 Open Access Article
Open Access ArticleNatural biomolecules for cell-interface engineering
Tong-Kai
Zhang†
a,
Zi-Qian
Yi†
a,
Yao-Qi
Huang
*ab,
Wei
Geng
*a and
Xiao-Yu
Yang
 *ac
*ac
aState Key Laboratory of Silicate Materials for Architectures & State Key Laboratory of Advanced Technology for Materials Synthesis and Processing & School of Chemistry, Chemical Engineering and Life Sciences & Laoshan Laboratory & School of Materials Science and Engineering, Wuhan University of Technology, Wuhan, 430070, China. E-mail: weigeng@whut.edu.cn; xyyang@whut.edu.cn
bSchool of Engineering and Applied Sciences, Harvard University, MA-02138, USA. E-mail: yhua@seas.harvard.edu
cNational Energy Key Laboratory for New Hydrogen-Ammonia Energy Technologies, Foshan Xianhu Laboratory, Foshan 528200, P. R. China
First published on 28th January 2025
Abstract
Cell-interface engineering is a way to functionalize cells through direct or indirect self-assembly of functional materials around the cells, showing an enhancement to cell functions. Among the materials used in cell-interface engineering, natural biomolecules play pivotal roles in the study of biological interfaces, given that they have good advantages such as biocompatibility and rich functional groups. In this review, we summarize and overview the development of studies of natural biomolecules that have been used in cell-biointerface engineering and then review the five main types of biomolecules used in constructing biointerfaces, namely DNA polymers, amino acids, polyphenols, proteins and polysaccharides, to show their applications in green energy, biocatalysis, cell therapy and environmental protection and remediation. Lastly, the current prospects and challenges in this area are presented with potential solutions to solve these problems, which in turn benefits the design of next-generation cell engineering.
1. Introduction
Cells are the true beginning of life, and they are also the most basic living system.1,2 Throughout the long evolutionary process, both single-cell and multicellular organisms have evolved various ways to better adapt to harsh living environments, and survive to continue their species.3 Among these ways, the spontaneous formation of protective nanoshells around cells or organisms is an extremely common and important process in nature.4 The spores of some bacteria have strong resistance to adverse environments due to the increased thickness of their cell wall, which is composed of polysaccharides, proteins, enzymes and fatty acids.5,6 Spores of Bacillus subtilis (B. subtilis) can survive under high moist heat, up to 100 °C.7 For marine mollusks, a mineralized shell provides excellent protection against harsh environments and threats from natural enemies.8–10 Surprisingly, tumor cells in blood can utilize fibrinogen in the microenvironment to form a fibrin shell in situ on their surface to escape natural killer (NK) cell cytotoxicity, thus cunningly achieving hematogenous metastasis.11 Altogether, living organisms continuously synthesize and choose various bioactive molecules with various functions for the better survival and reproduction of their species in long-term evolution, which has inspired scientists to utilize similar functional materials to enhance natural biological cells.12–14 Guided by these fascinating natural behaviours, making an effective composite of living cells with functional nanomaterials can enhance cell stability and endow cells with required new functions.15–19 Therefore, for better exploration, manipulation and utilization of living cells, a non-genetic and simple strategy has been developed to construct protective and multifunctional nanoshells around cells with nanoscale materials,20 which has been widely applied in green energy,21–24 biological catalysis,25–28 environmental protection and remediation,29–32 and cell therapy (Table 1).33–37| Applications | Cell types | Materials | Functional features | Examples |
|---|---|---|---|---|
| Green energy | Shewanella oneidensis MR-1 (S. oneidensis MR-1) cells | CdS nanoparticles (NPs) | Improving hydrogen yield | S. oneidensis MR-1@CdS21 |
| Escherichia coli (E. coli) cells | CdS NPs | Improving hydrogen yield | E. coli@CdS22 | |
| Chlorella pyrenoidosa (Chlorella) cells | FeIII-doped polypyrrole (PPy)/CaCO3 nanocoating | Improving hydrogen yield | Chlorella@PPy/CaCO3 (ref. 24) | |
| Biocatalysis | S. oneidensis MR-1 cells | Ag NPs | Improving microbial fuel cell generation efficiency | S. oneidensis MR-1@Ag25 |
| Moorella thermoacetica (M. thermoacetica) cells | Au nanoclusters (Au NCs) | Improving CO2 fixation | M. thermoacetica@Au26 | |
| Saccharomyces cerevisiae (S. cerevisiae) cells | Tannic acid (TA)-modified InP NPs | Improving production of shikimic acid | S. cerevisiae@TA-InP27 | |
| Environmental protection and remediation | Chlorella cells | Glass-fiber (GF)@PVDF | Efficient high-ammonia nitrogen wastewater treatment and efficient biofouling control | Chlorella@GF@PVDF29 |
| Phanerochaete chrysosporium (P. chrysosporium) cells | ZnS NPs | Efficient removal of heavy metals Pb2+ and Cd2+ | P. chrysosporium@ZnS30 | |
| Bacillus megaterium Y-4 (B. megaterium) cells | Pd0 NPs | Efficient improved OTC degradation | B. megaterium@Pd32 | |
| Cell therapy | Bifidobacterium longum (B. longum) cells | Iron single-atom catalyst (Fe SA50)@C18-PEG-B@metal–organic-framework-encapsulated Fe precursor (B-SA50) | Efficient alleviation of intestinal inflammation and microbiota dysbiosis | B. longum@B-SA50 (ref. 33) |
| Chlamydomonas reinhardtii (C. reinhardtii) cells | DBCO-PEG4-NHS ester NPs (NP-robot) | Efficient treatment of acute bacterial pneumonia | C. reinhardtii@NP-robot34 | |
| Salmonella typhimurium VNP20009 (S. typhimurium), Escherichia coli 25922 (E. coli) cells | Glucose-polymer-conjugated and indocyanine-green-loaded silicon NPs (GPICG-SiNPs) | Efficient promotion of antitumor immune responses | S. typhimurium/E. coli@GPICG-SiNPs35 |
Indeed, in the design process of engineering cells, the selection of functional materials must be balanced against the complexity of the cell-surface morphology and cell-surface functional groups to achieve the required functions without affecting the cell activity.38–41 In fact, a variety of materials (inorganic materials and organic materials) have been developed with the goal of enhancing cells or endowing them with new functions. Before diving fully into natural-molecule-based cell-interface engineering, it is worth comparing purely inorganic materials, synthetic polymers, in-situ self-synthesized polymers and natural molecules for creating engineered cells, to give an overview of their strengths and identify key areas for improvement. Firstly, we cover the strengths and weaknesses of each material, as summarized in Fig. 1, prior to evaluating the unique accomplishments of natural molecules in creating engineered living cells. The ratings in this figure are a subjective ranking of the performance of the different types of materials in cell-interface engineering, relative to the others. The primary performance criteria include long-term stability (Fig. 1A), natural degradability (Fig. 1B), biocompatibility for living cells (Fig. 1C), potential for scaling up (Fig. 1D) and the application prospects (Fig. 1E) of cell-surface nanoshells based on purely inorganic materials, synthetic polymers, cell-surface in-situ self-synthesized polymers and natural molecules. Inorganic-material-based cell-surface engineering typically enhances living cells with high stability and photoelectric properties due to the excellent physical and chemical stability, optical properties and electrical properties of such materials. They include oxides (MnO2, SiO2 and TiO2 nanoparticles (NPs),42–44 carbon (graphene oxide nanosheets, carbon tubes and carbon quantum dots),45–47 metals (Au and Pd NPs),48–50 and salt-based materials.51–53 However, inorganic materials directly bind to the cell surface through covalent bonds or electrostatic adsorption in cell-surface engineering, which may stimulate cells and reduce cell viability. Inorganic nanomaterials can enter the cell through endocytosis, which affects cell viability, and the stability of inorganic materials leads to their poor biodegradability, which is very important in clinical applications.54 Organic materials are other materials widely used in cell-surface engineering, and include synthetic polymers, cell-surface in-situ self-synthesized polymers and natural biomolecules. Synthetic polymers are macromolecular compounds with relatively uniform molecular structures and regular repetitive structures formed by the polymerization of monomers, and have been applied in cell-surface engineering due to their stable mechanical strength, acid and alkali resistance, transparency and controllable modifications. They include polyethylene glycol (PEG), poly(propylene sulfide) (PPS) and polypyrrole (PPY).55–57 However, these synthetic polymers always have problems with poor biocompatibility, reduced signal transduction and mass transport, and poor degradation performance.58 Besides synthetic polymers, in-situ self-synthesized polymer-based cell-surface engineering is achieved by being directly initiated from the cell surface through controlled radical polymerization (CRP) techniques, which can alleviate the damage to living cells brought about by polymerization reactants and conditions.58 This technology may face complex processes of engineering and require complex conditions, such as special lighting, initiators and catalysts. Thus, the application of natural active biological molecules (such as natural polyphenols, polysaccharides and proteins) for cell-interface engineering seems to be a perfect choice .59–62 Natural active biological molecules, as perfect interfacing materials, have always been considered the best material for cell-interface engineering due to their advantages of natural biocompatibility, biodegradability, high permeability, inherent bioactivity, and diverse chemical properties, although their nanostructures are not well-defined compared to the traditional abiotic nanomaterials.63–67 Most importantly, these natural active biological molecules are all successful cases in terms of living organisms continuously synthesizing them for the better survival and reproduction of those species in long-term evolution, meaning great feasibility in various cell-interface engineering processes.68–71
In this review, the progress, challenges and opportunities in cell-interface engineering are mainly discussed from the viewpoint of natural biomolecule-based functional materials for biointerfaces assembled on living-cell surfaces with augmented functionality. The natural molecules used for cell-interface engineering mainly fall into five types, including DNA (deoxyribonucleic acid) polymers, amino acids, natural polyphenols, proteins and saccharides.72 These molecules can form different functional hybrid nanoshells around cells, with great application prospects in green energy, biocatalysts, environmental protection and remediation and cell therapy, as discussed in Fig. 2 and Table 1. Moreover, future prospects of cell-interface engineering are also provided. Finally, this review discusses presently faced challenges and provide suggestions for future research directions in this rapidly expanding area. We believe that timely information about natural-molecule-based cell-surface-engineering strategies will inspire further developments of gentler and more precise multifunctional cell-interface engineering strategies based on natural biomolecular materials.
 | ||
| Fig. 2 A schematic showing natural biomolecular materials that are used for the creation of nanofunctionalized cells with specific nanofunctions. | ||
2. Cell-interface engineering induced by a biomolecule interface
As the fundamental structural and functional units of life, biological cells have been widely exploited in devising systems for novel biotechnologies due to their unparalleled biofunctionalities.16 However, in non-natural applications, biological cells face naturally high fragility and complex and harsh environments induce cell instability.19 To address these issues, hybrid systems provided by cell-surface engineering can integrate the advantages of functional materials (mainly inorganic and organic materials) and living cells, endowing traditional materials with biological activity while significantly improving the stability and functionality of cells.73 As described previously, natural biomolecules with unique characteristics and functions, such as DNA, amino acids, polyphenols, proteins and saccharides, contain a variety of functional groups that have been utilized for cell-surface engineering to create living-cell–material hybrid systems. Numerous natural active molecules have been widely exploited in developing protocols for cell-interface engineering, as illustrated in Fig. 2 and Table 2. In all cell-interface engineering approaches, the functional materials and the properties of biological cells collectively determine the resulting stability and functionality of the engineered cells. In this section, we mainly discuss natural biomolecules and biomolecule-based hybrid inorganic–organic materials (including DNA polymers, amino acids, polyphenols, proteins and saccharides) that have been developed for cell-interface engineering.| Type of natural molecule for engineering | Natural molecules | Functional features | Cell types | Ref. |
|---|---|---|---|---|
| DNA molecules | ||||
| Deoxyribonucleic acid (DNA) | Precise manipulation (encoding, handling, and sorting) of encapsulated cells | E. coli, yeast and MCF-7 cells | 82 | |
| DNA | Protection and high viability | CCRF-CEM cells | 81 | |
| Amino acids | ||||
| L-Cysteine | Improving engineered cells’ desulfurizing activity and separation efficiency | Gordonia sp. WQ-01A cells | 70 | |
| L-Cysteine | Self-repair and protective shells | Yeast cells | 89 | |
| L-Cysteine | Durable protection | Yeast cells | 90 | |
| Polyphenols | ||||
| Dopamine (DA) | Protection and post-engineering | Yeast cells | 68 | |
| DA | Improving the catalytic activity of engineered cells | R. glutinis cells | 110 | |
| DA | Protection and efficient multienzyme cascade-based interfacial biocatalysis | E. coli cells | 85 and 165 | |
| Tannic acid (TA) | Protection and controllable degradation on-demand | Yeast cells | 119 | |
| TA | Protection and controllable degradation | HeLa cells | 96 | |
| TA, gallic acid or epigallocatechin gallate | Improving the viability and stability of the biotherapeutic B. thetaiotaomicron | E. coli and B. thetaiotaomicron cells | 120 | |
| TA | Improving hydrogen production in seawater | E. coli cells | 71 | |
| Proteins | ||||
| β-galactosidase proteins | Generating essential nutrients and protection | Yeast cells | 132 | |
| Silk proteins | Biocompatible engineering and biodegradability | L929 fibroblasts and hMSCs | 135 | |
| Protamine proteins | Enhancing the activity and stability of cyanobacteria | Cyanobacterium (Synechocystis sp. PCC 7002) | 10 | |
| Polysaccharides | ||||
| Glycopolymers | Biotinylated phosphine | HeLa/Jurkat cells | 145 | |
| Glycopolymers | Phosphine probe comprising a flag peptide | Splenocyte cells | 146 | |
| Glycopolymers | Difluorinated cyclooctyne (DIFO)-fluorophore probes | Zebrafish | 147 | |
| Chitosan (CHI) and carboxymethyl cellulose | Enhancing probiotic-cell survival in the gastrointestinal (GI) tract | Lactobacillus acidophilus (L. acidophilus) cells | 69 | |
| Chitosan and alginate (ALG) | Introducing probiotic species into the GI tract | B. coagulans cells | 61 | |
| Anionized dextran and bovine serum albumin | Protection, long-term storage, post-engineering and heritable behavior | Yeast cells | 141 |
2.1 Cell-interface engineering induced by natural DNA polymers
As a naturally synthesized biopolymer and natural genetic material, deoxyribonucleic acid (DNA) is produced by the molecularly precise assembly of nucleotides with faithful replication via specific DNA base pairing (A-T and G-C) and the assistance of DNA-modifying enzymes; this can provide a highly tunable and specific technique for generating synthetic materials with DNA as a generic building block.74,75 Due to its merits of reversibility, stability, biocompatibility, and biodegradability, DNA can create functional materials with high purities, controllability, specificities and affinities.76–78 Thus, DNA polymers have been widely used in cell-interface engineering with great applications in cell–cell recognition, cell regulation and protection, and sensing of the intracellular and extracellular microenvironment.79Bertozzi and co-workers demonstrated a metabolic labelling strategy to modify cells with azido sialic acid (SiaNAz) residues, which can be used for specific reaction with single DNA strands (Fig. 3A(a)).80 Cells decorated with oligonucleotides can form 3-dimensional multicellular structures through DNA duplex formation between those cells. The rate of multicellular-structure formation is related to the density of engineered cells and cell-surface DNA. As shown in Fig. 3A(b), a decrease in cell density can lead to a decrease in the t1/2 for the cell–cell assembly reaction. Moreover, this strategy has the ability to purify desired multicellular structures away from undesired cell structures via fluorescence-activated cell sorting (Fig. 3A(c)). A microtissue was constructed by the contact of a CHO cell line, labeled with green fluorescent protein and able to express the secreted growth factor interleukin-3 (IL-3), and the untransformed hematopoietic progenitor cell line (FL5.12), whose growth depends on the presence of IL-3 (Fig. 3A(d)). By controlling the secretion of the cytokine, the growth direction of the multicellular structures can be effectively controlled, which can emulate cytokine-dependent immune-cell enlargement and the proliferation of tumor cells at locations of inflammation.
 | ||
| Fig. 3 Creation of engineered cell interfaces based on natural DNA polymers. (A) (a) Schematic illustration of cell-surface oligonucleotide-linked DNA polymer for forming stable cell–cell contacts, and the confocal laser scanning fluorescence microscope (CLSM) images of cells engineered with fluorescein-conjugated DNA assembled with cells with nonfluorescent complementary DNA strands. (b) The kinetic profiles of the DNA-based assembly process. (c) Fluorescence-activated cell-sorting separation of different Jurkat cell assemblies based on fluorescence properties, and fluorescence images of those isolated structures. (d) Multicellular structures of different sizes synthesized based on cell-surface DNA structures. Reproduced with permission from ref. 80. Copyright 2009, National Academy of Sciences. (B) (a) Schematic illustration of biomimetic cell wall (BCW) synthesis with DNA polymers on the cell surface. (b) Confocal fluorescence images and TEM images of the BCW template on the cell surface. (c) The relationship between the shielding enhancement and centrifugal force. (d) Staining assay of endothelial cells with an anti-CD31 antibody. Reproduced with permission from ref. 81. Copyright 2019, Springer Nature. (C) (a) Schematic illustration of in situ polymerization reaction with DNA polymers for cell-interface engineering and CLSM images of engineered cells. (b) The polymer density of the DNA cocoons on the MCF-7 cells through branched primers (BPs) with different concentrations. (c) The flow cytometry analysis of cell viability and the encapsulation efficiency with DNA polymers. (d) Precise handling of cells by post-editing of the DNA polymer cocoons around cells. Reproduced with permission from ref. 82. Copyright 2019, Springer Nature. | ||
A supramolecular DNA frame-based biomimetic cell wall (BCW) has been designed to shield live mammalian cells, playing a role in protecting cells from physical and biological assaults (Fig. 3B(a)).81 A DNA initiator (conjugated with cholesterol) was designed to be inserted into the cell membrane via a cholesterol–lipid interaction; the initiator can be used as the origin of the formation of a template via a coculture with solutions of two kinds of DNA monomer. The fluorescence images show that a DNA frame and a crosslinked matrix of alginate formed on the cell membrane, and the TEM images confirmed that a 70–150 nm BCW formed around cells (Fig. 3B(b)). As shown in Fig. 3B(c), the shielding enhancement of the BCW on the cell surface linearly increases with the centrifugal force, indicating that the DNA-based BCW can protect cells in an intelligent and resilient approach for different centrifugation situations.
This biointerface can also protect the engineered cells from biological damage. An in vivo assay, comparing the engineered cells with native MSCs, shows that the transplanted DNA-based BCW on engineered MSCs can stimulate more angiogenesis on the transplantation sites, suggesting that the engineered MSCs release more angiogenic factors for promoting vessel formation (Fig. 3B(d)). The data demonstrates that DNA-based BCWs can protect mammalian cells from complex environmental assaults.
In another work, a DNA-orientated polymerization method was used to fabricate a DNA interface at the cell surface.82 In this procedure, an initiating primer (IP) is attached to the cell membrane, serving as the origin of the in situ DNA polymerization (Fig. 3C(a)). Then, rolling circle replication guided by the IP (for synthesizing long and periodic DNA polymers) and branched replication guided by a branched primer (BP) (for generating single-stranded DNA polymers) are carried out cooperatively to complete the interface engineering of the cells, with DNA cocooning them like a fabric. The CLSM images show that the DNA-based interface engineering approach can be applied to various cells, like bacterial (E. coli), fungal (yeast) and mammalian cells (MCF-7) cells. The flow cytometry analysis indicates that the fluorescence intensities of the grafted DNA increase with the concentration of the IP, which implies that a controllable and well-aligned DNA interface can be obtained by adjusting the IP incubation concentration (Fig. 3C(b)). After the in situ DNA polymer-based cell-interface engineering, over 95.6% of the MCF-7 cells are engineered and alive, and over 80% of the cells are singly engineered (Fig. 3C(c)). This result shows that the DNA-based engineering approach is very biocompatible and highly efficient. More significantly, this method can be used for intelligently manipulating cells. Through DNA-modifying enzymes, the DNA polymer can be manipulated with nucleotide-level precision. In this work, the encoding sequences (ESs) are selected as the cleavage sites of high-fidelity restriction endonucleases (EcoRI-HF, HindIII-HF, and PstI-HF) (Fig. 3C(d)). Thus, when incubated with those corresponding restriction endonucleases, the specific targeted DNA polymers linked with cells could be cleaved at the cleavage sites, and the designated cells could be smartly released from the capture zones of the patterned surface. In summary, the DNA-based interface-engineering method can endow cells with a functional interface, and also has great potential for controlled interactions between cells, specific assembly approaches, and precise targeted cell delivery.
2.2 Cell-interface engineering induced by amino acids
In nature, there are twenty common amino acids, and as extremely important biomolecules, these amino acids can serve as the building blocks for synthesizing peptides or proteins for living things.83,84 Normally, amino acids have a primary amine and a carboxylic acid, and can be primed for amide, or peptide, bond formation. For proteins, the side chains of amino acids always display aromatic, polar or aliphatic groups, which are responsible for stabilizing proteins’ three-dimensional and tertiary structures.85 As typical small biological molecules, amino acids, with abundant functional groups (such as amino, carboxyl and/or thiol groups) and good biocompatibility, can display similar physicochemical properties to small organic molecules, and have been applied in fabricating specific and controllable nanostructures via self-assembly with nanomaterials.85–88Due to their active groups, natural amino acids can very easily non-covalently bind with cell-surface groups and form a nanoshell. Therefore, our group strategically use L-cysteine as the key component in the creation of advanced biointerfaces to engineer desulfurizing bacterial living cells (Fig. 4A(a)).70 Moreover, the biohybrids around the bacteria surface enable an active platform to stably introduce different functional nanomaterials (TiO2 NPs, Fe3O4–SiO2 nanocomposites) onto the bacteria surface (Fig. 4A(b) and (c)). Initially, we designed an L-lysine–Au biohybrid system, which can be used as an interface around desulfurizing bacteria cells for post-nanofunctionalization with TiO2 NPs to enhance the desulfurization efficiency. As shown in Fig. 4A(d), the cell@biohybrid@TiO2 cells have the highest desulfurizing activity in dibenzothiophene (DBT) degradation (63%), compared with cell@biohybrid cells (48%) and native cells (39%).
 | ||
| Fig. 4 Creation of engineered cell interfaces based on natural amino acids. (A) (a) Schematic of L-cysteine-based cell-surface engineering. (b) SEM and TEM images and EDX analysis of cell@biohybrid-shell@TiO2-shell cells. (c) Post-functionalization with functional nanomaterials. (d) The desulfurizing activity of cells with different treatments. Reproduced with permission from ref. 70. Copyright 2014, Royal Society of Chemistry. (B) (a) Schematic of cell-surface engineering with L-cysteine-based biohybrids. (b) SEM and TEM micrographs of yeast@biohybrid interfaces. (c) Cell activity of biohybrid engineered yeast cells exposed to ultraviolet (UV) radiation. (d) Process of self-repairing biohybrid artificial interfaces during the whole process of cell division. Reproduced with permission from ref. 89. Copyright 2015, Royal Society of Chemistry. (C) (a) Schematic of cell-interface engineering with a bilayered interface around an S. cerevisiae cell. (b) TEM micrographs of a single yeast cell encapsulated with a bilayered interface. (c) Protection of bilayered-interface-engineered cells against UV radiation. Reproduced with permission from ref. 90. Copyright 2018, Royal Society of Chemistry. | ||
Subsequently, we found that hybrid coatings of L-cysteine with gold NPs can form a dynamic self-repairing artificial interface on yeast cell surfaces (Fig. 4B(a)).89 Under this protocol, the construction of the innovative bilayered interface begins with the formation of a biohybrid layer, which is initiated by exposing yeast cells to gold NPs that are functionalized with L-cysteine molecules. During this reaction, the L-cysteine forms robust hydrogen bonds with the hydroxyl groups of polysaccharides on the yeast cell surface. This interaction results in the formation of an inner layer of the bilayered interface on the cell surfaces. The SEM, OM and TEM micrographs clearly demonstrate that the yeast cells are individually and separately engineered with an integrated interface without changing the cells’ biological morphologies (Fig. 4B(b)). The EDX line confirms that the L-cysteine-modified gold-NP biohybrid, containing the Au element, are uniformly distributed around the cells. Moreover, the biological hybrid interface created in this manner has an excellent protective effect on the yeast cells under UV radiation and various culture conditions (Fig. 4B(c)). Above all, during the division of coated cells, specific hydrogen bonding interactions with L-cysteine cause deposition of gold NPs on the surfaces of daughter cells to form a supplemental interface and obtain an artificial cell with a self-repairing biointerface (Fig. 4B(d)).
Furthermore, our group found that L-cysteine can forge hydrogen bonds with the hydroxyl groups presented on the surface of the silica (Fig. 4C(a)),90 where the interaction contributes significantly to the subsequent formation of the outer layer. By serving as a molecular bridge between these distinct layers, L-cysteine facilitates the formation of a finely structured bilayered interface. The SEM and TEM images reveal the intricate nanoscale structure of the bilayered interface (Fig. 4C(b)), which features a dense outer silica layer and a porous inner biohybrid layer. This dual-layered structure not only provides protection but also enables efficient nutrient exchange, promoting the overall health of the encapsulated cells. One of the critical aspects of this development relates to its ability to enhance the viability of yeast cells when they are exposed to a complex environment with multiple simultaneous hostile stimuli. For instance, exposure to UV radiation is known to cause DNA damage in living cells and even causes cell death. The results depicted in Fig. 4C(c) demonstrate that yeast cells engineered with the bilayered interface (depicted by the black line) maintain significantly higher viability compared to native yeast cells (depicted by the yellow line). This remarkable resilience results from a combination of factors, including nutrient storage within the interface and the absorption of UV radiation. Beyond protection, the bilayered interface can be tailored to incorporate additional functionalities, such as the integration of graphene to enhance electrical conductivity, offering opportunities for applications like bioelectrodes and cellular response monitoring. The development of this bilayered interface, composed of a biohybrid layer formed by L-cysteine-coated gold NPs and self-assembled amorphous silica, represents a ground-breaking solution for safeguarding individual S. cerevisiae cells against a multitude of simultaneous and hostile environmental factors. This innovation opens possibilities for a wide range of applications in biotechnology and cellular protection, and inspires us that more types of amino acids can be utilized for cell engineering.
2.3 Cell-interface engineering induced by natural polyphenol molecules
As a category of omnipresent compounds distributed in nature, polyphenols have advantages of being biocompatible, acting as biological adhesives, and having antioxidant and antibacterial activity, these properties attracting widespread attention from scientists.91,92 All those properties are based on the unique polyphenolic structures with catechol or pyrogallol moieties, which allow strong non-covalent interactions (such as hydrogen bonding, electrostatic, and cation–π interactions) and strong covalent interactions (such as Michael addition/Schiff-base reaction, radical coupling reaction, and dynamic coordination interactions with boronate or metal ions).93–95 Among these compounds, dopamine (DA) and tannic acid (TA) have been widely used for generation of cell-interface engineering through complex binding reactions.96,97DA or 4-(2-aminoethyl)benzene-1,2-diol is an intermediate product produced by the metabolism of tyrosine through dihydroxyphenylalanine, which has been found to be present in animals and plants.98 DA has been confirmed to have various physiological roles in human, such as neurotransmission. Therefore, under alkaline conditions, DA is easily polymerizable to form PDA with soft, adhesive and biocompatible properties; as a biological catecholamine neurotransmitter, it is also the main component of mussel adhesive proteins.99 Above all, PDA nanocoatings show extremely strong adhesion properties due to their hydroxyl, amino, and catechol functional groups, as well as π–π interactions.100 Thus, due to these properties, PDA has been widely used in biotechnology, such as in flexible electronic equipment and nanomedicine, where it contains an abundance of phenolic hydroxy groups.101–103 PDA-based engineering approaches create an artificial interface that protects yeast cells, along with mammalian, fungal and bacterial cells.104–108 The idea of using dopamine for cell-interface engineering was firstly developed by Choi and coauthors for interface engineering of yeast cells (Fig. 5A(a)).68 In this work, polydopamine (PDA) was chosen as an engineering material for introducing an organic interface formed by strong covalent bonds, and the polydopamine interface is confirmed by TEM micrographs, which show a uniform thin film firmly coating the cell wall and big particulates (Fig. 5A(b)). The work shows that yeast cells after PDA engineering kept the capability of division, and were much more resistant against lysis than the native cells: over 70% of yeast@PD1 (single PDA interface) and ∼90% of yeast@PD2 (double PDA interface) cells still survived after 1 h, while more than 90% of native yeast was lysed (Fig. 5A(c)). In another work, DA was also applied to block antigenic epitopes on human red blood cells (RBCs), which can be applied in creating universal RBCs in health care.104 The SEM images show that the PDA engineering process has no significant impact on cell morphology (Fig. 5B(a)), and the Raman spectroscopy distinguishes the PDA interface formed on human RBCs (Fig. 5B(b)). Most importantly, the PDA biointerface around RBCs can effectively shield specific antigens belonging to those RBCs that can induce blood coagulation when mixed with other RBCs with anti-type antisera (Fig. 5B(c)). Besides this, DA has also been used to construct artificial nanocoatings for algal cells (diatom cells), which is proved by reflection microscopy and SEM images (Fig. 5C(a)).109 To study the capacity of PDA as a protective nanocoating for diatom cells under different harsh environmental conditions, native diatoms and PDA-engineered diatom cells were treated with HCl (10% v/v) and sodium dodecyl sulfate (SDS, 1% v/v) solution at 55 °C. Compared with native diatom cells, with no chloroplast fluorescence after treatment with the HCl/SDS solution, the chloroplasts of the PDA-engineered diatom cells still have good auto-fluorescence, and have higher viability (∼70%) (Fig. 5C(b) and (c)).
 | ||
| Fig. 5 Creation of engineered cell interfaces based on natural polydopamine (PDA). (A) (a) Schematic of cell-engineering procedure with PDA. (b) TEM images of the PDA-engineered yeast cells. (c) Growth curve of yeast with or without PDA engineering and protection against lyticase by the PDA interface around yeast cells. Reproduced with permission from ref. 68. Copyright 2011, American Chemical Society. (B) SEM images (a) and Raman spectra (b) of red blood cells (RBCs) without or with cell-interface engineering with PDA. (c) Antibody-mediated aggregation of human RBCs before and after PDA engineering. Reproduced with permission from ref. 104. Copyright 2014, Royal Society of Chemistry. (C) (a) Reflection microscopy and SEM images of native diatoms and diatoms with PDA engineering. CLSM images (b) and viability (c) of native diatoms and PDA-engineered diatoms after treatment with hot cleaning solutions. Reproduced with permission from ref. 109. Copyright 2022, Royal Society of Chemistry. | ||
Furthermore, our group have shown that PDA can be used for cell-interface engineering for Rhodotorula glutinis, a whole-cell biocatalyst to produce chiral alcohols (Fig. 6A(a)).110 In this work, the yeast cells are incorporated with a PDA interface, where the interface is formed by pH-triggered oxidative polymerization of DA monomers, which adhere on the cell wall via self-assembly between PDA and amine or thiol groups on the glycoproteins of cells. The TEM image confirms the nanocoating of PDA on the bacteria surface (Fig. 6A(b)). Moreover, the PDA-coated yeast cells can provide a basis to induce formation of interfaces with nanomaterials such as titanium dioxide (TiO2), silicon dioxide (SiO2) and iron oxide (Fe3O4) to protect the yeast cells in harsh environments. Confocal microscopy further confirmed the post-engineering of SiO2 NPs via the PDA interface (Fig. 6A(c)). The interface coated with PDA increases the catalytic activity of the yeast cells, where the yield percent of cell@PDA@SiO2 is 4 times higher than that of the native cell (Fig. 6A(d)). These observations indicate that artificial cells can have a significantly positive effect on the catalytic efficiency of biological cells.
 | ||
| Fig. 6 Creation of engineered cell interfaces based on natural polydopamine (PDA). (A) (a) Schematic of cell engineering with PDA. (b) TEM image showing the PDA-nanocoating-engineered cells. (c) Confocal image showing the post-engineering based on the PDA interface around cells. (d) (S)-1-Phenylethanol yield of the native cell and engineered cells. Reprinted with permission from ref. 110. Copyright 2017, Royal Society of Chemistry. (B) (a) Schematic of PDA-engineered EcN bacteria with further modification with proteins and antibodies. (b) TEM and confocal images of the PDA-based engineered cells. (c) SEM of engineered cells targeting tumor cells and the animal experiments on mice treated with different engineered cells. Reprinted with permission from ref. 111. Copyright 2023, Wiley-VCH. (C) (a) Schematic illustration of in situ PDA-mediated antigen presentation on the surface of dendritic cells (DCs, top). CLSM images of ovalbumin nanovaccine-deposited DCs (bottom). (b) Fluorescence images of subcutaneous tissues (top) and quantitative analysis of mice from different treated mice (bottom). (c) Fluorescence images of inguinal draining lymph nodes (DLNs) from mice with different treatments (top), and the percentage of IFN-γ-expressing CD8+ T cells in the DLNs (bottom). (d) Tumor growth curves of mice throughout the immunization period. Reprinted with permission from ref. 112. Copyright 2023, American Chemical Society. | ||
Recently, PDA-based cell-interface engineering has been used to functionalize Escherichia coli Nissle 1917 (EcN) cells for treating tumors by eliciting dual anticancer and antiviral immunity.111 Here, PDA interfaces have been introduced on the EcN cell surface, which further allows the modification with α-PD1 and S1 protein via covalent bonds (Fig. 6B(a)). In this system, the α-PD1 antibody and SARS-CoV-2 spike (S1) protein are an immune checkpoint inhibitor and virus-specific antigen, while EcN cells are used as a carrier to colonize hypoxic tumor sites. The TEM image of EcN-αPD1-S1 shows that the engineered cells have the same rod shape as native EcN cells, and display a uniform PDA interface, indicating the successful engineering of the PDA biointerface (Fig. 6B(b)). Moreover, CLSM images show that the (PE)-labeled αPD1 and fluorescein isothiocyanate (FITC)-labeled S1 protein are well co-localized with EcN cells, which reveals that PDA-engineered EcN cells are efficiently decorated with αPD1 and S1 protein. The SEM images show that the PDA-based biointerface-engineered EcN cells could be captured more efficiently by DC2.4 tumor cells than native cells (Fig. 6B(c)). Furthermore, animal experiments show that the EcN-αPD1-S1 cell-treated groups have the best inhibition of the volume of the tumor cells, which implies the activation of a humoral response by the engineered bacteria. The PDA-based functional-interface-engineered bacteria cells provide an exciting platform to design various living therapeutics for more efficient and intelligent means of cell therapy.
Moreover, previous advancements in PDA-based interface engineering show good capabilities to functionalize various cell types with precise applications.112 Recently, Liu et al. proposed a strategy of PDA-based in situ polymerization-mediated antigen presentation (IPAP) for immunization against various diseases. The PDA-based IPAP strategy is achieved by anchoring antigen-loaded nanovaccines stably onto dendritic cells (DCs) with co-deposition with PDA in vivo (Fig. 6C(a), top). The CLSM images show that compared with the OVA-incubated DCs (ODCs), only the ovalbumin (OVA) nanovaccine-deposited DCs (ONDCs) show fluorescence signals around the cells, confirming the stable conjunction of OVANPs (Fig. 6C(a), bottom). The PDA-based strategy can effectively increase the efficiency of antigen presentation by DCs through improving antigen uptake and reducing lysosomal degradation. An immunofluorescence assay confirmed the in situ deposition of OVANPs on DCs via the PDA polymerization in vivo (Fig. 6C(b), top). The results demonstrate that PDA-based IPAP maintains prolonged localization at the injection site, with 45% fluorescence signal retention after 7 hours, indicating improved antigen stability (Fig. 6C(b), bottom). Moreover, IPAP-treated mice show a 3-fold increase in the presence of IFN-γ+ CD8+ T cells, highlighting robust IFN-γ+ CD8+ T cell activation (Fig. 6C(c)). Those activated CD8+ T cells can secrete IFN-γ, which provides good antiviral and antitumor immune responses. Compared with the control groups, the tumor growth of IPAP-treated mice was effectively suppressed, which demonstrates notable therapeutic promise (Fig. 6C(d)). These findings underscore PDA's potential to engineer different cells, which has great applications in cell protection, biocatalysis and cell therapy.
As another important natural polyphenol, TA is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration. It is a mixture of polygalloyl glucose molecules with different degrees of esterification, and is widely present in various plants.113 The polyphenol structures of TA can bind or chelate with polysaccharides, proteins, alkaloids, and metal ions, and are the main reason for the general surface binding affinity that has been used to form films on a wide variety of substrates, including different biological cells.27,114–117 In the application of cell-surface engineering, metal–organic coordination complexes of TA–FeIII can be formed in situ on cell surfaces. In this process, the pyrogallol (1,2,3-trihydroxybenzene) moiety in TA plays the role of a bidentate ligand for FeIII and forms bis and/or tris complexes on the cell surface, which act as a protective functional nanocoating.118
For example, a metal–organic biointerface composed of tannic acid (TA) and FeIII has been used to construct a structurally robust yet responsive biointerface around cells, amenable to controlled degradation under cytocompatible conditions (Fig. 7A(a)).119 This TA-based interface engineering is achieved via the biocompatible coordination complexes of TA and FeIII. The TA–FeIII interface is formed by adding aqueous solutions containing fresh TA and FeCl3 to an aqueous yeast suspension, creating a biointerface around cells in the mixture in only 10 s. In the FE-SEM image of native yeast cells, their surface appears smooth and intact. However, in the image of Yeast@[TA-FeIII]4, a distinct and well-defined interface-like structure is tightly surrounding the yeast cells (Fig. 7A(b)). This interface exhibits uniformity and is further substantiated in the TEM image of Yeast@[TA–FeIII]4, represented by a dark layer encircling the yeast cells with approximately 40 nm thickness. The TA–FeIII interface displays remarkable protective attributes, effectively shielding the encapsulated cells from various stressors, including UV-C irradiation, lytic enzymes, and silver NPs. Fig. 7A(c) illustrates the protective potential of the TA–FeIII interface against UV-C irradiation. The results indicate that when subjected to 8 J of UV-C light, 92.0 ± 0.6% of native yeast cells succumbed, while 73.1 ± 1.9% of Yeast@[TA–FeIII]4 cells remained viable. This underscores the capacity of the TA–FeIII interface to efficiently screen and absorb UV-C radiation, safeguarding the enclosed yeast cells from its deleterious effects. Under mild conditions, such as exposure to diluted hydrochloric acid, the interface can be selectively degraded, enabling the resumption of cell division. Moreover, this pH-dependent property of the TA–FeIII structure can realize reversible nanofunctionalization on the cell surface (Fig. 7B(a)).114 After the formation of the TA–FeIII interface on the cell surface, the TA–FeIII interface could be removed by reducing the pH value (Fig. 7B(b) and (c)). Furthermore, SEM images and EDS analysis confirmed that this TA–FeIII interface can also be used to engineer bacteria (E. coli, Fig. 7B(d)) and mammalian (PC12, Fig. 7B(e)) cells.
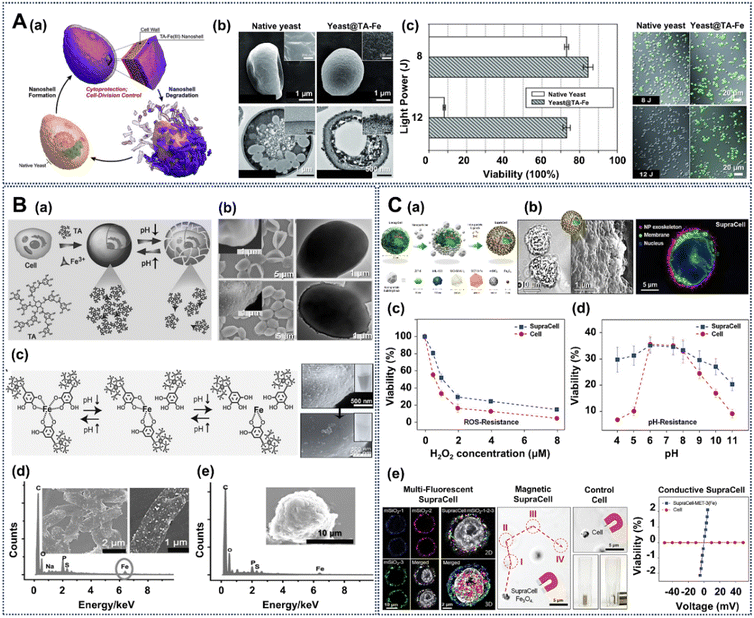 | ||
| Fig. 7 Creation of engineered cell interfaces based on TA. (A) (a) Schematic of the controlled formation and degradation of TA-FeIII-interface-engineered S. cerevisiae cells. (b) FE-SEM micrograph and TEM micrograph of TA-FeIII-engineered yeast cells. (c) Protection against UV-C irradiation of the TA-FeIII-engineered interface. Reprinted with permission from ref. 119. Copyright 2014, Wiley-VCH. (B) (a) The acid triggered reversible engineering of a TA-FeIII interface on yeast cells. (b) SEM and TEM images of native yeast cells (top) and TA-FeIII-interface-engineered yeast cells (bottom). (c) pH-dependent reversible formation of the TA-FeIII interface. SEM images and EDS spectrum of E. coli (d) and PC12 (e) cells engineered with a TA-FeIII interface. Reprinted with permission from ref. 114. Copyright 2015, Wiley-VCH. (C) (a) Schematic representation of SupraCell formation via immediate, TA-assisted formation of NP exoskeletons. (b) Bright-field (left), SEM (middle) and CLSM (right) images of HeLa SupraCells based on assembly of ZIF-8 NPs via TA interparticle ligands. Viability of native HeLa cells and SupraCells-MIL-100(Fe) against ROS stimulus (c) and pH change (d). (e) Multifluorescent labeling, magnetic, and conductive properties of SupraCells with different functional nanomaterials assembled via the cell-surface TA-FeIII biointerface. Reprinted with permission from ref. 96. Copyright 2019, Wiley-VCH. | ||
Brinker and co-workers introduced a stable and protective NP-based exoskeleton employing various NP building blocks (such as ZIF-8, SiO2 and Fe3O4 NPs) on the TA–FeIII interface around mammalian cells (called Supracells), which can avoid the typical endocytic nanoparticle internalization (Fig. 7C(a)).96 For example, the formation of a ZIF-8 NP-based exoskeleton on HeLa cells mediated by a TA–FeIII interface can be confirmed by the bright-field, SEM and confocal Z-stack images in Fig. 7C(b). After being functionalized with an NP-based exoskeleton, the HeLa cells have a higher viability than native HeLa cells in the presence of ROS (H2O2) stimuli, which is due to the antioxidant properties of the tannic acid of the TA–FeIII interface (Fig. 7C(c)). The cell-surface NP-based exoskeleton displays a good resistance to pH ranging from 4–11, mainly brought about by the ion-chelating effect of the cell-surface TA–FeIII-based exoskeleton framework (Fig. 7C(d)). Moreover, for different applications, NP-based exoskeletons with multifluorescent labeling, magnetism and electrical conductivity can be introduced on the cell surface as needed (Fig. 7C(e)). This TA-based biointerface holds the potential to propel the field of single-cell manipulation and chart new paths for innovative biotechnological applications.
TA and FeIII represent pioneering components in the establishment of biointerfaces for cell engineering, which implies potential for other polyphenolic compounds such as gallic acid (GA) and epigallocatechin gallate (EGCG) to engage with metal ions, thus facilitating the formation of metal-phenol networks (MPNs).120 Based on this point, Furst et al. explored different MPN biointerfaces for bacterial cell engineering. These comprise non-covalent coordination complexes of metal ions and different natural polyphenols (TA, GA and EGCG) (Fig. 8A(a)).120 The SEM images reveal that uncoated E. coli cells exhibit a notably smoother surface compared to their MPN-coated counterparts, which display an augmented surface roughness (Fig. 8A(b)). Changes in the morphology of the engineered cells are a direct result of the effective formation of an MPN layer. Lyophilization, a critical step in long-term cryopreservation and long-distance transportation, affects the activity of bacteria cells and thus influences follow-up applications.121 Furst et al’s work confirms that cells engineered with an MPN interface exhibit considerably higher survival rates post-lyophilization, even in the absence of traditional cryoprotectants. Furthermore, as shown in Fig. 8A(c), MPN-engineered E. coli and B. thetaiotaomicron display augmented optical density (OD) values after 48 h incubation, in contrast to control groups. Remarkably, even in the absence of conventional cryoprotectants, MPN-engineered E. coli suspended in phosphate citrate buffer (PC) exhibits a remarkable 3-fold increase in OD after 48 h, while B. thetaiotaomicron demonstrates exponential growth after 96 h, in stark contrast to the control groups, which exhibit no discernible growth (Fig. 8A(d)). The MPN-interface-based cell engineering provides a biocompatible and versatile solution with far-reaching implications for the development of microbial biotherapeutics, and has the potential to enable the production of a broader range of microbial strains and open new avenues for the delivery of living biotherapeutics to the gut through biointerface-engineered microorganisms.
 | ||
| Fig. 8 Creation of engineered cell interfaces based on TA. (A) (a) Illustration of the engineering of an MPN on microbes with FeIII ions and polyphenols. (b) SEM images of the MPN-engineered cells. (c) Enhanced survival and growth of MPN-coated E. coli and B. thetaiotaomicron. (d) The growth rates of native cells and MPN-coated cells. Reprinted with permission from ref. 120. Copyright 2022, American Chemical Society. (B) (a) Microscopy characterization of native E. coli, E. coli@TA–FeIII, and E. coli@TA–FeIII@CdS cells. (b) Quantitative assessment of hydrogen production by the E. coli@TA–FeIII system and control experiments within varying salinity environments. (c) Evaluation of the hydrogen production performance of the E. coli@TA–FeIII@CdS biohybrid system and control experiments under illumination. Reprinted with permission from ref. 71. Copyright 2023, Wiley-VCH. | ||
Moreover, a TA-based biointerface can operate as an intermediary medium, facilitating adhesion of various materials to microorganisms. Utilizing the TA–FeIII biological interface as a foundation, our group designed a functional biological hybrid system by attaching semiconductors (CdS NPs) to E. coli cells to enhance the stimulation of sustainable biological hydrogen production, especially under marine conditions.71 SEM images confirm that the E. coli surface exhibits a roughened appearance while still retaining structural integrity post-encapsulation (Fig. 8B(a)). TEM images further validate the successful engineering of E. coli within the TA–FeIII interface and corroborate the presence of CdS NPs on the cell surface. TEM elemental mapping unequivocally confirms an even dispersion of CdS NPs within the E. coli@TA–FeIII@CdS biohybrid system. Elevated salinity levels exert a marked detrimental effect on the hydrogen production capabilities of native E. coli; in striking contrast, E. coli@TA–FeIII displays notable resilience under such conditions, manifesting minimal fluctuations in hydrogen production (Fig. 8B(b)). Notably, experiments conducted within real seawater environments reveal that E. coli@TA–FeIII continues to exhibit efficient hydrogen production, surpassing native E. coli performance by an impressive 26.36%. This result attests to its efficacy in safeguarding cells from the adverse effects of heightened osmotic pressures in seawater to either sustain or enhance hydrogen production. Furthermore, the TA–FeIII interface facilitates the incorporation of CdS NPs onto the cell surface, which can bring more electrons. Under dark conditions, native E. coli and E. coli@TA–FeIII@CdS cells exhibit comparable hydrogen production levels, indicating that CdS loading has a negligible impact on the viability and activity of E. coli (Fig. 8B(c)). However, under intense illumination, cell damage may occur, leading to a decline in the hydrogen-producing activity of native E. coli cells. In contrast, E. coli@TA–FeIII@CdS consistently displays the highest hydrogen production levels, surpassing native E. coli by 36.15%. This suggests the presence of a photo-synergistic enhancement in the biohybrid system, where CdS NPs on the surface of E. coli amplify the catalytic activity for hydrogen generation in the presence of light. The result underscores the importance of the TA–FeIII biointerface as an adhesive interface to protect cells, enhance cell-surface electron transfer and adsorb functional nanomaterials, ultimately resulting in an enhancement of hydrogen production.
2.4 Cell-interface engineering induced by natural proteins
Proteins are the material foundation of all life and the main agents of activities in living systems, and are made up of amino acids.122 Every cell and all important components in the body involve proteins.123,124 Moreover, proteins exhibit a variety of naturally occurring biological functions such as catalyzing metabolic reactions, replicating DNA, responding to stimuli, and mediating molecule transportation.125 Thus, proteins with rich functional groups seem to be worthwhile biomolecules to employ in cell-interface engineering via complex bonding, such as covalent and noncovalent bonding.126,127 In fact, natural proteins have been widely used in cell-interface engineering via mild non-covalent bonds.128–130In tissue engineering, the stability of cells is affected by physical, chemical, and environmental stimuli. To overcome these issues, Akashi et al. introduced a protein-based extracellular matrix (ECM) using a layer-by-layer (LbL) approach on HepG2 cell surfaces, which can provide robust protection against physical stress (Fig. 9A(a)).131 In this process, fibronectin (FN) and gelatin (G) are sequentially layered onto the cell membrane using the LbL method, which can enhance cells’ structural integrity and resilience, essential for withstanding physical stress. Moreover, growth curves indicate that FN-G-engineered cells maintain good proliferation rates, similarly to uncoated cells, and still exhibit a typical morphology, which shows the high biocompatibility of this approach (Fig. 9A(b)). Importantly, the FN-G nanocoating around the HepG2 cells displays an excellent performance in preventing physical stress-induced damage. Compared with the 6% viability of uncoated HepG2 cells, FN-G-engineered cells retained a high viability rate exceeding 85% after 18 centrifugation cycles (Fig. 9A(c)). Besides that, lactate dehydrogenase (LDH) leakage assays further revealed that FN-G-coated cells showed minimal LDH leakage, demonstrating that the protein-based nanocoating effectively preserves membrane integrity and reduces cell damage during stress (Fig. 9A(d)).
 | ||
| Fig. 9 Creation of engineered cell interfaces based on natural proteins. (A) (a) The layer-by-layer (LbL) assembly of a fibronectin-gelatin (FN-G) biointerface on a human hepatocyte carcinoma (HepG2) cell surface to protect against physical stress. (b) Growth curves and phase-contrast images of HepG2 cells with different treatments after 8 days of culture. Viability of HepG2 cells with or without protein-based engineering against centrifugation (c) and lactate dehydrogenase (d) stresses. Reprinted with permission from ref. 131. Copyright 2013, American Chemical Society. (B) (a) Schematic of cells functionalized with β-gal and ZIF-8. (b) CLSM images of the β-gal and ZIF-8 engineered cells. (c) Protection from the β-gal and ZIF-8 hybrid nanocoating around cells in long term survival, and lyticase and protease environments. Reprinted with permission from ref. 132. Copyright 2017, Wiley-VCH. | ||
In another work, Falcaro et al. designed engineered yeast cells (yeast-β-gal/ZIF-8) with a functional β-galactosidase (β-gal) protein around cells, and then post-engineered them with a removable inorganic protective porous shell via the crystallization of a ZIF-8 metal–organic framework (MOF) film on the enzyme interface (Fig. 9B(a)).132 In the process, the cationic β-gal protein can be adsorbed onto the anionic cell surface, and then the protein interface around the cell can attract the MOF precursors and support the rapid biomimetic crystallization to form a ZIF-8 nanoshell. In this work, bioactive exogenous enzymes (β-gal protein) are used to engineer yeast cells to generate essential nutrients, and then the proteins and cells are protected with ZIF-8 to allow the cells to survive in a hostile environment. The surface coverage is confirmed by confocal laser scanning microscopy, where the living cells can catalyze fluorescein diacetate (FDA) into fluorescein (green), β-gal was labeled with Alexa Fluor 568 (purple), and the ZIF-8 coatings were labeled by infiltration of Alexa Fluor 647 (red) (Fig. 9B(b)). From Fig. 9B(c), compared with native cells, it is obvious that the yeast-β-gal/ZIF-8 cells show higher viability in various complex and harsh environments. For instance, in long-term preservation, the viability of yeast-β-gal/ZIF-8 cells only shows a 30% decrease after 7 days under different culture conditions, while most of the native cells died (Fig. 9B(c)). Moreover, yeast-β-gal/ZIF-8 cells retain over 70% viability after 7 days with culture with lyticase or protease, while native cells show a 90% decrease on day 1. The results suggest that the engineered interface of the yeast-β-gal/ZIF-8 cells can generate essential nutrients for supplying cells, and it also can be used for protecting cells against toxic enzymes.
Silk fibroin has wide applications in tissue engineering, regenerative medicine, drug delivery and medical devices due to its advantages of great biocompatibility and good mechanical and physicochemical properties (such as good flexibility and tensile strength, breathability and moisture permeability).128,133,134 Thus, researchers have used modified silk fibroin to design engineered cells.135 In this procedure, aminated silk and carboxylated silk proteins are designed to form an interface on mammalian cells using a layer-by-layer (LbL) assembly approach via electrostatic interactions, which can be confirmed by CLSM images (Fig. 10A(a)). As we can see from the CLSM images in Fig. 10A(b), the silk-based interface around cells shows good cytocompatibility, where the cell viability is not significantly different from that of the respective control groups. Furthermore, the metabolic activity estimated from the percent reduction of Almar Blue gradually increased over 8 days for both control and engineered cells, suggesting cell proliferation (Fig. 10A(c)). Dye reduction by silk-based engineered cells is significantly lower than that by the control cells at days 1 and 3, indicating lower metabolic activity and proliferation at early time points.
 | ||
| Fig. 10 Creation of engineered cell interfaces based on natural proteins. (A) (a) Schematic of mammalian cells engineered with aminated and carboxylated silk protein. (b) Fluorescence microscopy shows the cell viability of the silk-engineered cells. (c) Dye reduction rate of native and engineered cells. Reprinted with permission from ref. 135. Copyright 2020, American Chemical Society. (B) (a) Schematic of engineered cyanobacteria cells and post-functionalization with a silicon compound. (b) CLSM and TEM images indicate the success of the introduction of a silicon hybrid coating on the cyanobacteria. (c) The cell growth of the native and engineered cells. (d) Photosynthetic activities of native and engineered cyanobacteria, measured via oxygen production. Reprinted with permission from ref. 10. Copyright 2021, Oxford University Press. | ||
Recently, our group designed a protein-based interface system by introducing an ordered silicon interface around cyanobacterium cells (Synechocystis sp. PCC 7002).10 In this procedure, anionic cyanobacterium cells are engineered with cationic protamine to form a biointerface that can be used as an electrostatic template to obtain an ordered packed silica nanoshell with organized, uniform and tunable nanoporosity (Fig. 10B(a)). The complex ordered silica nanoshell induced by the protein interface around the cells can be confirmed by SEM micrographs, EDX mapping, CLSM micrographs and TEM micrographs (Fig. 10B(b)).
The engineered interface affects the growth kinetics with an increasing lag-time, but the growth rates of cell@protamine@ordered SiO2 (cell–ordered yolk–shell) shows the same trend as native cells (Fig. 10B(c)). In applications, the ordered-yolk–shell allows the cyanobacteria not only to maintain the regular biological activity of native cells at low photon flux densities, but also to exhibit significantly enhanced biological activity at higher photon flux densities as well (Fig. 10B(d)).
2.5 Cell-interface engineering induced by natural polysaccharides
Polysaccharides are a class of complex and large carbohydrate substances formed by the condensation and dehydration of multiple monosaccharide molecules, which are widely distributed and important in nature.136 Polysaccharides have various functions in living organisms, where peptidoglycans and cellulose can make up the cell walls of bacteria and plants, glycogen can be used as a nutrient store in animals and plants, and heparin has a good anticoagulant effect.137 As natural polymers, polysaccharides have been proven to be highly stable, nontoxic, hydrophilic, modifiable and biodegradable. Particularly, the hydrophilic groups of polysaccharides can interact with biological tissues in a noncovalent manner to enable high bioadhesion.138–140 Due to the advantages mentioned above, saccharides, such as chitosan and carboxymethyl cellulose, have been widely used in cell-interface engineering.For example, Raichur and coworkers provide a cell-interface engineering method by layer-by-layer (LbL) self-assembly of chitosan (CHI) and carboxymethyl cellulose (CMC) on the cell surface of the probiotic Lactobacillus acidophilus (L. acidophilus), which displays negative charge due to the ionized acid groups on the cell wall (Fig. 11A(a)).69 The images in Fig. 11A(b) show that in the native cells, the blue (DAPI) and green (FITC) fluorescence is found throughout the whole cells. But in the LbL-engineered cells, the blue fluorescence is basically inside the cell and the green fluorescence of FITC is around the cell surface. This indicates that the native cells can easily take up DAPI and the FITC-dextran, while the engineered cells can only take up DAPI and inhibit the larger FITC-dextran molecules, showing a selective permeability to molecules with different sizes. Thus, as shown in Fig. 11A(c), the number of native cells is reduced from 10.4 log cfu per 500 mg to 4 log cfu per 500 mg when cultured in simulated gastric fluid (SGF) for 120 min. Meanwhile, under the same conditions, the engineered cells just show a slightly decrease, where the number of engineered cells is changed from 9.4 log cfu per 500 mg to 8.2 log cfu per 500 mg. In summary, these results indicate that the interface around L. acidophilus cells has a selectively impermeability to large-scale enzyme molecules that could cause proteolysis of L. acidophilus cells and can enhance the stability of cells under gastric and intestinal pH conditions.
 | ||
| Fig. 11 Creation of engineered cell interfaces based on polysaccharides. (A) (a) Schematic of LbL assembly of polyelectrolytes of chitosan (CHI) and carboxymethyl cellulose (CMC) on bacterial cells walls. (b) CLSM micrographs of native and engineered L. acidophilus cells. (c) Survivability of native and engineered L. acidophilus in simulated gastric and intestinal conditions. Reprinted with permission from ref. 69. Copyright 2011, American Chemical Society. (B) (a) Schematic of LbL assembly of chitosan and alginate on a probiotic. (b) Bright-field and SEM images of uncoated-BC (Bacillus coagulans) and LbL-(CHI/ALG)2-BC cells. (c) Effect of LbL coatings on probiotic survival against acid and bile insults. (d) Representative in vivo imaging system (IVIS) images of plain-BC and LbL-BC after 1 h oral gavage. Reprinted with permission from ref. 61. Copyright 2016, Wiley-VCH. (C) (a) Procedure of the fabrication of a coacervate-based engineered artificial cell wall around cells (top) and TEM and SEM images of a coacervate-coated S. cerevisiae cell (bottom). (b), E. coli-induced agglutination assay (top) and the adsorption ability of native or engineered S. cerevisiae cells toward fluorescein after different times (bottom). (c) CLSM images of the engineered cells at different budding stages. Reprinted with permission from ref. 141. Copyright 2018, Wiley-VCH. | ||
In another work, the LbL approach of self-assembly of chitosan and alginate was also used to form a protective interface on the probiotic Bacillus coagulans (B. coagulans) via an electrostatic adsorption strategy (Fig. 11B(a)).61 Interestingly, after the interface engineering, the bright field images show that, compared with the native B. coagulans cells, the LbL engineered B. coagulans cells show aggregation (Fig. 11B(b)i and ii). SEM images show that after the interface engineering, compared with the native cells, there are no significant changes in cell morphology, likely due to the natural polysaccharides in the cell wall of Gram-positive bacteria cells (Fig. 11B(b)iii and iv). This work explores the protective effects of different LbL-engineered interfaces for B. coagulans cells against acid (SGF) and bile-salt insults. As we can see from Fig. 11B(c), compared with cells engineered with a single layer of chitosan (B. coagulans/(CHI)1), cells engineered with a single bilayer of chitosan and alginate (B. coagulans/(CHI/ALG)1 cells) provide a certain protective effect, with a 4 log reduction in CFU against SGF and bile salts. Furthermore, cells engineered with a double bilayer of chitosan and alginate (B. coagulans/(CHI/ALG)2 cells) demonstrate significant survival advantages, and just have 1 log reduction in CFU against SGF conditions after 2 h, and a 2 log reduction in CFU against 4% bile salts after 2 h (Fig. 11B(c)). Most importantly, the role of cell-interface engineering in survival and delivery of probiotics in vivo is determined by delivering native B. coagulans and B. coagulans/(CHI/ALG)2 cells by oral gavage. B. coagulans/(CHI/ALG)2 cells show an over 6 fold enhanced bioluminescence signal compared to native cells in the background of GI tract, at only 1 h after administration (Fig. 11B(d)).
In addition, a coacervate, composed of cytocompatible cationized protein (bovine serum albumin: BSA-NH2) and anionized polysaccharides (Dextran-COOH), was designed to construct a protective interface as an artificial cell wall around Saccharomyces cerevisiae (S. cerevisiae) cells via a direct in situ self-assembly method (Fig. 11C(a), top).141 After the cell-surface engineering, the TEM and SEM images confirmed a structurally intact composite biolayer, which firmly adheres to the surface of the cells (Fig. 11C(a), bottom). Due to the α-D-mannose recognition area on the cilia surface of E. coli, the native S. cerevisiae cells can aggregate within a few minutes via a co-culture with E. coli cells. But with the artificial interface, there is no clear aggregation, attributed to the protective interface between the E. coli and S. cerevisiae cells, which blocks the direct contact between them (Fig. 11C(b), top). Moreover, compared with the native cells, the biointerface-engineered cells display a high dye adsorption efficiency; over 70% of the dye could be easily captured within 10 s (Fig. 11C(b), bottom). Surprisingly, the fluorescence images demonstrate that during the fission of the engineered cells, the green and red fluorescence is found on the daughter cells, which suggests that this biointerface around the cells is soft and flexible with a good self-healing performance (Fig. 11C(c)).
In addition to directly engineering living cells with natural saccharides, artificial oligosaccharides containing unusual functional groups can also be introduced on the surface of biological cells via metabolic labelling approaches for engineering cell surfaces with special functions, which is considered as an important process in biorthogonal chemistry.142 This artificial-oligosaccharide-based strategy can incorporate the customized reaction into biological processes within the biological system without affecting normal biochemical processes, which can induce specific modifications for cells in living systems.143 The unusual specific groups of those cell-surface artificial oligosaccharides can be easily post-modified with versatile materials using efficient, simple, reliable and biocompatible click chemistry to realize cell-surface engineering. For example, Bertozzi and coworkers first proposed a metabolic labelling approach to treat mammalian cells (Jurkat, HL-60, and HeLa cells) with synthesized N-levulinoylmannosamine (ManLev) to introduce sialic acid with ketone groups on the cell surface. This can be cell-surface engineered via special covalent ligation with molecules containing hydrazide or other complementary reactive functional groups.144 Moreover, Bertozzi and coworkers developed a strategy to introduce artificial sialic acid with azido groups onto Jurkat cell surfaces by incubating them with N-azidoacetylmannosamine (Ac4ManNAz) (Fig. 12A(a)).145 Various biological probes or materials with carboxylic acid can be attached to cells based on the cell surface artificial azido groups (Fig. 12A(b)). These chemoselective reactions based on cell-surface artificial azido groups can be conducted according to the requirements of the design, with no reactions from nonspecific amine acylation (Fig. 12A(c)). This metabolic oligosaccharide engineering strategy can label unnatural sugars into cellular glycans for inducing abiotic functionality on the cell surface. Most importantly, this strategy can also be executed for specific labeling in living mice by daily intraperitoneal administration of Ac4ManNAz for 7 days, after which isolated splenocytes express azido glycans on the surface,146 which can be quantified with a special phosphine probe with a Flag peptide (Phos-Flag) via the Staudinger ligation (Fig. 12B(a)). After treatment with an anti-Flag antibody labeled with isothiocyanate, flow-cytometry analysis shows that the fluorescence of those isolated splenocytes with artificial azido glycans displays a dose-dependent increase (Fig. 12B(b)). Besides that, Zebrafish embryos can also be metabolically labeled with azides on the glycans of their cell surfaces by treatment with N-azidoacetylgalactosamine (Ac4GalNAz) to obtain a novel way to efficiently visualize and probe biomolecules in living systems (Fig. 12C(a)).147 After a copper-free click chemistry reaction with a fluorophore, those azido glycans of the zebrafish embryos can be stably visualized in a timely way at a subcellular resolution during their development in vivo (Fig. 12C(b)), which can provide a reliable spatiotemporal analysis of cell-surface glycan expression and trafficking of zebrafish embryos. As one type of glycosyltransferase isoenzyme, the N-acetylgalactosaminyl (GalNAc) transferase family (GalNAc-Ts) can also be used for generating unusual glycans with azido groups on living cell surfaces, which provides a way to probe cell-surface glycosylation (Fig. 12D(a)).148 Fluorescence images confirmed that the GalNAc-Ts co-localized with the Golgi compartment in cells, which means that the glycosylation is realized here and then transferred and relocated to the surface of the cell membrane (Fig. 12D(b)). Besides that, many synthetic glycopolymers with glycan structures have been designed to insert into cell membranes or to be used for cell-surface engineering.149,150 This metabolic oligosaccharide-based biorthogonal chemistry strategy provides stable and specific active sites and is recognized as an ideal biotechnology to create engineered cells with customized functions, which has created opportunities for fluorescent labeling, fluorescent probes, manipulation of cell behavior, accurate diagnosis and targeted therapy.151–153
 | ||
| Fig. 12 Creation of engineered cell interfaces based on Jurkat cell-surface artificial oligosaccharides. (A) (a) Metabolic delivery of artificial azido sialic acid to Jurkat cell surfaces with Ac4ManNAz. (b) Reaction of biotinylated phosphine and azides of artificial sialic acid on cell surfaces. (c) Specificity of the Staudinger reaction based on cell-surface unnatural azido sialic acid. Reprinted with permission from ref. 145. Copyright 2000, American Association for the Advancement of Science. (B) (a) The Staudinger ligation and metabolic oligosaccharide engineering with Ac4ManNAz in vivo in mice. (b) Mean fluorescence intensity (MFI) of the cells treated with different azido-sugar doses. Reprinted with permission from ref. 146. Copyright 2004, Springer Nature. (C) (a) The schematic of the metabolic labeling with Ac4GalNAz and click chemistry with a fluorescent probe for zebrafish in vivo. (b) Identification of temporally distinct artificial glycans during zebrafish development using fluorescence labeling. Reprinted with permission from ref. 147. Copyright 2008, American Association for the Advancement of Science. (D) (a) Schematic of the non-natural-substrate biosynthetic strategy of incorporating chemically tagged sugars onto the cell surface through engineered GalNAc-T glycosyl transferases. (b) Fluorescence images of HepG2 cells transfected with T2 constructs. Reprinted with permission from ref. 148. Copyright 2020, Elsevier. (E) (a) Schematic of preparation of glycopolymer-engineered dendritic cell vaccines (G-DCV) and confocal images of DC labeling with glycopolymer-DBCO after treatment with Ac4ManNAz. (b) The adhesion behavior between G-DCV and T cells. (c) The percentage of CD3+CD8+ T cells with or without G-DCV cells. (d) Survival rates of B16-OVA tumor-bearing mice with different treatments. Reprinted with permission from ref. 37. Copyright 2024, Wiley-VCH. | ||
Due to their powerful ability to stimulate tumor specific immune responses, dendritic cell vaccines (DCVs) have been widely used in tumor immunotherapy. Recently, Chen with coworkers developed a glycopolymer-engineered DCV (G-DCV), which has a great potency in antitumor immunotherapy.37 G-DCV was designed based on stable covalent copper-free click-chemistry between glycopolymers with dibenzocyclooctyne (DBCO) terminal groups and azido-labeled DCs, which were obtained by incubating bone-marrow-derived dendritic cells (BMDCs) with Ac4ManNAz for 3 days (Fig. 12E(a)).37 CLSM images show that the glycopolymers with fluorescent monomers can provide a stable and prolonged modification of DCs over 48 hours, which can efficiently induce T-cell activation for immunotherapy. Besides that, compared to native DCs, G-DCs have significantly more T cells adhered (Fig. 12E(b)). Moreover, flow cytometry analysis demonstrates that G-DCV allows stimulation of more CD8+ T cell expansion (Fig. 12E(c)). Notably, G-DCV has a good antitumor effect in a mouse B16-OVA tumor model and significantly prolongs the survival time of B16-OVA mice (Fig. 12E(d)).
Besides these natural molecules, polymers introduced via artificially initiated synthesis also represent a powerful means of directly engineering living cell surfaces under mild and biocompatible conditions. They can be called semi-natural molecules, which occupy the space between synthetic polymers and natural biomolecules. Due to the customized polymer synthesis process, this semi-natural polymer-based cell-surface engineering strategy always has good tunability, biocompatibility and precise functions. Unlike traditional cell-surface modification techniques, which often rely on pre-synthesized polymers or chemical conjugation, this strategy allows a controllable and localizable synthesis of polymers directly on cell surfaces. These polymers, synthesized directly on cell surfaces, combine the adaptability of synthetic polymers with the seamless compatibility of biological systems, paving the way for innovations in regenerative medicine, drug delivery, and cell therapy. For example, Hawker and coworkers have introduced an advanced method to engineer live cell surfaces based on polymers via artificially initiated synthesis.58 In this process, cells were treated with activated dibenzocyclooctyl ester to introduce a chain-transfer agent (CTA) onto the cell surfaces, which can provide the active sites to in situ graft synthetic polymers through controlled radical polymerization (CRP). The CLSM images demonstrate a stable, uniform shell on the cell surface, confirming the effectiveness of this technique for functionalizing cell surfaces (Fig. 13A(a)).58 Unlike traditional pre-synthesis methods, this self-synthesizing grafting-from approach initiates polymerization directly on cell surfaces, significantly enhancing grafting efficiency and preserving high cell viability; thus the growth curve of cells functionalized with the semi-natural polymer shows no difference in comparison to those of control groups (Fig. 13A(b)). Besides that, native yeast cells modified with these semi-natural polymers exhibit controlled aggregation in the presence of tannic acid (TA), highlighting the ability of the polymer coating to modulate cell behavior (Fig. 13A(c)). Furthermore, this strategy can also be used for mammalian cells, like Jurkat cells, confirming the adaptability and potential for broader biomedical applications, particularly where hybrid synthesis offers combined advantages of biocompatibility and customizable synthetic properties (Fig. 13A(d)).
 | ||
| Fig. 13 Creation of engineered cell interfaces based on cell-surface synthesized polymers. (A) (a) Schematic representation of yeast cells modified with surface-initiated photoinduced electron transfer–reversible addition fragmentation chain-transfer polymerization (PET-RAFT) and CLSM images of polymer-modified yeast cells. (b) Growth curves of yeast cells with or without engineering. (c) Bright-field microscope images of engineered yeast cells before and after TA treatment. (d) The surface-initiated PET-RAFT strategy used for engineering Jurkat cells, and the CLSM images of engineered Jurkat cells. Reprinted with permission from ref. 58. Copyright 2017, Springer Nature. (B) (a) Schematic illustration of cell-surface enzyme-mediated polymerization used for engineering yeast cells. (b) SEM images of yeast cells with or without the cell-surface polymerization. (c) Resistance of the encapsulated yeast cells to enzymatic degradation. (d) pH-responsive cell-surface polymerization on cell surface. Reprinted with permission from ref. 154. Copyright 2019, American Chemical Society. | ||
Moreover, Nash and coworkers developed a cell-surface enzyme-mediated polymer synthesis strategy to build a pH stimuli-responsive hydrogel capsule onto living cells, wherein the capsule is formed by cross-linking phenol-modified alginate and chitosan (Fig. 13B(a)).154 In the encapsulation process, SEM images show that engineered cells are covered by a smooth, uniform hydrogel layer (Fig. 13B(b)). Importantly, this strategy can selectively engineer living cells expressing glucose oxidase (GOx positive cells), a typical flavin adenine dinucleotide (FAD)-dependent oxidoreductase, and the enzyme-mediated polymerization is based on the enzymatic cascade of glucose oxidase (GOx) and horseradish peroxidase (HRP). When a heterogeneous population of 90% GOx-negative and 10% GOx-positive yeast cells is incubated with zymolyase for 1 h, only GOx-positive cells can survive, which confirmed the selective engineering by this strategy and the resistance of the cell-surface hydrogel capsules to cytotoxic agents (Fig. 13B(c)). Additionally, the cell-surface hydrogel capsules have a pH-responsive property from pH 6 to 8, which can be controlled by adjusting the pH value (Fig. 13B(d)). This adaptability emphasizes the dynamic nature of the synthetic material when combined with natural cellular processes, and underscores the potential of cell-driven synthesis approaches in developing polymers that integrate seamlessly with biological systems.
Similarly, Bruns et al. genetically modified yeast cells to express horseradish peroxidase (HRP) on their cell walls, which can drive the cell-surface localized polymerization of various monomers (Fig. 14A(a)).155 For the in situ cell-surface polymerization, grafting-from polymerization was conducted by conjugating a CTA for RAFT polymerization or an alkyl halide atom transfer radical polymerization (ATRP) initiator. After the cell-surface polymerization, the growth curve of PEGMA-engineered Yeast cells by ATRP shows a slower cell division rate due to the stable polymer nanoshell around cells, which limits nutrient uptake and cell expansion (Fig. 14A(b)). The cell-surface PEGMA-based nanoshell also has good resistance to zymolyase digestion (Fig. 14A(c)). Furthermore, based on the azide-group-bearing PEGMA nanoshell on yeast cells, those engineered cells can be quickly and gently labeled with a clickable fluorescent dye (Cy5-DBCO) on the cell surface using click chemistry. CLSM images demonstrate a bright, stable fluorescence localized around cells (Fig. 14A(d)).
 | ||
| Fig. 14 Creation of engineered cell interfaces based on cell-surface synthesized polymers. (A) (a) Schematic illustration of a horseradish peroxidase (HRP)-catalyzed, grafting-from method on yeast cell surfaces. Growth curve (b) and resistance to zymolyase (c) of native and PPEGMA-engineered yeast cells. (d) Schematic representation of Cy5 conjugation to PPEGMA-N3 on the cell surface, and CLSM images of Cy5-labeled yeast cells. Reprinted with permission from ref. 155. Copyright 2023, Royal Society of Chemistry. (B) (a) Schematic representation of visible-light-initiated radical graft polymerization of poly(PEGDA) to engineer yeast cells. (b) TEM images of native yeast and yeast-poly(PEGDA) cells. (c) Growth curves of yeast cells with different treatments. (d) Schematic illustration of the preparation process for Janus-Urease cells (top) and CLSM images (bottom) of those cells. (e) The velocity of Janus-Urease cells in urea solutions. Reprinted with permission from ref. 156. Copyright 2021, American Chemical Society. | ||
Yang et al. provide a visible-light-activated graft polymerization of poly(ethylene glycol) diacrylate (PEGDA) as a strategy to engineer yeast cells with a cross-linked nanoshell, which was initiated by cell-surface adsorbed polyethyleneimine (PEI) (Fig. 14B(a)).156 TEM images confirmed the effective engineering of yeast cells with the poly(PEGDA) nanoshell through the visible-light-induced graft polymerization on the cell surface, and indicated that the PEGDA-engineered yeast cells have an about 50 nm polymer nanoshell around them (Fig. 14B(b)). Light-dependent introduction of a cross-linked PEGDA nanoshell around cells can delay cell division due to the suppression of cells’ biochemical activity brought about by restricting the transmission of nutrients (Fig. 14B(c)). This means that the integrity and thickness of the polymer nanoshell synthesized on the cell surface can be controlled by adjusting the radiation time and intensity, thereby better manipulating cell behavior. Moreover, the poly(PEGDA) nanoshell around yeast cells can be used as a platform for secondary graft polymerization of sodium acrylate (AAS), which can be performed to modify half of the cell sphere with urease (called a Janus-Urease cell) (Fig. 14B(d)). The Janus-Urease cells exhibited good directional motion through self-propulsion by the cell-surface localized catalytic reactions in urea solutions, which can achieve a concentration gradient velocity control. The velocities of the Janus-Urease cells increase with the increase in urea concentration and can reach 4.04 μm s−1 in 200 mmol L−1 urea solution, while no obvious motion of the Janus-Urease cell was observed in the absence of urea (Fig. 14B(e)). The use of customized in situ semi-natural polymerization for cell-interface engineering represents a versatile and efficient approach to modifying cell surfaces. This technique combines the adaptability of synthetic polymers with the seamless compatibility of biological systems, opening new avenues for innovations in cell therapy, synthetic biology, and biocatalysis.
3. Remaining challenges
As an interdisciplinary scientific field with biology, physics, chemistry, materials science and engineering science, aimed at redesigning and constructing living-cell–material hybrid biological systems with new functions, cell-interface engineering technology has developed into a global topic, and has had a profound impact on multiple fields such as biomedicine, agriculture, industrial production, and even energy conversion. The development of science and bio/nanotechnology has made significant research progress in the field of customized synthetic cell-interface engineering. These studies not only advance our understanding of the essence of matter and life, but also open new paths for future technological development. As shown in Fig. 15, there are still some challenges that urgently need to be addressed: | ||
| Fig. 15 The challenges and future research outlook of next-generation cell-interface engineering systems. | ||
(1) Novel biointerfaces with precise controllability and functionality on the cell surface. This requires selecting suitable molecular components of materials and designing appropriate assembly mechanisms to construct interfaces with automatic assembly of fine structures and functions on the cell surface to achieve functional integration. This would ensure that engineered cells can perform specific functions (such as molecular transfer, or various biochemical triggers), thereby improving the functionality and stability of engineered cells.
(2) How to design self-sustaining and self-regenerating biological interfaces around cells. The stability of cell-surface nanocoatings brought about by current cell-surface engineering technology may disappear with cell proliferation, along with the functionality provided by the interface to the living cells. Finding how to develop a self-sustaining and self-healing interface structures around cells requires further advances in materials and synthesis methods, which can ensure that a cell-surface nanoshell can respond to environmental changes for self-healing and morphological changes, thereby maintaining the long-term stability of engineered cells.
(3) Enlarging the application scope for engineered cells. The functionality of the currently developed engineering cells is relatively focussed, and expanding the application scope of engineered cells will help promote the development of cell engineering research. Utilizing cell engineering technology to achieve efficient utilization of resources and sustainable management of the environment could promote the development of society towards a greener and more sustainable direction.
4. Future outlook
Cell-interface engineering using biomolecules has made significant progress and has wide applications in biotechnology.157–161 In order to promote the wider application and development of natural-molecule-based cell-interface engineering, sustained progress is ongoing in the design and improvement of these hybrid systems.162–166 We have raised the goals listed below as some of those that ought to be addressed in future studies within the field of cell-interface engineering (Fig. 15).(1) Developing new natural biomolecules to expand multi-function cell-interface engineering. Natural biomolecules have a unique advantage of biocompatibility, compared to other materials. Aside from the comparison with other materials, more biomolecules with special properties in living processes should be applied in cell-interface engineering. In one of the main pathological features of Alzheimer's disease, the amyloid-β peptide obtained by hydrolysis of amyloid precursor proteins can cause plaques, which possess a strong aggregation potential.167 In this case, amyloidosis occurs to transform three-dimensional proteins into planar peptides with the stable conformation of a β-pleated sheet lamellar structure and exposes more active binding sites. Inspired by this phenomenon, phase-transformed proteins are good candidates to construct a universal biointerface on living cell surfaces and induce nanomaterial assembly.
(2) Developing a new generation of biointerfaces around cells for efficient applications. Most efforts have focused on the construction of extracellular biointerfaces up to now. However, proliferation, a natural property of living cells, is an uncontrollable process, and would destroy the interface during the process or be inhibited by the rigid interface, affecting cell functions. Therefore, a new-generation design of intracellular biointerfaces around cells is most desired. Cell membrane-embedded interfaces have great potential in intracellular biointerface design.168,169 By embedding biomolecules into the cell membrane, the exogenous interface is most closely combined with cells to obtain a real sense of an artificial cell membrane for “living” functions. This new type of biointerface would minimize the impact on or by cells. Furthermore, to address the challenge of the disappearance of engineered cell-surface nanoshells and their functions caused by cell proliferation, it is necessary to select self-reproducing molecules and construct a self-sustaining nanoshell on cell surfaces.170 Thus, nanoshells on the surface of cells would have a self-reproducing effect with cell proliferation to maintain the stability of the nanoshell functions around cells, thereby obtaining true “living materials”.
(3) Developing engineered cells with integrated multiple functions to expand the applications of engineered cell-based biological systems. For example, Brinker et al. simultaneously introduced multiple functional materials onto the surface of HeLa cells, which provides the potential to introduce multiple functionalities for different applications.81 In addition, by coupling the effects of multiple physical fields, such as light, electrical and magnetic fields, materials can be utilized to activate and promote the application of engineered cells. For example, the use of electric-field stimulation can significantly enhance the hydrogen production performance of living cells.97
5. Conclusion
In this review, we introduce cell-interface engineering with functional materials based on natural biomolecules. These molecules include rich functional groups with great biocompatibility, and thus have been applied by researchers in recent years. We have mainly reviewed the history and the progress of 5 types of natural biomolecule (i.e., DNA polymers, amino acids, polyphenols, proteins and polysaccharides) that can be effectively integrated into cell interfaces. We explore challenges and prospects in the field of cell-interface engineering. We believe that our work will play a role in guiding next-generation cell-interface engineering, as well as better utilizing and manipulating cells, thereby creating new opportunities for the development of cell-based biotechnologies in the future.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review. Data available on request from the authors.Author contributions
Conceptualization: X. Y. Yang, Y. Q. Huang, W. Geng and T. K. Zhang; writing the original draft: T. K. Zhang, Z. Q. Yi and Y. Q. Huang; validation: T. K. Zhang, Z. Q. Yi and Y. Q. Huang; review and editing: X. Y. Yang, Y. Q. Huang and W. Geng.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study is supported by the National Key Research and Development Program of China (2022YFB3805600, 2022YFB3805604), National Natural Science Foundation of China (22293020), Major Program (JD) of Hubei Province (2023BAA003), Key R&D Program of Shandong Province, China (2023CXGC010314), Technology Innovation Program of Hubei Province (2023BIB018) and National 111 project (B20002).References
- M. Lynch, M. C. Field, H. V. Goodson, H. S. Malik, J. B. Pereira-Leal, D. S. Roos, A. P. Turkewitz and S. Sazer, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16990–16994 CrossRef CAS.
- D. Arendt, J. M. Musser, C. V. H. Baker, A. Bergman, C. Cepko, D. H. Erwin, M. Pavlicev, G. Schlosser, S. Widder, M. D. Laubichler and G. P. Wagner, Nat. Rev. Genet., 2016, 17, 744–757 CrossRef CAS PubMed.
- Z. Guo, J. Richardson, B. Kong and K. Liang, Sci. Adv., 2020, 6, eaaz0330 CrossRef CAS PubMed.
- J. Sun, L. Gao, L. Wang and X. Sun, Talanta, 2021, 234, 122671 CrossRef CAS PubMed.
- W. L. Nicholson, N. Munakata, G. Horneck, H. J. Melosh and P. Setlow, Microbiol. Mol. Biol. Rev., 2000, 64, 548–572 CrossRef CAS PubMed.
- P. Setlow, Bacterial Stress Responses, ed. G. Storz and R. Hengge, ASM Press, Washington, 2nd edn, 2010, pp. 319–332 Search PubMed.
- J. Errington, Nat. Rev. Microbiol., 2003, 1, 117–126 CrossRef CAS.
- L. Addadi, D. Joester, F. Nudelman and S. Weiner, Chem.–Eur. J., 2006, 12, 980–987 CrossRef CAS PubMed.
- J. Prywer, Science, 2022, 376, 240–241 CrossRef CAS.
- L. Wang, Y. Li, X.-Y. Yang, B.-B. Zhang, N. Ninane, H. J. Busscher, Z.-Y. Hu, C. Delneuville, N. Jiang, H. Xie, G. Van Tendeloo, T. Hasan and B.-L. Su, Natl. Sci. Rev., 2021, 8, nwaa097 CrossRef CAS.
- S. Zheng, J. Shen, Y. Jiao, Y. Liu, C. Zhang, M. Wei, S. Hao and X. Zeng, Cancer Sci., 2009, 100, 859–865 CrossRef CAS.
- B. Ma and A. Bianco, Nat. Rev. Mater., 2023, 8, 403–413 CrossRef CAS.
- H. Yang, L. Yao, Y. Wang, G. Chen and H. Chen, Chem. Sci., 2023, 14, 13325–13345 RSC.
- Y. Wang, Z. Li, F. Mo, T. J. Chen-Mayfield, A. Saini, A. M. LaMere and Q. Hu, Chem. Soc. Rev., 2023, 52, 1068–1102 RSC.
- S. Yao, B. Jin, Z. Liu, C. Shao, R. Zhao, X. Wang and R. Tang, Adv. Mater., 2017, 29, 1605903 CrossRef PubMed.
- W. Geng, L. Wang, N. Jiang, J. Cao, Y. X. Xiao, H. Wei, A. K. Yetisen, X. Y. Yang and B. L. Su, Nanoscale, 2018, 10, 3112–3129 RSC.
- Q. W. Chen, J. Y. Qiao, X. H. Liu, C. Zhang and X. Z. Zhang, Chem. Soc. Rev., 2021, 50, 12576–12615 RSC.
- H. Lee, N. Kim, H. B. Rheem, B. J. Kim, J. H. Park and I. S. Choi, Adv. Healthcare Mater., 2021, 10, e2100347 CrossRef PubMed.
- T.-K. Zhang, W. Geng, Y.-Q. Huang, F.-Z. Wang, G. Tian and X.-Y. Yang, Coord. Chem. Rev., 2024, 498, 215471 CrossRef CAS.
- Z. C. Lu, R. Zhang, H. Z. Liu, J. X. Zhou and H. F. Su, Trends Biotechnol., 2024, 42, 91–103 CrossRef CAS.
- H. X. Han, L. J. Tian, D. F. Liu, H. Q. Yu, G. P. Sheng and Y. Xiong, J. Am. Chem. Soc., 2022, 144, 6434–6441 CrossRef CAS PubMed.
- B. Wang, C. Zeng, K. H. Chu, D. Wu, H. Y. Yip, L. Ye and P. K. Wong, Adv. Energy Mater., 2017, 7, 1700611 CrossRef.
- Y. Honda, H. Hagiwara, S. Ida and T. Ishihara, Angew. Chem., Int. Ed., 2016, 55, 8045–8048 CrossRef CAS PubMed.
- Z. Xu, J. Qi, S. Wang, X. Liu, M. Li, S. Mann and X. Huang, Nat. Commun., 2023, 14, 1872 CrossRef CAS PubMed.
- B. Cao, Z. Zhao, L. Peng, H.-Y. Shiu, M. Ding, F. Song, X. Guan, C. K. Lee, J. Huang, D. Zhu, X. Fu, G. C. L. Wong, C. Liu, K. Nealson, P. S. Weiss, X. Duan and Y. Huang, Science, 2021, 373, 1336–1340 CrossRef CAS.
- H. Zhang, H. Liu, Z. Tian, D. Lu, Y. Yu, S. Cestellos-Blanco, K. K. Sakimoto and P. Yang, Nat. Nanotechnol., 2018, 13, 900–905 CrossRef CAS PubMed.
- J. Guo, M. Suástegui, K. Sakimoto Kelsey, M. Moody Vanessa, G. Xiao, G. Nocera Daniel and S. Joshi Neel, Science, 2018, 362, 813–816 CrossRef CAS PubMed.
- K. Sakimoto, A. Wong and P. Yang, Science, 2016, 351, 74–77 CrossRef CAS PubMed.
- L. Qin, M. Gao, M. Zhang, L. Feng, Q. Liu and G. Zhang, Water Res., 2020, 187, 116430 CrossRef CAS PubMed.
- H. Qin, T. Hu, Y. Zhai, N. Lu and J. Aliyeva, J. Hazard. Mater., 2020, 400, 123161 CrossRef CAS PubMed.
- H. Hu, X. Liang, S. Wang, Z. Xu, J. Li, H. Chen, D. Su, Y. Yin, Z. Huang and X. Huang, Macromol. Biosci., 2020, 20, e2000185 CrossRef PubMed.
- Y. Jia, Y. Chen and Y. Hu, ACS ES&T Water, 2021, 1, 2412–2422 Search PubMed.
- F. Cao, L. Jin, Y. Gao, Y. Ding, H. Wen, Z. Qian, C. Zhang, L. Hong, H. Yang, J. Zhang, Z. Tong, W. Wang, X. Chen and Z. Mao, Nat. Nanotechnol., 2023, 18, 617–627 CrossRef CAS PubMed.
- F. Zhang, J. Zhuang, Z. Li, H. Gong, B. E. de Avila, Y. Duan, Q. Zhang, J. Zhou, L. Yin, E. Karshalev, W. Gao, V. Nizet, R. H. Fang, L. Zhang and J. Wang, Nat. Mater., 2022, 21, 1324–1332 CrossRef CAS.
- R. Sun, M. Liu, J. Lu, B. Chu, Y. Yang, B. Song, H. Wang and Y. He, Nat. Commun., 2022, 13, 5127 CrossRef CAS.
- C. Sigl, E. M. Willner, W. Engelen, J. A. Kretzmann, K. Sachenbacher, A. Liedl, F. Kolbe, F. Wilsch, S. A. Aghvami, U. Protzer, M. F. Hagan, S. Fraden and H. Dietz, Nat. Mater., 2021, 20, 1281–1289 CrossRef CAS PubMed.
- H. Yang, Z. Xiong, X. Heng, X. Niu, Y. Wang, L. Yao, L. Sun, Z. Liu and H. Chen, Angew. Chem., Int. Ed., 2024, 63, e202315782 CrossRef CAS.
- H. Wei, X.-Y. Yang, W. Geng, H. C. van der Mei and H. J. Busscher, Nanoscale, 2021, 13, 7220–7233 RSC.
- J. Park, B. Andrade, Y. Seo, M.-J. Kim, S. C. Zimmerman and H. Kong, Chem. Rev., 2018, 118, 1664–1690 CrossRef CAS PubMed.
- J. Almeida-Pinto, M. R. Lagarto, P. Lavrador, J. F. Mano and V. M. Gaspar, Adv. Sci., 2023, 10, 2304040 CrossRef CAS.
- S. Yang, H. Choi, D. T. Nguyen, N. Kim, S. Y. Rhee, S. Y. Han, H. Lee and I. S. Choi, Adv. Ther., 2023, 6, 2300037 CrossRef CAS.
- W. Geng, N. Jiang, G. Y. Qing, X. Liu, L. Wang, H. J. Busscher, G. Tian, T. Sun, L. Y. Wang, Y. Montelongo, C. Janiak, G. Zhang, X. Y. Yang and B. L. Su, ACS Nano, 2019, 13, 14459–14467 CrossRef CAS.
- W. Youn, E. H. Ko, M. H. Kim, M. Park, D. Hong, G. A. Seisenbaeva, V. G. Kessler and I. S. Choi, Angew. Chem., Int. Ed., 2017, 56, 10702–10706 CrossRef CAS.
- W. Li, Z. Liu, C. Liu, Y. Guan, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2017, 56, 13661–13665 CrossRef CAS PubMed.
- P. Zhang, C. Yang, Z. Li, J. Liu, X. Xiao, D. Li, C. Chen, M. Yu and Y. Feng, Electrochim. Acta, 2022, 421, 140490 CrossRef CAS.
- A. Yulaev, A. Lipatov, A. X. Lu, A. Sinitskii, M. S. Leite and A. Kolmakov, Adv. Mater. Interfaces, 2017, 4, 1600734 CrossRef PubMed.
- R. Kempaiah, A. Chung and V. Maheshwari, ACS Nano, 2011, 5, 6025–6031 CrossRef CAS PubMed.
- H. Di, H. Liu, M. Li, J. Li and D. Liu, Anal. Chem., 2017, 89, 5874–5881 CrossRef CAS PubMed.
- V. Maheshwari, D. E. Fomenko, G. Singh and R. F. Saraf, Langmuir, 2010, 26, 371–377 CrossRef CAS.
- T.-K. Zhang, Z.-Q. Yi, K.-Q. Ye, W. Geng, Y.-Q. Huang, G. Tian and X.-Y. Yang, Chem. Phys. Lett., 2023, 829, 140750 CrossRef CAS.
- Z. Jiang, Y. Liu, R. Shi, X. Feng, W. Xu, X. Zhuang, J. Ding and X. Chen, Adv. Mater., 2022, 34, e2110094 CrossRef PubMed.
- N. Tang, H. Li, L. Zhang, X. Zhang, Y. Chen, H. Shou, S. Feng, X. Chen, Y. Luo, R. Tang and B. Wang, Angew. Chem., Int. Ed., 2021, 60, 6509–6517 CrossRef CAS PubMed.
- C. Chen, H. Zhang, L. Yang, W. Lv, L. Zhang, X. Jiang and D. Sun, ACS Sustainable Chem., 2021, 9, 9854–9860 CrossRef CAS.
- L. Yue, K. Yang, X.-Y. Lou, Y.-W. Yang and R. Wang, Matter, 2020, 3, 1557–1588 CrossRef.
- J. T. Wilson, W. Cui, V. Kozlovskaya, E. Kharlampieva, D. Pan, Z. Qu, V. R. Krishnamurthy, J. Mets, V. Kumar, J. Wen, Y. Song, V. V. Tsukruk and E. L. Chaikof, J. Am. Chem. Soc., 2011, 133, 7054–7064 CrossRef CAS PubMed.
- J. Liu, Y. Wang, W. J. Heelan, Y. Chen, Z. Li and Q. Hu, Sci. Adv., 2022, 8, eabp8798 CrossRef CAS PubMed.
- Y. Niu, D. Xue, X. Dai, G. Guo, X. Yang, L. Yang and Z. Bai, Nat. Sustain., 2024, 7, 1182–1189 CrossRef.
- J. Niu, D. J. Lunn, A. Pusuluri, J. I. Yoo, M. A. O'Malley, S. Mitragotri, H. T. Soh and C. J. Hawker, Nat. Chem., 2017, 9, 537–545 CrossRef CAS.
- L. Wang, Z. Cao, M. Zhang, S. Lin and J. Liu, Adv. Mater., 2022, 34, e2106669 CrossRef PubMed.
- Z. Zhao, D. C. Pan, Q. M. Qi, J. Kim, N. Kapate, T. Sun, C. W. t. Shields, L. L. Wang, D. Wu, C. J. Kwon, W. He, J. Guo and S. Mitragotri, Adv. Mater., 2020, 32, e2003492 CrossRef PubMed.
- A. C. Anselmo, K. J. McHugh, J. Webster, R. Langer and A. Jaklenec, Adv. Mater., 2016, 28, 9486–9490 CrossRef CAS PubMed.
- S. Kaushik, P. D. Thungon and P. Goswami, ACS Biomater. Sci. Eng., 2020, 6, 4337–4355 CrossRef CAS PubMed.
- P. Auffinger, F. A. Hays, E. Westhof and P. S. Ho, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16789–16794 CrossRef CAS.
- J. Clardy and C. Walsh, Nature, 2004, 432, 829–837 CrossRef CAS.
- L. S. Hui Chong, J. Zhang, K. S. Bhat, D. Yong and J. Song, Biomaterials, 2021, 266, 120473 CrossRef CAS PubMed.
- E. Tvrda, F. Benko, T. Slanina and S. S. du Plessis, Molecules, 2021, 26, 5196 CrossRef CAS PubMed.
- Y. Ju, H. Liao, J. J. Richardson, J. Guo and F. Caruso, Chem. Soc. Rev., 2022, 51, 4287–4336 RSC.
- S. H. Yang, S. M. Kang, K. B. Lee, T. D. Chung, H. Lee and I. S. Choi, J. Am. Chem. Soc., 2011, 133, 2795–2797 CrossRef CAS.
- A. J. Priya, S. P. Vijayalakshmi and A. M. Raichur, J. Agric. Food Chem., 2011, 59, 11838–11845 CrossRef CAS PubMed.
- N. Jiang, G. L. Ying, S. Y. Liu, L. Shen, J. Hu, L. J. Dai, X. Y. Yang, G. Tian and B. L. Su, Chem. Commun., 2014, 50, 15407–15410 RSC.
- Z. Yi, S. Tian, W. Geng, T. Zhang, W. Zhang, Y. Huang, H.-N. Barad, G. Tian and X.-Y. Yang, Chem.–Eur. J., 2023, 29, e202203662 CrossRef CAS PubMed.
- K. Adebowale, R. Liao, V. C. Suja, N. Kapate, A. Lu, Y. Gao and S. Mitragotri, Adv. Mater., 2023, 2210059 Search PubMed.
- W. Xiong, Y. Peng, W. Ma, X. Xu, Y. Zhao, J. Wu and R. Tang, Natl. Sci. Rev., 2023, 10, nwad200 CrossRef CAS PubMed.
- Y. H. Roh, R. C. Ruiz, S. Peng, J. B. Lee and D. Luo, Chem. Soc. Rev., 2011, 40, 5730–5744 RSC.
- M. R. Jones, N. C. Seeman and C. A. Mirkin, Science, 2015, 347, 1260901 CrossRef PubMed.
- X. Wang, W. E. Allen, M. A. Wright, E. L. Sylwestrak, N. Samusik, S. Vesuna, K. Evans, C. Liu, C. Ramakrishnan, J. Liu, G. P. Nolan, F.-A. Bava and K. Deisseroth, Science, 2018, 361, eaat5691 CrossRef PubMed.
- X. Xiong, H. Liu, Z. Zhao, M. B. Altman, D. Lopez-Colon, C. J. Yang, L. J. Chang, C. Liu and W. Tan, Angew. Chem., Int. Ed., 2013, 52, 1472–1476 CrossRef CAS PubMed.
- Y. Chen, R. Tian, Y. Shang, Q. Jiang and B. Ding, Adv. NanoBiomed Res., 2022, 2, 2100126 CrossRef CAS.
- P. Shi and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 11580–11591 CrossRef CAS PubMed.
- Z. J. Gartner and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4606–4610 CrossRef CAS.
- P. Shi, N. Zhao, J. Coyne and Y. Wang, Nat. Commun., 2019, 10, 2223 CrossRef PubMed.
- T. Gao, T. Chen, C. Feng, X. He, C. Mu, J. I. Anzai and G. Li, Nat. Commun., 2019, 10, 2946 CrossRef PubMed.
- Y. Hou and G. Wu, Adv. Nutr., 2018, 9, 849–851 CrossRef PubMed.
- G. Wu, Amino acids biochemistry and nutrition, CRC Press, Texas, USA, 2021 Search PubMed.
- T. A. King, J. Mandrup Kandemir, S. J. Walsh and D. R. Spring, Chem. Soc. Rev., 2021, 50, 39–57 RSC.
- B. Kelly and E. L. Pearce, Cell Metab., 2020, 32, 154–175 CrossRef CAS PubMed.
- R. P. Wilson, in Fish Nutrition, ed. J. E. Halver, R. W. Hardy, Academic Press, San Diego, 3rd edn, 2003, pp. 143–179 Search PubMed.
- A. Meister, Science, 1973, 180, 33–39 CrossRef CAS PubMed.
- N. Jiang, X. Y. Yang, G. L. Ying, L. Shen, J. Liu, W. Geng, L. J. Dai, S. Y. Liu, J. Cao, G. Tian, T. L. Sun, S. P. Li and B. L. Su, Chem. Sci., 2015, 6, 486–491 RSC.
- N. Jiang, G. L. Ying, A. K. Yetisen, Y. Montelongo, L. Shen, Y. X. Xiao, H. J. Busscher, X. Y. Yang and B. L. Su, Chem. Sci., 2018, 9, 4730–4735 RSC.
- J. G. Handique and J. B. Baruah, React. Funct. Polym., 2002, 52, 163–188 CrossRef CAS.
- D. Wu, J. Zhou, M. N. Creyer, W. Yim, Z. Chen, P. B. Messersmith and J. V. Jokerst, Chem. Soc. Rev., 2021, 50, 4432–4483 RSC.
- X. Zhang, Z. Li, P. Yang, G. Duan, X. Liu, Z. Gu and Y. Li, Mater. Horiz., 2021, 8, 145–167 RSC.
- Y. Guo, Q. Sun, F. G. Wu, Y. Dai and X. Chen, Adv. Mater., 2021, 33, 2007356 CrossRef CAS PubMed.
- J. Zhou, Z. Lin, Y. Ju, M. A. Rahim, J. J. Richardson and F. Caruso, Acc. Chem. Res., 2020, 53, 1269–1278 CrossRef CAS PubMed.
- W. Zhu, J. Guo, S. Amini, Y. Ju, J. O. Agola, A. Zimpel, J. Shang, A. Noureddine, F. Caruso, S. Wuttke, J. G. Croissant and C. J. Brinker, Adv. Mater., 2019, 31, 1900545 CrossRef PubMed.
- W. Zhou, W. Zhang, W. Geng, Y. Huang, T. K. Zhang, Z. Q. Yi, Y. Ge, Y. Huang, G. Tian and X. Y. Yang, ACS Nano, 2024, 18, 10840–10849 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- C. Pan, J. Li, W. Hou, S. Lin, L. Wang, Y. Pang, Y. Wang and J. Liu, Adv. Mater., 2021, 33, 2007379 CrossRef CAS.
- H. Cao, L. Yang, R. Tian, H. Wu, Z. Gu and Y. Li, Chem. Soc. Rev., 2022, 51, 4175–4198 RSC.
- X. He, E. Obeng, X. Sun, N. Kwon, J. Shen and J. Yoon, Mater. Today Bio, 2022, 15, 100329 CrossRef CAS.
- Z. Jin, L. Yang, S. Shi, T. Wang, G. Duan, X. Liu and Y. Li, Adv. Funct. Mater., 2021, 31, 2103391 CrossRef CAS.
- H. A. Lee, E. Park and H. Lee, Adv. Mater., 2020, 32, 1907505 CrossRef CAS.
- B. Wang, G. Wang, B. Zhao, J. Chen, X. Zhang and R. Tang, Chem. Sci., 2014, 5, 3463–3468 RSC.
- H. Guo, Z. Cao, J. Li, Z. Fu, S. Lin, L. Wang and J. Liu, ACS Nano, 2023, 17, 5059–5071 CrossRef CAS PubMed.
- L. Wang, B.-B. Zhang, X.-Y. Yang and B.-L. Su, Colloids Surf., B, 2022, 214, 112454 CrossRef CAS PubMed.
- Y. Zhao, M. Fan, Y. Chen, Z. Liu, C. Shao, B. Jin, X. Wang, L. Hui, S. Wang, Z. Liao, D. Ling, R. Tang and B. Wang, Sci. Adv., 2020, 6, eaaw9679 CrossRef CAS PubMed.
- Y. Y. Yu, Y. Z. Wang, Z. Fang, Y. T. Shi, Q. W. Cheng, Y. X. Chen, W. Shi and Y. C. Yong, Nat. Commun., 2020, 11, 4087 CrossRef CAS PubMed.
- D. Vona, S. R. Cicco, R. Ragni, C. Vicente-Garcia, G. Leone, M. M. Giangregorio, F. Palumbo, E. Altamura and G. M. Farinola, Photochem. Photobiol. Sci., 2022, 21, 949–958 CrossRef CAS PubMed.
- L. Wang, Z. Y. Hu, X. Y. Yang, B. B. Zhang, W. Geng, G. Van Tendeloo and B. L. Su, Chem. Commun., 2017, 53, 6617–6620 RSC.
- Y. Liu, M. Zhang, X. Wang, F. Yang, Z. Cao, L. Wang and J. Liu, Adv. Mater., 2023, 35, 2210949 CrossRef CAS PubMed.
- C. Pan, L. Wang, M. Zhang, J. Li, J. Liu and J. Liu, J. Am. Chem. Soc., 2023, 145, 13261–13272 CrossRef CAS PubMed.
- Y. Li, Y. Miao, L. Yang, Y. Zhao, K. Wu, Z. Lu, Z. Hu and J. Guo, Adv. Sci., 2022, 9, 2202684 CrossRef CAS PubMed.
- W. Li, W. Bing, S. Huang, J. Ren and X. Qu, Adv. Funct. Mater., 2015, 25, 3775–3784 CrossRef CAS.
- H. Ejima, J. Richardson, K. Liang, J. Best, M. van Koeverden, G. Such, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed.
- C. Zhang, X. Gao, X. Ren, T. Xu, Q. Peng, Y. Zhang, Z. Chao, W. Jiang, L. Jia and L. Han, ACS Nano, 2023, 17, 6886–6898 CrossRef CAS PubMed.
- J. Pan, G. Gong, Q. Wang, J. Shang, Y. He, C. Catania, D. Birnbaum, Y. Li, Z. Jia, Y. Zhang, N. S. Joshi and J. Guo, Nat. Commun., 2022, 13, 2117 CrossRef CAS PubMed.
- B. J. Kim, S. Han, K. B. Lee and I. S. Choi, Adv. Mater., 2017, 29, 1700784 CrossRef PubMed.
- J. H. Park, K. Kim, J. Lee, J. Y. Choi, D. Hong, S. H. Yang, F. Caruso, Y. Lee and I. S. Choi, Angew. Chem., Int. Ed., 2014, 53, 12420–12425 CrossRef CAS PubMed.
- G. Fan, P. Wasuwanich, M. R. Rodriguez-Otero and A. L. Furst, J. Am. Chem. Soc., 2022, 144, 2438–2443 CrossRef CAS.
- Y. Miyamoto-Shinohara, T. Imaizumi, J. Sukenobe, Y. Murakami, S. Kawamura and Y. Komatsu, Cryobiology, 2000, 41, 251–255 CrossRef CAS PubMed.
- U. T. Bornscheuer, B. Hauer, K. E. Jaeger and U. Schwaneberg, Angew. Chem., Int. Ed., 2019, 58, 36–40 CrossRef CAS PubMed.
- P. Guo, D. Wang, S. Zhang, D. Cheng, S. Wu, X. Zuo, Y. B. Jiang and T. Jiang, Nano Lett., 2023, 23, 6386–6392 CrossRef CAS PubMed.
- M. Mahmoudi, M. P. Landry, A. Moore and R. Coreas, Nat. Rev. Mater., 2023, 8, 422–438 CrossRef PubMed.
- M. J. Harrington and P. Fratzl, Prog. Mater. Sci., 2021, 120, 100767 CrossRef CAS.
- Y. Zhang, Y. Liu, Y. Liu, P. Zuo, S. Miao, B. Hu, Y. Kang, W. Liu, Q. Yang, H. Ren and P. Yang, J. Am. Chem. Soc., 2023, 145, 17125–17135 CrossRef CAS.
- X. Zhang, C. Long, X. Zhu, X. Zhang, J. Li, J. Luo, J. Li and Q. Gao, ACS Nano , 2023, 17, 18850–18863 CrossRef CAS.
- I. Drachuk, O. Shchepelina, S. Harbaugh, N. Kelley-Loughnane, M. Stone and V. V. Tsukruk, Small, 2013, 9, 3128–3137 CrossRef CAS PubMed.
- J. Yang, J. Li, X. Li, X. Wang, Y. Yang, N. Kawazoe and G. Chen, Biomaterials, 2017, 133, 253–262 CrossRef CAS PubMed.
- R. Liu, J. Zhao, Q. Han, X. Hu, D. Wang, X. Zhang and P. Yang, Adv. Mater., 2018, 30, 1802851 CrossRef PubMed.
- A. Matsuzawa, M. Matsusaki and M. Akashi, Langmuir, 2013, 29, 7362–7368 CrossRef CAS PubMed.
- K. Liang, J. J. Richardson, C. J. Doonan, X. Mulet, Y. Ju, J. Cui, F. Caruso and P. Falcaro, Angew. Chem., Int. Ed., 2017, 56, 8510–8515 CrossRef CAS PubMed.
- J. Liu, L. Shi, Y. Deng, M. Zou, B. Cai, Y. Song, Z. Wang and L. Wang, Biomaterials, 2022, 287, 121638 CrossRef CAS PubMed.
- J. K. Sahoo, O. Hasturk, T. Falcucci and D. L. Kaplan, Nat. Rev. Chem, 2023, 7, 302–318 CrossRef CAS PubMed.
- O. Hasturk, J. K. Sahoo and D. L. Kaplan, Biomacromolecules, 2020, 21, 2829–2843 CrossRef CAS PubMed.
- S. Boddohi and M. J. Kipper, Adv. Mater., 2010, 22, 2998–3016 CrossRef CAS PubMed.
- S. Mizrahy and D. Peer, Chem. Soc. Rev., 2012, 41, 2623–2640 RSC.
- J. F. Wardman, R. K. Bains, P. Rahfeld and S. G. Withers, Nat. Rev. Microbiol., 2022, 20, 542–556 CrossRef CAS PubMed.
- E. Drula, M.-L. Garron, S. Dogan, V. Lombard, B. Henrissat and N. Terrapon, Nucleic Acids Res., 2022, 50, D571–D577 CrossRef CAS PubMed.
- M. F. Chaplin, Carbohydrate analysis, Wiley-VCH, Weinheim, 2006, pp. 243–275 Search PubMed.
- D. Su, X. Liu, L. Liu, L. Wang, H. Xie, H. Zhang, X. Meng and X. Huang, Adv. Funct. Mater., 2018, 28, 1705699 CrossRef.
- K. K. Palaniappan and C. R. Bertozzi, Chem. Rev., 2016, 116, 14277–14306 CrossRef CAS PubMed.
- V. K. Tiwari, M. K. Jaiswal, S. Rajkhowa and S. K. Singh, in Click Chemistry, ed. V. K. Tiwari, M. K. Jaiswal, S. Rajkhowa and S. K. Singh, Springer Nature Singapore, Singapore, 2024, pp. 175–203 Search PubMed.
- L. K. Mahal, K. J. Yarema and C. R. Bertozzi, Science, 1997, 276, 1125–1128 CrossRef CAS PubMed.
- E. Saxon and C. R. Bertozzi, Science, 2000, 287, 2007–2010 CrossRef CAS PubMed.
- J. A. Prescher, D. H. Dube and C. R. Bertozzi, Nature, 2004, 430, 873–877 CrossRef CAS PubMed.
- S. T. Laughlin, J. M. Baskin, S. L. Amacher and C. R. Bertozzi, Science, 2008, 320, 664–667 CrossRef CAS PubMed.
- B. Schumann, S. A. Malaker, S. P. Wisnovsky, M. F. Debets, A. J. Agbay, D. Fernandez, L. J. S. Wagner, L. Lin, Z. Li, J. Choi, D. M. Fox, J. Peh, M. A. Gray, K. Pedram, J. J. Kohler, M. Mrksich and C. R. Bertozzi, Mol. Cell, 2020, 78, 824–834 CrossRef CAS PubMed.
- J. R. Kramer, B. Onoa, C. Bustamante and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 12574–12579 CrossRef CAS PubMed.
- D. Rabuka, M. B. Forstner, J. T. Groves and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 5947–5953 CrossRef CAS PubMed.
- J. C. Jewett and C. R. Bertozzi, Chem. Soc. Rev., 2010, 39, 1272–1279 RSC.
- B. A. H. Smith and C. R. Bertozzi, Nat. Rev. Drug Discovery, 2021, 20, 217–243 CrossRef CAS PubMed.
- G. Ahn, N. M. Riley, R. A. Kamber, S. Wisnovsky, S. Moncayo von Hase, M. C. Bassik, S. M. Banik and C. R. Bertozzi, Science, 2023, 382, eadf6249 CrossRef CAS PubMed.
- R. Vanella, A. Bazin, D. T. Ta and M. A. Nash, Chem. Mater., 2019, 31, 1899–1907 CrossRef.
- A. Belluati, D. Happel, M. Erbe, N. Kirchner, A. Szelwicka, A. Bloch, V. Berner, A. Christmann, B. Hertel, R. Pardehkhorram, A. Reyhani, H. Kolmar and N. Bruns, Nanoscale, 2023, 15, 19486–19492 RSC.
- C. Jiao, C. Zhao, Y. Ma and W. Yang, ACS Nano, 2021, 15, 15920–15929 CrossRef CAS PubMed.
- S. Lin, F. Wu, Y. Zhang, H. Chen, H. Guo, Y. Chen and J. Liu, Chem. Soc. Rev., 2023, 52, 6617–6643 RSC.
- B. Hou, T. Zhang, H. Yang, X. Han, L. Liu, L. Li, C. Grazioli, X. Wu, N. Jiang and Y. Wang, Interdiscip. Mater., 2023, 2, 511–528 Search PubMed.
- W. Geng, L. Wang and X. Y. Yang, Trends Biotechnol., 2022, 40, 974–986 CrossRef CAS PubMed.
- A. Rodrigo-Navarro, S. Sankaran, M. J. Dalby, A. del Campo and M. Salmeron-Sanchez, Nat. Rev. Mater., 2021, 6, 1175–1190 CrossRef.
- Z. Li, L. Xue, J. Yang, S. Wuttke, P. He, C. Lei, H. Yang, L. Zhou, J. Cao, A. Sinelshchikova, G. Zheng, J. Guo, J. Lin, Q. Lei, C. J. Brinker, K. Liu and W. Zhu, Adv.Sci., 2024, 11, 2305126 CrossRef CAS PubMed.
- L. Wen, G. Li, T. Huang, W. Geng, H. Pei, J. Yang, M. Zhu, P. Zhang, R. Hou, G. Tian, W. Su, J. Chen, D. Zhang, P. Zhu, W. Zhang, X. Zhang, N. Zhang, Y. Zhao, X. Cao, G. Peng, X. Ren, N. Jiang, C. Tian and Z. J. Chen, Innovation, 2022, 3, 100342 CAS.
- A. Noureddine, A. Maestas-Olguin, L. Tang, J. I. Corman-Hijar, M. Olewine, J. A. Krawchuck, J. Tsala Ebode, C. Edeh, C. Dang, O. A. Negrete, J. Watt, T. Howard, E. N. Coker, J. Guo and C. J. Brinker, ACS Nano, 2023, 17, 16308–16325 CrossRef CAS PubMed.
- S. Guo, C. J. Brinker and W. Zhu, Nat. Rev. Bioeng., 2024, 2, 282–283 CrossRef CAS.
- Z. Sun, R. Hubner, J. Li and C. Wu, Nat. Commun., 2022, 13, 3142 CrossRef CAS PubMed.
- X. Wei, Y. Wang, Y. Liu, K. Ji, K. Li, J. Wang and Z. Gu, Matter, 2024, 7, 826–854 CrossRef CAS.
- S. Pospich and S. Raunser, Science, 2017, 358, 45–46 CrossRef CAS PubMed.
- Q. Lei, J. Guo, F. Kong, J. Cao, L. Wang, W. Zhu and C. J. Brinker, J. Am. Chem. Soc., 2021, 143, 6305–6322 CrossRef CAS PubMed.
- Y. Deng, T. Wu, X. Chen, Y. Chen, Y. Fei, Y. Liu, Z. Chen, H. K. Liang, J. J. Richardson, C. J. Doonan, X. Mulet, Y. Ju, J. Cui, F. Caruso and P. Falcaro, Angew. Chem. Int. Ed., 2017, 56, 8510–8515 CrossRef PubMed.
- A. Kahana and D. Lancet, Nat. Rev. Chem., 2021, 5, 870–878 CrossRef CAS PubMed.
Footnote |
| † Tong-Kai Zhang and Zi-Qian Yi contributed to this work equally. |
| This journal is © The Royal Society of Chemistry 2025 |






