Open Access Article
Open Access ArticleScaling up lignin-based polyols for PU coatings†
Leire
Lorenzo
a,
Walter
Pitacco
b,
Nour
Mattar
c,
Ibrahima
Faye
c,
Belén
Maestro
a and
Pablo
Ortiz
 *a
*a
aTECNALIA R&I, Basque Research and Technology Alliance (BRTA), Leonardo da Vinci 11, Parque Tecnológico de Alava, E-01510 Vitoria-Gasteiz, Spain. E-mail: pablo.ortiz@tecnalia.com
bAEP POLYMERS, AREA Science Park, SS 14, km 163, 5–34149 Basovizza, Trieste, Italy
cECOAT, Av. Louison Bobet 1, 06130 Grasse, France
First published on 14th January 2025
Abstract
Lignin, a promising sustainable feedstock, has been utilized to produce polyols through a novel anionic ring opening polymerization of oxiranes. This approach overcomes the limitations of lignin's heterogeneity, enabling the synthesis of aliphatic polyols with tailored properties at room temperature and atmospheric pressure. By optimization of reaction conditions, polyols with specific characteristics suited for polyurethane dispersion coatings have been achieved. Notably, the process has been successfully scaled up by a factor of 330, from 15 mL to 5 L reactors, while the desired properties have been maintained. The resulting polyols have been used to partially substitute traditional polyols in polyurethane dispersions, demonstrating their potential in wood coating applications. This breakthrough has paved the way for the large-scale production of lignin-based polyols, offering a more sustainable alternative for the coatings industry.
1. Introduction
Lignin is one of the constituents of lignocellulosic biomass, and it is particularly abundant in wood. Currently, the main source of lignin is the pulp and paper industry which employs wood as raw material to extract cellulose. In this process lignin is a by-product and has been historically considered of low value and burnt for energy and material balance. However, the lignin availability is growing1 driven by the increase in demand.2 Lignin has already multiple applications and intense research is being carried out for developing new ones.3,4One of the applications that is attracting greatest interest is the synthesis of biopolyols, thanks to the hydroxyl functionality of lignin.5,6 However, lignin is a heterogeneous biomacromolecule, and contains, among other functional groups, phenolic and aliphatic hydroxyl groups, whose different reactivity affects the polymerization, for example the polyurethane reaction.7 Therefore, making a homogeneous polyol from lignin has been pursued by researchers. The principal route for this conversion is the oxyalkylation reaction, in which lignin is reacted with alkylene oxides (e.g., propylene oxide).8–11 The oxyalkylation is typically done at harsh reaction conditions, leading to degradation and bad odor.
There is an alternative to the anionic ring opening polymerization, the cationic ROP, that has been recently developed by Tecnalia.12–15 This method allows room temperature and no pressure synthesis of lignin polyols (LPO) (Scheme 1).
The various reports for lignin polyol (LPO) synthesis target gram synthesis, and few reports go to multigram scale.8 Here we report to what is, to the best of our knowledge, the first report on the production of LPOs at industrially relevant environment, using 5 L batch reactors and obtaining 1–1.5 kg of product. Moreover, we show a proof of concept of the application of the synthesized polyols in polyurethane dispersion (PUD) coatings for wood in a sector with high interest in sustainable solutions.16
2. Experimental
2.1. Materials
Lineo ® Lignin was kindly provided by Stora Enso. For lab-scale synthesis butylene oxide (BO, 99%) was purchased from TCI, boron trifluoride etherate (BF3OEt2, 48%) was purchased from Acros Organic, tetrahydrofuran (THF, 99%) and N,N-dimethylethylamine (99%) were purchased from ThermoFisher Scientific. For the up-scaling, all the reagents (with same purity as those used at lab-scale) were purchased from Sigma Aldrich.2.2. Methods
2.2.2.1. Lab-scale synthesis of LPO. The synthesis was carried out in a 500 mL four-necked glass jacketed reactor equipped with a mechanical stirrer at room temperature and at atmospheric pressure (close reactor without inert atmosphere). Previously, the lignin was dried at 55 °C in a vacuum oven (under 10 mbar pressure) for 24 h. Then, a solution of the lignin in tetrahydrofuran (THF) was placed into the reactor, and the desired amount of catalyst was added under stirring. Next, the stablished amount of the selected oxirane was added continuously using a syringe pump at 73 mL of oxirane per lignin hydroxyl groups per hour. Once oxirane addition was completed the reaction was kept under stirring for an additional 1–2 h of post-addition time to ensure its total conversion. Finally, the reaction was quenched with an excess of N,N-dimethylethylamine (1.66 equivalents referred to the catalyst) and the solvent was removed in a rotary evaporator until constant weight yielding the desired LBP, generally, as a dark brown viscous liquid. The lignin content was determined as the mass of the lignin used divided by the mass of the final polyol.
2.2.2.2. Scale-up of LPO synthesis. Based on the lab-scale process, the syntheses were carried out in three-necked glass reactors of increasing sizes (from 0.25 mL to 5 L) equipped with a mechanical stirrer at room temperature and atmospheric pressure (close reactor without inert atmosphere). Lignin, previously dried at 50 °C in a drying oven, was added into the reactor, then blended with tetrahydrofuran under moderate stirring. Once the reaction mixture was homogeneous, the catalyst was added under stirring at room temperature. Then, butylene oxide was added continuously at 73 mL of oxirane per lignin hydroxyl groups per hour. The temperature of the reaction was controlled to stay below 30 °C through a refrigerated bath circulator. Once completed the oxirane addition, the reaction was kept under stirring for an additional 1 h to ensure its total conversion. Finally, the catalyst was quenched with an excess of N,N-dimethylethylamine (1.66 equivalents referred to the catalyst) and the solvent was removed under reduced pressure at 60 °C yielding the desired LPO.
2.2.2.3. Waterborne alkyd-polyurethane dispersions (PUD) based on LPO. Preparation of an alkyd resin (the pre-polymer): A typical alkyd resins consists of polyhydric alcohols (3 or more hydroxyls per molecule), polybasic acids, and monobasic fatty acids (saturated and unsaturated). The mixture is heated up to 220–240 °C under an inert atmosphere to initiate the esterification reaction. Reaction progress was checked by acid number determination according to ASTM D4462-98 until an acid number of below 10 was achieved. The alkyd resin used for this study had a short oil length and a bio-based content up to 60%.
Polyurethane modification: The obtained alkyd resin (45.3%) was combined with the LPO (13.2%) and are stirred at 90 °C for an hour under vacuum in order to remove moisture that may react with isocyanate. The mixture was then cooled down to 50 °C and a cooling column is fixed to the reactor before adding dimethylolpropionic acid (DMPA). Acetone was then added to the mixture to dissolve the component all together and to control the viscosity of the mixture. The mixture was stirred for about 15 minutes before adding the neutralizing agent, the Triethylene amine (TEA). This latter is used to neutralize the carboxylic acid groups of DMPA, resulting in the formation of carboxylates salt. The carboxylates enhance the hydrophilicity of the PUD, improving its compatibility with water and facilitating the formation of a stable and finely dispersed product. The TEA was left to stir for approximatively 15 to 30 minutes. Isophorone diisocyanate (IPDI) was then added. At this stage, the reaction was allowed to proceed for 8 hours at 50 °C. The last step is the dispersion of polyurethane anionomers, in which deionized water was added slowly with a peristaltic pump under constant agitation. This process should be carried out for approximately one hour to ensure proper dispersion. An aqueous dispersion of 40 wt% solids was obtained after removal of the solvent and filtration of the dispersion.
2.2.3.1. Lab-scale LPO. The hydroxyl (OH) value was determined according to American Society for Testing and Materials (ASTM) E-1899-02 standard using THF as a solvent in a 702 SM Titrino equipment from Metrohm (Herisau, Swizerland). Gel permeation chromatography (GPC) analysis was done with Knauer Azura GPC machine equipped with refractive index detector and couple to a RESIPORE column of 300 mm of length, 7.5 mm of diameter and 3 μm of particle size. The temperature of the column, as well as the detector was set to 40 °C. The mobile phase was THF at 1 mL min−1 flow and the calibration curve was done using PS standards in the range of 162–278
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da. Both the samples and the standards to construct the calibration curves were prepared in THF at a 2000 mg L−1 concentration. The hydroxyl and carboxylic acid functionalities were determined by 31P NMR based on the method developed by Argyropoulos.18 It was measured immediately after preparation at 30 °C on a 300 Bruker AVANCE III HD spectrometer under Topspin 3.5 and equipped with a Bruker bbfo z-gradient 5 mm probe head. Chemical shifts are reported relative to the sharp signal (132.2 ppm) originating from the reaction between water and phosphorylating agents. Following NMR parameters were used: scans = 1024, pulse delay = 5 s, 90° pulse and line broadening = 2 and default baseline correction.
000 Da. Both the samples and the standards to construct the calibration curves were prepared in THF at a 2000 mg L−1 concentration. The hydroxyl and carboxylic acid functionalities were determined by 31P NMR based on the method developed by Argyropoulos.18 It was measured immediately after preparation at 30 °C on a 300 Bruker AVANCE III HD spectrometer under Topspin 3.5 and equipped with a Bruker bbfo z-gradient 5 mm probe head. Chemical shifts are reported relative to the sharp signal (132.2 ppm) originating from the reaction between water and phosphorylating agents. Following NMR parameters were used: scans = 1024, pulse delay = 5 s, 90° pulse and line broadening = 2 and default baseline correction.
LPO composition: There are three components in the final LPOs: lignin, butylene oxide and copolymerized solvent (THF) (Scheme 1). To determine the composition of the LPO, excess solvent was removed until a constant weight of the LBP was obtained. The lignin wt% in the LPO was calculated by dividing the initial mass of lignin employed in the reaction by the total weight of the LPO obtained and multiplying the result by 100. The BO weight content in the LPO (BO wt%) was calculated by dividing the difference between the BO weight used in the reaction and the unreacted BO mass by the total weight of LBP obtained and multiplying the result by 100. Copolymerized THF contents (THF wt%) were calculated by subtracting the sum of the two above amounts from 100.
2.2.3.2. Upscaling of LPOs. The hydroxy value was determined according to ASTM D4274 acetylation method using a Mettler Toledo automatic titrator. Gel permeation chromatography (GPC) analysis was performed with a Hitachi Chromaster system coupled to a Tosoh TSKgel column (150 mm length, 6 mm diameter, 3 μm particle size). The temperature of the column, was set to 40 °C. The mobile phase was THF at 0.4 mL min−1 flow and the calibration curve was based on PS standards in the range of 162–22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 Da. Both the samples and the standards were prepared in THF. The viscosity was measured with a Fungilab Smart Series rotational viscometer equipped with a small-sample adapter connected to a thermostatic bath set at 25 °C. The THF content was determined as the total volatile content by thermogravimetric analysis at 80 °C for 60 minutes carried out using a Kern DBS 60-3 thermobalance.
790 Da. Both the samples and the standards were prepared in THF. The viscosity was measured with a Fungilab Smart Series rotational viscometer equipped with a small-sample adapter connected to a thermostatic bath set at 25 °C. The THF content was determined as the total volatile content by thermogravimetric analysis at 80 °C for 60 minutes carried out using a Kern DBS 60-3 thermobalance.
2.2.3.3. Waterborne alkyd-polyurethane dispersions (PUD) based on LPO. After ensuring that the developed dispersions are in the correct pH range (6.5–8) and Brookfield viscosity (<5000 cPs), the PUD performance is determined by the properties of the coating film. One coating of each type of resin was prepared following this procedure: An iron based drier (0.3% based on the dispersion at 40% solid content) was added into the PUD under stirring 15 minutes. The mixture is left overnight then 100 μm thick wet films were applied on glass plates for tack free time measurement and on metal plates for other tests (Adhesion, Flexibility, Impact resistance)
• Tack free time: Tack-free refers to the amount of time it takes for a coating to dry on the surface after its application. It is the moment when a coating is completely dry with little or no moisture left after application on the surface to be protected.
• Adhesion: ASTM D3359 standard is used. This test method covers procedures to assess the adhesion of coating films to metallic substrates. This is done by applying and removing pressure-sensitive tape over cuts made in the film.
• Flexibility: To determine the flexibility of the coatings films, a conical mandrel is used according to ASTM D522 standard. The cured coated films are bent over a conical mandrel and the resistance to cracking of the coating is determined.
• Impact resistance: This test consists of determining the resistance of coatings to the effects or rapid deformation and is based on ASTM D2794 standard. During the test, a flat-coated panel is placed under a weighted spherical ball assembly. Then the weighted ball is dropped onto the panel from different heights. The impact causes a dimple to form in the test panel, and it is examined visually or with a ten-power lens to determine the extent of cracking or other failures.
3. Results and discussion
3.1. LPO synthesis at lab-scale
The target specifications for the LPO were those of the current polyol used by in the alkyd PUD. The commercial polyols used by ECOAT for the manufacturing of PUD have an OH value between 60 and 200 mg KOH per g and a molecular mass ranging between 500 and 2000 g mol−1. Hence, these specifications were also set for the LPO. The relatively low Mw of the target LPO and the high Mw (1318) of the starting kraft lignin (KL) (Stora Enso Lineo®) made this process unachievable, considering that the polyol synthesis involves grafting polyether chains on the lignin molecules, thus increasing their molecular weight, at least 3-fold and up to 30-fold.15 However, maintaining an appropriate molecular weight range is crucial to ensure the desired film formation and drying characteristics of the PUD. Consequently, a fractionation step had to be introduced. The fractionation with pure ethanol (FKL100) resulted in a soluble lignin with lower molecular weight, lower polydispersity (D) and higher OH value (Table 1).The optimization of the experiments was focused on obtaining a low Mw polyol, as the target OH range was relatively broad (Table 2). The experiments began with testing different lignin concentrations, which is linked with the abundance of OH groups in the reaction mixture (LPO 1–3). From these, the concentration of 165 g l−1 was chosen as the best as it gave the lowest Mw. An experiment to synthesize an LPO with lower amount of butylene oxide (BO) was carried out (LPO4) since it is known to decrease the Mw.15 It indeed resulted in a lower Mw, but due to an incomplete reaction. This could be visually seen (granular instead of liquid) and was also confirmed by both NMR and OH value, being the later higher than the original lignin. LPO5 was run with lower amount of catalyst, and this led to a liquid, lower Mw polymer with good Mw. However, these reaction conditions proved to be on the verge of the viability of the reaction, and the reproducibility was low, leading in some cases to a partial reaction like that witnessed with LPO4 (see ESI†). Therefore, a longer post addition time (2 hours instead of one) was implemented to allow longer time for the reaction to be completed, but it led to no improvement. Finally, a catalyst loading in between the standard and the reduced (LPO5) was tested in combination with two-hour post-addition time (LPO6). In the best result obtained, LPO2, a slightly higher than 30% of lignin was incorporated in the LPO. It has to be noted that if higher percentages are attempted, solid products are obtained, which cannot be further processed.
| Parametersa | LPO1 | LPO2 | LPO3 | LPO4 | LPO5 | LPO6 |
|---|---|---|---|---|---|---|
| a FKL100 lignin. Addition rate (mL BO per mol OH-L per h) = 73. Scale of experiments: 1–2 g lignin, 100 mL reactor. b See section 2.2.3.1 for the formula used. c Determined by GPC. d Determined by titration. | ||||||
| Lignin concentration (g L−1) | 110 | 165 | 220 | 165 | 165 | 165 |
| BO/OH-L molar ratio | 1 | 1 | 1 | 0.5 | 1 | 1 |
| % of mol of OH in lignin per mol THF | 6 | 9 | 12 | 9 | 9 | 9 |
| Molar ratio BF3/OH-L | 0.125 | 0.125 | 0.125 | 0.125 | 0.03 | 0.06 |
| Temperature (°C) | 25 | 25 | 25 | 25 | 25 | 25 |
| Post addition time | 1 | 1 | 1 | 1 | 1 | 2 |
| Lignin mass in polymerb (%) | 24 | 32 | 25 | 29 | 19 | 28 |
| BO massb (%) | 12 | 16 | 12 | 7 | 9 | 14 |
| THF massb (%) | 64 | 52 | 64 | 64 | 71 | 58 |
Polymer Mw![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (g mol−1) c (g mol−1) |
5148 | 3075 | 4514 | 2557 | 2007 | 4145 |
| Polymer D | 3.9 | 3.2 | 4.0 | 3.8 | 2.6 | 3.5 |
| OH valued | 66 | 103 | 99 | 427 | 146 | 108 |
3.2. LPO scale-up
For the larger scale synthesis of the LPOs greater quantities of fractionated lignin were needed. Fractionated lignin was supplied by the Technical Research Centre of Finland (VTT), who performed it at pilot scale.17 Contrary to the method used at lab-scale in TECNALIA, the process implemented at VTT used a 65/35 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture. The resulting lignin (FKL65) had the following characteristics: a Mw of 1380, a PD of 1.7 and a OH value of 7.72 mmol g−1. The fact that these numbers do not perfectly fit between the values of Lineo® and FKL100 are mainly due to the inherent variability of the different lignin batches.
water mixture. The resulting lignin (FKL65) had the following characteristics: a Mw of 1380, a PD of 1.7 and a OH value of 7.72 mmol g−1. The fact that these numbers do not perfectly fit between the values of Lineo® and FKL100 are mainly due to the inherent variability of the different lignin batches.
AEP Polymers undertook the upscaling of the most promising result, LPO2, and the result, LPO7, showed less lignin incorporation, higher Mw, PD and lower OH value, which can be explained by the fact that the FKL65 had itself higher Mw, PD and OH value than FKL100 (Table 3). To try to reduce the Mw, the previous strategy of lowering the catalyst loading was investigated. LPO8, analogous to previously tested LPO5 resulted in a solid product due to only partial reaction. Then a catalyst loading between that of unsuccessful LPO8 and successful LPO7 was explored in LPO9 and LPO10. LPO10 with a catalyst loading of 0.08 (molar ratio BF3/OH groups in lignin) gave a good result and was scaled up at 1 L (LPO11).
| Parametera | LPO2c | LPO7 | LPO8 | LPO9 | LPO10 | LPO11 |
|---|---|---|---|---|---|---|
| a BO/OH-L molar ratio 1, % of mol of OH in lignin per mol THF = 9, reaction temperature: r.t., addition rate (mL BO per mol OH-L per h) = 73. b Scale of the reactor. c The slight difference between the OH values, Mw and D reported for LPO2 from those shown in Table 2 is due to being measured by different equipment. | ||||||
| Lignin | FKL100 | FKL65 | FKL65 | FKL65 | FKL65 | FKL65 |
| Scaleb (mL) | 100 | 500 | 500 | 250 | 250 | 1000 |
| Molar ratio BF3/OH-L | 0.125 | 0.125 | 0.06 | 0.1 | 0.08 | 0.08 |
| Post addition time | 1 | 1 | 2 | 1 | 1 | 1 |
| Lignin mass in polymer (%) | 32 | 26 | 47 | 28 | 30 | 28 |
| Polymer Mw (g mol−1) | 2961 | 4347 | N.D. | 3243 | 3023 | 3325 |
| Polymer D | 4.2 | 5.2 | N.D. | 4.5 | 4.5 | 4.7 |
| OH value | 96 | 88 | 164 | 98 | 108 | 94 |
LPO11 exhibited for the first time a minor – although measurable – exothermal effect reaching 40 °C due to the higher scale of the experiment. This catalysed the reaction further with respect to smaller scale experiments, leading to a slight increase in the molecular weight and a lower lignin content. Whereas on the small-scale the exothermal heat of reaction was not observed, the progressive increase of batch size made it appreciable. As enthalpy of reaction speeds up kinetic of this polymerization, this can be the root cause of significantly higher molecular weight than expected. Therefore, for further upscaling the temperature of the reaction was controlled thanks to the cooling jacket. In the reaction temperature profile for experiment LPO13 a maximum temperature of 30 °C can be seen after the end of the addition of the epoxide (Fig. 1). The peak at the end of the reaction (around 2 hours) correspond to the neutralisation of the catalyst.
The upscaling of the was carried out performing the synthesis on 2 L and 5 L reactors (Table 4), reaching a scale larger than 330 times the initial lab-scale experiments (15 mL).
| LPO | Lignin fraction | Reaction scale | Lignin mass in polymer (%) | OH value (mg KOH per g) | M w (g mol−1) | Polymer D |
|---|---|---|---|---|---|---|
| Reaction conditions: BO/OH-L molar ratio 1, % of mol of OH in lignin per mol THF = 9, molar ratio BF3/OH-L 0.08, reaction temperature: r.t., addition rate (mL BO per mol OH-L per h) = 73. | ||||||
| LPO12 | FKL65 | 2 L reactor | 30% | 106 | 2881 | 4.3 |
| LPO13 | FKL65 | 5 L reactor | 32% | 109 | 2609 | 3.9 |
In terms of shape, geometry and configuration, the 5 L reactor system used is representative of bigger stirred batch reactors used in pre-industrial and industrial applications (Chart 1). Moreover, an optimisation was also performed in the solvent removal step, and instead of using the rotary evaporator (as in the lab-scale protocol), the solvent was distilled directly from the reactor in a more efficient way, avoiding crude reaction transfer that could prevent quantitative recovery of the product.
In order to have another quality control parameter in addition to OH value and lignin content, viscosity was selected due to its simple execution and widespread use in the chemical industry. On the other hand, viscosity is clearly affected by the presence of residual solvent (THF). In the polyol synthesis, THF acts both as reagent and as solvent, and therefore the unreacted THF at the end of the reaction needs to be removed to obtain the pure polyol and to recover and recycle the solvent.
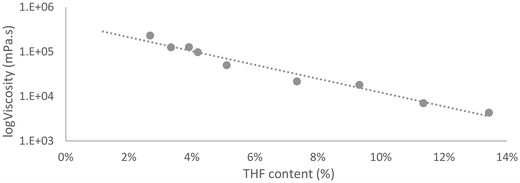
The residual THF is difficult to remove completely, but nevertheless, the comparison with the graph obtained for various repetitions of the definitive protocol with different THF residual content, can be useful for qualitative determination of the product obtained knowing the volatile content (Fig. 2).
The content of unreacted THF after the end of butylene oxide addition, was also used to qualitatively monitor the reaction. As discussed THF acts both as reagent and as solvent, therefore the reaction can be followed by measuring the reduction of the THF content. At the beginning of the reaction, THF is 80 wt% of the reaction mixture and then it is gradually incorporated in the product. When the volatile content is above 70 wt% the reaction is incomplete, and the product is a solid (as observed in LPO8). If the residual THF content is around 40 wt% a higher Mw than desired is obtained. A THF content of 52 ± 1%, corresponding to around 40% conversion of the initial THF, was identified as the value that gave the required final properties in terms of OH value and Mw. This can be therefore considered as a simple method to follow the reaction even if the results are susceptible to minor errors due to the volatile nature of THF and should be used only as an indication to be confirmed through the other analytical methods previously described.
As a summary of this section, the protocol for the synthesis of LPOs was successfully adapted from lab-scale (15–500 mL reactors) to 2–5 L reactors. The challenges encountered in this transfer and the approach followed to overcome them are summarized in Table 5.
| Challenges | Impact | Improvement in scaled-up process |
|---|---|---|
| Heat generated by the exothermic reaction | Increase in temperature catalyzes the reaction further than desired producing higher molecular weights | Controlling the temperature through a cooling jacket fed by a refrigerated bath circulator allowed to maintain the properties within the requirements |
| Monitoring the reaction using a method simpler than P-NMR and GPC | Missing an in-process control method, fixed reaction times were used without considering minor inter-batch variations and leading to replicability issues | THF content was identified as a possible parameter to monitor the reaction based on comparison with collected data |
| Avoid losses of material caused by the high viscosity of the LPO | Multiple steps involving raw materials and LPO transfer resulted in loss of yield | Integration of the process, combining all the steps sequentially in the same reactor, led to higher efficiency |
3.3. LPO-based PUD
With the obtained LPO different percentages of substitution of the reference polyol in the PUD were attempted and a 22.5% substitution was chosen since higher percentages led to gelation. The substitution of the reference polyol by the LPO resulted in a brown PUD (Chart 2), significantly more viscous and with higher gloss (Table 6).The PUD were applied on wood (Chart 3) and their properties measured. The addition of LPO did not lead to significant differences in certain key performance parameters. Specifically, the comparison showed similarity in terms of tack-free time, adhesion on metal, flexibility, and impact resistance (see ESI†). It is noteworthy that both the reference PUD and the PUD with LPO exhibited similar drying characteristics, with both formulations becoming tack-free after 30 minutes in the presence of a drier. Also, the results showed that both formulations achieved the same adhesion class (Class 0), indicating excellent adhesion to metal surfaces. Flexibility is a crucial property in coatings, especially in wood applications for instance. In this case, the results suggest that both the reference PUD and the PUD with LPO exhibit a similar level of flexibility, indicating that the addition of LPO did not compromise this aspect. Surprisingly, the impact resistance of both formulations was reported to be less than 5 cm, suggesting that the addition of LPO did not lead to an improvement in impact resistance.
4. Conclusions
We have proven that lignin-derived polyols can be produced at kilogram scale, demonstrating the viability of the upscaling and confirming the robustness of the developed protocol. The process was validated in an industrially relevant environment considering the reactor geometry, the fully controlled temperature (monitoring the reaction, heating and cooling during distillation) and the fact that that the upscaled process is integrated, with all the steps sequentially combined.Lignin polyols could partially replace standard polyols used in alkyd-PUD systems. However, there was an important limitation on the percentage that could be used due to the gelation effect caused by the LPO. In the future, depolymerized lignin might be available in large quantities and with its lower molecular weight is likely to reduce the viscosity of the resulting LPO.
Current efforts are ongoing in further exploring the application of the LPOs for PUD coatings.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was funded by the Bio-Based Industries Joint Undertaking (JU) under the European Union's Horizon 2020 research and innovation programme through the LIGNICOAT project (grant agreement No. 101023342). The JU receives support from the European Union's Horizon 2020 research and innovation programme and the Bio-based Industries Consortium. We kindly acknowledge Stora Enso for providing the Lineo® lignin and VTT (Technical Research Centre of Finland) for providing the fractionated lignin.References
- L. Dessbesell, M. Paleologou, M. Leitch, R. Pulkki and C. Xu (Charles), Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers, Renewable Sustainable Energy Rev., 2020, 123, 109768, DOI:10.1016/j.rser.2020.109768.
- P. C. A. Bruijnincx, R. Rinaldi and B. M. Weckhuysen, Unlocking the Potential of a Sleeping Giant: Lignins as Sustainable Raw Materials for Renewable Fuels, Chemicals and Materials, Green Chem., 2015, 17(11), 4860–4861, 10.1039/C5GC90055G.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact, Ind. Crops Prod., 2019, 139, 111526, DOI:10.1016/j.indcrop.2019.111526.
- O. Yu and K. H. Kim, Lignin to Materials: A Focused Review on Recent Novel Lignin Applications, Appl. Sci., 2020, 10(13), 4626, DOI:10.3390/app10134626.
- S. Mastrolitti, E. Borsella, A. Giuliano, M. T. Petrone, I. D. Bari, R. J. A. Gosselink, G. v. Erven, E. Annevelink, K. S. Traintafyllidis and H. Stichnothe, Sustainable Lignin Valorization: Technical Lignin, Processes and Market Development, 2021 Search PubMed.
- M. H. Tran and E. Y. Lee, Production of Polyols and Polyurethane from Biomass: A Review, Environ. Chem. Lett., 2023, 21, 2199–2223, DOI:10.1007/s10311-023-01592-4.
- M. Rubens, M. V. Wesemael, E. Feghali, L. L. Lufungula, F. Blockhuys, K. Vanbroekhoven, W. Eevers and R. Vendamme, Exploring the Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Lignin Hydrogenolysis Oil towards Urethane Bond Formation, Ind. Crops Prod., 2022, 180, 114703, DOI:10.1016/j.indcrop.2022.114703.
- A. Duval, D. Vidal, A. Sarbu, W. René and L. Avérous, Scalable Single-Step Synthesis of Lignin-Based Liquid Polyols with Ethylene Carbonate for Polyurethane Foams, Mater. Today Chem., 2022, 24, 100793, DOI:10.1016/j.mtchem.2022.100793.
- C. A. Cateto, M. F. Barreiro, A. E. Rodrigues and M. N. Belgacem, Ind. Eng. Chem. Res., 2009, 48(5), 2583–2589, DOI:10.1021/ie801251r.
- H. Nadji, C. Bruzzèse, M. N. Belgacem, A. Benaboura and A. Gandini, Oxypropylation of Lignins and Preparation of Rigid Polyurethane Foams from the Ensuing Polyols, Macromol. Mater. Eng., 2005, 290(10), 1009–1016, DOI:10.1002/mame.200500200.
- J. P. S. Aniceto, I. Portugal and C. M. Silva, Biomass-Based Polyols through Oxypropylation Reaction, ChemSusChem, 2012, 5(8), 1358–1368, DOI:10.1002/cssc.201200032.
- J. R. Ochoa Gómez, J. Pérez Arce, B. Maestro Madurga and E. J. García Suárez, Lignin-Based Polyols, WO2020109460, June 4, 2020.
- J. Perez-Arce, A. Serrano, J.-L. Dauvergne, A. Centeno-Pedrazo, S. Prieto-Fernandez, E. Palomo Del Barrio and E. J. Garcia-Suarez, Sustainable Lignin-Based Polyols as Promising Thermal Energy Storage Materials, J. Appl. Polym. Sci., 2021, 138(46), 51356, DOI:10.1002/app.51356.
- J. Perez-Arce, A. Centeno-Pedrazo, J. Labidi, J. R. Ochoa-Gómez and E. J. Garcia-Suarez, A Novel and Efficient Approach to Obtain Lignin-Based Polyols with Potential Industrial Applications, Polym. Chem., 2020, 11(46), 7362–7369, 10.1039/D0PY01142H.
- J. Perez-Arce, A. Centeno-Pedrazo, J. Labidi, J. R. Ochoa-Gomez and E. J. Garcia-Suarez, Lignin-Based Polyols with Controlled Microstructure by Cationic Ring Opening Polymerization, Polymers, 2021, 13, 651, DOI:10.3390/polym13040651.
- G. Pourhashem, Coating a Sustainable Future, Coatings, 2020, 10(8), 713, DOI:10.3390/coatings10080713.
- A. Ghosh, O. Fearon, M. Agustin, S. Alonso, E. Cámara Balda, S. Franco and A. Kalliola, Fractionation of Kraft Lignin for Production of Alkyd Resins for Biobased Coatings with Oxidized Lignin Dispersants as a Co-Product, ACS Omega, 2024, 9(46), 46276–46292, DOI:10.1021/acsomega.4c07187.
- X. Meng, C. Crestini and H. Ben, et al., Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy, Nat. Protoc., 2019, 14, 2627–2647, DOI:10.1038/s41596-019-0191-1.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lp00328d |
| This journal is © The Royal Society of Chemistry 2025 |