged acceptor-based compounds. Reproduced with permission from ref. 79. Copyright © 2019, Wiley-VCH. | ||
The nitrogen–boron–nitrogen (N–B–N) MR motif has also enabled efficient, solution-processable TADF emission. Duan and co-workers developed three dendric TADF emitters, 5CzBN-ESF, 5CzBN-BSF and 5CzBN-HSF, with high horizontal orientation and PLQYs in neat films, serving as efficient hosts for MR molecules. With 2 wt% S-Cz-BN doping, the devices exhibit narrow-blue EL at 492–496 nm with a small FWHM of 29 nm and a high EQEMAX of 22.1–25.6%.82 Shao and co-workers developed three dendritic host materials, D1-tBu, D1-OBu and D2-OBu. The 5 wt% m-Cz-BNCz:D2-OBu-based solution-processed OLEDs exhibit green EL at 519 nm with high color purity (FWHM = 42 nm) and a high EQEMAX of 24.2%.83 Yang and co-workers designed two small-molecule host materials, 3CzAcPy and 9CzAcPy, featuring high T1 (2.72 eV), good thermal stability and excellent film morphology. With 1 wt% BN-CP1 doping, the devices exhibited blue EL with high satisfactory color purity (FWHM = 28 nm) and a high EQEMAX of 23.5–26.6%.84 Wang and co-workers employed bulky and efficient TADF sensitizers, 5tBuCzTRZ and 5CzTRZ, with kRISC > 107 s−1, as the assistant dopants for DtBuCzBN to enhance exciton harvesting. A ternary emissive layer (mCP: 20 wt% 5tBuCzTRZ: 2 wt% DtBuCzBN) delivered narrow blue EL with a small FWHM of 28 nm, an EQEMAX of 23.9% and extremely small efficiency roll-off (EQE1000 = 21.5%), surpassing the performance of the unsensitized control device.85 Ren and co-workers reported a high-efficiency asymmetric TADF host, DMAC-BP-tBu3Cz, which possessed a high PLQY of 94%, together with a high kRISC of 1.3 × 106 s−1. A ternary system of 2 wt% tBu3Cz-BN: 30 wt% DMAC-BP-tBu3Cz: mCPCN provides a high EQEMAX of 18.4% and small efficiency roll-off with EQE1000 = 17.5% and narrow green EL at 524 nm with an FWHM of 39 nm.86
Hatakeyama and co-workers reported a solution-processable MR-TADF material, OAB-ABP-1, with an extended π-skeleton and bulky substituents. The MR effect of B, N, and O atoms enforces alternating HOMO/LUMO localization, endowing it with attractive photophysical properties. Finally, solution-processed OLEDs using OAB-ABP-1 with a polymer host deliver pure green EL at 505 nm (FWHM = 33 nm) and a high EQEMAX of 21.8% with minimum efficiency roll-off.87 The same group reported another solution-processable MR-TADF emitter, V-DABNA-Mes, featuring three boron and six nitrogen atoms that give rise to a strong MR effect. This design enables narrowband sky-blue TADF with an ultra-narrow FWHM of 16 nm in a 1 wt% doped PMMA film. When applied in a solution-processed OLED, V-DABNA-Mes delivered a sharp EL peak at 480 nm, a high EQEMAX of 22.9% and a slightly broader EL with an FWHM of 27 nm.88 Further structural optimization of BCzBN is likewise applicable to the development of more advanced solution-processable MR emitters. Choi and co-workers replaced the tert-butyl groups in BCzBN with bulky 9-phenyl-fluorene (Fl) units to afford 4FlCzBN. The enhanced steric shielding reduces intermolecular interactions and suppresses the undesired concentration quenching. As a result, X (2–16) wt% 4FlCzBNZ: mCP-based OLEDs show an EQEMAX of 10.1–10.9% and narrow blue EL with an FWHM of 28–30 nm. By contrast, the BCzBN analogue exhibits lower efficiencies and broader emission as doping concentration increases.89 Interestingly, they integrated a host segment (tmCP) with the CzBN unit via a non-conjugated cyclohexane linker, yielding Cy-tmCPBN, which showed efficient intramolecular energy transfer from tmCP to CzBN, delivering more than twice LMAX and an improved EQEMAX of 12.3% compared to Cy-CzBN-based OLEDs at the same CzBN concentration.90 Notably, such a molecular design, doubling host material20 or emitter90,91via a non-conjugated cyclohexane linker while preserving the core electronic structure, improves solubility and film-forming ability, and can be broadly applicable to solution-processed materials. Wang and co-workers replaced the tert-butyl groups of BCzBN with electron-donating BPPA units, yielding BN-Y with red-shifted emission at ∼567 nm. Then, they appended TRZ and various pyrimidine derivatives at the para-positions of boron-bound phenyl rings of BN-Y, affording BN-R,92 BN-R1, BN-R2, and BN-R3.93 Especially, in non-sensitized doped solution-processed OLEDs, BN-R exhibits pure-orange-red EL at 617 nm with a FWHM of 47 nm, an EQEMAX of 22.0%, and a long operational lifetime (T50) of 113.5 hours at 1000 cd m−2. Zhang and co-workers appended heavy-atom-containing side chains to the DtBuCzBN core, yielding TCzBN-S and TCzBNSO. In solution-processed OLEDs, these emitters deliver narrow green emission at 500 nm and 516 nm, high EQEMAX values of 23.3% and 25.5%, respectively. Although TCzBN-S exhibits a large kRISC, roughly twice that of TCzBN-SO, their efficiency roll-off remains comparable.94 The same group designed a novel double boron (B)-containing MR-TADF paradigm by appending a a triarylboron-moiety at the para-positions of boron-bound phenyl rings of the DtBuCzBN core, BNB′-1.95 The optimized solution-processed OLED fabricated using a ternary system (1.6 wt% BNB′-1: 10 wt% 4CzIPNZ: PhCzBCz) shows pure-green emission at 540 nm (an FWHM of 30 nm), with an impressively high EQEMAX of 36.2% (Fig. 13). It should be noted that the efficiency roll-off is large with an EQE1000 of only 10.4%, which can partly be attributed to the low kRISC of ∼104 s−1. They replaced the tert-butyl groups of BNB′-1 with TPA units, producing another novel double boron MR-TADF emitter, BNCz-DPAB. Notably, at doping concentrations of 2–14 wt%, the solution-processed OLEDs achieve EL at 624–637 nm with an FWHM of 52–23 nm with EQEMAX values of 22.2–27.7% (Fig. 14).96 Yang and co-workers incorporated two BN units with 3,6- or 2,7-substituted carbazole bearing long alkyl chains, producing BN-36Cz-BN and BN-27Cz-BN. This well-defined design effectively suppresses the intermolecular aggregation, while largely preserving the MR character of the BN core. As a result, solution-processed doped OLEDs based on BN-36Cz-BN and BN-27Cz-BN exhibit high a EQEMAX of 20.1% and 27.1% and narrow blue EL with an FWHM of 33 nm and 31 nm, respectively.97 Wang and co-workers reported two carbazole-decorated BN emitters, Cz-DABNA and t-BuCz-DABNA; impressively, t-BuCz-DABNA simultaneously realized a high EQEMAX of 29.2% and narrow blue EL at 472 nm with an extremely small FWHM of 16.6 nm (Fig. 12).98
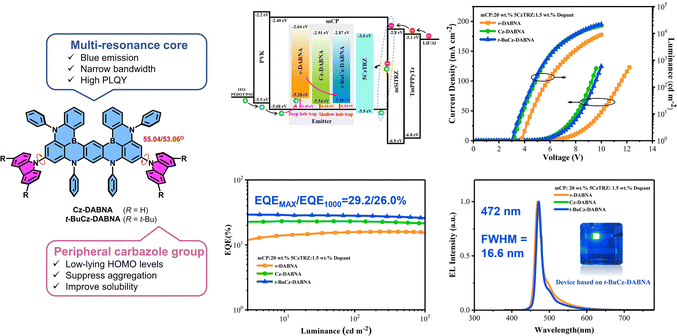 | ||
| Fig. 12 Molecular design and device performance of the most efficient boron-based solution-processable MR TADF emitters in the emission range of <500 nm. Reproduced with permission from ref. 98. Copyright © 2023, Wiley-VCH. | ||
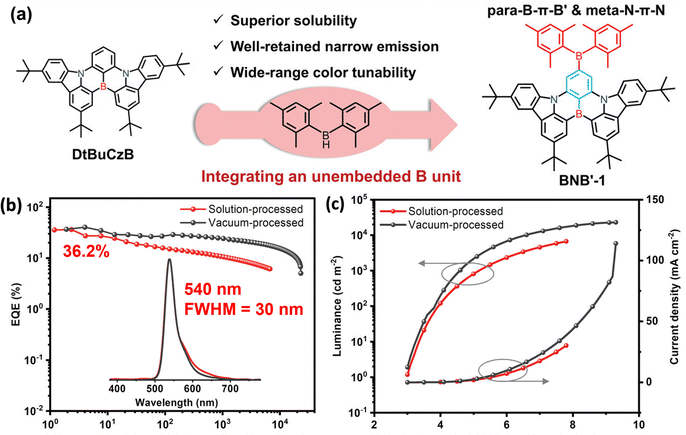 | ||
| Fig. 13 (a) Molecular design of BNB′-1, (b) and (c) device performance of the most efficient boron-based solution-processable MR TADF emitters in the emission range of 500–600 nm. Reproduced with permission from ref. 95. Copyright © 2023, Wiley-VCH. | ||
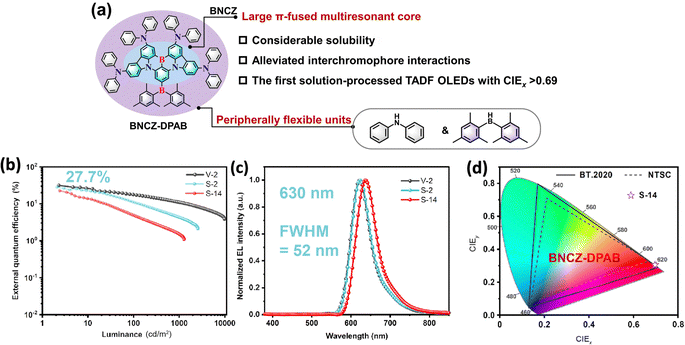 | ||
| Fig. 14 (a) Molecular design of DMCZ-DPAB and (b)–(d) device performance of the most efficient boron-based solution-processable MR TADF emitters in the emission range of >600 nm. Reproduced with permission from ref. 96. Copyright © 2024 Elsevier B.V. | ||
Tetracoordinate boron-based compounds are also effective acceptors in designing solution-processable TADF emitters. Wan and co-workers developed two TADF emitters, PMB-SPAc and spiro-PMB-SPAc, by introducing bulky four-coordinate boron into pyrimidine acceptors. The spiro structures enhance molecular rigidity and suppress nonradiative decay, achieving near-unity PLQYs in 10 wt% doped mCP films. A binary system of 1 wt% PMB-SPAc in CzAcSF achieves an EQEMAX of 26.04% at 492 nm, whereas spiro-PMB-SPAc exhibits improved performance with an EQEMAX of 34.68% at 496 nm. A ternary system (mCP: 20 wt% CzAcSF: 20 wt% spiro-PMB-SPAc) yields a higher EQEMAX of 38.2% (Fig. 15). Notably, spiro-PMB-SPAc delivers the highest EQEs reported for both blue and green solution-processed OLEDs to date.99 The same group employed a rigid triazatruxene (DI) donor to develop spiro-PMB-DI. In solution-processed OLEDs using mCP as the host, spiro-PMB-DI achieves a high EQEMAX of 24.86% with a minimal efficiency roll-off (EQE1000 = 23.1%).100 Chen and co-workers reported two red TADF emitters, DPhCzB and DTPAB, based on a highly soluble, flexible difluoroboron β-diketonate unit with outward-facing, readily accessible fluorine atoms.101 Adachi and co-workers reported two boron-difluoride curcuminoid TADF emitters using the same acceptor, achieving near-infrared EL at 721 nm and an EQEMAX of 9.7%,102 a further red-shifted EL at 758 nm and a high EQEMAX of 5.1%103 in doped solution-processed OLEDs.
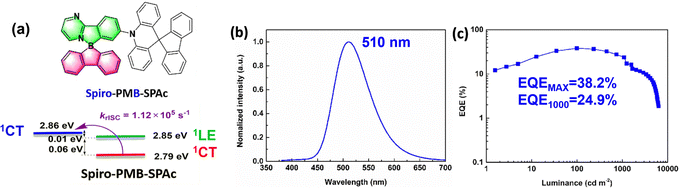 | ||
| Fig. 15 (a) Molecular design of spiro-PMB-SPAc and (b) and (c) device performance of the most efficient solution-processable tetracoordinate boron-based TADF emitters. Reproduced with permission from ref. 99. Copyright © 2025 Elsevier B.V. | ||
All these results demonstrate the versatility of boron-based molecular design in advancing high-performance solution-processable TADF materials. These emitters cover a broad range across the whole visible spectrum, also showing great potential in achieving both exceptional device performance and high color purity.
3.6 Others
In addition to the aforementioned categories, several other classes of efficient solution-processable small-molecule TADF emitters have also been developed, as summarized in Fig. 16. Yang and co-workers developed three red TADF emitters, NAI_R1, NAI_R2, and NAI_R3, by phenyl encapsulation and tert-butyl substitution on a prototypical 1,8-naphthalimide-acridine hybrid. All exhibit high solubility, robust thermal stability, and good film-forming ability. With optimized hosts, doped solution-processed OLEDs show 616 nm/15.1% for NAI_R2 and 622 nm/22.5% for NAI_R3.104 They designed two novel acceptors, ANOP and ANTP, which enable deep-red emission ranging 630–672 nm.105 Jiang and co-workers proposed a red TADF emitter, DBP-2MOTPA, with a through-space configuration. The non-doped device shows deep-red EL at 676 nm with an EQEMAX of 0.73%, and the doped device emits at 645 nm with an EQEMAX of 6.09%.106Thus far we have considered neutral TADF emitters. Ionic TADF materials, by comparison, offer distinctive advantages in solution-processed OLEDs due to their inherent ionic nature, exceptional solubility, and luminescent properties that can be readily adjusted through both cation and anion manipulation. Lu and co-workers developed two ionic TADF molecules, DMAC-TPP[PF6] and 2DMAC-TPP[PF6], featuring D–A+ and D–A+–D topologies that employ a phosphonium cation. The 2DMAC-TPP[PF6]-based solution-processed OLED shows green emission at 512 nm, a high EQEMAX of 18.3% and a small efficiency roll-off of 7.1% (EQE1000 = 17%).107 Building on anion engineering, three related iTADF materials, DMAC-TPP[Br], DMAC-TPP[BF4] and DMAC-TPP[BArF24], are reported. It is found that the anions will affect the photophysical properties via molecular configuration, intra-/inter-molecular interactions and molecular stacking. They also produce marked differences in EL performance, as charge-recombination dynamics are shaped by field-driven anion migration and their hole-trapping ability.108 In parallel, cation modification by incorporating O or S into the phosphonium framework yields DMAC-TPPO[BF4] and DMAC-TPPS[BF4], which show high PLQYs of 77% and 83% and kRISC values of 2.60 × 106 and 2.73 × 106 s−1, respectively. Eventually, solution-processed OLEDs utilizing DMAC-TPPS[BF4] realize an EQEMAX of 17.8% and show a minor efficiency roll-off of 7.3% (EQE1000 = 16.5%).109 Collectively, these results underscore the promise of systematic anion/cation engineering for advancing high-performance iTADF materials in solution-processed devices.
4. Conclusion
Overall, small-molecule solution-processable TADF emitters have emerged as one of the most promising classes for achieving highly efficient solution-processed OLEDs. Through rational molecular design and systematic device engineering, including host optimization for improved charge balance, HIL and HTM optimization for enhanced hole injection and transport, and electron-transport material (ETM) selection for efficient electron mobility, remarkable EQEMAX values exceeding 30% have been achieved in the blue and green regions, while reaching 27.7% in the deep-red region and 28.5% in the orange-red band. However, significant efficiency roll-off remains a major issue, leading to poor performance at practical luminance levels and limiting their potential for commercialization. Another critical issue lies in the unsatisfactory color purity in the solution-processed OLEDs. Although many MR motifs exhibit intrinsically narrow emission in dilute solutions, their EL spectra often broaden significantly in solution-processed devices due to severe molecular aggregation in the solid state, thereby diminishing color purity. This issue becomes even more pronounced for red emitters. To date, only a limited number of efficient MR emitters have been reported with emission wavelengths beyond 600 nm, all of which exhibit FWHM values exceeding 40 nm in their solution-processed OLEDs. Furthermore, it is important to note that currently most of solution-processed OLEDs are only partially solution-processed; typically, the HIL, HTL, and EML are deposited by a solution process, while other layers, such as the hole block layer, ETL, and electron-injection layer, are still introduced via vacuum deposition. As a result, key issues associated with vacuum processing, including high production costs and device size limitations due to small vacuum chambers, still partially remain. Consequently, there is a clear and pressing need to develop novel solution-processable small-molecule TADF emitters that integrate high solubility, suppressed aggregation, and improved morphological stability. In parallel, advancements in fully solution-processable multilayer architectures, compatible ETMs, and scalable coating techniques will be essential to unlock the full potential of this technology for commercial OLED applications.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
The authors greatly appreciate the financial support from the National Key R&D Program of China (2020YFA0714600) and the National Natural Science Foundation of China (52273179 and U23A20594).References
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef
.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef
.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed
.
- X. Cai and S.-J. Su, Adv. Funct. Mater., 2018, 28, 1802558 CrossRef
.
- J. U. Kim, I. S. Park, C. Y. Chan, M. Tanaka, Y. Tsuchiya, H. Nakanotani and C. Adachi, Nat. Commun., 2020, 11, 1765 CrossRef CAS PubMed
.
- X. Cai, B. Gao, X. L. Li, Y. Cao and S. J. Su, Adv. Funct. Mater., 2016, 26, 8042–8052 CrossRef
.
- L. Gan, Z. Xu, Z. Wang, B. Li, W. Li, X. Cai, K. Liu, Q. Liang and S.-J. Su, Adv. Funct. Mater., 2019, 29, 1808088 CrossRef
.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680 CrossRef PubMed
.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef
.
- Q. He, M. Li and S.-J. Su, ChemPhysChem, 2025, 26, e202400955 CrossRef PubMed
.
- Y. H. Kim, C. Wolf, H. Cho, S. H. Jeong and T. W. Lee, Adv. Mater., 2015, 28, 734–741 CrossRef PubMed
.
- T. Zhou, G. Xie, S. Gong, M. Huang, J. Luo and C. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 34139–34145 CrossRef
.
- M. R. Nagar, A. Choudhury, D. Tavgeniene, R. Beresneviciute, D. Blazevicius, V. Jankauskas, K. Kumar, S. Banik, S. Ghosh and S. Grigalevicius,
et al.
, J. Mater. Chem. C, 2022, 10, 3593–3608 RSC
.
- Y. J. Cho, K. S. Yook and J. Y. Lee, Adv. Mater., 2014, 26, 6642–6646 CrossRef PubMed
.
- M. Godumala, S. Choi, S. K. Kim, S. W. Kim, J. H. Kwon, M. J. Cho and D. H. Choi, J. Mater. Chem. C, 2018, 6, 10000–10009 RSC
.
- M. Godumala, J. Yoon, C. H. Jeong, C. Lee, J.-E. Jeong, S. Park, H. Y. Woo, M. J. Cho and D. H. Choi, J. Mater. Chem. C, 2019, 7, 13930–13938 RSC
.
- J. Yoon, C. Lee, S. H. Park, D. W. Kang, H. Kim, J.-E. Jeong, H. Y. Woo, C. S. Hong, S. Park and M. J. Cho,
et al.
, J. Mater. Chem. C, 2020, 8, 2196–2204 RSC
.
- J. Hwang, J. Yoon, C. Y. Kim, S. Choi, H. Kang, J. Y. Kim, D.-W. Yoon, C. W. Han, S. Park and M. J. Cho,
et al.
, Dyes Pigm., 2020, 179, 108403 CrossRef
.
- D. W. Lee, J. Hwang, H. J. Kim, H. Lee, J. M. Ha, H. Y. Woo, S. Park, M. J. Cho and D. H. Choi, ACS Appl. Mater. Interfaces, 2021, 13, 49076–49084 CrossRef
.
- N. Y. Kwon, S. H. Park, C. W. Koh, J. Y. Park, M. J. Kang, H. I. Baek, J. Youn, S. Park, C. W. Han and M. J. Cho,
et al.
, ACS Appl. Mater. Interfaces, 2023, 15, 28277–28287 CrossRef
.
- S. Cho, N. Y. Kwon, C. W. Kim, H. Lee, J. M. Ha, H. J. Kim, H. Y. Woo, S. Park, M. J. Cho and D. H. Choi, Polym. Chem., 2022, 13, 1824–1830 RSC
.
- H. Je, M. J. Cho, N. Y. Kwon, S. H. Park, M. J. Kang, H. I. Baek, J. Youn, C. W. Han and D. H. Choi, ACS Appl. Mater. Interfaces, 2023, 15, 9792–9799 CrossRef
.
- Y. J. Cho, B. D. Chin, S. K. Jeon and J. Y. Lee, Adv. Funct. Mater., 2015, 25, 6786–6792 CrossRef CAS
.
- Z. He, C. Wang, J. Zhao, X. Du, H. Yang, P. Zhong, C. Zheng, H. Lin, S. Tao and X. Zhang, J. Mater. Chem. C, 2019, 7, 11806–11812 RSC
.
- L. Chen, Y. Chang, S. Shi, S. Wang and L. Wang, Mater. Horiz., 2022, 9, 1299–1308 RSC
.
- Y. J. Cho, S. K. Jeon and J. Y. Lee, Adv. Opt. Mater., 2016, 4, 688–693 CrossRef CAS
.
- S. K. Jeon, H. J. Park and J. Y. Lee, ACS Appl. Mater. Interfaces, 2018, 10, 5700–5705 CrossRef CAS PubMed
.
- D. Zhang, M. Cai, Y. Zhang, D. Zhang and L. Duan, Mater. Horiz., 2016, 3, 145–151 RSC
.
- Y. He, Z. Qiao, X. Cai, M. Li, W. Li, W. Xie, W. Qiu, L. Wang and S. J. Su, ACS Appl. Mater. Interfaces, 2020, 12, 49905–49914 CrossRef CAS
.
- D. Liu, Y. He, W. Qiu, X. Peng, M. Li, D. Li, J. Pu, J. Yang, Y. Gan and G. Yang,
et al.
, Adv. Funct. Mater., 2023, 33, 2301327 CrossRef CAS
.
- T. Zhou, K. Zhang, Q. Cao, H. Xu, X. Ban, P. Zhu, Q. Li, L. Shi, F. Ge and W. Jiang, J. Mater. Chem. C, 2022, 10, 2109–2120 RSC
.
- K. Sun, D. Liu, W. Tian, F. Gu, W. Wang, Z. Cai, W. Jiang and Y. Sun, J. Mater. Chem. C, 2020, 8, 11850–11859 RSC
.
- F.-M. Xie, Z.-D. An, M. Xie, Y.-Q. Li, G.-H. Zhang, S.-J. Zou, L. Chen, J.-D. Chen, T. Cheng and J.-X. Tang, J. Mater. Chem. C, 2020, 8, 5769–5776 RSC
.
- H.-N. Shi, F.-M. Xie, H.-Z. Li, Y.-Q. Li and J.-X. Tang, J. Mater. Chem. C, 2025, 13, 5582–5590 RSC
.
- X. Zheng, R. Huang, C. Zhong, G. Xie, W. Ning, M. Huang, F. Ni, F. B. Dias and C. Yang, Adv. Sci., 2020, 7, 1902087 CrossRef CAS
.
- Y. J. Cho, K. S. Yook and J. Y. Lee, Sci. Rep., 2015, 5, 7859 CrossRef CAS
.
- G. Kreiza, D. Berenis, D. Banevičius, S. Juršėnas, T. Javorskis, E. Orentas and K. Kazlauskas, Chem. Eng. J., 2021, 412, 128574 CrossRef CAS
.
- J. Wang, C. Liu, C. Jiang, C. Yao, M. Gu and W. Wang, Org. Electron., 2019, 65, 170–178 CrossRef CAS
.
- Y. Wada, K. Shizu, S. Kubo, K. Suzuki, H. Tanaka, C. Adachi and H. Kaji, Appl. Phys. Lett., 2015, 107, 183301 CrossRef
.
- Y. Wada, K. Shizu, S. Kubo, T. Fukushima, T. Miwa, H. Tanaka, C. Adachi and H. Kaji, Appl. Phys. Express, 2016, 9, 032102 CrossRef
.
- K. W. Tsai, M. K. Hung, Y. H. Mao and S. A. Chen, Adv. Funct. Mater., 2019, 29, 1901025 CrossRef
.
- Y. Yin, Y. Wu, B. He, Z. Xia, W. Zhu, J. Y. Lee and Y. Wang, ACS Mater. Lett., 2024, 6, 4738–4747 CrossRef CAS
.
- Z. Ma, W. Dong, J. Hou, Q. Duan, S. Shao and L. Wang, J. Mater. Chem. C, 2019, 7, 11845–11850 RSC
.
- Z. Ma, W. Dong, X. Lü, P. Chen, J. Hou, Q. Duan and S. Shao, Dyes Pigm., 2020, 174, 108097 CrossRef CAS
.
- B. Li, D. Song, Z. Xu, B. Qiao, W. Zheng, J. Yang, W. Jing and S. Zhao, Org. Electron., 2020, 83, 105721 CrossRef CAS
.
- J. Pu, X. Nie, D. Li, X. Peng, W. Qiu, W. Li, D. Li, G. Sun, C. Shen and S. Ji,
et al.
, Chem. Eng. J., 2023, 471, 144508 CrossRef CAS
.
- C. C. Peng, S. Y. Yang, H. C. Li, G. H. Xie, L. S. Cui, S. N. Zou, C. Poriel, Z. Q. Jiang and L. S. Liao, Adv. Mater., 2020, 32, 2003885 CrossRef CAS PubMed
.
- Y. Wada, S. Kubo and H. Kaji, Adv. Mater., 2018, 30, 1705641 CrossRef
.
- Q. Zhang, D. Tsang, H. Kuwabara, Y. Hatae, B. Li, T. Takahashi, S. Y. Lee, T. Yasuda and C. Adachi, Adv. Mater., 2015, 27, 2096–2100 CrossRef PubMed
.
- K. Wu, Z. Wang, L. Zhan, C. Zhong, S. Gong, G. Xie and C. Yang, J. Phys. Chem. Lett., 2018, 9, 1547–1553 CrossRef PubMed
.
- G. Xie, X. Li, D. Chen, Z. Wang, X. Cai, D. Chen, Y. Li, K. Liu, Y. Cao and S. J. Su, Adv. Mater., 2015, 28, 181–187 CrossRef PubMed
.
- G. Xie, D. Chen, X. Li, X. Cai, Y. Li, D. Chen, K. Liu, Q. Zhang, Y. Cao and S.-J. Su, ACS Appl. Mater. Interfaces, 2016, 8, 27920–27930 CrossRef
.
- Y. Li, T. Chen, M. Huang, Y. Gu, S. Gong, G. Xie and C. Yang, J. Mater. Chem. C, 2017, 5, 3480–3487 RSC
.
- X. Li, K. Wang, Y.-Z. Shi, M. Zhang, G.-L. Dai, W. Liu, C.-J. Zheng, X.-M. Ou and X.-H. Zhang, J. Mater. Chem. C, 2018, 6, 9152–9157 RSC
.
- M. Zhang, G.-L. Dai, C.-J. Zheng, K. Wang, Y.-Z. Shi, X.-C. Fan, H. Lin, S.-L. Tao and X.-H. Zhang, Org. Electron., 2021, 99, 106312 CrossRef
.
- S. Gao, X. Chen, X. Ge, Z. Chen, J. Zhao and Z. Chi, Chem. Res. Chin. Univ., 2022, 38, 1526–1531 CrossRef
.
- M. Zhang, G. Dai, C. Zheng, K. Wang, Y. Shi, X. Fan, H. Lin, S. Tao and X. Zhang, Chin. Chem. Lett., 2022, 33, 1110–1115 CrossRef
.
- Y. Liu, L. Hua, Z. Zhao, S. Ying, Z. Ren and S. Yan, Adv. Sci., 2021, 8, 2101326 CrossRef
.
- X. Cai, D. Chen, K. Gao, L. Gan, Q. Yin, Z. Qiao, Z. Chen, X. Jiang and S.-J. Su, Adv. Funct. Mater., 2018, 28, 1704927 CrossRef
.
- Y. Wang, A. Ying and S. Gong, J. Polym. Sci., 2023, 62, 241–265 CrossRef
.
- C. Zhang, H. Yan, Y. He, Y. Chai and D. Zhou, Mater. Chem. Front., 2022, 6, 3442–3449 RSC
.
- J.-X. Chen, W.-W. Tao, Y.-F. Xiao, K. Wang, M. Zhang, X.-C. Fan, W.-C. Chen, J. Yu, S. Li and F.-X. Geng,
et al.
, ACS Appl. Mater. Interfaces, 2019, 11, 29086–29093 CrossRef CAS
.
- J. X. Chen, Y. F. Xiao, K. Wang, D. Sun, X. C. Fan, X. Zhang, M. Zhang, Y. Z. Shi, J. Yu and F. X. Geng,
et al.
, Angew. Chem., Int. Ed., 2021, 60, 2478–2484 CrossRef CAS PubMed
.
- C. Si, D. Sun, T. Matulaitis, D. B. Cordes, A. M. Z. Slawin and E. Zysman-Colman, Sci. China: Chem., 2024, 67, 1613–1623 CrossRef CAS
.
- D. Jiang, H. Sasabe, H. Arai, K. Nakao, K. Kumada and J. Kido, Adv. Opt. Mater., 2022, 10, 2102774 CrossRef CAS
.
- S. Kothavale, S. C. Kim, K. Cheong, S. Zeng, Y. Wang and J. Y. Lee, Adv. Mater., 2023, 35, 2208602 CrossRef CAS
.
- S. Kothavale, K. Cheong, S. C. Kim, S. Zeng, Y. Wang and J. Y. Lee, Chem. Eng. J., 2023, 477, 146897 CrossRef CAS
.
- S. Kothavale, R. K. Konidena, W. J. Chung, U. Jo, S. Zeng, Y. Wang and J. Y. Lee, Chem. Eng. J., 2024, 496, 154048 CrossRef CAS
.
- X. Liu, L. Hua, X. Lai, J. H. Kim, Q. Zhu, J. Y. Lee, W. Zhu and Y. Wang, Adv. Mater., 2025, 37, 2200690 Search PubMed
.
- X. Zhou, Y. Xiang, S. Gong, Z. Chen, F. Ni, G. Xie and C. Yang, Chem. Commun., 2019, 55, 14190–14193 RSC
.
- Y. Liu, G. Xie, K. Wu, Z. Luo, T. Zhou, X. Zeng, J. Yu, S. Gong and C. Yang, J. Mater. Chem. C, 2016, 4, 4402–4407 RSC
.
- Y. Liu, H. Huang, T. Zhou, K. Wu, M. Zhu, J. Yu, G. Xie and C. Yang, J. Mater. Chem. C, 2019, 7, 4778–4783 RSC
.
- Q. Chen, Y. Xiang, X. Yin, K. Hu, Y. Li, X. Cheng, Y. Liu, G. Xie and C. Yang, Dyes Pigm., 2021, 188, 109157 CrossRef CAS
.
- H. J. Kim, M. Godumala, S. K. Kim, J. Yoon, C. Y. Kim, H. Park, J. H. Kwon, M. J. Cho and D. H. Choi, Adv. Opt. Mater., 2020, 8, 1902175 CrossRef CAS
.
- J. Hwang, H. Kang, J.-E. Jeong, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Chem. Eng. J., 2021, 416, 129185 CrossRef CAS
.
- H. J. Kim, H. Kang, J. E. Jeong, S. H. Park, C. W. Koh, C. W. Kim, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Adv. Funct. Mater., 2021, 31, 2102588 CrossRef CAS
.
- B. Chen, C. Liao, D. Li, H. Liu and S. Wang, J. Mater. Chem. C, 2023, 11, 8767–8775 RSC
.
- Y. Xie, L. Hua, Z. Wang, Y. Liu, S. Ying, Y. Liu, Z. Ren and S. Yan, Sci. China: Chem., 2023, 66, 826–836 CAS
.
- L. Chen, J. Lv, S. Wang, S. Shao and L. Wang, Adv. Opt. Mater., 2021, 9, 2100752 CrossRef CAS
.
- Y. Liu, B. Du, X. Han, X. Wu, H. Tong and L. Wang, Chem. Eng. J., 2022, 446, 137372 CrossRef CAS
.
- G. Liao, J. Lei, S. Li, M. Liu, Y. Qiao, K. Liu, N. Wang, Q. Niu and X. Yin, Adv. Opt. Mater., 2023, 12, 2301242 CrossRef
.
- G. Zhao, D. Liu, P. Wang, X. Huang, H. Chen, Y. Zhang, D. Zhang, W. Jiang, Y. Sun and L. Duan, Angew. Chem., Int. Ed., 2022, 61, 202212861 CrossRef PubMed
.
- R. Ma, Z. Ma, X. Wang, Z. Si, Q. Duan and S. Shao, Chem. Eng. J., 2022, 447, 137517 CrossRef CAS
.
- N. Li, Z. Chen, C. Zhou, F. Ni, Z. Huang, X. Cao and C. Yang, Adv. Mater., 2023, 35, 2300510 CrossRef CAS
.
- L. Chen, Y. Chang, H. Shu, Q. Li, S. Shi, S. Wang and L. Wang, Adv. Opt. Mater., 2022, 11, 2201898 CrossRef
.
- K. Shi, Y. Xie, L. Hua, S. Li, Z. Yang, Y. Yin, Z. Wang, S. Ying, Y. Liu and Z. Ren,
et al.
, ACS Mater. Lett., 2024, 6, 1491–1503 CrossRef CAS
.
- N. Ikeda, S. Oda, R. Matsumoto, M. Yoshioka, D. Fukushima, K. Yoshiura, N. Yasuda and T. Hatakeyama, Adv. Mater., 2020, 32, 2004072 CrossRef CAS PubMed
.
- S. Oda, B. Kawakami, Y. Yamasaki, R. Matsumoto, M. Yoshioka, D. Fukushima, S. Nakatsuka and T. Hatakeyama, J. Am. Chem. Soc., 2022, 144, 106–112 CrossRef CAS PubMed
.
- N. Peethani, N. Y. Kwon, C. W. Koh, S. H. Park, J. M. Ha, M. J. Cho, H. Y. Woo, S. Park and D. H. Choi, Adv. Opt. Mater., 2023, 12, 2301217 CrossRef
.
- N. Y. Kwon, H. Kwak, H. Y. Kim, S. H. Park, J. Y. Park, M. J. Kang, C. W. Koh, S. Park, M. J. Cho and D. H. Choi, Chem. Sci., 2024, 15, 12361–12368 RSC
.
- J.-Y. Park, N.-Y. Kwon, C.-W. Koh, S.-H. Park, M.-J. Kang, H. Kwak, C.-Y. Park, W.-S. Chae, C. S. Hong and S. Park,
et al.
, ACS Appl. Mater. Interfaces, 2024, 16, 16553–16562 CrossRef CAS PubMed
.
- X. Cai, Y. Xu, Y. Pan, L. Li, Y. Pu, X. Zhuang, C. Li and Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202216473 CrossRef CAS PubMed
.
- X. Cai, Y. Pan, X. Song, C. Li, Y. Pu, X. Zhuang, H. Bi and Y. Wang, Adv. Opt. Mater., 2023, 12, 2302811 CrossRef
.
- F. Huang, Y.-C. Cheng, H. Wu, X. Xiong, J. Yu, X.-C. Fan, K. Wang and X.-H. Zhang, Chem. Eng. J., 2023, 465, 142900 CrossRef CAS
.
- H. Wang, X.-C. Fan, J.-X. Chen, Y.-C. Cheng, X. Zhang, H. Wu, X. Xiong, J. Yu, K. Wang and X.-H. Zhang, Adv. Funct. Mater., 2023, 33, 2306394 CrossRef CAS
.
- H. Wang, Y.-C. Cheng, X.-C. Fan, D.-Y. Chen, X. Xiong, X.-Y. Hao, Y.-Z. Shi, J. Yu, D. Huang and J.-X. Chen,
et al.
, Sci. Bull., 2024, 69, 2983–2986 CrossRef CAS
.
- T. Wang, X. Yin, X. Cao and C. Yang, Angew. Chem., Int. Ed., 2023, 62, e202301988 CrossRef CAS
.
- K. Zhang, X. Wang, Y. Chang, Y. Wu, S. Wang and L. Wang, Angew. Chem., Int. Ed., 2023, 62, e202313084 CrossRef CAS
.
- B. Li, Z. Ding, Z. Wu, M. Liu, S. Chen, D. Chen, Z. Li, L. Sang, Y. Liu and L. Lin,
et al.
, Chem. Eng. J., 2023, 476, 146484 CrossRef CAS
.
- Z. Pan, J. Pan, L. Sang, Z. Ding, M. Liu, L. Fu, M. Wang, X. Huang, B. Li and S. Chen,
et al.
, Chem. Eng. J., 2024, 483, 149221 CrossRef CAS
.
- J. Jin, W. Wang, P. Xue, Q. Yang, H. Jiang, Y. Tao, C. Zheng, G. Xie, W. Huang and R. Chen, J. Mater. Chem. C, 2021, 9, 2291–2297 RSC
.
- D.-H. Kim, A. D’Aléo, X.-K. Chen, A. D. S. Sandanayaka, D. Yao, L. Zhao, T. Komino, E. Zaborova, G. Canard and Y. Tsuchiya,
et al.
, Nat. Photonics, 2018, 12, 98–104 CrossRef CAS
.
- H. Ye, D. H. Kim, X. Chen, A. S. D. Sandanayaka, J. U. Kim, E. Zaborova, G. Canard, Y. Tsuchiya, E. Y. Choi and J. W. Wu,
et al.
, Chem. Mater., 2018, 30, 6702–6710 CrossRef CAS
.
- W. Zeng, T. Zhou, W. Ning, C. Zhong, J. He, S. Gong, G. Xie and C. Yang, Adv. Mater., 2019, 31, 1901404 CrossRef
.
- X. Gong, Y. Xiang, W. Ning, L. Zhan, S. Gong, G. Xie and C. Yang, J. Mater. Chem. C, 2022, 10, 15981–15988 RSC
.
- J. Wu, X. Wang, W. Tian, J. Chen, Y. Liu, Y. Tian, X. Tang, Z. Cai, K. Sun and W. Jiang, ACS Appl. Mater. Interfaces, 2024, 16, 69670–69678 CrossRef CAS PubMed
.
- X.-L. Chen, X.-D. Tao, Y.-S. Wang, Z. Wei, L. Meng, D.-H. Zhang, F.-L. Lin and C.-Z. Lu, CCS Chem., 2023, 5, 589–597 CrossRef CAS
.
- Y.-S. Wang, T. Zhao, J.-H. Song, X.-D. Tao, D.-H. Zhang, L. Meng, X.-L. Chen and C.-Z. Lu, Chem. Eng. J., 2023, 460, 141836 CrossRef CAS
.
- Y.-S. Wang, X. Lu, J.-H. Song, X. Li, X.-D. Tao, L. Meng, X.-L. Chen and C.-Z. Lu, Chem. Eng. J., 2024, 482, 148865 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |